Tissue-specific regulation of SIRT1 by calorie restriction (original) (raw)
Abstract
Calorie restriction (CR) has been reported to increase SIRT1 protein levels in mice, rats, and humans, and elevated activity of SIRT1 orthologs extends life span in yeast, worms, and flies. In this study, we challenge the paradigm that CR induces SIRT1 activity in all tissues by showing that activity of this sirtuin in the liver is, in fact, reduced by CR and activated by a high-caloric diet. We demonstrate this change both by assaying levels of SIRT1 and its small molecule regulators, NAD and NADH, as well as assessing phenotypes of a liver-specific SIRT1 knockout mouse on various diets. Our findings suggest that designing CR mimetics that target SIRT1 to provide uniform systemic benefits may be more complex than currently imagined.
Keywords: Aging, calorie restriction, SIRT1
Caloric intake influences life span, and the incidence of diseases in animals (Koubova and Guarente 2003). Food excess accounts for the recent historic increase in metabolic disorders in humans. Conversely, calorie restriction (CR) promotes metabolic fitness, long life, and disease protection in rodent models (Weindruch 1988). Several genetic pathways have been identified that govern diet, metabolism, and life span (Van Remmen et al. 2001; Koubova and Guarente 2003; Kenyon 2005; Sinclair 2005).
Genes related to yeast SIR2, called sirtuins, encode NAD-dependent deacetylases, and promote longevity in yeast, worms, and flies (Chen and Guarente 2007). In model systems ranging from yeast to mice, sirtuins have also been associated with the salutary effects of CR. The mammalian Sir2 ortholog SIRT1 targets numerous regulatory factors affecting stress management and metabolism (Sinclair 2005; Chen and Guarente 2007). The levels of SIRT1 have been reported to increase in rodent and human tissues in response to CR (Cohen et al. 2004; Nisoli et al. 2005; Civitarese et al. 2007), and this increase is proposed to cause favorable changes in metabolism and stress tolerance triggered by this diet. The polyphenol resveratrol has also been proposed to partially mimic CR by activating SIRT1 to induce beneficial effects on health (Baur et al. 2006; Lagouge et al. 2006).
Results and Discussion
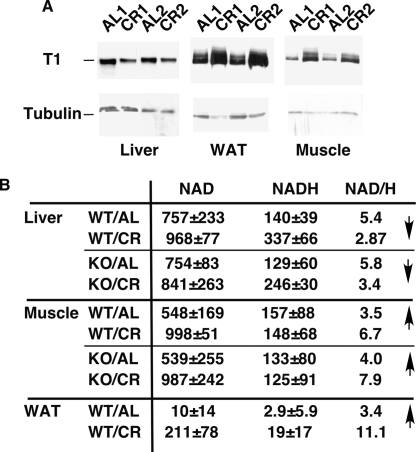
To address the relationship between SIRT1 activity and the diet, we first analyzed SIRT1 protein levels in the liver, white adipose tissue (WAT), and skeletal muscle in mice fed ad libitum (AL) or CR. While SIRT1 was induced in the WAT and muscle, as previously reported (Cohen et al. 2004; Nisoli et al. 2005; Civitarese et al. 2007), levels surprisingly were lower in CR liver (Fig. 1A). It is not clear whether the increased SIRT1 expression in CR liver reported by Cohen et al. (2004) is specific to rat or to the time point when the tissue samples are removed from animals after daily feeding. Because SIRT1 activity is also regulated by the NAD/NADH ratio (Lin et al. 2004), we determined NAD and NADH levels in these tissues. Whereas the NAD/NADH ratio increased significantly in the muscle during CR, it decreased in the liver (Fig. 1B). In the WAT, both NAD and NADH were found in extremely low amounts in AL mice, and there was a striking increase in their absolute levels in CR, suggesting an up-regulation of metabolic activity. These findings suggest that SIRT1 activity in the liver may decrease in CR, opposite to what occurs in the muscle and the WAT. Very similar changes in NAD and NADH were observed in the same tissues derived from whole-body SIRT1 KO mice (Fig. 1B), indicating that diet-induced metabolic changes that influence NAD and NADH occur upstream of SIRT1, similar to what was observed in the case of yeast Sir2 (Lin et al. 2002).
Figure 1.
