Regulation of Nuclear Positioning and Dynamics of the Silent Mating Type Loci by the Yeast Ku70/Ku80 Complex (original) (raw)
Abstract
We have examined the hypothesis that the highly selective recombination of an active mating type locus (MAT) with either _HML_α or HMRa is facilitated by the spatial positioning of relevant sequences within the budding yeast (Saccharomyces cerevisiae) nucleus. However, both position relative to the nuclear envelope (NE) and the subnuclear mobility of fluorescently tagged MAT, HML, or HMR loci are largely identical in haploid a and α cells. Irrespective of mating type, the expressed MAT locus is highly mobile within the nuclear lumen, while silent loci move less and are found preferentially near the NE. The perinuclear positions of HMR and HML are strongly compromised in strains lacking the Silent information regulator, Sir4. However, _HML_α, unlike HMRa and most telomeres, shows increased NE association in a strain lacking yeast Ku70 (yKu70). Intriguingly, we find that the yKu complex is associated with HML and HMR sequences in a mating-type-specific manner. Its abundance decreases at the _HML_α donor locus and increases transiently at MATa following DSB induction. Our data suggest that mating-type-specific binding of yKu to _HML_α creates a local chromatin structure competent for recombination, which cooperates with the recombination enhancer to direct donor choice for gene conversion of the MATa locus.
Long-range interactions between two genomic loci in distinct nuclear and chromatin environments are thought to influence recombination and transcription and to contribute to gene control during cell type differentiation in multicellular organisms (1, 17, 48, 50). In Saccharomyces cerevisiae, a- or α-cell type is determined by two homeobox-containing genes transcribed at the MAT locus. The ability of yeast cells to switch mating type requires transcriptionally silent copies of both a and α information, which are generally found at the homologous mating type loci HML and HMR (19). Genes at the HM loci, like genes in subtelomeric regions, are prone to position-dependent transcriptional repression. This repression is mediated by the recruitment and spreading of a Silent information regulatory (Sir) complex of Sir2, Sir3, and Sir4 (45).
For subtelomeric genes, Sir-mediated repression is facilitated by the clustering of telomeres near the nuclear envelope (NE) (18, 21, 51), which generates a high local concentration of Sir factors. Since Sir factors are limiting for repression (32), the juxtaposition of a reporter gene near such clusters favors repression (2). Telomere anchoring itself is mediated by two redundant pathways: one that requires the yeast Ku70/Ku80 (yKu70/80) complex, and a second that is mediated by Sir4 interaction with the membrane-associated Enhancer of silent chromatin 1 (Esc1) (21, 51). In contrast to the repression that is enhanced by NE association, the interaction of active genes with nuclear pore complexes can increase transcript levels for some inducible genes, possibly by providing a barrier between active and inactive chromatin domains (1, 48).
In this study, we have examined whether there is cell-type-specific nuclear positioning for either MAT or the donors of mating type information, HML and HMR, all of which are found on one of the smallest yeast chromosomes, chromosome 3 (Chr3). HM loci are positioned near the left and right telomeres of Chr3, while MAT is found midway along the longer right arm (Fig. 1A). Budding yeast has a sophisticated mechanism for directed recombination that allows a haploid cell of one mating type to preferentially recombine with donor sequences of the opposite mating type (reviewed in references 5 and 19). This results in a mating type switch that can occur as often as once per cell division. The directional recombination event is triggered by targeted cleavage at the MAT locus by the HO endonuclease, which is normally expressed only in late-G1-phase cells (26, 38). The DNA double-strand break (DSB) created by HO initiates the excision of the Ya- or Yα-specific sequences, which are replaced by sequences provided by one of the two silent HM donor loci, in a unidirectional gene conversion event (11). In addition to repressing transcription, Sir/nucleosome complexes also block cleavage by HO at HML and HMR (42, 43, 56).
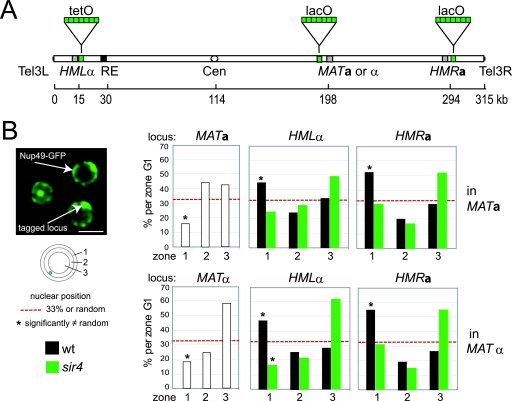
FIG. 1.
MAT adopts a central nuclear position, while HM loci assume a peripheral, Sir4-dependent location. (A) Schematic representation of Saccharomyces cerevisiae Chr3. GFP-LacI or GFP-TetR fusions allow visualization of the lacO and tetO arrays inserted near one of the three mating type loci. Distances are indicated in kb from the left telomere. (B) Positions were mapped relative to the NE in strains of GFP-tagged MAT loci (white), as well as HML and HMR loci in wt (black) and sir4 (green) cells. Data are represented in bar graphs as the percentage of spots in one of three concentric zones of equal surface area. The numbers of G1-phase MATa cells analyzed for the MAT, HML, and HMR loci were as follows: 80 for MAT, 181 (wt) and 135 (sir4) for HML, and 122 (wt) and 146 (sir4) for HMR. The numbers of G1-phase _MAT_α cells analyzed for the MAT, HML, and HMR loci were 69 for MAT, 103 (wt) and 87 (sir4) for HML, and 172 (wt) and 67 (sir4) for HMR. Confidence values (P) for the χ2 analysis between random (33% in each zone) and test distributions are summarized in Fig. 2. *, value significantly different from random (P < 0.05). Bar, 2 μm.
To ensure preferential gene conversion with the opposite mating type information, Chr3 contains a _cis_-acting enhancer of recombination (RE) located 15 kb centromere proximal from HML (Fig. 1A). The RE has been shown to be necessary and sufficient to render a large region of the left arm of Chr3 (Chr3L) more accessible for recombination, but only in a-type-specific cells (reviewed in reference 19). In its presence, _HML_α outcompetes HMRa for recombination with MATa (58). The RE harbors binding sites for Mcm1 and Fkh1/2 transcription factors, which alter chromatin structure at the RE to affect donor preference (13, 57). Intriguingly, the nucleosomal organizations of Chr3L are identical in a and α cells, with exception of a 3- to 4-kb region that spans the RE (14, 55).
It is not clear how donor preference is established or whether the RE is the only element that influences this event. Indeed, it was proposed that the preference of MATa for _HML_α could be achieved by a cell-type-specific subnuclear juxtaposition of the relevant loci. To test this, Simon et al. (49) examined the spatial relationship between the HM loci and MAT by fluorescently tagging pairs of loci in diploid cells in which one allele of the active MAT was deleted. They found that the relative positions of the three mating type loci are identical in MATa and _MAT_α cells. In particular, they demonstrated that _HML_α and MATa or HMRa and _MAT_α are not prealigned in G1 in either the presence or the absence of the RE (49). These loci only associate upon initiation of the recombination event. In a subsequent study, Bressan et al. (4) examined the relative distance between two green fluorescent protein (GFP)-tagged HML loci over time in diploids engineered to have one active mating type locus. The spatial relationship between the two HML loci appeared more constrained in α cells, and in a cells lacking the RE, as compared to wild-type (wt) a cells (4). They proposed that the RE increases Chr3L mobility uniquely in a-type cells, although the distances separating the two HMR loci are independent of both the mating type and the RE (4). In other words, the directional bias during mating type switching appeared to be independent of the relative position of the loci but correlated with variation in the dynamic constraint of the HML donor locus. These studies did not, however, examine the subnuclear position of the HM donor loci or the cleavage site at MAT, nor did they test mutants that might control locus mobility. Moreover, both previous studies monitored the spatial organization of MAT and HM loci in diploid cells, whereas mating type switching is normally a haploid-specific event (19).
Here, we have tagged MAT, HML, or HMR independently in haploid cells of wt and mutant MATa or _MAT_α strains to determine their absolute positioning and dynamic behavior relative to the fluorescently labeled NE. We show that the movement and radial positions of HML and HMR are maintained by distinct molecular mechanisms. Peripheral anchoring of HMR is Sir4 dependent, but it is independent of yKu. In contrast, the yKu70/80 complex participates in the constraint imposed on HML mobility while reducing its perinuclear anchoring, a mechanism distinct from that involved in telomere tethering (21, 51). Our data suggest that rather than a cell-type-specific subnuclear position, differential, cell-type-specific binding of yKu to _HML_α in MATa cells contributes to donor choice during mating type switching. We propose that the mating-type-specific binding of yKu at HML engenders a local chromatin structure competent for recombination that cooperates with the RE to enhance exchange with MATa.
MATERIALS AND METHODS
Plasmid, strains, and yeast methods.
