Aire controls the differentiation program of thymic epithelial cells in the medulla for the establishment of self-tolerance (original) (raw)
Abstract
The roles of autoimmune regulator (Aire) in the expression of the diverse arrays of tissue-restricted antigen (TRA) genes from thymic epithelial cells in the medulla (medullary thymic epithelial cells [mTECs]) and in organization of the thymic microenvironment are enigmatic. We approached this issue by creating a mouse strain in which the coding sequence of green fluorescent protein (GFP) was inserted into the Aire locus in a manner allowing concomitant disruption of functional Aire protein expression. We found that Aire+ (i.e., GFP+) mTECs were the major cell types responsible for the expression of Aire-dependent TRA genes such as insulin 2 and salivary protein 1, whereas Aire-independent TRA genes such as C-reactive protein and glutamate decarboxylase 67 were expressed from both Aire+ and Aire− mTECs. Remarkably, absence of Aire from mTECs caused morphological changes together with altered distribution of mTECs committed to Aire expression. Furthermore, we found that the numbers of mTECs that express involucrin, a marker for terminal epidermal differentiation, were reduced in Aire-deficient mouse thymus, which was associated with nearly an absence of Hassall's corpuscle-like structures in the medulla. Our results suggest that Aire controls the differentiation program of mTECs, thereby organizing the global mTEC integrity that enables TRA expression from terminally differentiated mTECs in the thymic microenvironment.
Autoimmune diseases are mediated by sustained adaptive immune responses specific for self-antigens (Ags) through unknown pathogenic mechanisms. Although breakdown of self-tolerance is considered to be the key event in the disease process, the mechanisms that allow the production of autoantibodies and/or autoreactive lymphocytes are largely enigmatic (1). Autoimmune-polyendocrinopathy-candidiasis ectodermal dystrophy (APECED; OMIM 240300) is a rather rare autoimmune disease affecting mainly the endocrine glands. Because mutation of a single gene, autoimmune regulator (AIRE), is solely responsible for the development of APECED, understanding the relationship between AIRE gene malfunction and the breakdown of self-tolerance promises to help unravel the pathogenesis of not only APECED but also other types of autoimmune diseases (2, 3).
One of the most important aspects of AIRE in the context of autoimmunity is its limited tissue expression in medullary thymic epithelial cells (mTEC) (4, 5). mTECs are believed to play major roles in the establishment of self-tolerance by eliminating autoreactive T cells (negative selection) and/or by producing immunoregulatory T cells, which together prevent CD4+ T cell–mediated organ-specific autoimmune diseases (6, 7). For this purpose, mTECs appear to express a set of self-Ags encompassing many or most of the self-Ags expressed by parenchymal organs. Supporting this hypothesis, analysis of gene expression in the thymic stroma has demonstrated that mTECs are a specialized cell type in which promiscuous expression of a broad range of peripheral tissue-restricted Ag (TRA) genes (i.e., promiscuous gene expression) is an autonomous property (8). Aire in mTECs has been suggested to regulate this promiscuous gene expression (9–11) through as yet undetermined mechanisms.
From a mechanistic viewpoint, there are two possible models to explain the function of Aire in the thymic organogenesis required for the establishment of self-tolerance. First, Aire may play a tolerogenic role within the types of mTECs characterized by Aire expression. In other words, the presence of Aire within cells is necessary in order for them to function normally as tolerance-establishing cells. Consistent with this idea, the current prevailing view on the roles of Aire in establishing self-tolerance is that Aire-positive cells are the major cell types that show promiscuous gene expression and that the lack of Aire protein within cells impairs their tolerogenic function because of the reduced transcription of TRA genes, although the developmental process of mTECs is otherwise unaltered in the absence of Aire (model 1). The second model hypothesizes that Aire is necessary for the developmental program of mTECs, including Aire-positive cells themselves. In this case, we assume that what are called Aire-positive mTECs and other Aire-dependent cell-types do not develop normally in the absence of Aire. Given that acquisition of the properties of promiscuous gene expression depends on the maturation status of mTECs (see Results and Discussion), impaired promiscuous gene expression from Aire-deficient mice can be associated with a defect of such an Aire-dependent developmental program in mTECs (model 2). Although it is still controversial whether reduced transcription of particular TRA genes in Aire-deficient mTECs can account for the development of autoimmunity targeting the corresponding self-Ags in Aire-deficient mice by itself (11–15), it is critical to determine which model provides a more appropriate explanation of Aire-dependent promiscuous gene expression to further elucidate the molecular aspects of Aire (16). Model 1 would direct research toward the mechanisms underlying how a single Aire gene can regulate a large number of target genes (i.e., TRA genes), whereas model 2 would accelerate studies of the developmental program of mTECs in which Aire plays a pivotal role. These two models can be tested if we can monitor the developmental process of mTECs committed to Aire expression in both the presence and absence of functional Aire protein.
This issue regarding the roles of Aire in thymic organogenesis is also directly linked to the fundamental question of how mTECs acquire their unique ability to express a broad range of self-Ags (i.e., promiscuous gene expression). The terminal differentiation model assumes that mTECs eventually acquire the capacity for promiscuous gene expression by becoming differentiated, more mature, and more promiscuous (7, 10). This model suggests that mTECs, especially Aire-positive cells, are specialized cell types that have acquired this ability through differentiation. In this context, it is noteworthy that the transcriptional machinery necessary for promiscuous gene expression other than Aire protein is considered to be acquired by mTECs independent of Aire expression in this model. The model suggests that the transcriptional unit for promiscuous gene expression becomes fully active when Aire starts to be expressed in terminally differentiated mTECs. In contrast, the developmental model considers that promiscuous gene expression is a reflection of the multipotency of immature mTECs before the developmental fate of particular cell types is determined (17). In this model, expression of a broad spectrum of TRA genes is regulated by conserved developmental programs that are active in developing mTECs, and Aire and/or Aire+ cells control this process (18). Accordingly, the developmental model considers that Aire acts at the early developmental stage of mTEC differentiation, which is in marked contrast to the timing of Aire expression proposed in the terminal differentiation model. Thus, the terminal differentiation model and the developmental model favor models 1 and 2, respectively, proposed for the roles of Aire in promiscuous gene expression and self-tolerance (19).
To investigate in more detail the roles of Aire in thymic organogenesis, we have used a knock-in mouse strategy in which the coding sequence of GFP was inserted into the Aire gene locus in a manner allowing concomitant disruption of functional Aire protein expression. This strategy allowed us to distinguish mTECs committed to expressing Aire from Aire-nonexpressing mTECs, in both the presence and absence of functional Aire protein. In addition, with the use of knock-in mice in which thymic TRA (i.e., glutamate decarboxylase 67 [_GAD67_]) expression can be monitored by GFP expression we also examined the cell types of mTECs responsible for promiscuous gene expression in situ. The results suggest that Aire promotes the differentiation program of mTECs and that promiscuous gene expression is accomplished in terminally differentiated mTECs that have fully matured in the presence of Aire protein.
RESULTS
Establishment of Aire/GFP knock-in mice
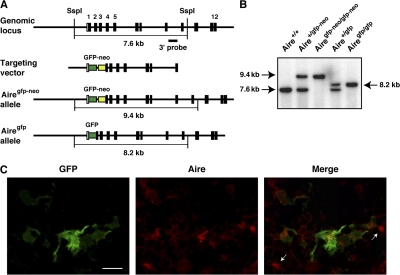
To examine the molecular and cellular contribution of Aire to thymic organogenesis, we established Aire/GFP knock-in mice in which expression of the GFP gene is under the transcriptional control of the endogenous Aire gene. In this strategy, modification of the Aire gene locus was minimized by inserting a GFP-neomycin resistance (neor) gene cassette (gfp-neo) (20) between exon 1 and exon 2 (Fig. 1 A). After establishing Aire+/gfp-neo mice, they were crossed with a general deleter Cre recombinase-expressing transgenic line (21) to remove the neor gene cassette, which contains the herpes simplex virus thymidine kinase gene promoter for efficient neor gene expression. After confirming the removal of the neor gene cassette (Fig. 1 B), mice were crossed with C57BL/6 mice to select a line containing the GFP knock-in allele but not the Cre recombinase-expressing transgenic allele. Aire+/gfp mice were then crossed to obtain Airegfp/gfp mice, which have a null mutation for the Aire gene because of disruption of the Aire gene by insertion of the GFP gene (Fig. 1 B). As expected, Airegfp/gfp mice, but not Aire+/gfp mice, showed no expression of endogenous Aire in the thymus, as detected with polyclonal anti-Aire antibody (Ab) recognizing peptides located within the proline-rich region of Aire (unpublished data).