Differential regulation of SIRT1 in the tissues of CR mice. (A) The expression of SIRT1 is upregulated in the muscle and WAT but downregulated in the liver of CR mice. The expression of SIRT1 in the liver, muscle, and WAT of mice fed ad libtum or calorie restricted was determined by Western blotting with the anti-SIRT1 antibody. Tubulin was used as a loading control. (B) The NAD/NADH ratio is increased in the muscle and WAT but decreased in the liver of CR mice. Note that levels of both NAD and NADH are increased in WAT by CR. The NAD and NADH concentrations in the liver, muscle, and WAT of wild-type and SIRT1 knockout mice fed ad libitum or calorie restricted are expressed as nanomole per gram of tissue.
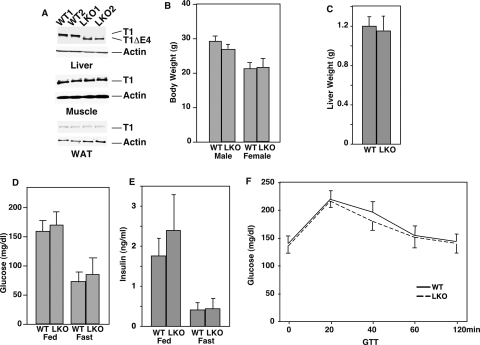
We sought to devise a functional test of the hypothesis that the activity of SIRT1 in the liver is directly proportional to caloric intake. This hypothesis would predict that the elimination of SIRT1 in the liver would generate an extreme phenotype under dietary conditions in which this sirtuin is normally most active (i.e., high caloric intake), but would be without a strong phenotype under conditions where it is least active (i.e., low caloric intake). Thus, we bred a SIRT1 allele containing a floxed exon 4 (encoding a catalytic domain of the protein) (Cheng et al. 2003) into the C57/BL6 background. Crossing this floxed allele to a whole-body Cre expressing mouse recapitulated the SIRT1-null phenotype (Cheng et al. 2003). Subsequent crossing to C57 mice expressing Cre under the liver-specific albumin promoter generated littermates that were homozygous for this SIRT1 allele and expressed Cre. Tissues of these mice showed the loss of the SIRT1 protein and the appearance of a slightly smaller protein missing exon 4 specifically in the livers of Cre-expressing mice, compared with littermates homozygous for the floxed allele but not expressing Cre (wild type) (Fig. 2A). Unlike the whole-body SIRT1 KO mice, liver-specific KO mice (LKO) did not display any overt phenotype; i.e., the LKO mice showed no difference in body weight, liver weight, blood insulin, blood glucose, or glucose tolerance on the standard AL chow diet (Fig. 2B–F).
Figure 2.
SIRT1 liver-specific knockout mice have no overt phenotype when fed a chow diet. (A) SIRT1 is specifically knocked out in the livers of SIRT1 LKO mice. Expression of SIRT1 in the liver, the muscle, and the WAT was detected by Western blotting with an anti-SIRT1 antibody. Actin was used as a loading control. (B–F) Body weight, liver weight, blood glucose, and insulin levels (fed and fasted), and glucose tolerance were compared between wild-type and SIRT1 LKO mice on a chow diet.
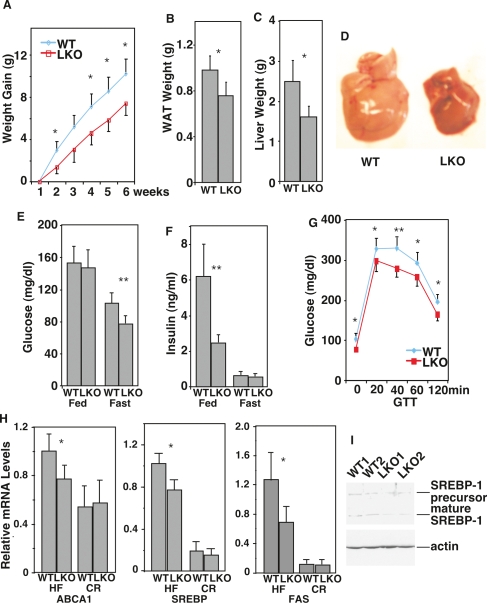
Next we tested the LKO mice on a high-calorie/high-fat Western diet versus a CR diet. LKO mice showed a highly significant difference on the Western diet compared with wild type (Fig. 3). Weight gain over time, which is indicative of accumulation of body fat, was reduced in LKO mice (Fig. 3A), even though food intake was not affected (data not shown). Correspondingly, the accumulation of fat in the WAT and the liver was significantly reduced in LKO mice (Fig. 3B–D). In addition, the LKO mice were protected from the physiological decline induced by the Western diet in wild-type mice; they were more glucose tolerant and had lower levels of blood glucose and insulin (Fig. 3E–G). We conclude that LKO mice are at least partially protected from fat accumulation and accompanying metabolic deficits on the Western diet. The SIRT1 whole-body KO mice on a high-fat diet are also protected from body weight gain and fat accumulation in the liver (X. Li and L. Guarente, unpubl.), consistent with our findings using the LKO mice.