The yeast strains used are listed in Table 1. Yeast cells were grown at 30°C in rich glucose media (yeast extract-peptone-dextrose [YPD]) unless otherwise indicated. Plasmids used to integrate the tet or lac operator arrays and repressors were as described previously (6). The following PCR-amplified genomic fragments (SGD coordinates) were used for insertion within 1 to 2 kb from the respective loci: 15160 to 15773 for HML, 294892 to 295241 for HMR, and 197194 to 196910 for MAT on Chr3. LacI-GFP, TetR-GFP, and, where indicated, GFP-Nup49 fusions were introduced as described previously (6, 24). Complete y_ku70_, sir4, and RE deletions were obtained using a PCR-based gene deletion technique (30). The region containing the RE comprised between coordinates 28717 and 30035 of Chr3 was replaced by the TRP1 gene. Plasmid SG151 (3) contains a replacement of HML with the URA3 gene flanked by endogenous sequences for integration at the HML locus after HindIII excision. Deletion of HML sequences was verified by PCR and Southern blotting (not shown). The YCp-based pGAL-HO plasmid was previously described (23). The DSB at MAT was induced in yeast cells carrying the HO plasmid or a galactose-inducible HO transgene by addition of 2% galactose. Cells were grown for 18 h in glycerol lactate medium to 0.4 × 107 cells/ml. After 30 to 60 min in galactose, cells were washed and transferred to glucose-containing medium or plated on YPD, except for chromatin immunoprecipitation (ChIP) experiments, in which cells were kept in galactose-containing medium. Switching efficiency was assessed by colony mating tests (23).
TABLE 1.
Yeast strains used in this study
| Name(s) | Parent strain | Genotype (change over parent) | Source or reference |
|---|---|---|---|
| GA-180 | MATa_HML_α HMRa_ade2_-_1 can1_-_100 his3_-_11,15 leu2_-_3,112 trp1_-1 ura3-1 | W303-1A | |
| GA-1320 | GA-180 | MATa_ade2_-_1 can1_-_100 his3_-11,15::_HIS3p_-GFP-_LacI-HIS3 trp1_-_1 ura3_-_1 leu2_-3,112 nup49::NUP49-GFP | 24 |
| GA-1320 α | GA-1320 | _MAT_α | This study |
| GA-2254 | GA-180 | MATa_ade2_-1::_URA3p-tetR_-GFP-_ADE2 can1_-_100 his3_-_11,15 trp1_-_1 ura3_-_1 leu2_-3,112 nup49::NUP49-GFP | 7 |
| GA-2254 α | GA-2254 | _MAT_α | This study |
| GA-2909 (MRG2253) | W303-1A | MATa RS::E-HMR-I_-TRP1 lacO::RS Δ_sir3::HIS3 (HMR::lacO-TRP1) | 15 |
| GA-2194 (MRG2251) | GA-2909 | MATaSIR3::URA3::Δ_sir3_::HIS3 | 15 |
| GA-2910 | GA-2194 | MATasir4::KanMX | This study |
| GA-2911 | GA-2194 | MATayku70::KanMX | This study |
| GA-2196 | GA-1320 a | MATaMAT::lacO-TRP1 | 7 |
| GA-2915 | GA-1320 α | _MAT_α MAT::lacO-TRP1 | This study |
| GA-2193 | GA-2254 | MATaHML::tetO-LEU2 | 7 |
| GA-2323 | GA-2254 α | _MAT_α HML::tetO-LEU2 | This study |
| GA-2324 | GA-2193 | MATayku70::HIS3 | This study |
| GA-2325 | GA-2323 | _MAT_α yku70::HIS3 | This study |
| GA-2326 | GA-2193 | MATasir4::KanMX | This study |
| GA-2327 | GA-2323 | _MAT_α sir4::KanMX | This study |
| GA-2328 | GA-2324 | MATayku70::HIS3 sir4::KanMX | This study |
| GA-2913 | GA-2193 | MATahml::URA3 | This study |
| GA-4592 | GA-2913 | MATasir4::HIS3 | This study |
| GA-2914 | GA-2913 | MATayku70::HIS3 | This study |
| GA-2330 | GA-2193 | MATare::TRP1 | This study |
| GA-2331 | GA-2323 | _MAT_α re::TRP1 | This study |
| GA-2332 | GA-2330 | MATayku70::HIS3 | This study |
| GA-2333 | GA-2331 | _MAT_α yku70::HIS3 | This study |
| GA-426 | W303-1A | MATa_HML_α HMRa_ade2_-1 can1::_hisG his3_-_11,15 leu2_-_3,112 trp1_-_1 ura3_-1VR-ADE2-TEL | H. Renauld |
| GA-1009 | GA-426 | MATayKu80-myc-KanMX | 34 |
| GA-3092 | GA-426 | MATayKu70-myc-TRP1 | This study |
| GA-2293 | W303-1A | MATaMcm1-myc | 9 |
| GA-2166 | JKM154 | MATa_ade1 leu2_-_3,112 lys5 trp1 ura3_-52 ade3::GAL HO | 27 |
| GA-3339 | GA-2166 | MATaYKU70-myc-TRP1 | This study |
| GA-3340 | GA-2166 | MATaMCM1-myc-TRP1 | This study |
| GA-1061 | GA-1009 | MATasir4::HIS3 | 34 |
| GA-3930 | _MAT_α _trp1_-_1 his3_-_11 his3_-_15 ura3_-_1 leu2_-_3 leu2_-112 RAD52-GFP | R. Rothstein | |
| GA-5194 | GA-3930 | NUP49::URA3::_NUP49_-GFP | This study |
| GA-5196 | GA-5194 | _NUP49_-GFP | This study |
| GA-5239 | GA-5196 | MATa | This study |
Microscopy.
For live imaging, cultures grown in YPD to 0.2 × 107 to 0.4 × 107 cells/ml were imaged on synthetic complete (SC) agar plus 4% glucose patches or in a Ludin chamber at 30°C. Subnuclear position assignment was performed on 21-image (190-nm step size) stacks of living cells acquired on an Olympus IX70 microscope as described previously (21). Cell cycle phase was determined by visual inspection of transmission images. Radial positions of tagged loci were determined as a percentage of fluorescent spots in one of three concentric nuclear zones of equal surface in the plane bearing the brightest GFP-lacI, GFP-tetR or GFP-Rad52 focus. Only the 10 core focal planes were scored (22). The outermost zone, zone 1, extends from the middle of the Nup49-GFP ring inwards to 0.816 r, with r being the radius of the nucleus in the relevant focal plane (Fig. 1B), while the limit between zones 2 and 3 is fixed at 0.578 r. More pronounced enrichment at the periphery was observed for HMR and many other loci (unpublished observations) in cells grown in galactose instead of glucose, explaining why we see lower enrichment in zone 1 for HMR (compare Fig. 1B to Fig. 1 in reference 15). Time-lapse imaging was performed on a Zeiss LSM510 confocal microscope using a 100× Plan-Apochromat objective (numerical aperature, 1.4). Live imaging was performed as described previously (7) with closed pinhole (1 to 1.2 Airy units; GFP at 488 nm with 0.1 to 1.0% transmission). Two-dimensional time-lapse series were analyzed with the spot tracking plug-in for Image J (47). For each strain, we analyzed 8 to 12 independent two-dimensional time-lapse series of 100 confocal images acquired at 1.5-s intervals of G1-phase nuclei, following the tagged foci by adjusting the focal plane (22, 25). Locus mobility and the parameters of constraint can be characterized by plotting the mean squared displacement (MSD), or d(Δ_t_)2 = [r(t + Δ_t_) − r(t)]2, over increasing time intervals (Δ_t_) from 0 to 105s. In this study, we monitored spatial constraints based on measurements that reflect the actual distances, d, of the tagged locus covered from any one time point to all others after an alignment of nuclear centers in all frames.
ChIP.
ChIP was performed and quantified as described previously (34, 53; http://www.epigenome-noe.net). Strains were either grown in YPD-adenine medium to 0.5 × 107 to 1 × 107 or in rich medium containing 3% glycerol, 2% lactic acid, and 0.05% glucose to 1 × 106 to 5 × 106 cells/ml, after which 2% galactose (to induce HO) or 2% glucose (to repress HO) was added. Wash buffers contained 0.5 M LiCl. Monoclonal antibodies against Myc (9E10) for the Myc-tagged proteins and hemagglutinin (HA; 12CA5) for the negative control were prebound to Dynabeads (Dynal Biotech). The amplified DNA regions were quantified by real-time PCR performed using the Perkin-Elmer ABI Prism 7000 sequence detector system and software. Amplified regions in the Saccharomyces cerevisiae genome correspond to SGD Chr3 coordinates 13124 to 13151 for _HML/MAT_α, 29184 to 29214 for RE (which includes the Mcm1 binding site), and 294114 to 294138 for HMR/MATa and Chr6 coordinates 269582 to 269614 for telomere 6R (Tel6R). Efficiency of the PCR and linearity of the signal with input DNA quantity were ensured for all primer pairs used. ChIP experiments were done in duplicate, quantitative PCR (QPCR) runs were done in triplicate, and threshold cycle values for all amplicons, samples, and runs were carefully monitored to ensure that they fall within the linear range of amplification. When strains carrying Myc-tagged copies of yKu70, yKu80, or Mcm1 but lacking an HA epitope were used (see Fig. 6B, C, E, and F), enrichment was calculated by first normalizing the test sample to a control locus (SMC2) for both anti-Myc and anti-HA and then dividing the Myc-dependent signal by the HA background. In Fig. 6D, we compared the anti-Myc immunoprecipitate from a yKu80-Myc strain with a nontagged control strain, after the test/SMC2 value from the anti-Myc immunoprecipitate was normalized to the same ratio from the input material. ChIP results are presented as the mean of two to three experiments (with real-time PCR performed in triplicate) ± the standard error of mean. The efficiency of DSB induction was determined by PCR on input DNA as described, except that primers SG1298 5′-AGTCACATCAAGATCGTTTATGG-3′ and SG1300 5′-ACTCCACTTCAAGTAAGAGTTTG-3′, which flank the HO cut site at MATa, were used (53).