Figure 1.
Establishment of Aire/GFP knock-in mice. (A) Targeted insertion of the GFP gene into the Aire gene locus by homologous recombination. SspI, SspI restriction site. (B) Southern blot analysis of genomic DNA from offspring of Aire/GFP knock-in mice. Tail DNA was digested with SspI and hybridized with the 3′ probe shown in A. (C) Concomitant expression of GFP (green) and endogenous mouse Aire (red) assessed by immunohistochemistry of a thymus section from an Aire+/gfp mouse. Cells positive for Aire staining but negative for GFP expression are marked with arrows. Bar, 20 μm. One representative experiment from a total of four repeats is shown.
Using immunohistochemistry, we first examined whether GFP expression from Aire+/gfp mouse thymus reflects endogenous Aire gene expression. Stromal cells showing variable extents of GFP expression in the cytoplasm and nucleus were scattered throughout the thymic medulla (Fig. 1 C). The medullary region was identified by staining with Ulex europaeus agglutinin 1 (UEA-1) (Fig. 2 A), anti–epithelial cell adhesion molecule 1 (EpCAM) mAb (Fig. 2 B), or anti–keratin 5 (K5) Ab (Fig. S1 A, available at http://www.jem.org/cgi/content/full/jem.20080046/DC1). GFP-expressing cells from Aire+/gfp mouse thymus showed a dendritic to fibroblastic morphology and were enriched at the cortico-medullary junction (Fig. 1 C; Fig. 2, A and B; and Fig. S1 A). When doubly stained with anti-Aire Ab, most of the GFP-expressing cells contained variable amounts of Aire nuclear dots within their nuclei (Fig. 1 C), indicating that GFP expression is under the transcriptional control of the authentic Aire gene. However, a few cells showed Aire nuclear dots without any detectable GFP expression (Fig. 1 C, arrows) or expressed GFP without obvious Aire nuclear dots (not depicted). As expected, Aire+/+ mouse thymus showed no GFP signals (Fig. 2, A and B). Notably, most of the CD11c-positive DCs in the thymus were GFP negative (Fig. S1 B), suggesting that Aire expression from thymic DCs is negligible compared with that from mTECs.
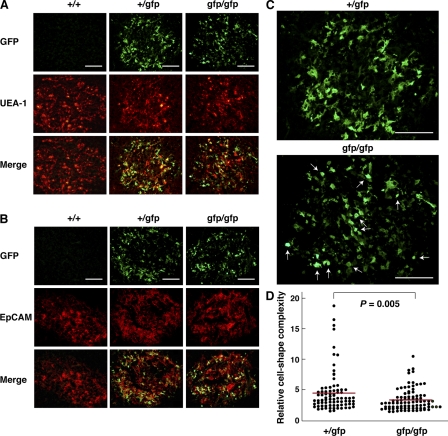
Figure 2.
Altered morphology and distribution of mTECs committed to express Aire in the absence of functional Aire protein. (A and B) mTECs active in Aire gene transcription were visualized by immunohistochemistry with anti-GFP Ab (green). The medullary region was identified by staining with UEA-1 (A) or anti-EpCAM mAb (B; red). Bars, 100 μm. One representative experiment from a total of five repeats is shown. (C) Enlargement of the staining with anti-GFP Ab from A for demonstration of altered morphology and distribution of mTECs committed to express Aire in Airegfp/gfp mouse thymus. There were more GFP+ cells with globular shapes (bottom, arrows) in Airegfp/gfp thymus than in Aire+/gfp thymus. GFP+ cells from Aire+/gfp thymus were enriched at the cortico-medullary junction (top), whereas GFP+ cells from Airegfp/gfp thymus tended to be localized more evenly within each medulla or even enriched at the center of the medulla (bottom). Bars, 100 μm. One representative experiment from a total of five repeats is shown. (D) Morphological changes in the shape of GFP+ cells from Airegfp/gfp mouse thymus demonstrated in C were analyzed statistically. Each circle corresponds to the relative cell shape complexity of a single GFP+ cell calculated with a computer program (see Materials and methods). A total of 80 and 88 GFP+ cells from Aire+/gfp and Airegfp/gfp thymi, respectively, were evaluated. Red lines represent mean values. Two mice for each group were analyzed, and similar results were obtained from a total of three repeats.
Altered thymic organization in Aire-deficient mice
We then examined the effect of Aire deficiency on thymic organization in Airegfp/gfp mouse thymus sections, focusing on the production of cells genetically marked with GFP and, therefore, active in Aire gene transcription but lacking functional Aire protein. There were many GFP+ “Aire-less” TECs within the medulla (Figs. 2, A and B; and Fig. S1 A), indicating clearly that Aire protein itself is not necessary for the production of particular mTEC lineages committed to express Aire. However, detailed inspection demonstrated that the morphology and location of GFP+ cells from Airegfp/gfp thymus were altered compared with those of GFP+ cells containing functional Aire protein from Aire+/gfp mouse thymus. First, we noticed that the cell shape of GFP+ mTECs lacking functional Aire protein was altered; in Airegfp/gfp thymus, more GFP+ cells exhibited a globular shape instead of a dendritic to fibroblastic morphology, compared with Aire+/gfp thymus (Fig. 2 C, arrows). The lower preponderance of a dendritic shape of GFP+ Aire-less mTECs was verified by statistical analysis. We calculated the level of cell shape complexity for each GFP+ cell by dividing the length of the cellular periphery by the cell area using a computer program (i.e., the higher the value, the more complex the cell shape). GFP+ cells from Airegfp/gfp thymus showed lower values (i.e., less complexity per cell) and a narrower distribution of values (i.e., less heterogeneity of cell shape) than those from Aire+/gfp thymus (Fig. 2 D). Because a gene-dosage effect has been noticed at the Aire gene locus (11), we carefully excluded the possibility that the altered shape of GFP+ cells from Airegfp/gfp thymus was due simply to higher GFP protein expression within each cell, i.e., imposing a potentially toxic burden on the cells. For this purpose, we crossed Aire+/gfp mice with _Aire_−/− mice (12) to establish Aire−/gfp mice in which the gfp allele is single, as in Aire+/gfp mice (Fig. S2 A). Similarly to the Airegfp/gfp thymus analysis, GFP+ cells from Aire−/gfp thymus demonstrated less complexity of cell shape than those from Aire+/gfp thymus, as confirmed using the same method of statistical analysis (Fig. S2 B). Although we analyzed the thymic organization of Airegfp/gfp mice before the onset of autoimmune pathology (i.e., 4–6 wk after birth), we also excluded the possibility that the altered cell shape of GFP+ cells from Airegfp/gfp thymus was secondary to the autoimmune phenotypes by establishing Aire−/gfp mice expressing the OT-II TCR transgene in which the autoreactive T cell repertoire is absent (Fig. S3 A). Morphological changes in GFP+ cells were similarly observed in these mice (Fig. S3 B), suggesting that the altered shape of GFP+ cells lacking Aire protein was independent of autoimmune phenotype.