Figure 3.
SIRT1 LKO mice are protected from physiological decline when fed a high-fat diet. (A–G) Body weight gain over time, WAT weight, liver weight, blood glucose, and insulin levels (both fed and fasted) and glucose tolerance were compared between wild-type and LKO mice on a high-fat diet. (H) Expression of ABCA1, FAS, and SREBP1c were compared between wild-type and LKO mice fed a high-fat diet or a CR diet by QRT–PCR. (I) Expression of SREBP1c was compared between wild-type and LKO mice fed a high-fed diet by Western blotting. Statistics was calculated by Student’s _t_-test. (*) P < 0.05; (**) P < 0.01.
We next investigated the mechanism by which LKO mice are resistant to effects of the Western diet. A prior study showed that the nuclear receptor LXR was deacetylated and activated by SIRT1 (Li et al. 2007). LXR is most active in the liver in a high-calorie diet. Indeed, the LXR targets, the ABCA1 transporter involved in cholesterol transport to blood LDL, and SREBP1c, the master regulator of fat synthesis, were not fully activated in LKO mice on the Western diet (Fig. 3H,I). Activation of the SREBP1c target, fatty acid synthase (FAS), encoding the rate-limiting step in fatty acid synthesis, was also reduced. As expected, levels of all three targets were low and not altered in LKO mice on CR. Thus, a failure to coactivate LXR can explain the reduction in fat production and accumulation in the liver, as well as the protection from metabolic deficits, in LKO mice fed the Western diet.
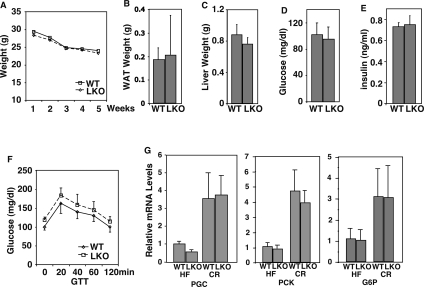
The fact that the LKO had a phenotypic effect in mice fed a high-calorie diet but not mice fed a normal chow diet suggested that the activity of the liver SIRT1 may be directly proportional to caloric intake, and predicted that this sirtuin may not be functional in the liver of wild-type mice on CR. Consistent with this idea, LKO mice showed weight loss and fat reduction over time on CR that paralleled wild type (Fig. 4A,B). Liver size reduction, which is triggered in part by apoptosis after imposition of CR (Grasl-Kraupp et al. 1994; James et al. 1998), also occurred normally in LKO mice (Fig. 4C). Further, metabolic parameters that were tested (blood glucose, insulin, and glucose tolerance) all changed similarly in LKO mice (Fig. 4D–F). Finally, the CR-induced up-regulation of two key gluconeogenic genes, PEPCK, and glucose 6-phosphatase (G6P), as well as their transcriptional coactivator PGC-1α, proceeded normally in LKO mice (Fig. 4G).
Figure 4.
SIRT1 LKO mice respond to CR normally. (A–F) Body weight, WAT weight, liver weight, blood glucose levels, insulin levels, and glucose tolerance were compared between wild-type and LKO mice on a CR diet. (G) Expression of PGC-1α, PEPCK, and G6P were compared between wild-type and LKO mice fed a high-fat diet or a CR diet by QRT–PCR.
We conclude that SIRT1 ablation in the liver does not alter the time course or steady-state levels of physiological changes during CR. This genetic finding, along with the demonstration that SIRT1 protein levels and the NAD/NADH ratio decrease in the CR liver, indicates that the activity of this sirtuin indeed goes down in the liver during CR. The recent observation that resveratrol induces glycogen synthesis in cultured HepG2 cells (Sun et al. 2007) is consistent with the idea that high SIRT1 activity in liver cells favors energy storage.