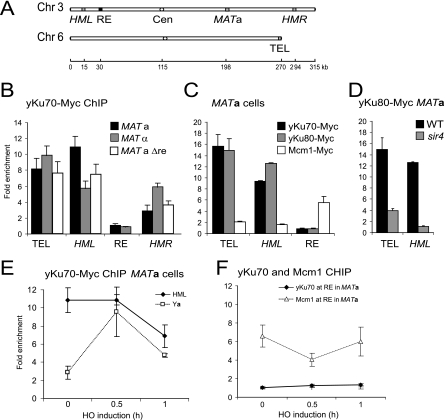
FIG. 6.
Mating-type-specific binding of yKu70/80 to the silenced HML locus, but not to the RE. ChIP was performed as described in Materials and Methods using strains bearing Myc-tagged copies of yKu70 (GA-3339; yKu70-Myc) and yKu80 (GA-1009; yKu80-Myc) and GA-3340 (Mcm1-Myc). (A) Real-time QPCR was performed with probe/primer sets that amplify the indicated loci of Chr3, a telomeric locus, and the SMC2 control locus on Chr6. Enrichment relative to the SMC2 control locus and a negative control ChIP (B, C, E, and F) or relative to a nontagged strain (D) as the mean ± standard error of the mean are shown. For calculation of enrichment, see Materials and Methods. (B) yKu70 binds to a telomere, HML, and HMR, but not the RE in exponentially growing MATa and _MAT_α cells. yKu70 has a preference for HML in MATa, but not in _MAT_α, cells. (C) Mcm1, but not yKu70 and yKu80, binds the RE in exponentially growing MATa cells. yKu70 and yKu80 bind to a telomere and HML, while Mcm1 does not. (D) yKu70 binding is abolished at both telomeric and HML loci in the absence of Sir4. ChIP was performed as described in Materials and Methods using a wt strain and an isogenic strain with sir4 deleted, each bearing a Myc-tagged copy of yKu80. (E) ChIP was performed for yKu70-Myc on exponentially growing MATa cells (GA-3339) before and after 30 min and 60 min of Gal1::HO expression. Efficiency of DSB induction at the MAT locus was determined by PCR using primers that span the HO cut site at MAT and primers that anneal to the SMC2 control locus on Chr6. DSB induction monitored as loss of the MAT product relative to the SMC2 signal yielded cleavage efficiencies of 86% and 96% at 0.5 h and 1 h after HO induction, respectively. At 60 min after HO induction, switching is nearly complete and yKu70 binding resembles that observed in the MATa strain (data not shown). (F) Mcm1 binds to the RE before and after HO induction, but yKu70 does not. Results are shown as in panel E, except that yKu70-Myc MATa, and Mcm1-Myc MATa cells (GA-3340) were used. Cleavage efficiencies were 86% and 96% (yKu70-Myc _MAT_a) and 60% and 82% (Mcm1-Myc _MAT_a) at 0.5 h and 1 h after HO induction, respectively.
Statistics and error calculation.
We determined that our measured frequencies of foci in each zone deferred from a theoretical random distribution by using a χ2 test for goodness of fit with a degree of freedom of 2 and a confidence limit of 95%. To establish whether the frequency of tagged loci in zone 1 is different between two genotypes, we used a proportional analysis with a confidence limit of 95% (40).
RESULTS
Radial positioning of MAT and HM loci is independent of mating type.
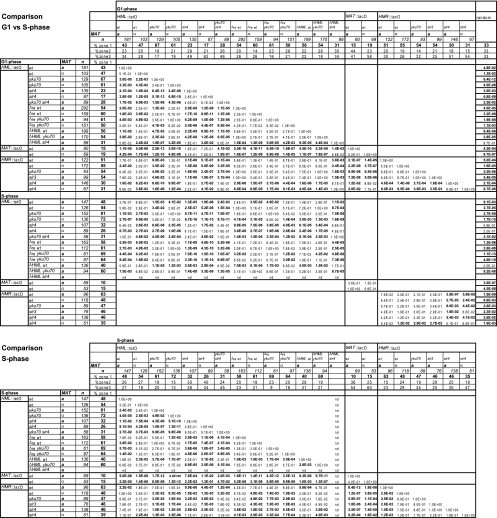
We examined the radial positions of the actively transcribed MAT locus situated near the center of Chr3 and of the silent HML and HMR loci located near the left or right telomere of the same chromosome in MATa and _MAT_α cells (Fig. 1A). Site-specific insertions of sequence repeats of lac or Tet operators (lacO or tetO) were visualized using LacI or TetR repressor-GFP fusion proteins. Data were acquired in three dimensions to determine the position of the resulting fluorescent spot relative to the GFP-tagged NE in the focal plane in which it was brightest (Fig. 1B; and see Materials and Methods). In both MATa and _MAT_α cells, radial positions of MAT, HML, and HMR were scored for cells grown in rich glucose-containing medium, as the percentage of fluorescent spots in one of three concentric nuclear zones of equal surface. We found that in either cell type the MAT locus was depleted from the outermost zone 1 (<20% versus 33% for a random distribution), indicating that MAT is preferentially positioned in the nuclear center in G1-phase cells (Fig. 1B). _MAT_α, furthermore, showed a preference for zone 3 (Fig. 1B and Fig. 2). In contrast, the positions of both silent HM loci were significantly enriched in the outermost zone in both mating types (≥43% spots in zone 1; for statistical significance, see Fig. 2). Generally HMR was more efficiently anchored at the nuclear periphery than HML (zone 1 HMR values were 51% and 55% in MATa and _MAT_α cells, respectively, versus 43% and 47% for HML), although the difference between HML and HMR was only statistically significant in S-phase MATa cells. In MATa cells in S phase, anchoring was improved for both HML and HMR over G1-phase values (Fig. 2), as had been reported for other telomeres (21). In the present study, we confirm slight variations between G1- versus S-phase results (Fig. 2), yet the conclusions that we draw from G1-phase data appear to hold true for S-phase cells as well. For simplicity, we will only discuss G1-phase data.
FIG. 2.
Summary of HML, MAT, and HMR locus positioning data from comparison of G1- and S-phase cells. Shown are the percentages of total cells counted in three equal-surface concentric nuclear zones of the indicated tagged loci in the wt and indicated mutant strains in the G1 and S phases. n, number of cells analyzed. Confidence values (P) using a proportional analysis between two test frequencies or a χ2 analysis between a predicted random distribution (33% in each zone) and test distributions are given for S- and G1-phase values. Bold numbers indicate P < 0.05.
Peripheral anchoring of the HM loci is Sir4 dependent.
The HML and HMR lacO tags are equidistant to the left and right ends of Chr3, being inserted at 17 kb and 20 kb from the TG repeats, respectively. This proximity suggested that their positions may be regulated by the same pathways that regulate telomere anchorage and, most probably, through the components of silent chromatin itself. Indeed, it has been shown that an excised ring of HMR chromatin associates with the NE in a Sir3-dependent manner (15). Sir4 is crucial for the nucleation of the Sir2/3/4 complex thanks to its affinities for Rap1 and yKu bound at telomeres and for Rap1/Abf1/ORC-Sir1 at HM silencers (31, 46). We therefore analyzed the dependence of HM positioning on Sir4-Esc1 and on yKu.
We first scored the impact of a complete sir4 deletion on the nuclear localization of HML and HMR in both cell types (Fig. 1B). As expected, in the absence of Sir4 we found that HML was strongly depleted from the outermost nuclear zone (drop in zone 1 occupancy in MATa from 43% to 23%, P = 2.1 × 10−4; and drop in zone 1 occupancy in _MAT_α from 47% to 17%, P = 1.2 × 10−5). Similarly, the derepressed HMR locus lost NE anchorage and was no longer enriched at the nuclear rim (P = 4.6 × 10−4 in MATa and P = 8.5 × 10−4 in _MAT_α when comparing sir4 and wt distributions). We note, however, that a significant degree of peripheral anchoring was retained for HMR in a sir3 mutant (54% occupancy in zone 1) (Fig. 2), suggesting that the binding of Sir4 to Orc1 or Rap1 at the HMR silencer elements or at nearby telomeric repeats (31, 46) is sufficient to maintain weak anchoring even in the absence of silencing.
yKu antagonizes HML association with the NE.