Second, we noticed that the distribution pattern of mTECs committed to Aire expression was also affected in the absence of functional Aire protein. In contrast with the enrichment of GFP+ cells from Aire+/gfp thymus at the cortico-medullary junction, GFP+ cells from Airegfp/gfp thymus tended to be localized more uniformly within each medulla or even enriched at the medulla center (Fig. 2 C and Fig. S1 A). Altered distribution of GFP+ Aire-less mTECs was also evident in Aire−/gfp mice (Fig. S2 A), as well as in Aire−/gfp mice expressing the nonautoreactive OT-II TCR transgene (Fig. S3 A). Collectively, production of a particular mTEC lineage committed to express Aire is not determined by Aire protein alone. However, Aire deficiency in these cells results in morphological changes together with altered location within the medulla, suggesting a role of Aire in the differentiation program of mTECs in a cell-intrinsic manner.
Analysis of embryonic thymus demonstrated that GFP+ cells were absent at embryonic day 13.5, but clearly present at embryonic day 16.5 in both Aire+/gfp and Airegfp/gfp mice (Fig. S1 C). Although the effect of absence of Aire protein on the location of GFP+ cells from Airegfp/gfp mice at the embryonic and early P1 (postneonatal) stages was difficult to evaluate because of the less organized thymic structure together with relatively small numbers of GFP+ cells at those stages, morphological alteration of each mTEC committed to Aire expression was already evident at the neonatal stage (P1; Fig. S4 A), as confirmed by the same statistical analysis applied to Fig. 2 D (Fig. S4 B). The properties of GFP− (i.e., Aire nonexpressing) mTECs as evaluated by immunohistochemistry with UEA-1, anti-EpCAM Ab (Fig. 2, A and B), anti-K5 Ab (Fig. S1 A), ER-TR5 Ab, anti–claudin 3/4 Abs, and MTS10 Ab (not depicted) showed no obvious difference between Aire+/gfp and Airegfp/gfp adult thymi.
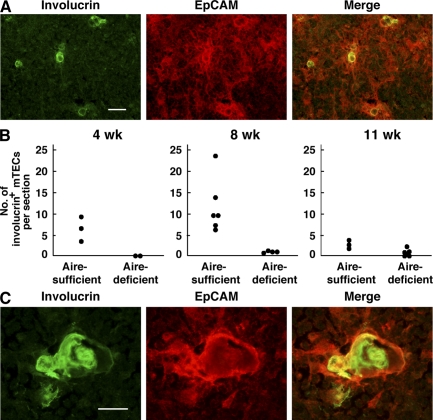
In addition to the histological evaluation of mTECs based on Aire/GFP expression, another possibility that Aire controls the differentiation program of mTECs has emerged from studies focusing on the cell differentiation markers expressed by mTECs. In the skin, involucrin expression is restricted to postmitotic epithelial cells and serves as a marker of epidermal and follicular terminal differentiation (22). Interestingly, immunohistochemistry of the human thymus using anti-involucrin Ab stains characteristic swirled epithelial structures known as Hassall's corpuscles (23), which is consistent with the fact that Hassall's corpuscles are composed of terminally differentiated mTECs (24). When thymus sections from Aire-sufficient mice were stained with anti-involucrin Ab, involucrin-expressing cells were scattered within the EpCAM+ thymic medulla (Fig. 3 A). The number of involucrin-expressing cells was age dependent and declined between 8 and 11 wk (Fig. 3 B and Table S1, available at http://www.jem.org/cgi/content/full/jem.20080046/DC1). In addition, we occasionally found larger involucrin-expressing structures with a hyalinized degenerated core in the thymic medulla from Aire-sufficient mice, which is reminiscent of Hassall's corpuscles in human thymus (Fig. 3 C). Remarkably, the numbers of mTECs expressing involucrin in Aire-deficient mice were significantly lower than those in Aire-sufficient mice, especially at 4 and 8 wk of age (Fig. 3 B). Furthermore, we observed no typical Hassall's corpuscle-like structures in the thymus of Aire-deficient mice at any age, which is in contrast to those seen in Aire-sufficient mice (Table S1). These results further support the notion that lack of Aire in mTECs alters their differentiation program, thereby altering mTEC integrity.
Figure 3.
Reduced numbers of terminally differentiated mTECs in the absence of Aire. (A) Involucrin-expressing mTECs (green) were scattered within the thymic medulla (red; stained with anti-EpCAM Ab) of Aire-sufficient mice. Bar, 50 μm. (B) Numbers of involucrin-expressing mTECs were reduced in Aire-deficient mice at 4 (left) and 8 (middle) wk of age. Numbers of involucrin-expressing mTECs in Aire-sufficient mice declined at 11 wk of age (right). Each circle corresponds to the mean number of involucrin-expressing mTECs per section examined in individual mice. Detailed information for the mice examined from a total of five experiments is presented in Table S1 (available at http://www.jem.org/cgi/content/full/jem.20080046/DC1). (C) Hassall's corpuscle-like structures seen in Aire-sufficient mouse thymus stained with anti-involucrin Ab (green) together with anti-EpCAM Ab (red). These discrete and larger involucrin-expressing structures were scarcely detectable in Aire-deficient mouse thymus. Bar, 20 μm. One representative experiment from a total of five repeats is shown.
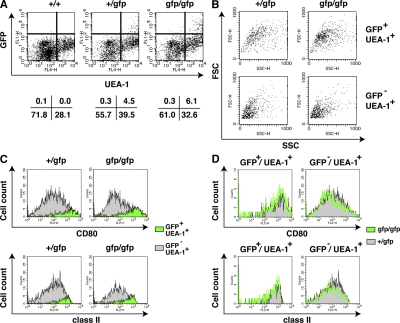
Next, we used flow cytometric analysis to examine GFP-expressing cells from the thymus. Thymic stromal cells were released enzymatically from adult thymi and stained with anti-CD45 mAb and UEA-1, together with anti-CD80 and anti–MHC class II mAbs. Aire+/gfp thymus contained 4.5% UEA-1+GFP+ (i.e., Aire+) cells (from here on simply designated GFP+ cells) in the population of CD45− stromal cells (Fig. 4 A). When forward scatter (FSC) and side scatter (SSC) parameters were compared between GFP+ cells and UEA-1+GFP− cells (from here on simply designated GFP− cells), GFP+ cells were larger and more broadly distributed compared with GFP− cells (Fig. 4 B, left), suggesting a distinct cellular morphology of Aire+ cells among mTECs. Airegfp/gfp thymus also contained GFP+ cells in the population of CD45− stromal cells (Fig. 4 A), as already observed by immunohistochemical analysis (Figs. 2 and S1). Interestingly, the proportion of GFP+ cells in Airegfp/gfp thymus was consistently 30–40% higher than in Aire+/gfp thymus (Fig. 4 A). Consequently, the ratio of GFP+ cells to GFP− cells was higher in Airegfp/gfp thymus (∼1:5) compared with that in Aire+/gfp thymus (∼1:10). Although the difference in FSC/SSC parameters between GFP+ and GFP− cells observed for Aire+/gfp mice was also seen in Airegfp/gfp mice (Fig. 4 B, right), FSC/SSC plots of GFP+ cells from Airegfp/gfp mice showed a more condensed profile over a narrower region compared with GFP+ cells from Aire+/gfp mice (Fig. 4 B, top), which might reflect the morphological changes in GFP+ mTECs observed by immunohistochemistry (Fig. 2 C). We recorded no GFP expression from CD45+ hematopoietic cells (not depicted) or from CD45−UEA-1− thymic stromal cells from either Aire+/gfp or Airegfp/gfp mice (Fig. 4 A).
Figure 4.
Global alteration of mTEC phenotypes in the absence of Aire. (A) Detection of GFP-expressing cells from thymic stroma by flow cytometric analysis. CD45− thymic stromal cells were analyzed for the expression of GFP together with binding of UEA-1. Percentages of cells from each fraction are indicated below. (B) mTECs committed to express Aire were larger than mTECs noncommitted to express Aire, irrespective of the presence of Aire protein. FSC/SSC profiles of mTECs committed to express Aire were altered in the absence of functional Aire protein (top). Each FSC/SSC profile was obtained by back gating the corresponding fractions from A based on the expression of GFP and UEA-1. (C) CD80 and MHC class II expression levels were higher in mTECs committed to express Aire than in mTECs noncommitted to express Aire, irrespective of the presence of functional Aire protein. Filled profiles in green and gray are from GFP+ and GFP− mTECs, respectively. (D) CD80 and MHC class II expression from mTECs committed to express Aire were indistinguishable between Aire+/gfp and Airegfp/gfp mice (left) but were reduced in mTECs noncommitted to express Aire in the absence of functional Aire protein (right). Filled profiles in gray and green lines are from Aire+/gfp and Airegfp/gfp mice, respectively. Flow cytometric profiles from C were merged for comparison. One representative result from a total of more than five repeats is shown.