Why is SIRT1 regulated by diet oppositely in the liver compared with other tissues; for example, the muscle or the WAT? CR increases metabolic activity in the muscle and the WAT, which likely promotes the entry of NADH into electron transport and may account for our observed increases in the NAD/NADH ratio. However, the liver is unique in carrying out key biosynthetic functions—the synthesis of the bulk of fat and cholesterol for the body (Canbay et al. 2007), which occurs in proportion to caloric intake. Since fat synthesis is a highly reductive process—i.e., consumes reducing equivalents—the highly fed liver should have a redox state that is oxidized and the CR liver a redox state that is reduced. This redox effect will dictate a high NAD/NADH ratio in the fed liver and a low ratio in the CR liver, which is supported by our findings. As we also showed above, changes in the NAD/NADH ratio lie upstream of SIRT1, and will therefore constrain the activity of this sirtuin to be high in the fed liver and low in the CR liver. Further, SIRT1 expression levels have been reported to be coordinated to the redox state in cells (Zhang et al. 2007), which may explain the strong positive correlation observed between SIRT1 protein levels and the NAD/NADH ratio in CR versus AL tissues.
Evolution may have adapted the function of SIRT1 in different tissues to the metabolic constraints on its activity imposed by CR. In the muscle, CR-activated SIRT1 deacetylates PGC-1α to induce mitochondria and fat oxidation (Gerhart-Hines et al. 2007), and also prevents apoptosis (Luo et al. 2001; Vaziri et al. 2001), while in the WAT it fosters fat mobilization (Picard et al. 2004). In the liver, the repression of SIRT1 by CR also makes physiological sense. First, because SIRT1 coactivates LXR, the reduced activity of this sirtuin in CR will decrease fat synthesis (Li et al. 2007). Second, reduced SIRT1 activity may activate liver apoptosis, which is known to occur in response to CR (Grasl-Kraupp et al. 1994; James et al. 1998). Third, while SIRT1 deacetylation of PGC-1α activates mitochondrial biogenesis in the muscle, deacetylation of this coactivator by SIRT1 does not affect mitochondrial gene expression in the liver (Rodgers et al. 2005). Thus, there should be no cost in energy production associated with the reduction in SIRT1 activity in the liver during CR.
It has been suggested that SIRT1 deacetylation of PGC-1α in the liver is important for the induction of gluconeogenesis during fasting (Rodgers et al. 2005; Rodgers and Puigserver 2007). However, our findings indicate that blood glucose and insulin are not altered in LKO mice, at least in a long-term fast (24 h). Moreover, during steady-state CR two key enzymes of gluconeogenesis, PEPCK and G6P, as well as PGC-1α itself, are normally induced in the LKO mice. Evidently the elevated level of PGC-1α during CR suffices for gluconeogenesis, even in the absence of SIRT1 coactivation. These findings are consistent with the recent demonstration that resveratrol and the newer classes of SIRT1 activators do not induce gluconeogenesis in dosed animals(Baur et al. 2006; Lagouge et al. 2006; Milne et al. 2007). It remains possible that SIRT1 is required for gluconeogenesis during shorter times after fasting, but this will require further study.
Our findings may have important implications for the development of SIRT1 activators as CR mimetics. Any systemic SIRT1 activator may have the unwanted effect of inducing, or at least failing to prevent, the synthesis of fat and cholesterol in the liver. This possibility may explain why several categories of SIRT1 activators do not reduce body weight (Milne et al. 2007). However, activation of SIRT1 in metabolic tissues like muscle and adipocytes may partially offset untoward effects of fat accumulation. For example, resveratrol-fed mice on a high-fat diet do not acquire fatty liver, and actually show improved liver physiology and metabolic function (Baur et al. 2006; Lagouge et al. 2006). Our findings do raise the interesting possibility that SIRT1 inhibitors specifically targeted to the liver may be of benefit in treating obesity. Such inhibitors may also help mitigate unwanted side effects in the liver of LXR agonists, which have been developed to increase reverse cholesterol transport and efflux from peripheral tissues.