Recent studies have shown that yKu can be detected at HM silencers and that silencing efficiency at HMR and HML is affected by deletion of either yKu subunit, albeit only in the absence of Sir1 (41, 54). This nonetheless suggested that yKu might act directly at HM loci. We therefore next examined whether the subnuclear position of HML is altered in a yku70 deletion strain. Consistent with data on the HMR chromatin ring (15), we found that the radial positions of HMR were almost identical in wt and yku70 deletion strains (P = 0.66) (Fig. 3). However, to our surprise, the perinuclear anchoring of HML was greatly improved in yku70 mutant cells in both mating types (67% and 61% in zone 1 versus 43% and 47% in wt MATa and _MAT_α cells) (Fig. 2 and 3). This change was significant in both cell types (P = 3.0 ×10−5 and P = 4.3 × 10−2 MATa and _MAT_α cells, respectively, compared to the wt) (Fig. 2).
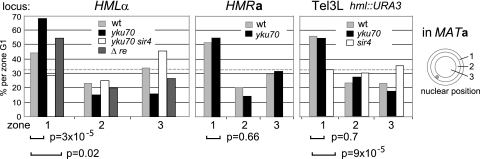
FIG. 3.
Anchoring of HML is increased in the absence of yKu70 and of the RE. Positions relative to the NE (percentage in zone 1) (Fig. 1) in MATa wt (light gray), yku70 (black), yku70 sir4 (white), and Δ_re_ (dark gray) strains GFP tagged at HML, HMR, or HML in the absence of the silenced HML locus (Tel3LΔ_hml_). The confidence values (P) for the proportional analysis between two test frequencies are given. For complete statistical data, see Fig. 2.
This unusual behavior could reflect unique features either of the left arm of Tel3 (Tel3L) or of HML. To test this, we replaced the HML locus including the E and I silencers with the URA3 gene, and monitored the position of the lacO array inserted 17 kb from Tel3L with or without HML. Intriguingly, the removal of the silent locus itself improved peripheral anchoring of this lacO array (56% in zone 1 versus 43% in wt MATa; P = 0.011) (Fig. 2 and 3). This result argues that the silent HML locus antagonizes association with the NE. As expected, the increased anchoring of Tel3LΔ_hml_ to the NE was fully dependent on Sir4 (31% in zone 1 in a sir4 mutant) (Fig. 3). However, it was independent of yKu, again suggesting that yKu association with HML antagonizes NE tethering by Sir4 (54% versus 56% in zone 1 in yku70 versus wt strains, respectively) (Fig. 3). The simplest explanation of these results is that the association of yKu antagonizes the interaction of _HML_α-bound Sir4 with Esc1. Alternatively yKu binding could promote association with an internal anchorage site, although the character of such putative internal binding site in yeast is unknown. In either case, the loss of yKu70 would allow the Sir complexes bound at HML to shift the locus to Esc1 at the nuclear periphery. In support of this model, we note that the double deletion of yku70 and sir4 led to the release of the HML locus from the NE (28% in zone 1) (Fig. 2 and 3).
HML anchorage is distinct from that of other telomere proximal sites.
We asked whether the unusual behavior of HML/Tel3L with respect to perinuclear binding might reflect the distance of the tag from the chromosome end. Based on a reservoir of data from our laboratory on telomere position determined in an identical fashion (7, 20, 21, 51), we plotted the distances of the lacO or tetO insertion sites in kb from the respective chromosome end against the percentage of fluorescent foci in zone 1 for 12 telomeres (Fig. 4). Zone 1 values varied from 35% to 71%, yet there was no correlation of anchorage efficiency with the distance of the tag from the TG repeat. Overall, loss of Sir4 reduced anchoring, as did loss of yKu70, albeit to different degrees. Only Tel5R, Tel3LΔ_hml_, and HMR were insensitive to the absence of yKu70, and HML is the only locus whose perinuclear anchoring increased significantly upon deletion of yku70. This behavior is characteristic of HML and not of distal Tel3L sequences, since upon deletion of HML Tel3L no longer increases its association with the NE in the yku70 mutant (Tel3LΔ_hml_). The unique behavior of HML with respect to the NE and yKu is true in S- as well as G1-phase cells (Fig. 2).
FIG. 4.
Influence of lac or tet operator repeat insertion site distance to the telomere on peripheral anchoring. The degree of peripheral anchoring of the HM loci compared with previously analyzed zone 1 values for telomere proximal sites (7, 20, 21, 51) in wt, yku70, and sir4 mutant strains. lac or tet operator insertion site distances in kb from the respective chromosome end plotted against scored positions relative to nuclear pores. HML_α values are in blue and are marked by asterisks. The left arm of Chr3 bearing the HML deletion (Tel3LΔ_hml) is in red and is labeled 3LΔ. 6Rt indicates Tel6R bearing a truncation of 5 kb.
The RE participates in HML positioning.
Although subnuclear positioning of the mating type loci relative to the NE is independent of mating type (Fig. 2), positioning might nonetheless be influenced by the RE, which is found 15 kb centromere proximal from HML. To test this, we deleted a 1.3-kb fragment encompassing the RE (Fig. 1A) and confirmed that the MATa Δ_re_ strain no longer used _HML_α as a donor for the mating type switch (Table 2) (58). Next we analyzed the subnuclear position of HML. Strikingly, we found that in the absence of the RE, HML shifted to a more peripheral position; zone 1 values in G1 cells increased from 43% to 54% in MATa and from 47% to 60% in MAT_α cells (P = 0.02 and P = 0.039 compared to wt) (Fig. 2 and 3). Thus, the presence of the RE, like yKu, negatively affects the perinuclear anchoring of HML. The increase in anchoring of HML provoked by yku70 deletion was more pronounced than the effect of deleting the RE (P = 0.013 comparing HML distributions in Δ_re and yku70 MATa strains) (Fig. 2 and 3). Nonetheless, the two were epistatic: combining the deletions had but a slight and statistically insignificant additive effect on the perinuclear anchoring of HML (P = 0.23 and P = 0.077 in MATa and _MAT_α, respectively) (Fig. 2). Taken together, these results suggest that the RE and yKu cooperate to limit the association of HML with the NE.
TABLE 2.
Effect of yku mutation on directional mating type switchinga
| MATa strain | % Switching efficiency | No. of colonies counted |
|---|---|---|
| wt | 78 | 680 |
| ku70 | 56 | 445 |
| Δ_re_ | 8 | 311 |
yKU and RE affect mating type switch preference.
The increased association of HML with the nuclear periphery in the Δ_re_ mutant correlates with a decreased use of _HML_α as a donor in MATa strains (19, 58). We next examined whether the directionality of mating type switching would be compromised in MATa yku70 cells as it is in cells lacking the RE. Using a quantitative mating type assay, we confirm a 28% reduction in the use of _HML_α in the MATa yku70 strain, compared to its use in a wt background (Table 2) (see also reference 44). While the drop due to the absence of yKu is clearly significant, loss of the RE reduces the switching preference even more (90% down; see also reference 58). It has been carefully documented that _yku_-deficient strains do not show a general decrease in homologous recombination (39); thus, it seems that yKu indeed contributes to the preference of _HML_α as a donor for MATa mating type switching. At the same time, yKu antagonizes the anchorage of Tel3L to the NE, in a manner dependent both on the silent mating type locus HML and the nearby RE. Although it is unlikely to be the only factor influencing donor choice, the sequestration of _HML_α at the NE correlates with its diminished use as a donor for mating type switching.
Rad52-mediated repair takes place in the nuclear lumen.
Given that mating type switching is a gene conversion event that relies on the homologous recombination (HR) machinery, we next asked where HR occurs in a normal cell. Recombination requires a cascade of events mediated by proteins that form foci during the repair process (28). Both the random induction of DSB by ionizing radiation and the targeted cleavage of DNA by HO endonuclease lead to the formation of a focus containing Rad52, a protein essential for both reciprocal exchange and gene conversion events. Rad52 foci also form spontaneously at low frequency in S-phase cells, when replication forks encounter DNA damage (29). To explore the significance of HML association with the NE in the context of recombination, we scored the subnuclear position of spontaneous, hydroxyurea (HU)- or methyl methanesulfonate (MMS)-induced Rad52 foci relative to the Nup49-tagged nuclear rim. In striking contrast to telomeric foci, we see that Rad52 foci localize preferentially in the nuclear interior under all conditions tested (Fig. 5A). This is also true for Rad52 foci induced by zeocin or by combinations of MMS and HU (data not shown). Although we cannot rule out transient interaction with the NE, these data suggest that HR itself does not occur at the nuclear periphery, consistent with the position scored for MAT (Fig. 1 and 3).
FIG. 5.
