Expression of H-RASV12 in a zebrafish model of Costello syndrome causes cellular senescence in adult proliferating cells (original) (raw)
Summary
Constitutively active, ‘oncogenic’ H-RAS can drive proliferation and transformation in human cancer, or be a potent inducer of cellular senescence. Moreover, aberrant activation of the Ras pathway owing to germline mutations can cause severe developmental disorders. In this study we have generated transgenic zebrafish that constitutively express low levels, or can be induced to express high levels, of oncogenic H-RAS. We observed that fish carrying the integrated transgene in their germline display several hallmarks of Costello syndrome, a rare genetic disease caused by activating mutations in the gene H-RAS, and can be used as a model for the disease. In Costello-like fish, low levels of oncogenic H-RAS expression are associated with both reduced proliferation and an increase in senescence markers in adult progenitor cell compartments in the brain and heart, together with activated DNA damage responses. Overexpression of H-RAS through a heat-shock-inducible promoter in larvae led to hyperproliferation, activation of the DNA damage response and tp53-dependent cell cycle arrest. Thus, oncogene-induced senescence of adult proliferating cells contributes to the development of Costello syndrome and provides an alternative pathway to transformation in the presence of widespread constitutively active H-RAS expression.
INTRODUCTION
Ras proteins are small GTPases which are essential components of cellular signaling pathways, which are responsible for growth, migration, adhesion, cytoskeletal integrity, survival and differentiation. They have been intensely studied in the last 30 years for their involvement in cancer; indeed, defects in Ras signaling may result in malignant transformation (Downward, 1996; Malumbres and Barbacid, 2003). It is estimated that approximately 30% of all human tumors have activating mutations in one of the RAS genes. In fact, somatic mutations, which lock Ras in an active GTP-bound conformation resulting in constitutive activation of the downstream pathways, are frequent in cancer – they are catalogued by the Cancer Genome Project in the Catalogue Of Somatic Mutations In Cancer (COSMIC) (www.sanger.ac.uk/genetics/CGP/Census/).
Moreover, the discovery of germline mutations in neurofibromin 1 (NF1) and protein tyrosine phosphatase, non-receptor type 11 (PTPN11) genes provided the first indication that aberrant Ras signaling might underlie a group of human developmental disorders, collectively known as cardio-facio-cutaneous syndromes (reviewed by Cichowski and Jacks, 2001; Tartaglia and Gelb, 2005). All of these disorders share similar phenotypic traits. Among them, it has emerged that Costello syndrome is caused by ‘de novo’ germline mutations in the H-RAS gene (Aoki et al., 2005). Patients with Costello syndrome have multiple congenital disorders, which, all together, are diagnostic for the disease, including a characteristic craniofacial dysmorphology, cardiac defects, mild mental retardation, and high birth weight followed by a failure to thrive and developmental delays (Costello, 1977) (reviewed by Rauen, 2007). These patients also have a tendency to develop multiple papillomas and various types of cancer, mainly rhabdomyosarcomas, neuroblastomas and bladder carcinomas (Gripp et al., 2006).
It is still unclear how the expression of activated H-RAS can induce oncogenic transformation and unrestricted growth as a consequence of somatic mutations in cancer, and induce developmental abnormalities affecting specific tissues in Costello patients who have inherited the same mutations through germline transmission. Perhaps, by understanding how activation of the Ras pathway is kept under control in Costello patients, we might be able to devise ways of controlling it in cancer. In contrast to the oncogenic activity of mutant H-RAS, the mutant protein can also induce a potent antiproliferative response, i.e. cellular senescence (Serrano et al., 1997). Cellular senescence is characterized by an irreversible proliferation block, which is accompanied by the expression of specific markers such as the upregulation of senescence-associated β-galactosidase (SA-βgal) activity (reviewed by Dimri and Campisi, 1994).
Oncogene-induced senescence (OIS) has been demonstrated in cells that overexpress H-RAS (Serrano et al., 1997) and in several benign tumors (Collado et al., 2005). It is believed to represent a protective mechanism against proliferation and transformation, and to be activated by H-RAS (Sun et al., 2007). In mouse models of breast and lung cancer, in which expression of the H-Ras or K-Ras oncogene is inducible (Sarkisian et al., 2007), the occurrence of OIS was found to be related to the level and duration of oncogenic Ras expression. In this system, low levels of K-Ras expression stimulate proliferation whereas high levels trigger an irreversible growth arrest (Sarkisian et al., 2007).
We investigated the effects of oncogenic H-RAS expression in zebrafish. Using germline integration, we generated zebrafish lines containing a mutated form of H-RAS, carrying the activating mutation G12V, that can be expressed in an inducible or constitutive manner, and found that these fish display several developmental defects that are hallmarks of the Costello syndrome. We then used these fish to gain insights into the mechanisms by which oncogenic H-RAS expression leads to the Costello phenotype, and found that it induces a DNA damage response (DDR) and initiates a senescence program in subpopulations of adult proliferating cells in the heart and brain. The occurrence of OIS in a zebrafish model of Costello syndrome suggests that OIS contributes to the severe developmental defects, and to the degenerative/premature aging phenotype, present in human patients.
RESULTS
Generation of zebrafish expressing oncogenic H-RASV12
It has been documented that oncogenic Ras proteins can perform different functions according to the cellular context. We asked what the effect of oncogenic Ras expression would be, in vivo, during the development of different organs and tissues. In fish, as well as in humans, three genes have been identified: h-ras, n-ras and k-ras (Rotchell et al., 2001). We decided to use the human H-RAS gene and in particular a constitutively active form carrying a mutation, in which a GGC codon is substituted with a GTC codon (G12V, or in short V12), because its roles in transformation and cellular senescence are well documented (Kraus et al., 1984; Barbacid, 1990; Downward, 1996; Di Micco et al., 2006).