We then analyzed the expression of CD80 and MHC class II from each of the populations separated on the basis of GFP expression and UEA-1 binding. GFP+ cells from Aire+/gfp mice expressed both CD80 and MHC class II at high levels (CD80hi/class IIhi), whereas GFP− cells from the same animals expressed intermediate to low levels of both CD80 and MHC class II (Fig. 4 C, left). GFP+ cells from Airegfp/gfp thymus were also CD80hi/class IIhi (Fig. 4 C, right), indicating that expression of these Ag presentation-related molecules was Aire independent. Indeed, expression levels of both CD80 and MHC class II from GFP+ cells were almost indistinguishable between Aire+/gfp and Airegfp/gfp mice when the two flow cytometric profiles were merged (Fig. 4 D, left). However, although difference was small, expression of both CD80 and MHC class II from GFP− cells from Airegfp/gfp mice was consistently lower than that from Aire+/gfp mice (Fig. 4 D, right). This result may indicate that the absence of normal Aire-expressing cells from the medulla is accompanied by phenotypic alteration of Aire-nonexpressing mTECs, which was not evident with the immunohistochemical analysis with the commonly used medullary epithelial cell markers (Fig. 2, A and B; and Fig. S1 A). Collectively, the results suggest that Aire deficiency results in a global alteration of the thymic microenvironment that involves not only mTECs committed to express Aire but also the Aire-nonexpressing mTECs that surround Aire+ cells.
Aire-dependent TRA gene expression
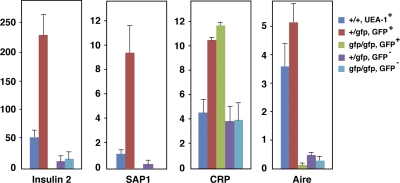
Although Aire has been suggested to regulate promiscuous gene expression in mTECs (9, 10), demonstration that Aire+ cells are the major source of promiscuous gene expression from mTECs is still incomplete in the absence of appropriate cell markers for Aire-expressing cell lineages. Existing data for promiscuous gene expression from mTECs were obtained by flow cytometric sorting using surrogate Aire+ cell markers such as CD80 and MHC class II. As a result, it is not yet clear which population of mTECs (i.e., Aire-expressing or Aire-nonexpressing mTECs) is deficient in promiscuous gene expression as a result of absence of functional Aire protein. To answer this question, we separated GFP+ and GFP− mTECs from both Aire+/gfp and Airegfp/gfp mice and examined the expression of several TRA genes, including both Aire-dependent (i.e., insulin 2 and salivary protein 1 [_SAP1_]) and Aire-independent (C-reactive protein [_CRP_]) TRA genes; expression of the former and the latter gene classes has been demonstrated to be reduced or unchanged, respectively, in CD80hi/class IIhi Aire-deficient mTECs (9, 10). GFP+ mTECs from Aire+/gfp mice showed the highest expression of insulin 2 and SAP1, and expression of those genes was much lower in GFP− mTECs from the same animals (Fig. 5). Remarkably, both GFP+ and GFP− mTECs from Airegfp/gfp mice expressed almost none of the Aire-dependent TRA genes insulin 2 and SAP1. mTECs defined by UEA-1 binding from Aire+/+ mice, which includes both Aire+ and Aire− cells, showed intermediate expression of those genes. These results clearly indicate two important features of promiscuous gene expression in mTECs. First, Aire+ mTECs are the major cell types responsible for the expression of Aire-dependent TRA genes. Second, mTECs cannot express Aire-dependent TRA genes in the absence of functional Aire protein, even though the lineage commitment to express Aire and the expression of Ag presentation-related molecules, such as CD80 and MHC class II, are preserved (Fig. 4 C). It is important to emphasize that the latter observation does not necessarily mean that Aire acts on the already existing transcriptional machinery required for TRA gene expression within established terminally differentiated mTECs. Rather, in the light of the fact that GFP+ Aire-less mTECs show defective development, as indicated by their altered morphology and distribution, we suggest that Aire+ mTECs acquire their unique machinery for promiscuous gene expression only when they have fully achieved maturation with the help of Aire protein (see Discussion and see Fig. 8).
Figure 5.
TRA gene expression from mTECs assessed by real-time PCR. Expression of insulin 2, SAP1, CRP, and Aire was examined from each fraction of mTECs sorted on the basis of the flow cytometric profile demonstrated in Fig. 4 A. Color bars corresponding to each fraction are indicated on the right. Aire+ mTECs were the major cell types responsible for the expression of Aire-dependent TRA genes (insulin 2 and SAP1), whereas an Aire-independent TRA gene (CRP) was expressed from both Aire+ and Aire− mTECs. Aire expression was assessed to verify the proper sorting of each mTEC fraction. Numbers are relative gene expression level compared with that of the Hprt gene. Results are expressed as the mean ± SEM for triplicate wells of one representative experiment from a total of three repeat experiments.
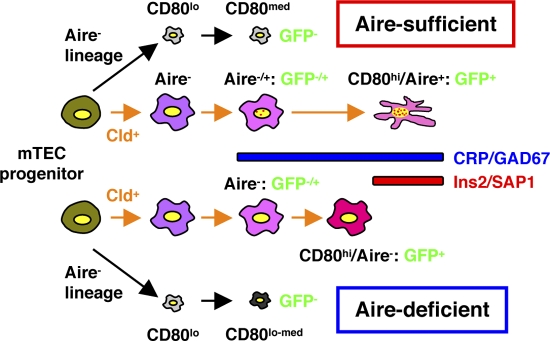
Figure 8.
Schematic representation of the roles of Aire in mTEC differentiation and TRA gene expression. Aire-expressing cell lineages develop from mTEC progenitor cells through concomitant expression of claudin (26). Expression of Aire-dependent TRA genes, such as insulin 2 and SAP1, can be accomplished in terminally differentiated mTECs showing a dendritic to fibroblastic morphology that have fully matured with the help of Aire protein (marked as Aire-sufficient). Lack of Aire in mTECs results in premature termination of differentiation, although claudin+ Aire-expressing cell lineages can still develop and pass the CD80-expressing maturation stage (marked as Aire-deficient). These CD80hi Aire-less mTECs have a more globular cell shape and lack transcriptional machinery for Aire-dependent TRA genes. Because Aire-independent TRA genes, such as CRP and GAD67, can be expressed before the terminal differentiation stages, lack of Aire has little impact on their expression. The possibility also remains that Aire is necessary for the maintenance of a terminally differentiated state, in which mTECs manifest a dendritic shape with fully competent promiscuous gene expression.
In marked contrast to Aire-dependent TRA genes, expression of an Aire-independent TRA gene, CRP, from GFP+ mTECs was indistinguishable between Aire+/gfp and Airegfp/gfp mice. CRP expression from GFP− mTECs was detectable, although the levels were lower than from GFP+ mTECs, and was also similar between Aire+/gfp and Airegfp/gfp mice (Fig. 5). As expected, the Aire gene was highly expressed from GFP+ mTECs of Aire+/gfp mice, although a low level of Aire gene expression was detected from GFP− mTECs, which is possibly a result of slight contamination by cells expressing a trace amount of GFP (i.e., Aire) in this fraction. Expression of the Aire gene from both GFP+ and GFP− cells of Airegfp/gfp mice was at background levels.