Gene conversion takes place in the nuclear lumen. (A) Spontaneous and damage-induced foci of Rad52-GFP were scored mainly in S-phase cells in asynchronous (asynchr) cultures of GA-5196. Cultures were grown to exponential phase and then cultured for 60 min or 120 min in the presence of DNA-damaging agents as indicated by the number of foci counted: spontaneous, n = 244; HU treated, n = 192 and n = 96 for 60 and 120 min, respectively; MMS treated, n = 249 and n = 268 for 60 and 120 min, respectively. For treatment in synchronized cultures (synchr), GA-5239 cells were grown to exponential phase and arrested in G1 by incubation with α-factor for 1.5 h. The cells were subsequently released into SC medium (spontaneous, n = 152) or into MMS (n = 130 and n = 105 for 60 and 120 min, respectively), prior to scoring Rad52 focus location. (B) Positions relative to the NE of MATa wt cells before (0 min; white) and during (60 min; black) gene conversion. (C) Positions relative to the NE of HML in MATa wt and yku and _MAT_α wt cells before (0 min; white) and during (60 min; black) gene conversion. Asterisks indicate statistically significant difference between distributions at 0 and 60 min (P = 4.5 × 10−3). Cells were grown in lactate-containing medium, and at 0 min, galactose was added to induce expression of the HO endonuclease from a plasmid. Gray bars indicate the position of HML relative to the NE in cells not bearing the HO plasmid but grown in galactose. For complete statistical data, see Table 3.
The positioning of the mating type loci reported above was determined in yeast strains that are rendered _ho_− in order to prevent mating type switching. To see if the induction of the HO-dependent DSB at MAT influences the subnuclear position of the break or HM donors, we scored the location of the DSB at MAT and of HM donor loci under conditions of repair, by inducing HO cleavage by the addition of galactose in pheromone-synchronized MATa cells. We note that gene conversion is already fairly pronounced by 20 and 60 min after HO induction, in that 18% and 62%, respectively, of the MATa cells have switched mating type (data not shown). This is achieved by using _HML_α as a donor to repair the HO-induced break. In all cells monitored, whether they expressed HO or not, MAT was preferentially retained in the center of the nucleus at both the 20-min (data not shown) and 60-min (Fig. 5B and Table 3) time points (37). These data suggest that the gene conversion event, and thus the interaction between MAT and HML, takes place in the nuclear lumen. Although transient contacts of MAT with the nuclear periphery undoubtedly occur, MAT does not accumulate there before or after cleavage. Considering the localization of MAT after cut induction, together with finding that Rad52 foci are enriched in zone 3 and depleted from the NE, we conclude that gene conversion most likely occurs in the nuclear lumen.
TABLE 3.
Summary of positioning data after DSB induction at MATa
| Induction time | Locus | Strain | MAT type | No. of cells analyzed | % of total cells counted in: | P for comparison: | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 min | 60 min + HO | ||||||||||
| Zone 1 | Zone 2 | Zone 3 | MAT wt | HML wt | MAT wt | HML wt | |||||
| 0 min | MAT | wt | a | 127 | 19 | 25 | 56 | 1 | |||
| HML | wt | a | 252 | 55 | 25 | 20 | 2.3E−11 | 1 | |||
| yku70 | a | 232 | 50 | 25 | 25 | 8.5E−09 | 2.7E−01 | ||||
| wt | α | 106 | 54 | 26 | 20 | 2.4E−08 | 8.6E−01 | ||||
| yku70 | α | 107 | 56 | 33 | 11 | 4.1E−09 | 8.6E−01 | ||||
| 60 min | |||||||||||
| +HO | MAT | wt | a | 74 | 14 | 16 | 70 | 3.6E−01 | 4.8E−10 | 1 | |
| HML | wt | a | 225 | 42 | 15 | 43 | 1.1E−05 | 4.5E−03 | 1.1E−05 | 1 | |
| yku70 | a | 279 | 56 | 20 | 24 | 3.4E−12 | 8.1E−01 | 1.2E−10 | 1.7E−03 | ||
| wt | α | 110 | 52 | 28 | 20 | 9.4E−08 | 5.9E−01 | 1.5E−07 | 8.4E−02 | ||
| yku70 | α | 107 | 60 | 18 | 22 | 1.1E−10 | 3.8E−01 | 6.3E−10 | 2.1E−03 | ||
| −HO | MAT | wt | a | 78 | 11 | 33 | 56 | 1.2E−01 | 8.4E−12 | 5.7E−01 | 6.3E−07 |
| HML | wt | a | 90 | 55 | 25 | 20 | 3.4E−08 | 1.0E+00 | 5.7E−08 | 3.6E−02 | |
| wt | α | 71 | 57 | 22 | 21 | 4.5E−08 | 7.6E−01 | 5.8E−08 | 2.6E−02 |
A similar experiment was performed in a MATa strain bearing a tagged _HML_α locus to monitor the position of the donor locus. Strikingly, the donor locus shifted to a more central position upon induction of the DSB (from 55% to 42% in zone 1) (Fig. 5C and Table 3). No shift was observed when galactose was added to cells lacking the HO plasmid. Indeed, _HML_α is equally peripheral in cells grown for 60 min in galactose and in glycerol/lactate irrespective of mating type (compare results at 0 and 60 min for −HO in Fig. 5C and Table 3). Importantly, after HO induction the shift in the nuclear position of _HML_α in a MATa strain was not observed in _MAT_α strains (52% of _HML_α spots in zone 1 (Fig. 5C and Table 3). Thus, despite the lack of correlation between radial position and mating type in wt G1-phase cells, the DSB induced shift of the donor locus toward the nuclear interior is mating type specific.
Finally, we scored the nuclear localization of HML following induction of a DSB at MATa in a yku70 strain. In the absence of yKu70, HML did not change position even after 60 min of HO endonuclease expression (56% of HML spots remained in zone 1) (Fig. 5C and Table 3). This correlates with a reduced efficiency of mating type switching in MATa cells and argues that the subnuclear position of HML may be relevant for efficient recombination. Tight anchoring to the nuclear periphery could delay and thus reduce interaction between the donor and the cleaved MAT locus.
yKu70/80 binds HML, but not the RE, in a mating type-specific manner.
Given that the loss of yKu perturbs HML localization (Fig. 3) and HMR anchoring in an esc1 deletion (15), we hypothesized that yKu may bind directly to the HM loci. To quantify this and compare its abundance in MATa versus _MAT_α cells, we used ChIP for Myc-tagged yKu70, followed by QPCR. We observed a significant enrichment for both yKu70 and yKu80 at _HML_α in MATa cells, consistent with recently published results (41, 54) (Fig. 6). The level detected was comparable to that found at Tel6R (11- to 13-fold over background) (Fig. 6B to D). In addition, yKu70 was slightly enriched at Ya sequences (representing both HMRa and MATa), most likely due to its association at HMR. The association of yKu with HML and Tel6R was abolished in a sir4 mutant (Fig. 6D), in agreement with recent observations on HMR (54). We note, however, that there was no significant binding of yKu to the RE, in contrast to an earlier report (44). Our system is able to detect strong enrichment of yKu at other internal sequences (e.g., _MAT_α after cleavage) (12), and we are able to detect the transcription factor Mcm1 robustly at the RE (Fig. 6C); therefore, we conclude that yKu is bound at both HM loci, but not at the RE, in MATa cells.
We next quantified yKu binding at the same loci in _MAT_α cells. Intriguingly, in MAT_α cells yKu binding of HML is only ∼50% of its level in MATa cells, while the amount of yKu70 recovered at HMR increased twofold, again in a mating-type-specific manner (Fig. 6B). Furthermore, deletion of the RE led to a reduction in yKu70 binding at HML but not at HMR (Fig. 6B). We note that the reduced binding of yKu70 detected at HML in the Δ_re strain correlates with increased perinuclear sequestration of HML (Fig. 3). Thus, yKu levels at HML are mating type specific and correlate with a more internal positioning of Tel3L in MATa cells. This suggests a mechanism through which yKu could participate directly in donor choice during mating type switching: the higher levels of yKu may impair sequestration of Tel3L by Sir4-Esc1 interactions, thereby promoting recombination.
Cooperative yKu binding to the HM loci and MAT during gene conversion.
To examine how yKu binding changes after induction of the DSB at MAT, we used strains carrying a chromosomal copy of a galactose-inducible HO endonuclease. Following the addition of galactose, HO-dependent cleavage efficiencies up to 86% and 96% by 30 and 60 min, respectively, were achieved (data not shown). yKu binding to _HML_α decreased rapidly after DSB induction (from 11- to 7-fold enrichment) (Fig. 6E), while its association with Ya sequences (MATa and HMRa) increased sharply in the MATa strain, in agreement with the documented recruitment of yKu to a DSB (12, 34). On the other hand, under these conditions, there was no detectable association of yKu at or near the RE before and after 30 or 60 min of cleavage (Fig. 6F). These results suggest that the impact of yKu on mating type switching does not stem from direct interaction with the RE as previously proposed (44) but rather reflects the binding of yKu at _HML_α prior to cleavage and possibly also the increase that occurs at MAT after DSB induction.
Chr3L constraint is found in both mating types independently of HML or RE.
It has been argued that the mobility of Chr3L changes in a mating-type-specific manner (4). Since previous mobility studies were performed in diploid cells, we next examined whether chromatin dynamics, which depend on the compaction and flexibility of the chromatin fiber (6) are altered in haploid cells in a cell-type-specific manner.