In order to generate fish lines that express H-RASV12 in different organs and tissues, we took advantage of the Tol2 gene trap system developed by Kawakami (Kawakami et al., 2000). We generated a chimeric H-RASV12 protein that was tagged with green fluorescent protein (GFP) at the N-terminus (Fig. 1A) in order to track developing tumors. We verified that the GFP tag did not affect the function of H-RASV12 in vitro and in vivo. We transfected NIH3T3 cells with the chimeric construct, or with an empty pEGFP vector as a control, and performed a focus formation assay. We confirmed that, both in vitro and in vivo, the oncogenic activity of H-RASV12 was not affected by the presence of the GFP tag (supplementary material Fig. S1A). Subsequently, we cloned the GFP-H-RASV12 chimeric construct into a modified version of the T2KSAG plasmid (Kawakami et al., 2000). We injected fertilized eggs with this plasmid (see Methods) and found that approximately 20% of the injected fish developed some type of neoplasia (mainly nevi, melanomas and lymphomas) (Fig. 1B), probably owing to somatic integration and expression of the GFP-H-RASV12 transgene, as demonstrated by the expression of GFP in the tumors (Fig. 1B).
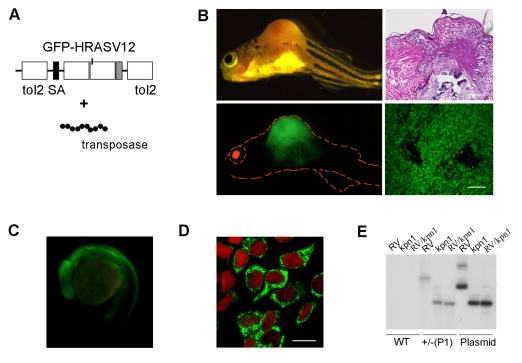
Fig. 1.
Embryos and adults with germline integration and constitutive expression of GFP-H-RASV12 are healthy, whereas somatic integrations generate tumors. (A) Diagram of the gene trap T2KSAG:GFP-H-RASV12 construct used in this study, containing Tol2 sequences, a splice acceptor (SA) and the sequence coding for the fusion protein GFP-H-RASV12. This construct is injected in combination with mRNA encoding the Tol2 transposase at the one-cell stage. (B) Formation of a malignant fibrosarcoma in injected 5-week-old fish. The presence of GFP-positive tumors in injected fish indicates that the GFP tag does not affect the oncogenic activity of H-RASV12. Hematoxylin and eosin (H&E) staining of a paraffin section of the tumor (upper right panel) and GFP expression in a cryostat section of the tumor (lower right panel). Bars, 40 μm. (C) GFP-H-RASV12 expression in the P1 line at 24 hpf, lateral view, head to the left. (D) Immunostaining with anti-GFP of cultured cells derived from P1 embryos shows that GFP-H-RASV12 localizes to the cell membrane and the Golgi complex. The nuclei are stained with Topro (red). Bar, 10 μm. (E) Southern blot analysis using genomic DNA extracted from 1-month-old wild-type (WT) zebrafish. Analysis of heterozygous (+/–) P1 fish DNA reveals that a single insertion occurred in the line. RV (_Eco_RV) and _Kpn_I are restriction enzymes. We used the T2KSAG:GFP-H-RASV12 plasmid as a control.
When fish reached adulthood we screened for germline integrations. We screened 142 genomes, by analyzing the progeny for GFP expression under a fluorescent stereomicroscope and by PCR (supplementary material Fig. S1B), and found that 22% (_n_=32/142) of the injected fish had integrated the transgene into their germline, but only 6.4% (_n_=2/32) of germline integrations gave rise to GFP-H-RASV12-positive progeny. The two different transgenic lines, full names: Gt(GFP-H-RASV12)io1 and Gt(GFP-H-RASV12)io2, were called P1 and P2, respectively. We analyzed the expression pattern of the fusion protein both in the embryos and in the juvenile fish from the two lines. Both P1 and P2 embryos display ubiquitous expression of the fusion protein (Fig. 1C; and data not shown), mainly confined to the cell membrane and Golgi apparatus, as visualized in fibroblast cultures derived from fluorescent embryos at 24 hours postfertilization (hpf) (Fig. 1D). As multiple insertions could have occurred, we evaluated the integration number in the P1 and P2 lines. Southern blot analysis showed that P1 and P2 carry single germline insertions at different genomic locations (Fig. 1E; supplementary material Fig. S1C).
Characterization of a zebrafish model of Costello syndrome
Hemizygous fish from the two lines were healthy and fertile. In the progeny of hemizygous crosses, approximately 25% of the fish showed an abnormal morphology that was visible by 5 weeks of age. The morphological phenotype includes: shorter body length, flattened head with increased interocular distance (Fig. 2A; Table 1), small heart (Fig. 2B) and scoliotic spine (Fig. 2A,E). Other visible phenotypes include an enlarged gill area (Fig. 2C) and swimming near the water surface, both of which are indicative of reduced blood oxygenation, and sterility. These features are indicative of the Costello phenotype and we refer to these abnormal fish as Costello-like fish (CS-like). Consistent with the human disease, we observed that the phenotype worsened with increasing age.
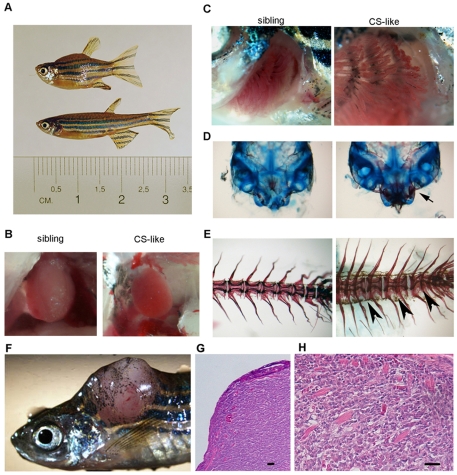
Fig. 2.
Homozygous P1 fish develop a Costello (CS)-like phenotype. Developmental defects in a 6-week-old CS-like fish. (A) CS-like fish have reduced body size compared with normal sibling fish. They also have a smaller heart (B), an enlarged gill area (C) and craniofacial dysmorphogenesis with increased ossification (Alizarin Red staining) of the Weberian complex (arrow) (D). (E) Alizarin Red staining also reveals fusion of vertebrae (arrowheads). An increase in cancer development is observed in transgenic fish. (F) Trunk rhabdomyosarcoma in a heterozygous P1 5-month-old fish. (G,H) H&E staining of a paraffin section of the tumor, showing the overall appearance (G) and the mature striated muscle component (H). Bars, 40 μm.