In summary, we show that the regulation of SIRT1 by the diet is more complicated than originally imagined. While it has been assumed that SIRT1 activity increases generally during CR, we show that in the liver the activity of this sirtuin actually decreases. The regulation of SIRT1 activity during CR is not only tissue-specific, but region-specific in nonhomogeneous tissues, such as the brain (D. Chen and L. Guarente, unpubl.). The reduction in SIRT1 activity in the CR liver correlates with the reduced role of this organ in fat synthesis. SIRT1 may thus figure prominently in the redistribution of resources during CR from growth,metabolism, and reproduction to maintenance and survival.
Materials and methods
Mice
SIRT1 knockout mice have been described previously (McBurney et al. 2003). SIRT1 liver-specific knockout mice were generated by crossing a SIRT1 allele containing a floxed exon 4 (Cheng et al. 2003) with Cre-expressing mice driven by the liver-specific albumin promoter. All mice were housed on a 12:12-h light:dark cycle at controlled temperature (25 ± 1°C). Three- to four-month-old animals (n = 10) were either fed ad libitum or subjected to a 40% calorie restricted diet for up to 3 mo, which was provided daily in the evening. The data of the CR experiments were collected in the morning. The high-fat Western diet was provided by OpenSource Diets (D12079B). All animal procedures were in accordance with the MIT animal care committee.
Blood was collected from the tail veins of 10 mice per group and kept on ice until centrifugation (1500_g_, 15 min at 4°C). The plasma was either used immediately for assays or stored at −80°C until analysis. Glucose concentrations were determined using OneTouch Ultra glucose meter (LifeScan). Insulin levels were measured using the Ultrasensitive Mouse Insulin EIA Kit (Alpco Diagnostics).
For the glucose tolerance test, mice were fasted overnight and injected intraperitoneally with a saline glucose solution at 1 g/kg body weight. Plasma glucose levels were measured before and 20, 40, 60, and 120 min after glucose injection.
RNA and protein preparation and analysis
Total RNA was extracted from tissues by TRIZOL (Invitrogen) and was further purified with RNeasy mini-kit (Qiagen). For real-time PCR analysis, cDNA was synthesized from total RNA by SuperScript III reverse transcriptase (Invitrogen) with random primers. cDNA was subjected to PCR analysis with gene-specific primers in the presence of CYBR green (Bio-Rad). Relative mRNA abundance was obtained by normalization to cyclophilin levels.
Proteins from mouse tissues were extracted in RIPA buffer (1% Triton X-100, 150 mM NaCl, 5 mM EDTA, 10 mM Tris 7.0) containing a protease inhibitor cocktail (Sigma). Protein extracts were subjected to centrifugation at 14,000 rpm for 10 min. SIRT1 antibody (1:1000 dilution; Upstate Biotechnologies). SREBP1c antibody (Santa Cruz Biotechnologies).
NAD and NADH measurement
NAD and NADH nucleotides were measured as described (Lin et al. 2004). About 10 mg of frozen tissues were homogenized in 300 μL of acid extraction buffer to obtain NAD concentration or alkali buffer to obtain NADH concentration. Two-hundred-forty microliters of supernatant were neutralized with 120 μL of buffer. The concentration of nucleotides was measured fluorimetrically in an enzymatic cycling reaction using 2–5 μL of sample.
Acknowledgments
We thank S. Liebert, A. Chalkiadaki, and K. Zainabadi for comments on the manuscript. This work is supported by a Leukemia and Lymphoma Society post-doctoral fellowship to D.C. (5168-06) and NIH grant AG15339-09 and Paul F. Glenn Foundation to L.G. L.G. is a consultant for Sirtris Pharmaceuticals and Elixir Pharmaceuticals. This work is supported by an Ellison Medical Foundation Senior Scholar award to F.W.A. F.W.A. is an Investigator of the Howard Hughes Medical Institute. F.W.A. is a member of the Scientific Advisory Board of Sirtris Pharmaceuticals.