Nontelomeric chromosomal loci in yeast move in a random walk with a radius of constraint of 0.6 to 0.7 μm2 (15). Telomeres are more restricted in their movement, particularly relative to the nuclear periphery (21, 24), and it is to be expected that the HML and HMR lacO locus mobility will be influenced by the adjacent telomere. To test whether mating type specificity imposes constraints on locus dynamics, we used live time-lapse imaging to follow the movement of tagged loci relative to the interpolated center of the nucleus (Fig. 7A). For each strain, we plotted the MSD, or d(Δ_t_)2 = [r(t + Δ_t_) − r(t)]2, over all possible time periods from 0 to 100 s using 1.5-s intervals. We found that the actively transcribed MATa moves much like other transcribed regions in both cell types, while the movement of _MAT_α is slightly more constrained (Fig. 7B). The radius of constraint (rc) for MATa, calculated from the plateau achieved at Δ100 s, is 0.65 μm (15). The subdiffusive movement of either HML or HMR is limited to an rc of ≤0.5 μm, which is calculated from the MSD value of 0.2 μm2 (Fig. 7B). Importantly, the dynamic constraint of HML and HMR in wt cells did not change significantly between MATa and _MAT_α cells (Fig. 7B and C). This mating type independence differs from that reported by Bressan et al. (4), who found HML more mobile in MATa than in _MAT_α cells. These authors computed relative movement between two HML loci in a diploid cell, while we have determined the movement of a single site relative to a fixed point of reference in haploid cells. While the systems analyzed are clearly distinct, it is not clear what exactly the source of the discrepancy is.
FIG. 7.
Movement of the left arm of Chr3 is constrained, but is relieved by yku70 mutation. (A) Time-lapse microscopy was performed by taking single-frame images every 1.5 s on a Zeiss LSM 510 confocal microscope. Representative single-frame images of GFP-tagged HML in wt, sir4, and yku70 MATa strains relative to the NE visualized by GFP-Nup49 pore components. The path obtained by aligning the spot position of 100 frames was superposed in red on the respective right panel. Bar, 1 μm. (B) MSD for MAT, HML, and HMR in wt MATa and MAT_α strains using MSD, where d represents the spot-to-spot distance for each frame as a function of the time interval (Δ_t = 1.5 to 100 s). For each trace, six to eight movies were averaged. (C). Normalized MSD values for wt and mutant strains. The MSD (in μm2) from time-lapse series for HML in MATa and MAT_α wt, sir4, yku70, Δ_re, and Δ_re_ y_ku70_ strains and for Tel3L in the absence of the HML locus (Δ_hml_) in wt and yku70 strains as a function of the time interval (Δ_t_ = 1.5 to 100 s) was calculated. The MSD at Δ100 s was calculated for all movies of one genotype and set to 1 for the wt. The increase (fold) in MSD at 100 s relative to the MSD of each wt strain is shown for the MSD of mutant strains (thick bars) and all individual movies recorded (open circles). (D) Same as panel C, but the movement of the tagged HMR locus was monitored. The MSD (in μm2) from time-lapse series for HMRa in MATa wt, sir4, yku70 and sir3 strains as a function of the time interval (Δ_t_ = 1.5 to 100 s) was calculated and is presented after normalization to the wt.
We next calculated similar MSD plots for HML and HMR in various mutants and compared the mean and spread of the data graphically to wt values (Fig. 7C and D). As expected, in the absence of Sir4 the mobility of HMR increased, since its association with the NE was compromised. The mean MSD for HMR in the MATa sir4 strain was equivalent to those scored for MAT (compare the maximum MSD of HMR and MAT in the wt in Fig. 7B to that of HMR in sir4 in Fig. 7D). In contrast, the mobility of HMR was unaffected by the yku70 deletion and showed an intermediate value in cells lacking Sir3 (Fig. 7D). These results nicely parallel the effects of these mutations on HMR subnuclear position and confirm that in the absence of yKu the silent HMR locus remains NE bound by Sir4-Esc1 interactions (15, 21). The situation is quite different for HML. The mobility of _HML_α increased only incrementally in a sir4 mutant (1.3× the wt mean), indicating that _HML_α constraint is imposed by a mechanism independent of silencing (Fig. 7C). This is consistent with the proposed internal attachment mediated by yKu. Consistently, the deletion of yku70 led to a noticeable increase in HML mobility, even though HML movement was restricted to a radial trajectory along the inner face of the nuclear envelope (Fig. 7A and C). The average MSD of HML doubles in a yku70 background relative to the wt, suggesting yKu indeed imposes constraint on HML movement (Fig. 7C). We note that there is also enhanced variability in locus movement from cell to cell in a yku population. This correlates with earlier reports of an epigenetic state that alters silencing behavior in genetically identical yku70 cells (33).
We next asked whether these dynamics reflect Tel3L or the presence of HML. Consistent with localization data, the dynamic constraint that restricts movement of Tel3L persists in a wt background even when the HML locus is deleted (Fig. 7C, center panel). This reflects intact telomere anchoring pathways. However, the increase in mobility that occurs upon yku70 deletion no longer occurred in the absence of HML (Tel3LΔ_hml_). This argues that increased mobility requires sequences at HML, just as the restriction of mobility requires yKu. We then explored whether the RE influenced HML mobility, as proposed by Bressan et al. (4). As shown in Fig. 7C, in our hands, the dynamic constraint on Chr3L is independent of the RE, just as it is independent of mating type. Nonetheless, as shown above for positioning, the effect of deletion of yku70 on the dynamics of HML was indeed epistatic to Δ_re_. In other words, in MATa Δ_re_ cells the deletion of yku70 no longer led to increased mobility (Fig. 7C). Thus, the RE is required for the increase in HML movement, possibly by increasing the flexibility of the chromatin fiber. This increase was observed when the constraint imposed by yKu binding is relieved, which appears to occur in vivo following DSB induction (Fig. 6).
Based on these results, we propose a model in which mating-type-specific enrichment of the yKu complex at HML keeps the locus away from the nuclear periphery, thereby enabling the RE to favor recombination with the cleaved MATa locus. The cooperation with the RE is mating type specific, although positioning per se is not. Consistent with this model of an internal mating type switch, we can show that diverse HR events take place in the nuclear lumen.
DISCUSSION
What regulates long-range DNA interactions that are necessary for recombination-mediated repair of DSBs remains a mystery. Even less clear is the mechanism through which a DSB preferentially recognizes one of two equivalent donor sites. Nonetheless this occurs in HO+ yeast cells once per cell cycle with remarkable fidelity. Previous studies have tried without success to identify mating-type-specific spatial clues that predispose one HM locus and not the other to interact with the cleaved MAT locus (4, 49). Although neither found mating-type-specific juxtaposition, it was argued that Chr3L has a distinct mobility in MATa versus _MAT_α cells, which might favor exchange with MATa (4). Our results confirm and extend these previous studies, showing that in G1- and in S-phase cells prior to cleavage by HO, the radial subnuclear position and dynamics of HML and HMR are largely mating type independent. Thus, it is unlikely that a predetermined spatial juxtaposition determines the directionality of the mating type switch.
On the other hand, we do find distinct mating-type specific characteristics that correlate with donor preference. First, yKu is enriched at the _HML_α locus in MATa cells compared to _MAT_α cells. yKu binding to the silent mating type loci provides an alternate pathway to Sir1 for recruiting Sir4 to establish heterochromatin (41). We confirm that in a sir4 deletion strain, yKu binding to HML is abolished and as a consequence HML relocates to the center of the nucleus. Intriguingly, upon DSB induction in a recombination-competent strain _HML_α moves to a more internal position in MATa, but not in _MAT_α, cells. This movement is yKu dependent and thus correlates with mating-type-specific donor preference.
The yKu heterodimer is a highly conserved factor that is implicated in the repair of DSBs by nonhomologous end joining, a repair pathway that involves direct ligation of unresected DSB ends. Although mating type switching does not require nonhomologous end joining, being a form of HR, we did score an effect of yku70 deletion on the directionality of recombination: efficient mating type exchange is reduced by >20% in the absence of yKu. Our analysis of the effect of yKu70 on HML position and mobility may be able to explain this effect.
Through a careful quantitative analysis of locus position in a range of mutants, we attribute a unique position and behavior to Tel3L, which is dependent on the presence of _HML_α. Whereas Tel3R and HMR behave like other telomere proximal loci, HML obeys other rules. First, perinuclear anchoring and dynamic constraint appear to be uncoupled, so that in the absence of yKu, the locus is shifted to the NE thanks to Sir4-Esc1 interactions, yet the constraints on its mobility are reduced. Indeed, in the absence of yKu70, the HML locus moves readily along a radial trajectory but remains associated with the NE, a property earlier noted for Tel14L in a wt background (47) or for the active GAL1 gene (8). The distinct behavior of HML may be related to the general lack of recombination shown on the left arm of Chr3, an incompetence that can be overcome by the action of the RE (59). Consistent with a model in which yKu and the RE work in parallel to ensure that recombination of _HML_α with MATa is favored, Coic et al. (10) have recently proposed that the targets of RE function may be anchorage sites that tether Chr3L in MATa cells. This is in full agreement with our quantitative microscopic analysis. We propose that the RE acts at the time of DSB cleavage to overcome a natural inaccessibility of _HML_α, enabling fruitful recombination with MATa.