Table 1.
Morphometric parameters (mean±s.d.) of CS-like fish at 3 months of age
| Genotype | Normal siblings | CS-like fish |
|---|---|---|
| Number | 10 | 10 |
| Body length (cm) | 3.03±0.22 | 2.46±0.12 (P<0.01) |
| Cranium length (cm) | 0.32±0.01 | 0.31±0.03 (_P_=0.01) |
| Cranium width (cm) | 0.13±0.01 | 0.18±0.01 (P<0.01) |
| Width/length | 9.52±0.66 | 8.07±0.88 (P<0.01) |
We performed an analysis of the craniofacial and spinal abnormalities of CS-like fish at 5-6 weeks of age using cartilage and bone staining. The analysis of several bones and cartilages at this stage showed extensive alterations in ossification, with precocious ossification in almost all examined bones including both endochondral and intramembranous bones [for classification, see Elizondo et al. (Elizondo et al., 2005)]. Examples of the ossification state of the skull and Weberian complex in CS-like fish and normal siblings are shown in Fig. 2D. In the spine, we observed a progressive compression/disappearance of some intervertebral cartilagineous discs, leading to fusion of vertebrae and severe scoliosis between 5 and 12 weeks of age (Fig. 2E).
Since Costello patients are prone to developing tumors, we analyzed the predisposition of the CS-like fish to tumor formation. In older fish (5–12 months of age), we noticed a higher frequency of tumor formation (_n_=12/200 P1 fish – corresponding to 1 in 16) compared with wild-type populations (1 in 500) and that tumors developed at an earlier stage than in wild-type fish (5 months vs >2 years). Half of the tumors (_n_=6/12) were lymphatic infiltrations of the gut, liver or interstitial tissue; however, two transgenic fish developed metastatic melanomas, one had gut carcinomas, two had hepatocarcinoma and one developed rhabdomyosarcoma (Fig. 2F-H).
To show that the phenotype observed in CS-like fish was not the result of disruption of a gene at the insertion site, we mapped the insertion site in the P1 line using inverse PCR (Detrich, 2004). The location of the insertion was in a gene-free region in chromosome 15 (supplementary material Fig. S1D) (see Methods). Moreover, semiquantitative Southern blot analysis of genomic DNA copy number (Gabellini et al., 2006) showed that, in the CS-like fish genome, two copies of the transgene are present (supplementary material Fig. S1E). In addition, the same CS-like phenotype was also observed in the other gene trap line (P2), which, as shown in the supplementary material Fig. S1C, has a different insertion site.
There is a high frequency of heart defects in Costello patients; therefore, we investigated the occurrence of signs indicating a heart malformation in CS-like fish. We found that the mutants’ hearts were smaller (Fig. 2B) and had thicker walls (supplementary material Fig. S2D) compared with their normal siblings. Next, we investigated heart morphogenesis at 16 hpf in groups of fish from hemizygous intercrosses using a heart-specific probe, tbx20 (Szeto et al., 2002). We observed a delay in heart morphogenesis in approximately 25% of the embryos, as shown by a large and flat _tbx20_-positive heart disc that was centrally located as opposed to the majority of embryos which, at the same stage, had a left-oriented _tbx20_-positive heart tube (supplementary material Fig. S2A). Defects in heart morphogenesis were also visible at 32 hpf, with a significant reduction in the tbx20 expression area (supplementary material Fig. S2B,C). The defects in heart morphogenesis led to myocardial hypertrophy and defective functions including reduced oxygenation of peripheral tissues, which was seen at later stages. In conclusion, we propose that a delay in heart morphogenesis is an early sign of a CS-like phenotype.
Activation of RAS downstream pathways does not occur in CS-like fish
The G12V mutation of the H-RAS gene is known to lead to RAF1 activation and to increased ERK and AKT phosphorylation (Barbacid, 1990). Thus, we investigated the state of these pathways in CS-like fish and compared the results with the amount of H-RASV12 present in the tissue.
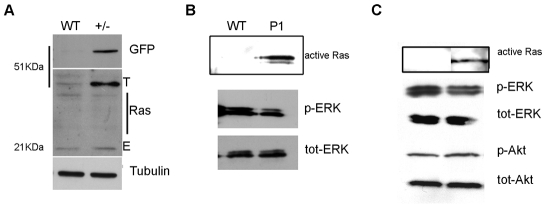
We found that the level of GFP-H-RASV12 expression was slightly higher (approximately 1.4 times) than the level of endogenous Ras expression (Fig. 3A). We then evaluated the presence of RAS in its active form (RAS-GTP) by testing its ability to bind to Raf1. Using an immunoprecipitation protocol that uses protein extracts from pools of 4-day-old fluorescent P1 larvae (including both heterozygous and homozygous transgenic larvae) (Fig. 3B), we detected the presence of active GTP-bound GFP-H-RASV12. In addition, we detected active GFP-H-RASV12 in protein extracts prepared from the brains of 3-month-old CS-like fish (Fig. 3C). Next, we investigated the presence of phosphorylated forms of ERK1/2 and Akt – the latter results from activation of the Mek and PI3 kinase pathways by active RAS. Despite the presence of active GTP-bound H-RASV12, overt phosphorylation of ERK1/2 or Akt was not be detected in the samples (Fig. 3B,C). Therefore, active H-RASV12, present in CS-like fish, is unable to sustain ERK1/2 or Akt phosphorylation, although it is possible that a local or temporary activation of these downstream pathways could have occurred in transgenic larvae or juveniles and escaped our analysis.
Fig. 3.
Ras targets are not activated in embryos and adult P1/CS-like zebrafish. (A) Western blot analysis shows that, in heterozygous transgenic (+/–) fish, the expression level of GFP-H-RASV12 from the transgene (T) is slightly higher than the expression of endogenous (E) Ras proteins. The antibodies used are indicated on the right of each blot and molecular weights are indicated on the left. (B,C) Protein extracts from 4 dpf P1 larvae (B) and adult CS-like fish (C) show active Ras (immunoprecipitated with Raf1), but there is no increase in ERK or Akt phosphorylation.