Aire-independent TRA gene expression in situ from mTECs
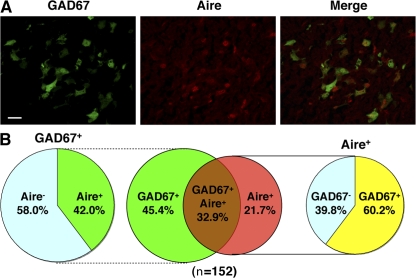
The results in the previous section suggest that individual mTECs do not express a broad array of TRA genes. Rather, each mTEC seems to express a different spectrum of TRA genes. Some TRA genes, such as insulin 2 and SAP1 (previously recognized as Aire-dependent genes; references 9, 10), were predominantly expressed from cells of the Aire+ mTEC lineage only when Aire protein was present within the cells, and other TRA genes, such as CRP (previously recognized as an Aire-independent gene; references 9, 10), were expressed from both Aire+ and Aire− mTECs irrespective of the presence of Aire protein. The latter situation was further investigated with the use of GAD67/GFP knock-in mice (GAD67+/gfp mice). GAD67, an Aire-independent TRA gene that is expressed in the brain and pancreas, is also active in mTECs from GAD67+/gfp mice (25). Using immunohistochemistry, we examined the expression of GAD67 together with Aire in GAD67+/gfp mouse thymus sections. There were three types of TECs: GAD67+Aire− (45.4%), GAD67+Aire+ (32.9%), and GAD67−Aire+ (21.7%) (Fig. 6, A and B). Among the GAD67+ mTECs, 42.0% expressed Aire and the rest did not (Fig. 6 B), consistent with the Aire-independent nature of GAD67 gene expression (9, 10). Conversely, among the Aire+ mTECs, 60.2% expressed GAD67 and the rest did not, suggesting that Aire expression is not sufficient for TRA expression, at least for this Aire-independent TRA gene.
Figure 6.
Expression of the Aire-independent TRA gene GAD67 and of Aire from mTECs in situ. (A) Expression of the GAD67 gene and Aire was detected by immunohistochemistry with anti-GFP Ab (green) and anti-Aire Ab (red), respectively, in thymus sections from GAD67/GFP knock-in mice. Bar, 20 μm. (B) Results obtained as described for A were calculated for a total of 152 mTECs expressing the GAD67 gene and/or Aire. One representative experiment from a total of three repeats is shown.
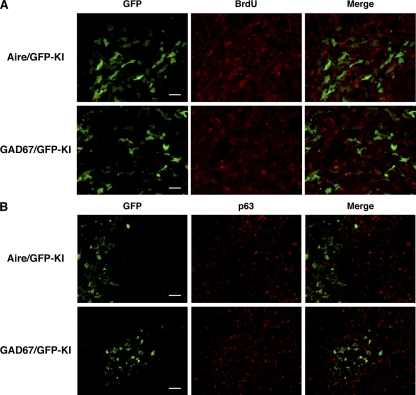
Expression of Aire and Aire-independent TRA genes by nonproliferating mTECs
Previous studies suggested that Aire is predominantly expressed by terminally differentiated cells on the basis of their poor incorporation of BrdU (26, 27). We confirmed this finding by injecting BrdU into Aire+/gfp mice. BrdU incorporation was scarce in GFP+ mTECs (Fig. 7 A, top). We similarly examined which type of mTECs, immature proliferating or mature nonproliferating, express GAD67 by injecting BrdU into GAD67/GFP knock-in mice. We found that GFP+ mTECs incorporated BrdU only weakly (Fig. 7 A, bottom), suggesting that expression of this Aire-independent TRA gene is also imposed on terminally differentiated cells rather on immature proliferating mTECs.
Figure 7.
Expression of the Aire and GAD67 genes by nonproliferating mTECs. (A) BrdU incorporation by _Aire_- and _GAD67_-expressing mTECs was evaluated 4 h after i.p. injection of BrdU into Aire+/gfp and GAD67+/gfp mice, respectively. The thymus sections were stained with anti-GFP (green) and anti-BrdU (red) Abs. Bars, 20 μm. (B) p63 (red) was not detected in mTECs expressing the Aire and GAD67 genes (green). Bars, 40 μm. One representative experiment from a total of four repeats is shown.
p63 is strongly expressed in epithelial stem cells of the thymus and specifically functions to maintain their extraordinary proliferative capacity (28). To examine whether mTECs expressing the Aire and GAD67 genes have this high proliferative capacity, we examined p63 expression from thymi of Aire/GFP knock-in and GAD67/GFP knock-in adult mice. mTECs expressing GFP from both mouse strains showed little p63 expression by immunohistochemistry (Fig. 7 B), suggesting that neither of these genes is expressed in mTECs with high proliferative capacity. Instead, Aire seems to function within mTECs in the later stages of differentiation, when the cells are also responsible for TRA gene expression.
DISCUSSION
In the present study, we addressed fundamental questions regarding how mTECs acquire the capacity for promiscuous gene expression with the participation of Aire, with the hope that understanding the roles of Aire in thymic organogenesis will help to unravel the molecular mechanisms responsible for expression of immunological self in the thymic microenvironment. The issues include the following: first, whether Aire itself is necessary for the production and/or differentiation program of Aire+ cell lineages; second, whether Aire+ mTECs are necessary for the structural and/or functional organization of other types of mTECs; third, to what extent Aire+ mTECs contribute to the expression of TRA genes; and fourth, the nature of the maturation status of mTECs that express Aire and are responsible for TRA expression. Because Aire-specific Ab cannot be used to investigate the differentiation process of mTECs committed to express Aire in the absence of Aire protein, we established Aire/GFP knock-in mice in which the GFP marker gene was inserted into the Aire gene locus in a manner allowing concomitant disruption of functional Aire protein expression. In Aire+/gfp mice, this strategy also enables us to distinguish Aire-expressing cells from Aire-nonexpressing cells without introducing any cell markers incompletely unique to Aire-expressing cells. Accordingly, mTECs committed to Aire expression were faithfully GFP marked with this strategy; mTECs transcriptionally active for the Aire gene were mostly positive for staining with anti-Aire Ab by immunohistochemistry. There were, however, small numbers of cells that were either positive for Aire staining but negative for Aire gene transcription (i.e., GFP−) or, conversely, positive for Aire gene transcription (i.e., GFP+) but negative for Aire staining. The former cell type could result from different half-lives of the two proteins (GFP vs. Aire), whereas the latter cell type could result from Aire protein being present as a diffuse nucleoplasmic form (more difficult to recognize) instead of the typical nuclear-dot form (29). Alternatively, these discrepancies could simply be accounted for by differences in detection sensitivity. Indeed, RT-PCR analysis of flow cytometry–sorted cell fractions showed the expected patterns of Aire gene expression.
With Aire/GFP knock-in mice, we have clearly demonstrated that Aire+ mTECs are the major cell types responsible for the expression of so-called Aire-dependent TRA genes such as insulin 2 and SAP1 (9, 10). These genes were almost exclusively expressed from GFP+ mTECs of Aire+/gfp mice but not of Airegfp/gfp mice. In contrast, expression of Aire-independent genes, such as CRP, was not affected by the absence of Aire. CRP expression from GFP+ cells was similar between Aire+/gfp and Airegfp/gfp mice. CRP expression, although at lower levels, was also observed from GFP− cells and, again, was indistinguishable between Aire+/gfp and Airegfp/gfp mice. Expression of GAD67 in an Aire-independent manner (9, 10) was also supported by immunohistochemistry of GAD67/GFP knock-in thymus, demonstrating GAD67 expression irrespective of the presence of Aire protein in each mTEC. We speculate that the Aire dependency of TRAs reflects, in part, the cell types in which TRAs are expressed; expression of Aire-dependent genes is confined to Aire+ mTECs, whereas expression of Aire-independent genes occurs from both Aire+ and Aire− mTECs. It is of note that mTECs do not uniformly express the overlapping spectrum of TRAs, as exemplified by the scattered expression of the GAD67 gene in GAD67/GFP knock-in mouse thymus. Similarly, although Aire+ mTECs are the major cell types responsible for the expression of Aire-dependent TRA genes, this does not mean that all Aire+ mTECs express Aire-dependent TRA genes uniformly. Indeed, single-cell analysis has demonstrated that expression of Aire in mTECs is not sufficient for simultaneous coexpression of Aire-dependent TRA genes (17). Thus, we favor the notion that promiscuous gene expression reflects the thymus-wide summation of expression of a small number of self-Ags by individual mTECs rather than expression of the complete spectrum of self-Ags by each cell (17, 18).