We argue that the effect of yKu on the nuclear positioning of HML and MAT contributes to the regulation of mating type exchange. In MATa cells, yKu binds to _HML_α in both the absence and presence of a DSB, while MAT is bound only after cleavage (34). We propose that high levels of yKu at _HML_α favor recruitment of _HML_α to the cleaved MATa locus, which in turn would facilitate gene conversion thanks to the immediate juxtaposition of two homologous loci. Our observation that the amount of yKu binding to HML and HMR is mating type specific reinforces the idea of an unexpected role for the yKu complex in donor choice. Thus, yKu may both directly facilitate long-range interactions of sites to which it binds, as well as establish a nuclear organization conducive to selective sequence exchange.
In the absence of yKu, HML is more efficiently sequestered at the nuclear periphery and this correlates with less efficient switching. This suggests that the tight perinuclear anchoring of HML inhibits its recruitment to the MAT locus during gene conversion. However, since the perinuclear anchoring of HMRa does not interfere with its recombination with _MAT_α, we propose that the function of the RE is partially impaired when HML is sequestered at the NE (i.e., in the yku70 mutant). To reconcile this, we propose that HMRa and _HML_α associate with distinct subnuclear anchorage sites, which affect the gene conversion event differently. This is supported by the fact that HMR position is not affected by yku70 deletions. Our results further imply that the role or complex formed by yKu at HML is not functionally equivalent to telomere-associated yKu. This is not unexpected, given the multiplicity of functions ascribed to the yKu heterodimer.
We have recently demonstrated that the probability with which two chromosomal loci interact is influenced by nuclear geometry and is reduced when the loci are tethered at the periphery (16). Consistent with the notion that peripheral sequestration negatively affects HML-MAT interaction, computer-driven simulations of spontaneous interaction between randomly moving loci argue that HML tethering at an internal position leads to a twofold increase in the probability of spontaneous collision with MAT, although contact was infrequent in all cases analyzed (L.G., K.B., and S.M.G., data not shown). This is particularly relevant since we find MAT strongly enriched in the nuclear lumen even after induction of the DSB, as long as the strain is competent for recombinational repair. Only when an irreparable DSB is induced at MAT, i.e., cleavage in a strain bearing deletions of HML and HMR, does MAT become sequestered at the nuclear periphery (37). These observations are consistent with enrichment of Rad52 foci in the nuclear lumen under a wide range of damage conditions (Fig. 5).
The nuclear and chromosomal architecture in budding yeast seems to offer a compromise that ensures the efficient regulation of two different processes at the same locus: transcriptional repression and a competence for selective recombination. Given that mating type interconversion occurs every cell cycle, yeast has opted for only one architectural plan for the two cell types, and it must therefore regulate the recombination event not by global positioning, but by quickly and locally activating the appropriate donor. This may be ensured by mating-type-specific yKu binding at HML.
This simple but plausible model is reminiscent of an idea proposed for higher eukaryotic nuclear organization (reviewed in reference 35). Namely, it was proposed that elements of nuclear structure and the stable juxtaposition of chromosomal domains provide a scaffold for these sites during transient assembly of functional complexes during active chromosomal repair and transcription processes. The dynamic association and tethering of factors involved in HR, such as Rad51, to nuclear matrix-like structures may facilitate the repair of DNA damage (36). Moreover, BRCA2-dependent association of Rad51 with a nuclear matrix was correlated with the formation of Rad51 nuclear foci in response to DNA damage (52). In conclusion, we have identified a mechanism through which the yeast nucleus can sequester a specific locus in a reversible manner, possibly releasing it upon DSB induction to promote interaction with an internal DSB. The identification of further mechanisms that confer spatial constraint on specific DNA loci may yield new insights into the control of genome stability.
Acknowledgments
The Gasser laboratory acknowledges the Swiss Cancer League, the Swiss National Science Foundation, NCCR Frontiers in Genetics, and the Novartis Research Foundation, as well as fellowships from the European Molecular Biology Organization (EMBO) to H.V.A. and V.D. and from the Human Frontiers Science Program to H.V.A. K.B. further acknowledges financial support from the University Paul Sabatier, Toulouse, and the ANR (project JC05-42116).
We thank M. Tsai for assistance with Myc-tagged strain construction, V. Kalck for assistance with positioning data acquisition, and O. Gadal for fruitful discussions.
Footnotes
▿
Published ahead of print on 1 December 2008.
REFERENCES
- 1.Akhtar, A., and S. M. Gasser. 2007. The nuclear envelope and transcriptional control. Nat. Rev. Genet. 8507-517. [DOI] [PubMed] [Google Scholar]
- 2.Andrulis, E. D., A. M. Neiman, D. C. Zappulla, and R. Sternglanz. 1998. Perinuclear localization of chromatin facilitates transcriptional silencing. Nature 394592-595. [DOI] [PubMed] [Google Scholar]
- 3.Boscheron, C., L. Maillet, S. Marcand, M. Tsai-Pflugfelder, S. M. Gasser, and E. Gilson. 1996. Cooperation at a distance between silencers and proto-silencers at the yeast HML locus. EMBO J. 152184-2195. [PMC free article] [PubMed] [Google Scholar]
- 4.Bressan, D. A., J. Vazquez, and J. E. Haber. 2004. Mating type-dependent constraints on the mobility of the left arm of yeast chromosome III. J. Cell Biol. 164361-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broach, J. R. 2004. Making the right choice—long-range chromosomal interactions in development. Cell 119583-586. [DOI] [PubMed] [Google Scholar]
- 6.Bystricky, K., P. Heun, L. Gehlen, J. Langowski, and S. M. Gasser. 2004. Long-range compaction and flexibility of interphase chromatin in budding yeast analyzed by high-resolution imaging techniques. Proc. Natl. Acad. Sci. USA 10116495-16500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bystricky, K., T. Laroche, G. van Houwe, M. Blaszczyk, and S. M. Gasser. 2005. Chromosome looping in yeast: telomere pairing and coordinated movement reflect anchoring efficiency and territorial organization. J. Cell Biol. 168375-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabal, G. G., A. Genovesio, S. Rodriguez-Navarro, C. Zimmer, O. Gadal, A. Lesne, H. Buc, F. Feuerbach-Fournier, J. C. Olivo-Marin, E. C. Hurt, and U. Nehrbass. 2006. SAGA interacting factors confine sub-diffusion of transcribed genes to the nuclear envelope. Nature 441770-773. [DOI] [PubMed] [Google Scholar]
- 9.Chang, V. K., M. J. Fitch, J. J. Donato, T. W. Christensen, A. M. Merchant, and B. K. Tye. 2003. Mcm1 binds replication origins. J. Biol. Chem. 2786093-6100. [DOI] [PubMed] [Google Scholar]
- 10.Coic, E., G. F. Richard, and J. E. Haber. 2006. Saccharomyces cerevisiae donor preference during mating-type switching is dependent on chromosome architecture and organization. Genetics 1731197-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connolly, B., C. I. White, and J. E. Haber. 1988. Physical monitoring of mating type switching in Saccharomyces cerevisiae. Mol. Cell. Biol. 82342-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubrana, K., H. van Attikum, F. Hediger, and S. M. Gasser. 2007. The processing of double-strand breaks and binding of single-strand-binding proteins RPA and Rad51 modulate the formation of ATR-kinase foci in yeast. J. Cell Sci. 1204209-4220. [DOI] [PubMed] [Google Scholar]
- 13.Ercan, S., J. C. Reese, J. L. Workman, and R. T. Simpson. 2005. Yeast recombination enhancer is stimulated by transcription activation. Mol. Cell. Biol. 257976-7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ercan, S., and R. T. Simpson. 2004. Global chromatin structure of 45,000 base pairs of chromosome III in _a_- and α-cell yeast and during mating-type switching. Mol. Cell. Biol. 2410026-10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gartenberg, M. R., F. R. Neumann, T. Laroche, M. Blaszczyk, and S. M. Gasser. 2004. Sir-mediated repression can occur independently of chromosomal and subnuclear contexts. Cell 119955-967. [DOI] [PubMed] [Google Scholar]
- 16.Gehlen, L., A. Rosa, K. Klenin, J. Langowski, S. M. Gasser, and K. Bystricky. 2006. Spatially confined polymer chains: implication of chromatin fiber flexibility and peripheral anchoring on telomere-telomere interaction. J. Phys. Condensed Matter 18S245-S252. [Google Scholar]
- 17.Gellert, M. 2002. V(D)J recombination: RAG proteins, repair factors, and regulation. Annu. Rev. Biochem. 71101-132. [DOI] [PubMed] [Google Scholar]