Footnotes
References
- Baur J.A., Pearson K.J., Price N.L., Jamieson H.A., Lerin C., Kalra A., Prabhu V.V., Allard J.S., Lopez-Lluch G., Lewis K., et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canbay A., Bechmann L., Gerken G. Lipid metabolism in the liver. Z. Gastroenterol. 2007;45:35–41. doi: 10.1055/s-2006-927368. [DOI] [PubMed] [Google Scholar]
- Chen D., Guarente L. SIR2: A potential target for calorie restriction mimetics. Trends Mol. Med. 2007;13:64–71. doi: 10.1016/j.molmed.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Cheng H.L., Mostoslavsky R., Saito S., Manis J.P., Gu Y., Patel P., Bronson R., Appella E., Alt F.W., Chua K.F. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc. Natl. Acad. Sci. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitarese A.E., Carling S., Heilbronn L.K., Hulver M.H., Ukropcova B., Deutsch W.A., Smith S.R., Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H.Y., Miller C., Bitterman K.J., Wall N.R., Hekking B., Kessler B., Howitz K.T., Gorospe M., de Cabo R., Sinclair D.A. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Gerhart-Hines Z., Rodgers J.T., Bare O., Lerin C., Kim S.H., Mostoslavsky R., Alt F.W., Wu Z., Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1α. EMBO J. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasl-Kraupp B., Bursch W., Ruttkay-Nedecky B., Wagner A., Lauer B., Schulte-Hermann R. Food restriction eliminates preneoplastic cells through apoptosis and antagonizes carcinogenesis in rat liver. Proc. Natl. Acad. Sci. 1994;91:9995–9999. doi: 10.1073/pnas.91.21.9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S.J., Muskhelishvili L., Gaylor D.W., Turturro A., Hart R. Upregulation of apoptosis with dietary restriction: Implications for carcinogenesis and aging. Environ. Health Perspect. 1998;106 (Suppl. 1):307–312. doi: 10.1289/ehp.98106s1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: Insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Koubova J., Guarente L. How does calorie restriction work? Genes & Dev. 2003;17:313–321. doi: 10.1101/gad.1052903. [DOI] [PubMed] [Google Scholar]
- Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Li X., Zhang S., Blander G., Tse J., Krieger M., Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol. Cell. 2007;28:91. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- Lin S.J., Kaeberlein M., Andalis A.A., Sturtz L.A., Defossez P.A., Culotta V.C., Fink G.R., Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- Lin S.J., Ford E., Haigis M., Liszt G., Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes & Dev. 2004;18:12–16. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Nikolaev A.Y., Imai S., Chen D., Su F., Shiloh A., Guarente L., Gu W. Negative control of p53 by Sir2α promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- McBurney M.W., Yang X., Jardine K., Hixon M., Boekelheide K., Webb J.R., Lansdorp P.M., Lemieux M. The mammalian SIR2α protein has a role in embryogenesis and gametogenesis. Mol. Cell. Biol. 2003;23:38–54. doi: 10.1128/MCB.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne J., Lambert P., Westphal C., Milne J., Lambert P., Schenk S., Carney D., Smith J., Gagne D., Jin L., et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisoli E., Tonello C., Cardile A., Cozzi V., Bracale R., Tedesco L., Falcone S., Valerio A., Cantoni O., Clementi E., et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- Picard F., Kurtev M., Chung N., Topark-Ngarm A., Senawong T., Machado De Oliveira R., Leid M., McBurney M.W., Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers J.T., Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc. Natl. Acad. Sci. 2007;104:12861–12866. doi: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers J.T., Lerin C., Haas W., Gygi S.P., Spiegelman B.M., Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Sinclair D.A. Toward a unified theory of caloric restriction and longevity regulation. Mech. Ageing Dev. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Sun C., Zhang F., Ge X., Yan T., Chen X., Shi X., Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Van Remmen H., Guo Z., Richardson A. The anti-ageing action of dietary restriction. Novartis Found. Symp. 2001;235:221–230. doi: 10.1002/0470868694.ch18. [DOI] [PubMed] [Google Scholar]
- Vaziri H., Dessain S.K., Ng Eaton E., Imai S.I., Frye R.A., Pandita T.K., Guarente L., Weinberg R.A. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- Weindruch R., Walford R.L. The retardation of aging and disease by dietary restriction. Charles C. Thomas; Springfiled, IL: 1988. [Google Scholar]
- Zhang Q., Wang S.Y., Fleuriel C., Leprince D., Rocheleau J.V., Piston D.W., Goodman R.H. Metabolic regulation of SIRT1 transcription via a HIC1:CtBP corepressor complex. Proc. Natl. Acad. Sci. 2007;104:829–833. doi: 10.1073/pnas.0610590104. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]