Cellular senescence and persistent activation of DDR markers in CS-like fish
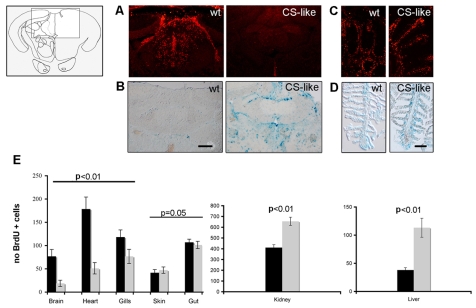
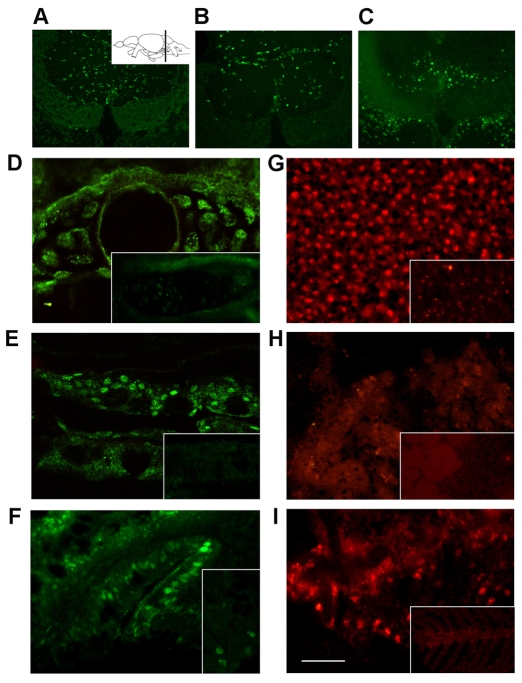
It has been reported that cells respond to oncogenic H-RAS activation by engaging in a senescence program (Bartkova et al., 2006; Di Micco et al., 2006), which protects them from proliferating in the presence of oncogene-induced DNA damage. We asked whether constitutive expression of the H-RAS oncogene activates a senescence program in CS-like fish. Thus, we analyzed the proliferative state and the presence of SA-βgal activity in several organs from wild-type and CS-like fish at 4–5 weeks and at 2–3 months of age. Cell proliferation takes place in certain tissues and in progenitor niches throughout the body of adult zebrafish (Poss et al., 2002; Adolf et al., 2006; Grandel et al., 2006), which are well known for their ability to regenerate several tissues, including brain, heart and fin (Kaul et al., 2007; Becker et al., 2005; Koster and Fraser, 2006). We analyzed two areas in the brain that are renowned for being sites of adult neurogenesis and neural progenitor proliferation, namely the preoptic/precommissural area of the ventral telencephalon and the valvula cerebelli. In addition, we examined the whole ventricular region in the heart. We found that, at both ages, bromodeoxyuridine (BrdU)-positive (BrdU+) cells in the brain and heart of adult CS-like fish were reduced to approximately half of the original population (Fig. 4A; Fig. 5A,B; supplementary material Fig. S3A). In contrast, we noticed that there was little or no change in BrdU uptake in the skin, gills (Fig. 4B) and gut, and an increase in BrdU+ cells in the liver and kidney (Fig. 4E; supplementary material Fig. S3B). The increase in cell proliferation in the kidney and liver of CS-like fish leads to a net expansion in organ size. We also detected an increased number of SA-βgal-positive cells in the brain, heart, liver and bone of CS-like fish (Fig. 4C,D; supplementary material Fig. S3C-G).
Fig. 4.
DDR and cellular senescence in proliferating cells from the adult brain and heart of CS-like fish. CS-like fish show signs of cellular senescence in areas of adult neurogenesis as indicated by reduced BrdU incorporation (A) and increased SA-βgal staining (B) in the cerebellum. The area of the valvula cerebelli analyzed here is shown in the line diagram (left panel). In contrast, no changes in BrdU incorporation (C) and SA-βgal staining (D) were observed in the gills of CS-like fish. The immunostaining and SA-βgal staining were performed on cryosections from 6-week-old CS-like fish and their sibling controls. Bars, 50 μm (B); 20 μm (D). (E) Quantification of proliferating cells (BrdU+ cells) in the brain, heart, gills, skin, gut, liver and kidney. Black bars indicate wild-type adult control fish and gray bars represent CS-like fish (_n_=5, mean±s.d.; analysis of variance).
Fig. 5.
DDR markers in tissues of CS-like zebrafish. BrdU staining on cryosections from the valvula cerebelli region of the brain. The inset in (A) shows the plane of the sections in a lateral view of the adult zebrafish brain. BrdU+ cells in the valvula cerebelli of normal sibling (A) and CS-like (B) 2-month-old fish compared with γH2AX immunofluorescence in CS-like fish (C). (D-I) γH2AX (green) and pAtm (red) immunofluorescence in the following tissues from adult CS-like and wild-type controls (insets): bone (D); skin (E); gut (F); liver (G); heart (H) and gills (I). Bar, 40 μm.