- 18.Gotta, M., T. Laroche, A. Formenton, L. Maillet, H. Scherthan, and S. M. Gasser. 1996. The clustering of telomeres and colocalization with Rap1, Sir3, and Sir4 proteins in wild-type Saccharomyces cerevisiae. J. Cell Biol. 1341349-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haber, J. E. 1998. Mating-type gene switching in Saccharomyces cerevisiae. Annu. Rev. Genet. 32561-599. [DOI] [PubMed] [Google Scholar]
- 20.Hediger, F., A. S. Berthiau, G. van Houwe, E. Gilson, and S. M. Gasser. 2006. Subtelomeric factors antagonize telomere anchoring and Tel1-independent telomere length regulation. EMBO J. 25857-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hediger, F., F. R. Neumann, G. Van Houwe, K. Dubrana, and S. M. Gasser. 2002. Live imaging of telomeres: yKu and Sir proteins define redundant telomere-anchoring pathways in yeast. Curr. Biol. 122076-2089. [DOI] [PubMed] [Google Scholar]
- 22.Hediger, F., A. Taddei, F. R. Neumann, and S. M. Gasser. 2004. Methods for visualizing chromatin dynamics in living yeast. Methods Enzymol. 375345-365. [DOI] [PubMed] [Google Scholar]
- 23.Herskowitz, I., and R. E. Jensen. 1991. Putting the HO gene to work: practical uses for mating-type switching. Methods Enzymol. 194132-146. [DOI] [PubMed] [Google Scholar]
- 24.Heun, P., T. Laroche, M. K. Raghuraman, and S. M. Gasser. 2001. The positioning and dynamics of origins of replication in the budding yeast nucleus. J. Cell Biol. 152385-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heun, P., T. Laroche, K. Shimada, P. Furrer, and S. M. Gasser. 2001. Chromosome dynamics in the yeast interphase nucleus. Science 2942181-2186. [DOI] [PubMed] [Google Scholar]
- 26.Klar, A. J., S. Fogel, and K. Lusnak. 1979. Gene conversion of the mating-type locus in Saccharomyces cerevisiae. Genetics 92777-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, S. E., J. K. Moore, A. Holmes, K. Umezu, R. D. Kolodner, and J. E. Haber. 1998. Saccharomyces Ku70, mre11/rad50 and RPA proteins regulate adaptation to G2/M arrest after DNA damage. Cell 94399-409. [DOI] [PubMed] [Google Scholar]
- 28.Lisby, M., J. H. Barlow, R. C. Burgess, and R. Rothstein. 2004. Choreography of the DNA damage response: spatiotemporal relationships among checkpoint and repair proteins. Cell 118699-713. [DOI] [PubMed] [Google Scholar]
- 29.Lisby, M., U. H. Mortensen, and R. Rothstein. 2003. Colocalization of multiple DNA double-strand breaks at a single Rad52 repair centre. Nat. Cell Biol. 5572-577. [DOI] [PubMed] [Google Scholar]
- 30.Longtine, M. S., A. McKenzie III, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14953-961. [DOI] [PubMed] [Google Scholar]
- 31.Luo, K., M. A. Vega-Palas, and M. Grunstein. 2002. Rap1-Sir4 binding independent of other Sir, yKu, or histone interactions initiates the assembly of telomeric heterochromatin in yeast. Genes Dev. 161528-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maillet, L., C. Boscheron, M. Gotta, S. Marcand, E. Gilson, and S. M. Gasser. 1996. Evidence for silencing compartments within the yeast nucleus: a role for telomere proximity and Sir protein concentration in silencer-mediated repression. Genes Dev. 101796-1811. [DOI] [PubMed] [Google Scholar]
- 33.Maillet, L., F. Gaden, V. Brevet, G. Fourel, S. G. Martin, K. Dubrana, S. M. Gasser, and E. Gilson. 2001. Ku-deficient yeast strains exhibit alternative states of silencing competence. EMBO Rep. 2203-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin, S. G., T. Laroche, N. Suka, M. Grunstein, and S. M. Gasser. 1999. Relocalization of telomeric Ku and SIR proteins in response to DNA strand breaks in yeast. Cell 97621-633. [DOI] [PubMed] [Google Scholar]
- 35.Misteli, T. 2007. Beyond the sequence: cellular organization of genome function. Cell 128787-800. [DOI] [PubMed] [Google Scholar]
- 36.Mladenov, E., B. Anachkova, and I. Tsaneva. 2006. Sub-nuclear localization of Rad51 in response to DNA damage. Genes Cells 11513-524. [DOI] [PubMed] [Google Scholar]
- 37.Nagai, S., K. Dubrana, M. Tsai-Pflugfelder, M. B. Davidson, T. M. Roberts, G. W. Brown, E. Varela, F. Hediger, S. M. Gasser and N. J. Krogan. 2008. Functional localization of DNA damage to pore complex bound to Slx5/8 SUMO-dependent E3 ligase. Science 322597-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nasmyth, K. 1993. Regulating the HO endonuclease in yeast. Curr. Opin. Genet. Dev. 3286-294. [DOI] [PubMed] [Google Scholar]
- 39.Palancade, B., X. Liu, M. Garcia-Rubio, A. Aguilera, X. Zhao, and V. Doye. 2007. Nucleoporins prevent DNA damage accumulation by modulating Ulp1-dependent sumoylation processes. Mol. Biol. Cell 182912-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parker, R. E. 1997. Introductory statistics for biology, 2nd ed. Cambridge University Press, Cambridge, United Kingdom.
- 41.Patterson, E. E., and C. A. Fox. 2008. The Ku complex in silencing the cryptic mating-type loci of Saccharomyces cerevisiae. Genetics 180772-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ravindra, A., K. Weiss, and R. T. Simpson. 1999. High-resolution structural analysis of chromatin at specific loci: Saccharomyces cerevisiae silent mating-type locus _HMR_a. Mol. Cell. Biol. 197944-7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reimer, S. K., and A. R. Buchman. 1997. Yeast silencers create domains of nuclease-resistant chromatin in an SIR4-dependent manner. Chromosoma 106136-148. [DOI] [PubMed] [Google Scholar]
- 44.Ruan, C., J. L. Workman, and R. T. Simpson. 2005. The DNA repair protein yKu80 regulates the function of recombination enhancer during yeast mating type switching. Mol. Cell. Biol. 258476-8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rusche, L. N., A. L. Kirchmaier, and J. Rine. 2003. The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Annu. Rev. Biochem. 72481-516. [DOI] [PubMed] [Google Scholar]
- 46.Rusche, L. N., A. L. Kirchmaier, and J. Rine. 2002. Ordered nucleation and spreading of silenced chromatin in Saccharomyces cerevisiae. Mol. Biol. Cell 132207-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sage, D., F. R. Neumann, F. Hediger, S. M. Gasser, and M. Unser. 2005. Automatic tracking of individual fluorescence particles: application to the study of chromosome dynamics. IEEE Trans. Image Process. 141372-1383. [DOI] [PubMed] [Google Scholar]
- 48.Schneider, R., and R. Grosschedl. 2007. Dynamics and interplay of nuclear architecture, genome organization, and gene expression. Genes Dev. 213027-3043. [DOI] [PubMed] [Google Scholar]
- 49.Simon, P., P. Houston, and J. Broach. 2002. Directional bias during mating type switching in Saccharomyces is independent of chromosomal architecture. EMBO J. 212282-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spector, D. L. 2003. The dynamics of chromosome organization and gene regulation. Annu. Rev. Biochem. 72573-608. [DOI] [PubMed] [Google Scholar]
- 51.Taddei, A., F. Hediger, F. R. Neumann, C. Bauer, and S. M. Gasser. 2004. Separation of silencing from perinuclear anchoring functions in yeast Ku80, Sir4 and Esc1 proteins. EMBO J. 231301-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tarsounas, M., D. Davies, and S. C. West. 2003. BRCA2-dependent and independent formation of RAD51 nuclear foci. Oncogene 221115-1123. [DOI] [PubMed] [Google Scholar]
- 53.van Attikum, H., O. Fritsch, B. Hohn, and S. M. Gasser. 2004. Recruitment of the INO80 complex by H2A phosphorylation links ATP-dependent chromatin remodeling with DNA double-strand break repair. Cell 119777-788. [DOI] [PubMed] [Google Scholar]
- 54.Vandre, C. L., R. T. Kamakaka, and D. H. Rivier. 2008. The DNA end-binding protein Ku regulates silencing at the internal HML and HMR loci in Saccharomyces cerevisiae. Genetics 1801407-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weiss, K., and R. T. Simpson. 1997. Cell type-specific chromatin organization of the region that governs directionality of yeast mating type switching. EMBO J. 164352-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weiss, K., and R. T. Simpson. 1998. High-resolution structural analysis of chromatin at specific loci: Saccharomyces cerevisiae silent mating type locus _HML_α. Mol. Cell. Biol. 185392-5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu, C., K. Weiss, C. Yang, M. A. Harris, B. K. Tye, C. S. Newlon, R. T. Simpson, and J. E. Haber. 1998. Mcm1 regulates donor preference controlled by the recombination enhancer in Saccharomyces mating-type switching. Genes Dev. 121726-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu, X., and J. E. Haber. 1996. A 700 bp cis-acting region controls mating-type dependent recombination along the entire left arm of yeast chromosome III. Cell 87277-285. [DOI] [PubMed] [Google Scholar]
- 59.Wu, X., and J. E. Haber. 1995. MATa donor preference in yeast mating-type switching: activation of a large chromosomal region for recombination. Genes Dev. 91922-1932. [DOI] [PubMed] [Google Scholar]