Because expression of transcription factors associated with developmental plasticity of progenitor cells (i.e., Nanog, Oct4 and Sox2) is Aire-dependent in mTECs (18), the developmental model predicts that Aire acts early in the development of mTECs. The developmental model also suggests that promiscuous gene expression represents coordinated gene expression reflecting an alternate program of epithelial differentiation among actively proliferating mTECs at their progenitor or immature stages (19). However, accumulating data together with the results of the present study do not support such a view (26, 27). Rather, it is likely that Aire is acting at the late differentiation stages of mTECs. Accordingly, Aire-dependent processes for achieving promiscuous gene expression might also be active at the late differentiation stages of mTECs (see the subsequent paragraph). Clearly, this does not involve mTECs gaining the ability to express CD80 from CD80lo precursors (30) because GFP+ mTECs from Airegfp/gfp mice demonstrated normal levels of CD80 expression. It is necessary to dissect the developmental process of mTECs, thereby precisely identifying the Aire-dependent steps of mTEC differentiation.
Given that Aire-expressing cells are terminally differentiated, the demonstration that Aire+ mTECs are the major cell types responsible for expression of TRA genes, at least for Aire-dependent genes, apparently favors the terminal differentiation model for Aire-dependent promiscuous gene expression from mTECs (7, 10, 11). However, our results do support a key aspect of a role for Aire in the developmental model (17–19): absence of Aire in mTECs causes morphological changes together with altered distribution of mTECs committed to express Aire. Indeed, the difference in appearance of GFP-expressing cells was distinct enough to allow discrimination between Aire+/gfp and Airegfp/gfp mouse sections by blind analysis. Interestingly, Gillard et al. (18) noted that globular mTECs without visible cellular projections were more prominent in Aire-deficient thymus, which could represent the GFP+ globular mTECs we observed in Airegfp/gfp mice. Furthermore, expression of functional molecules, such as CD80 and MHC class II from mTECs noncommitted to express Aire, was also affected by the absence of Aire, suggesting that Aire and/or Aire+ mTECs influence the organization of mTECs beyond simply controlling promiscuous gene expression within Aire-expressing cell lineages. We do not believe that the demonstration that terminally differentiated Aire-expressing cells are the major source of promiscuous gene expression (apparently favoring the terminal differentiation model) and the demonstration that Aire and/or Aire+ cells controls thymic organogenesis (consistent with the developmental model; reference 18 and present study) are mutually exclusive. Instead, Aire could both promote the differentiation program of mTECs committed to express Aire, ensuring that they become fully equipped with the necessary machinery for promiscuous gene expression, and be an efficient driver of promiscuous gene expression in such cells. Thus, promiscuous gene expression seems to be accomplished in terminally differentiated mTECs that have matured in the presence of Aire protein (Fig. 8). Alternatively, Aire might be necessary for maintenance of a terminally differentiated state in which mTECs manifest a dendritic shape with fully competent promiscuous gene expression.
We found that the numbers of mTECs expressing involucrin, a marker of epidermal differentiation (22), were reduced in Aire-deficient mouse thymus. It was noteworthy that involucrin-expressing mTECs themselves were negative for Aire expression with immunohistochemistry (unpublished data), thus making it unlikely that involucrin gene expression in mTECs is under direct transcriptional control by Aire as a part of TRA gene expression. Similarly, it is unknown whether impaired involucrin expression is specific to mTECs committed to Aire expression or whether lack of Aire+ mTECs affects the differentiation of other type(s) of mTECs that would otherwise express involucrin at their terminally differentiated stages. Based on the fact that GFP+ Aire-less mTECs showed alterations in their morphology as well as distribution, we assume that the former possibility is more likely. Interestingly, we found that reduction of involucrin-expressing mTECs in Aire-deficient mice was associated with a nearly absence of Hassall's corpuscle-like structures, although the exact relevance of this phenotype to the breakdown of central tolerance in Aire-deficient mice remains unknown (31). Together with the fact that formation of thymic cysts is a predominant feature of Aire-deficient mice (18, 26), it seems likely that Aire exerts more global control of the differentiation program of mTECs than was initially thought.
Finally, although we have demonstrated that Aire organizes the global mTEC integrity that facilitates promiscuous gene expression in the thymic microenvironment, the exact nature of the mTEC differentiation program under the control of Aire protein still remains unknown. We have demonstrated that both Aire and an Aire-independent TRA gene, GAD67, are predominantly expressed by nonproliferative cells, although we cannot completely exclude the possibility that expression of these genes is associated with immature cells that turn over slowly and, thus, would be poorly labeled by BrdU. The results prompt us to propose a fascinating hypothesis that promiscuous gene expression is achieved by induction of heterogeneity among terminally differentiated mTECs rather than by multipotentiality of mTEC progenitors. We speculate that Aire may contribute to mTEC heterogeneity by acting on mTECs at the late differentiation stages and that lack of Aire may result in failure to create this heterogeneity. According to this scenario, additional mechanisms for the development of Aire-dependent autoimmunity might be possible beyond reduced TRA expression from Aire-deficient mTECs, for instance, altered Ag processing and/or presentation capacity by Aire-deficient mTECs (12) and/or altered T cell development affecting establishment of the complete T cell repertoire. Study of the mechanisms underlying the Aire-dependent production of heterogeneity among mature mTECs might be a rewarding approach to elucidating the nature of the negative selection niche in the thymus.
MATERIALS AND METHODS
Mice.
Aire/GFP knock-in mice (RIKEN Center for Developmental Biology accession No. CDB0483K) were generated by gene targeting as described previously (32). In brief, the targeting vector was constructed by replacing the genomic Aire locus starting from exon 1 (immediately after the Kozak sequence) to exon 2 with a GFP-neomycin resistance (neor) gene cassette (20). The neor gene cassette harbors loxP sites at both ends. The targeting vector was introduced into TT2 embryonic stem cells (33), and the homologous recombinant clones were first identified by PCR and confirmed by Southern blot analysis. After the targeted cells had been injected into morula-stage embryos, the resulting chimeric male mice were mated with C57BL/6 females (CLEA) to establish germ-line transmission. Aire+/gfp-neo mice were crossed with Ayu1-Cre mice (21), a general deleter Cre recombinase-expressing transgenic line, to remove the neor gene cassette. After confirming removal of the neor gene cassette, mice were crossed with C57BL/6 mice to select the line containing the GFP knock-in allele but not the Cre recombinase-expressing transgene. Aire+/gfp mice were then crossed to obtain Airegfp/gfp mice, which have the null mutation for the Aire gene. GAD67/GFP knock-in mice were heterozygous for GAD67-GFP (Δneo) as described previously (34). OT-II transgenic mice (35) were purchased from The Jackson Laboratory. The mice were maintained under pathogen-free conditions. The protocols used in this study were in accordance with the Guidelines for Animal Experimentation of Tokushima University School of Medicine and were conducted with the approval of the RIKEN Kobe Animal Experiment Committee.
Immunohistochemistry.