The occurrence of OIS in CS-like fish suggests that it might be the result of persistent DNA damage caused by prolonged exposure to oncogenic H-RAS. We investigated the presence of markers of an activated DDR in CS-like fish; as positive controls, we used wild-type fish of the same age that had been exposed to X-rays. Canonical DDR markers include activation of the upstream ataxia telangiectasia mutated (Atm) kinase which autophosphorylates in response to damage, several substrates of pAtm, which can be detected collectively with a phosphorylated S/TQ antibody (pS/TQ), and the phosphorylated histone variant H2AX known as γH2AX (Campisi and d’Adda di Fagagna, 2007). Massive upregulation of γH2AX, phosphorylated Atm (pAtm) and pS/TQ were detected through western blot analysis (data not shown) and immunohistochemistry, with fluorescent detection in tissue sections of irradiated fish (supplementary material Fig. S4E). These results demonstrate that these DDR activation markers can be reliably used in vivo in adult zebrafish. In CS-like fish, but not in wild-type controls, distinct nuclear foci containing γH2AX and pAtm were detected in various tissues (Fig. 5; supplementary material Fig. S4D-F,I). Strikingly, we noticed that the nuclei that stained for γH2AX and pAtm in the brain and heart of CS-like fish are localized in specific regions (Fig. 5A-C; supplementary material Fig. S4F,I) and throughout the heart ventricle (Fig. 5H), corresponding to areas enriched with adult proliferating cells (Adolf et al., 2006; Grandel et al., 2006; Poss, 2007). There was a clear inverse correlation between the decrease in BrdU+ cells and the increase of SA-βgal and DDR markers in the brain and heart of CS-like fish (Table 2; Fig. 5A-C; supplementary material Fig. S4G-I). These results suggest that constitutive expression of oncogenic H-RAS in a developing vertebrate organism leads to DNA damage in subpopulations of adult progenitor cells in different organs.
Table 2.
Number of BrdU-positive cells, and SA-βgal and DDR markers in tissues of CS-like fish
| Tissue | BrdU | SA-βgal | DDR |
|---|---|---|---|
| Brain | – | + | ++ |
| Heart | – | + | ++ |
| Gills | +/– | +/– | + |
| Liver | +/+ | + | + |
| Kidney | + | +/– | – |
| Gut | +/– | +/– | + |
| Skin | +/– | + | + |
OIS and apoptosis are dependent on tp53 activation (Schmitt et al., 2002; Sarkisian et al., 2007), which mediates many of the cell defenses in response to oncogenic stress. We investigated whether CS-like fish exhibited activation of tp53 through tp53 target gene expression. We found an increase in the expression of the tp53 target gene cdkn1a/p21 in RNA extracted from whole CS-like fish and from specific tissues (supplementary material Fig. S5A), and tissue sections processed for in situ hybridization, from CS-like fish (supplementary material Fig. S5B-E).
Overexpression of GFP-H-RASV12 in an inducible transgenic line leads to tp53-dependent OIS and DNA damage in vivo
As in humans, the CS-like phenotype develops over time and we can distinguish affected fish at just 5–6 weeks of age. In order to elucidate the development of the Costello phenotype, we generated an inducible H-RASV12 transgenic line Tg(hsp70I:GFP-H-RASV12) io3 (see Methods) (supplementary material Fig. S6), in which we can overexpress GFP-H-RASV12. This inducible line helped us to address the question of how constitutive expression of oncogenic H-RASV12 in CS-like fish leads to the development of DDR and OIS.
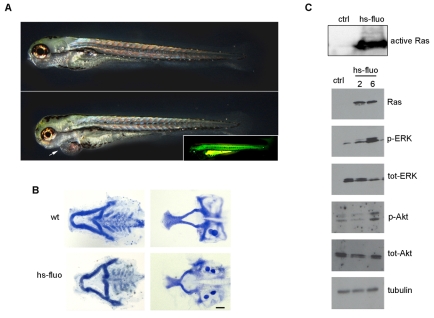
In this transgenic line, higher levels of oncogenic H-RASV12 expression were obtained through the exposure of developing embryos, larvae and adults to 39°C for 30 minutes (Fig. 6; supplementary material Fig. S6B). We asked whether transgenic larvae from this line developed traits of the CS-like phenotype and, if so, during what stage of development. We induced the expression of the chimeric protein in embryos at 24 hpf and analyzed the phenotype over time. We observed that larvae display an abnormal phenotype at 4–6 days postfertilization (dpf), characterized by heart edema (Fig. 6A) and craniofacial abnormalities (Fig. 6B). This embryonic phenotype mimics the CS-like phenotype observed in homozygous juveniles of the P1 line at 4–5 weeks of age, but is rather common in manipulated zebrafish embryos. To ensure that it was the result of H-RASV12 overexpression, we analyzed the activation of Ras downstream targets and observed a strong and prompt phosphorylation of ERK1/2 and Akt, and an increase in the amount of active GTP-bound GFP-H-RASV12 at 2 and 6 hours following heat shock (Fig. 6C).
Fig. 6.
Heat-shock-induced H-RASV12 expression causes a CS-like phenotype within 5 days and ERK/Akt phosphorylation. Embryos were subject to heat shock at 24 hpf. At 5 days, fluorescent transgenic larvae (inset) display abnormal phenotypes, characterized by (A) strong pericardial edema (arrow) and (B) craniofacial defects as revealed by Alcian Blue-stained splanchnocranium (right) and neurocranium (left). Bar, 50 μm. (C) Western blot analysis revealed that, in 4 dpf larvae, the inducible and high expression of H-RASV12 causes an increase in active Ras and the phosphorylation of ERK/Akt at 2 and 6 hours following heat shock, respectively. ‘WT’ or ‘ctrl’ indicates heat-shocked non-fluorescent sibling control fish and ‘hs-fluo’ indicates heat-shocked fluorescent transgenic fish.
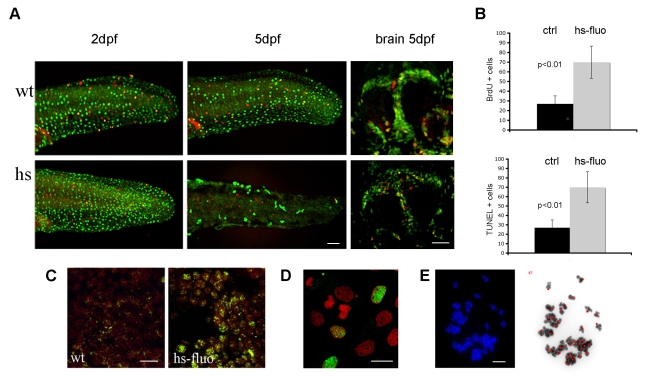
We asked whether the overexpression of H-RASV12 induced hyperproliferation, apoptosis or cellular senescence. BrdU incorporation in embryos subjected to heat activation at 24 hpf showed an increase in the number of proliferating cells up to 3 dpf (Fig. 7A). However, by 5 dpf the number of BrdU+ cells dropped to less than half of that observed in controls (Fig. 7A,B). We also checked whether H-RASV12 expression induced apoptosis and at 5 dpf we observed an increase in the number of TUNEL-positive cells (Fig. 7C). Together with the proliferation block and the increase in apoptosis, we detected an increase in the number of γH2AX- and pAtm-positive nuclei in sections from 5 dpf heat-shocked transgenic larvae (hs-fluo) (Fig. 7D), as well as in fibroblast cultures derived from these embryos (Fig. 7E). Next, we asked whether high levels of GFP-H-RASV12 could have any impact on genome stability and found that aneuploidy in mitotic spreads from hs-fluo embryos at 32 hpf (Fig. 7F; supplementary material Fig. S7A) was similar to that observed in irradiated embryos (supplementary material Fig. S7A). This suggests that cells which overexpress GFP-H-RASV12 in zebrafish transgenic lines are subjected to replicative stress that leads to genome instability.