Mice were killed and the thymus tissues were fixed as described previously (25, 36). Immunohistochemical analysis of the thymus with UEA-1 (Vector Laboratories), rat anti-EpCAM mAb (BD), and rabbit polyclonal anti-K5 Ab (Covance) was performed as described previously (37). Rabbit polyclonal anti-Aire Ab was produced as described previously (13). Goat polyclonal anti-GFP Ab (Novus Biologicals) and rabbit polyclonal anti-GFP Ab (Invitrogen) were used for the detection of GFP-expressing cells. BrdU incorporation by mTECs was examined 4 h after i.p. injection of 1 mg BrdU/mouse, and the detection of BrdU incorporation was performed with anti-BrdU Ab (BD), as described previously (26). Rabbit polyclonal anti-p63 Ab was purchased from Santa Cruz Biotechnology, Inc. The level of cell shape complexity for each GFP+ cell was calculated by dividing the length of the cellular periphery by the cell area (i.e., periphery/area × 1/4π) measured by the WinROOF program (Mitani Corporation). After obtaining photos of the thymus sections stained with anti-GFP Ab, the photos were subjected to analysis with the software. Immunohistochemistry of the thymus sections and statistical analysis of cell shape complexity from different genotype of mice for comparison were processed simultaneously in the same set of experiment to minimize variability between the assays. Numbers of involucrin-expressing mTECs were assessed after staining the thymus sections with rabbit polyclonal Ab against mouse involucrin (Covance). Well developed EpCAM+ thymic medullas were examined for the presence of involucrin-expressing cells from several thymus sections obtained from individual mice.
TEC preparation and flow cytometric analysis.
TECs were prepared as described previously (12). In brief, thymic lobes were isolated from mice and cut into small pieces. The fragments were gently rotated in RPMI 1640 medium (Invitrogen) supplemented with 10% heat-inactivated FCS (Invitrogen), 20 mM Hepes, 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μM 2-ME at 4°C for 30 min and dispersed further with pipetting to remove the majority of thymocytes. The resulting thymic fragments were digested with 0.125% collagenase D (Roche) and 10 U/ml DNase I (Roche) in RPMI 1640 at 37°C for 15 min. The supernatants, containing dissociated TECs, were saved, and the remaining thymic fragments were further digested with collagenase D and DNase I. This step was repeated twice, and the remaining thymic fragments were digested with 0.125% collagenase/dispase (Roche) and DNase I at 37°C for 30 min. The supernatants from this digest were combined with the supernatants from the collagenase digests, and the mixture was centrifuged for 5 min at 450 g. The cells were suspended in PBS, containing 5 mM EDTA and 0.5% FCS, and kept on ice until the staining. The cells were stained with anti-CD45 mAb (BD) and UEA-1 and subjected to flow cytometric cell sorting with a FACS Vantage (BD). Flow cytometric analysis was performed after staining the cells with anti-CD45 mAb, UEA-1, anti-I-Ab (eBioscience), and anti-CD80 (eBioscience) mAbs with a FACSCalibur (BD) as described previously (13, 37).
Real-time PCR.
RNA was extracted from sorted mTECs with RNeasy Mini kits (QIAGEN) and made into cDNA with cDNA Cycle kits (Invitrogen) according to the manufacturer's instructions. Real-time PCR for quantification of the insulin 2, SAP1, CRP, and Hprt genes was performed as described previously (12, 13). The primers and the probes are as follows: insulin 2 primers, 5′-AGACCATCAGCAAGCAGGTC-3′ and 5′-CTGGTGCAGCACTGATCCAC-3′; insulin 2 probe, 5′-FAM-CCCGGCAGAAGCGTGGCATT-3′; SAP1 primers, 5′-ACTCCTTGTGTTGCTTGGTGTTT-3′ and 5′-TCGACTGAATCAGAGGAATCAACT-3′; SAP1 probe, 5′-FAM-TTCACCAGCAGAATCAGCAGTTCCAGAA-3′; CRP primers, 5′-TACTCTGGTGCCTTCTGATCATGA-3′ and 5′-GGCTTCTTTGACTCTGCTTCCA-3′; CRP probe, 5′-FAM-CAGCTTCTCTCGGACTTTTGGTCATGA-3′; Hprt primers, 5′-TGAAGAGCTACTGTAATGATCAGTCAAC-3′ and 5′-AGCAAGCTTGCAACCTTAACCA-3′; and Hprt probe, 5′-FAM-TGCTTTCCCTGGTTAAGCAGTACAGCCC-3′.
Statistical analysis.
All results are expressed as mean ± SEM. Statistical analysis was performed using Student's two-tailed unpaired t test for comparisons between two groups. Differences were considered significant if p-values were 0.05 or less.
Online supplemental materials.
Fig. S1 shows Aire-expressing cells in adult and embryonic thymi. Fig. S2 shows altered morphology together with the distribution of GFP+ Aire-less mTECs in Aire−/gfp mice. Fig. S3 shows altered morphology together with the distribution of GFP+ Aire-less mTECs in Aire−/gfp mice expressing the nonautoreactive OT-II TCR transgene. Fig. S4 shows altered morphology of GFP+ Aire-less mTECs in Airegfp/gfp mice at neonatal stage P1. Table S1 shows detailed information for mice analyzed for involucrin-expressing mTECs. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20080046/DC1.
Supplementary Material
[Supplemental Material Index]
Acknowledgments
We thank Drs. Y. Hamazaki, E.A. Robey and A.G. Farr for suggestions on immunohistochemistry.
This work was supported in part by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science and from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by Health and Labor Sciences Research Grants, Research on Psychiatric and Neurological Diseases and Mental Health (M. Matsumoto).
The authors have no conflicting financial interests.
Abbreviations used: Ab, antibody; Ag, antigen; Aire, autoimmune regulator; APECED, autoimmune-polyendocrinopathy-candidiasis ectodermal dystrophy; CRP, C-reactive protein; EpCAM, epithelial cell adhesion molecule 1; FSC, forward scatter; GAD67, glutamate decarboxylase 67; K5, keratin 5; mTEC, medullary thymic epithelial cell; SAP1, salivary protein 1; SSC, side scatter; TRA, tissue-restricted Ag; UEA-1, Ulex europaeus agglutinin 1.
References
- 1.Kamradt, T., and N.A. Mitchison. 2001. Tolerance and autoimmunity. N. Engl. J. Med. 344:655–664. [DOI] [PubMed] [Google Scholar]
- 2.Björses, P., J. Aaltonen, N. Horelli-Kuitunen, M.L. Yaspo, and L. Peltonen. 1998. Gene defect behind APECED: a new clue to autoimmunity. Hum. Mol. Genet. 7:1547–1553. [DOI] [PubMed] [Google Scholar]
- 3.Pitkänen, J., and P. Peterson. 2003. Autoimmune regulator: from loss of function to autoimmunity. Genes Immun. 4:12–21. [DOI] [PubMed] [Google Scholar]
- 4.Björses, P., M. Pelto-Huikko, J. Kaukonen, J. Aaltonen, L. Peltonen, and I. Ulmanen. 1999. Localization of the APECED protein in distinct nuclear structures. Hum. Mol. Genet. 8:259–266. [DOI] [PubMed] [Google Scholar]
- 5.Heino, M., P. Peterson, J. Kudoh, K. Nagamine, A. Lagerstedt, V. Ovod, A. Ranki, I. Rantala, M. Nieminen, J. Tuukkanen, et al. 1999. Autoimmune regulator is expressed in the cells regulating immune tolerance in thymus medulla. Biochem. Biophys. Res. Commun. 257:821–825. [DOI] [PubMed] [Google Scholar]
- 6.Hogquist, K.A., T.A. Baldwin, and S.C. Jameson. 2005. Central tolerance: learning self-control in the thymus. Nat. Rev. Immunol. 5:772–782. [DOI] [PubMed] [Google Scholar]
- 7.Kyewski, B., and L. Klein. 2006. A central role for central tolerance. Annu. Rev. Immunol. 24:571–606. [DOI] [PubMed] [Google Scholar]
- 8.Derbinski, J., A. Schulte, B. Kyewski, and L. Klein. 2001. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat. Immunol. 2:1032–1039. [DOI] [PubMed] [Google Scholar]
- 9.Anderson, M.S., E.S. Venanzi, L. Klein, Z. Chen, S.P. Berzins, S.J. Turley, H. von Boehmer, R. Bronson, A. Dierich, C. Benoist, and D. Mathis. 2002. Projection of an immunological self shadow within the thymus by the aire protein. Science. 298:1395–1401. [DOI] [PubMed] [Google Scholar]
- 10.Derbinski, J., J. Gabler, B. Brors, S. Tierling, S. Jonnakuty, M. Hergenhahn, L. Peltonen, J. Walter, and B. Kyewski. 2005. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J. Exp. Med. 202:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liston, A., D.H. Gray, S. Lesage, A.L. Fletcher, J. Wilson, K.E. Webster, H.S. Scott, R.L. Boyd, L. Peltonen, and C.C. Goodnow. 2004. Gene dosage–limiting role of Aire in thymic expression, clonal deletion, and organ-specific autoimmunity. J. Exp. Med. 200:1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuroda, N., T. Mitani, N. Takeda, N. Ishimaru, R. Arakaki, Y. Hayashi, Y. Bando, K. Izumi, T. Takahashi, T. Nomura, et al. 2005. Development of autoimmunity against transcriptionally unrepressed target antigen in the thymus of Aire-deficient mice. J. Immunol. 174:1862–1870. [DOI] [PubMed] [Google Scholar]
- 13.Niki, S., K. Oshikawa, Y. Mouri, F. Hirota, A. Matsushima, M. Yano, H. Han, Y. Bando, K. Izumi, M. Matsumoto, et al. 2006. Alteration of intra-pancreatic target-organ specificity by abrogation of Aire in NOD mice. J. Clin. Invest. 116:1292–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson, M.S., E.S. Venanzi, Z. Chen, S.P. Berzins, C. Benoist, and D. Mathis. 2005. The cellular mechanism of Aire control of T cell tolerance. Immunity. 23:227–239. [DOI] [PubMed] [Google Scholar]
- 15.Devoss, J., Y. Hou, K. Johannes, W. Lu, G.I. Liou, J. Rinn, H. Chang, R. Caspi, L. Fong, and M.S. Anderson. 2006. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J. Exp. Med. 203:2727–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto, M. 2007. Transcriptional regulation in thymic epithelial cells for the establishment of self tolerance. Arch. Immunol. Ther. Exp. (Warsz.). 55:27–34. [DOI] [PubMed] [Google Scholar]
- 17.Gillard, G.O., and A.G. Farr. 2006. Features of medullary thymic epithelium implicate postnatal development in maintaining epithelial heterogeneity and tissue-restricted antigen expression. J. Immunol. 176:5815–5824. [DOI] [PubMed] [Google Scholar]
- 18.Gillard, G.O., J. Dooley, M. Erickson, L. Peltonen, and A.G. Farr. 2007. Aire-dependent alterations in medullary thymic epithelium indicate a role for Aire in thymic epithelial differentiation. J. Immunol. 178:3007–3015. [DOI] [PubMed] [Google Scholar]
- 19.Gillard, G.O., and A.G. Farr. 2005. Contrasting models of promiscuous gene expression by thymic epithelium. J. Exp. Med. 202:15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moriguchi, T., M. Hamada, N. Morito, T. Terunuma, K. Hasegawa, C. Zhang, T. Yokomizo, R. Esaki, E. Kuroda, K. Yoh, et al. 2006. MafB is essential for renal development and F4/80 expression in macrophages. Mol. Cell. Biol. 26:5715–5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niwa, H., K. Araki, S. Kimura, S. Taniguchi, S. Wakasugi, and K. Yamamura. 1993. An efficient gene-trap method using poly A trap vectors and characterization of gene-trap events. J. Biochem. 113:343–349. [DOI] [PubMed] [Google Scholar]
- 22.Fuchs, E. 1990. Epidermal differentiation. Curr. Opin. Cell Biol. 2:1028–1035. [DOI] [PubMed] [Google Scholar]
- 23.Hale, L.P., and M.L. Markert. 2004. Corticosteroids regulate epithelial cell differentiation and Hassall body formation in the human thymus. J. Immunol. 172:617–624. [DOI] [PubMed] [Google Scholar]
- 24.Patel, D.D., L.P. Whichard, G. Radcliff, S.M. Denning, and B.F. Haynes. 1995. Characterization of human thymic epithelial cell surface antigens: phenotypic similarity of thymic epithelial cells to epidermal keratinocytes. J. Clin. Immunol. 15:80–92. [DOI] [PubMed] [Google Scholar]
- 25.Maemura, K., Y. Yanagawa, K. Obata, T. Dohi, Y. Egashira, Y. Shibayama, and M. Watanabe. 2006. Antigen-presenting cells expressing glutamate decarboxylase 67 were identified as epithelial cells in glutamate decarboxylase 67-GFP knock-in mouse thymus. Tissue Antigens. 67:198–206. [DOI] [PubMed] [Google Scholar]
- 26.Hamazaki, Y., H. Fujita, T. Kobayashi, Y. Choi, H.S. Scott, M. Matsumoto, and N. Minato. 2007. Medullary thymic epithelial cells expressing Aire represent a unique lineage derived from cells expressing claudin. Nat. Immunol. 8:304–311. [DOI] [PubMed] [Google Scholar]
- 27.Gray, D., J. Abramson, C. Benoist, and D. Mathis. 2007. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J. Exp. Med. 204:2521–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Senoo, M., F. Pinto, C.P. Crum, and F. McKeon. 2007. p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 129:523–536. [DOI] [PubMed] [Google Scholar]
- 29.Akiyoshi, H., S. Hatakeyama, J. Pitkänen, Y. Mouri, V. Doucas, J. Kudoh, K. Tsurugaya, D. Uchida, A. Matsushima, K. Oshikawa, et al. 2004. Subcellular expression of autoimmune regulator (AIRE) is organized in a spatiotemporal manner. J. Biol. Chem. 279:33984–33991. [DOI] [PubMed] [Google Scholar]
- 30.Rossi, S.W., M.Y. Kim, A. Leibbrandt, S.M. Parnell, W.E. Jenkinson, S.H. Glanville, F.M. McConnell, H.S. Scott, J.M. Penninger, E.J. Jenkinson, et al. 2007. RANK signals from CD4+3− inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J. Exp. Med. 204:1267–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, Y.J. 2006. A unified theory of central tolerance in the thymus. Trends Immunol. 27:215–221. [DOI] [PubMed] [Google Scholar]
- 32.Murata, T., K. Furushima, M. Hirano, H. Kiyonari, M. Nakamura, Y. Suda, and S. Aizawa. 2004. ang is a novel gene expressed in early neuroectoderm, but its null mutant exhibits no obvious phenotype. Gene Expr. Patterns. 5:171–178. [DOI] [PubMed] [Google Scholar]
- 33.Yagi, T., T. Tokunaga, Y. Furuta, S. Nada, M. Yoshida, T. Tsukada, Y. Saga, N. Takeda, Y. Ikawa, and S. Aizawa. 1993. A novel ES cell line, TT2, with high germline-differentiating potency. Anal. Biochem. 214:70–76. [DOI] [PubMed] [Google Scholar]
- 34.Tamamaki, N., Y. Yanagawa, R. Tomioka, J. Miyazaki, K. Obata, and T. Kaneko. 2003. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J. Comp. Neurol. 467:60–79. [DOI] [PubMed] [Google Scholar]
- 35.Barnden, M.J., J. Allison, W.R. Heath, and F.R. Carbone. 1998. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76:34–40. [DOI] [PubMed] [Google Scholar]
- 36.Kusser, K.L., and T.D. Randall. 2003. Simultaneous detection of EGFP and cell surface markers by fluorescence microscopy in lymphoid tissues. J. Histochem. Cytochem. 51:5–14. [DOI] [PubMed] [Google Scholar]
- 37.Kajiura, F., S. Sun, T. Nomura, K. Izumi, T. Ueno, Y. Bando, N. Kuroda, H. Han, Y. Li, A. Matsushima, et al. 2004. NF-κB-inducing kinase establishes self-tolerance in a thymic stroma-dependent manner. J. Immunol. 172:2067–2075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Material Index]