Fig. 7.
Overexpression of H-RASV12 leads to OIS of DNA damaged cells in vivo. (A) 24 hpf larvae subjected to a 30-minute heat shock display an initial increase in proliferation, represented by the green BrdU+ cells and shown here at 2 dpf in the skin (left panels), followed by a decrease in proliferation at 5 dpf in both skin (middle panels) and brain (right panels). The percentage of mitotic, PH3-positive, cells (red) is unchanged. (B) Quantification of BrdU+ cells shows a reduced number in 5-day-old transgenic larvae (_n_=5; mean±s.d.) following heat shock. Quantification of apoptotic cells revealed an increase in TUNEL-positive cells in heat-shocked transgenic larvae (_n_=5; mean±s.d.). Nuclear γH2AX immunostaining in the brain of control (wt) or heat-shocked transgenic (hs-fluo) 5 dpf larvae (C), and in cells dissociated from transgenic larvae and heat shocked in vitro (D). GFP-H-RASV12 expression induces aneuploidy, a sign of genomic instability, in mitotic spreads from 32 hpf embryos (E). Bars, 40 μm (A,C,D); 10 μm (E).
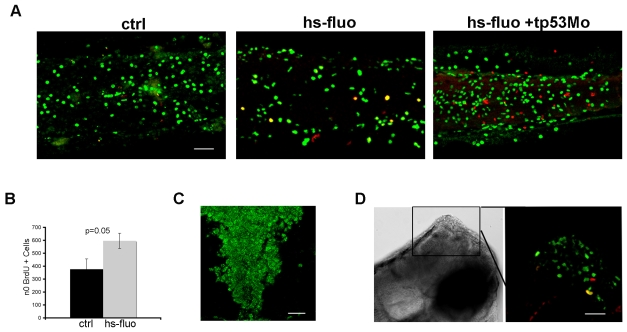
Finally, we analyzed the contribution of tp53 to the DDR and the proliferation block that occur in larvae overexpressing GFP-H-RASV12. Using a tp53 morpholino (mo) (Langheinrich et al., 2002), we were able to prevent the proliferation block, which occurred 4 days after induction of oncogene expression (Fig. 8A,B). In contrast, development of the heart and cranial cartilage defects was not prevented by tp53 mo injection (data not shown) suggesting that tp53 is not involved in the early response to H-RASV12 expression, but rather it controls the response to DNA damage that occurs after prolonged oncogene expression and, therefore, promotes cellular senescence. In support of this hypothesis, hs-fluo embryos injected with tp53 mo show increased γH2AX and pAtm nuclear staining (Fig. 8C). Moreover, as expected, the absence of tp53 completely prevented both upregulation of cdkn1a/p21, mdm2 and bax expression, and the decrease in ccnd1 expression, which are seen 4 days after heat shock in the transgenic larvae (supplementary material Fig. S7B). Interestingly, embryos that express an activated H-RAS oncogene in the absence of tp53 developed overgrowths of the central nervous system that are composed of proliferating BrdU-and phosphoH3-positive cells (Fig. 8D).
Fig. 8.
The OIS phenotype is rescued by a tp53 morpholino. (A) BrdU incorporation in the inducible GFP-H-RASV12 line injected with the tp53 mo. Persistence of hyperproliferation is observed in hs-fluo embryos injected with the tp53 mo at 5 dpf (hs-fluo+tp53 mo), lateral view of the tail region. (B) Quantification of BrdU+ cells in the skin of heat-shocked 5 dfp transgenic larvae (_n_=5; mean±s.d.) injected with the tp53 mo (gray bar, hs-fluo) compared with control wild-type larvae (_n_=5; mean±s.d.) injected with the same morpholino (black bar, ctrl). (C) Immunofluorescence for γH2AX shows a diffuse DDR in the hindbrain of an hs-fluo larva at 5 dpf. (D) GFP-H-RASV12 is able to induce brain overgrowths in the absence of tp53 (left), lateral view, dorsal to the top. The right panel shows an enlargement of the boxed area stained for BrdU (green) and PH3 (red). Bars, 40 μm.
DISCUSSION
Despite the fact that Ras proteins have been studied for years, it is clear that a complete understanding of their function is still missing. The observation that oncogenic H-RAS can lead to two rather different cell behaviors, i.e. transformation and senescence, is particularly puzzling. Moreover, owing to germline activating mutations, the same oncogenic H-RAS also leads to developmental disorders when constitutively expressed. In order to dissect the role of oncogenic H-RAS in vivo, we generated transgenic zebrafish lines expressing different levels of the oncogene. Our study shows that: (1) fish expressing oncogenic H-RAS in their germline display traits of Costello syndrome; (2) low but prolonged exposure of a developing vertebrate organism to the H-RAS oncogene causes a DDR followed by senescence (OIS) in adult proliferating cells of target organs (heart and brain); (3) the process can be recapitulated in embryos using a _GFP-H-RASV12_-inducible line, where OIS depends on the ability of tp53 to block H-RASV12 hyperproliferation.
Zebrafish as a model for Costello syndrome
During the generation of fish lines that express oncogenic H-RAS in different organs or tissues, we identified fish that expressed GFP-H-RASV12 ubiquitously. We observed that these fish displayed hallmarks of the Costello syndrome, which is a rare, congenital, multiple abnormality syndrome associated with a failure to thrive and with developmental delays. The syndrome is caused by de novo germline activating mutations in the H-RAS gene (Aoki et al., 2005). Moreover, the appearance of this phenotype in fish carrying two copies of both the transgene and the endogenous h-ras gene is consistent with the genetic situation in affected humans, where the presence of the mutated H-RAS gene is balanced by the presence of a wild-type allele (Aoki et al., 2005). Recently, a mouse model of Costello syndrome was reported (Schuhmacher et al., 2008); in this model, the endogenous H-Ras allele was replaced by a mutant allele carrying the G12V mutation. The phenotype of the mouse Costello model resembles the human disorder, but with two important differences. First, an increase in tumor formation was not observed in the mouse model, which, unlike human patients, developed angiotensin II-dependent systemic blood hypertension (Schuhmacher et al., 2008). In the zebrafish model, we found a remarkable increase in tumor incidence, with tumor types similar to those reported to occur in human Costello patients. Surprisingly, in mouse and zebrafish models, phosphorylation of ERK or Akt, two of the most commonly activated Ras downstream targets, was not observed. We cannot exclude the possibility that ERK or Akt activation could have occurred at certain times or in restricted places in the transgenic fish and escaped our analysis; however, it is clear that if such activation occurs, it is not maintained. What is surprising is that in both studies we were able to detect GTP-bound GFP-H-RASV12 which did not lead to phosphorylation of ERK1/2 or Akt. The presence of active H-RAS suggests that other Ras downstream pathways should be investigated, because these might be responsible for the OIS phenotype detected in our fish. We also observed an increase in cdkn1a/p21 expression in different organs and tissues in the CS-like fish. This strongly correlates with the fact that OIS requires cdkn1a/p21 to arrest cell cycle progression; in fact, human fibroblasts lacking CDKN1A/p21 fail to arrest in response to DNA damage (Brown, 1997).
The predominant view on the pathogenesis of Costello syndrome was that activation of the ERK and/or AKT pathways owing to H-RAS mutations could be the main molecular driver(s) of the disease phenotype, a concept reinforced by the study of mutant H-RAS activity in vitro (Aoki et al., 2005; Rauen, 2007; Zampino et al., 2007). In this study, we propose a zebrafish model for this syndrome and show that, like in the mouse model (Schuhmacher et al., 2008), sustained ERK and/or Akt phosphorylation are unlikely to be solely responsible for the abnormalities observed in Costello patients.
DNA damage and senescence may be responsible for the age-related worsening of the Costello phenotype
It has already been documented that high levels of oncogenic RAS can cause hyperproliferation and increased DNA damage, followed by cellular senescence (Di Micco et al., 2006). We propose that very similar outcomes can be obtained with low but prolonged exposure to H-RASV12. In fact, the relatively low and ubiquitous expression of oncogenic RAS leads to a DDR in cells that continue to proliferate throughout adult stages. The cells that are more affected belong to pools of adult progenitors in the brain and heart, where oncogenic H-RAS expression paralyzes cellular functions leading to OIS in response to DDR. The reason why some organs, although engaged in a hyperproliferative response to H-RASV12, do not undergo senescence even in the presence of elevated DNA damage (i.e. kidney and liver) is not known. Perhaps the increase in organ size that we detected in the liver and kidney of CS-like fish reflects defective tp53 activation, which would allow proliferation to continue even in the presence of massive DDR, or the specific functions of these organs. The zebrafish kidney serves as the primary site for adult hematopoiesis (de Jong and Zon, 2005), which may be increased in response to oncogenic H-RAS; whereas, in the liver, hepatocytes may be involved in metabolic responses to H-RASV12 expression, including detox functions to deal with the increase in reactive oxygen species that is often reported in response to oncogenic RAS. So, our model shows that the CS-like phenotype is the result of a low and prolonged expression of oncogenic H-RAS, which uncovers a specific weakness of adult progenitor cells. Interestingly, a similar abnormal phenotype was also observed in the inducible line where higher H-RASV12 expression leads to activation of ERK/Akt signaling and to a robust DDR in a shorter time. Usually these larvae do not survive for more than 5–6 days following heat shock, and this prevented us from using them to study the appearance of cellular senescence following DDR activation. However, a different protocol for H-RASV12 induction, involving multiple, short heat shocks in juveniles/adults, leads to a dose-dependent DDR with senescence of adult progenitor cells, similar to what we observe in CS-like fish, and a little heart and bone phenotype (data not shown). Thus, we propose that the Costello syndrome phenotype might result from two different concerted and linked actions of oncogenic H-RAS: activation of tissue-specific pathways and OIS in progenitor/stem cells. We suggest that the age-related worsening of the CS-like phenotype might be the result of OIS affecting progenitors pools. When these cells undergo senescence they can no longer repair damaged or apoptotic tissues, generate specialized cells, or maintain the normal turnover in regenerating organs. As a consequence, tissues and organs carrying senescent progenitor cells undergo irreparable damage under normal conditions of stress and become dysfunctional earlier.
We predict that the tumor suppressor tp53 orchestrates the oncogene-induced DDR and cell cycle arrest, but not the tissue-specific responses to H-RAS activation. Our results show that the hyperproliferation observed in the inducible line is not the result of tp53 activation. Interestingly, we observed that in the absence of tp53, the induction of oncogenic H-RAS causes overgrowths in the CNS. Besides the frequent appearance of glioblastomas in human Costello patients (Gripp et al., 2006), it has recently been shown that when activating H-RAS mutations and p53 inactivation occur simultaneously, they promote the generation of brain cancer stem-like cells in vitro (Lee et al., 2008).
In summary, exhaustion of the replication abilities of adult progenitor cells, owing to oncogene-induced DNA damage and senescence, might lead to the age-related worsening of the Costello phenotype.
METHODS
Raising fish and generation of the transgenic fish lines
Zebrafish were maintained and crossed according to standard methods (Westerfield, 2000). For the generation of the gene trap lines, we used a modified version of the T2KSAG plasmid designed by Kawakami (Kawakami et al., 2000), in which the GFP is replaced by the fusion protein eGFP-H-RASG12V (indicated as GFP-H-RASV12) using the restriction enzymes _Bam_HI and _Bgl_II. Fertilized eggs were injected with 2 nl of a mixture containing 25 ng/μl of the circular plasmid T2KSAG:GFP-H-RASV12 and 25 ng/μl of T2 transposase mRNA. Injected embryos were raised to adulthood and crossed with either wild-type fish or fish injected with the same construct. F1 embryos were observed under a fluorescent dissecting microscope from day 1 to 5 after fertilization. For generation of the inducible line, the same GFP-H-RASG12V construct was cloned in a Tol2 vector containing the Hsp70 promoter.
NIH3T3 cell transfection and focus formation assay
NIH3T3 cells were transfected with the pEGFP-C2 vector that carries a neo selective marker (Clontech) containing the full-length active H-RASV12 sequence or the empty pEGFP-C2 vector as an internal control. The transfection was carried out by the calcium phosphate coprecipitation method, as described previously (Cox and Der, 1994), followed by drug selection with G418 at 0.8 mg/ml. The focus formation assay was performed as described previously (Kraus et al., 1984). Culture plates containing foci or colonies were fixed with ice-cold methanol and stained with Crystal Violet then scanned on a flatbed scanner and quantified using ImageJ.
The two constructs generated comparable numbers of G418-resistant colonies; therefore, differences in focus-forming ability are not the result of differences in transfection efficiency between the constructs.
Screening for GFP-RAS insertions
For PCR analysis of F1 embryos, sixty embryos at 24 hpf were pooled and used for DNA genomic extraction. Embryos were digested in 300 μl of a solution containing 10 mM Tris, 2 mM EDTA, pH 8, 0.2% Triton and 200 μg/ml proteinase K, incubated overnight at 50°C and heated for 5 minutes at 95°C. DNA was subsequently purified by phenol-chloroform extraction and precipitation with ethanol. The first round of PCR used 300 ng of DNA for each template and the following primers: eGFP-RAS F (5′-AGCTGACCCTGAAGTTCATCT-3′) and eGFP-RAS R (5′- GTACTGGTGGATGTCCTCAAAAG-3′). Twenty cycles of PCR were carried out as follows: 96°C for 30 seconds, 55°C for 30 seconds, 72°C for 1 minute. 0.5 μl of DNA from the first PCR was used as a template for the second round, which used the primers eGFP-F2 (5′-CACATGAAGCAGCACGACTT-3′) and eGFP-R3 (5′ -ATCAATGACCACCTGCTTCC-3′). Twenty-eight cycles of PCR were carried out as follows: 96°C for 30 seconds, 55°C for 30 seconds, 72°C for 50 seconds. A DNA band of 725 nucleotides was present when the construct was inserted into the genome.
Southern blot analysis
Genomic DNA was prepared according to Westerfield (Westerfield, 2000). About 7 μg of DNA per fish was digested with _Eco_RV, _Kpn_I or a combination of the two enzymes. Following electrophoresis with 1% TAE-agarose gel, the DNA was transferred to a Hybond N+ membrane (Amersham) and hybridized with a probe designed upon the GFP-HRAS sequence. The GFP-RAS probe was prepared for use in a PCR using the following primers: forward (5′-CCACTACCTGAGCACCCAGT-3′) and reverse (5′-GTCTCCCCATCAATGACCAC-3′). We used 10 ng of pT2SAG:GFP-HRAS plasmid as a template. The 337-nucleotide PCR product was gel purified and labeled with 32P-dCTP (Amersham) using the Rediprime II random primer labeling kit (GE healthcare, Amersham).
RT-PCR analysis
RNA was purified using the Trizol extraction protocol. After purification, RNA was digested with DNase I according to the RNeasy kit (Qiagen). 2 μg of RNA was used, in combination with oligo(dT) primers, for reverse transcription with Superscript III (Invitrogen). We used 2 μl of cDNA for each PCR reaction; the oligonucleotides used are described by Ghiselli (Ghiselli, 2006). β-actin oligonucleotides are as follows: forward (5′-ACC TCA TGA AGA TCC TGA CC-3′) and reverse (5′-TGC TAA TCC ACA TCT GCT GG-3′).
Inverse PCR strategy
The full T2KSAG:GFP-H-RASV12 plasmid was found integrated into the zebrafish germline. We identified the break point in the plasmid, which occurred between nucleotides 6850 and 7221, and used a modified version of Kawakami’s protocol (Kawakami et al., 2000) in which genomic DNA was digested with _Xma_I and the self-ligation reaction was used directly as a PCR template. We used the following primers: I-NR2 (5′-CTG TGT TCC GAA GAT GAA CGG AGG TGT-3′), I-NR3 (5′-CCT CTA CAA ATG TGG TAT GGC TGA TTA-3′), I-F1 (5′ -CCC GTG GAC CTA ACG TTA CCA ATT ACA-3′) and I-NF2 (5′-GCG GGA GAC AGA GGA CAC ATT TCA TCT-3′).
Detection of apoptotic cells
Apoptotic cells in 5 dpf embryos were detected using an ApopTag Red in situ apoptosis detection kit (S7165, Chemicon), which is based on a terminal transferase dUTP nick-end labeling (TUNEL) assay. Samples were mounted in Vectashield (Vector Laboratories) with DAPI.
In situ hybridization
In situ hybridization on whole-mounts and cryostat sections was performed as described previously (Costagli et al., 2002).
Zebrafish primary cell cultures
Zebrafish cell cultures from transgenic embryos of Gt(GFP-H-RASV12) io1 and Tg(hsp70I:GFP-H-RASV12) io3 lines were generated and maintained as described previously (Vallone et al., 2007).
Fish ionizing radiation treatment
Adult fish were exposed to 42 Gy using a Faxitron X-ray machine (Faxitron, Lincolnshire, Illinois). Protein extracts were prepared between 2 hours and 1 day after irradiation.
Paraffin embedding and H&E staining
The fish were dehydrated in ethanol, decalcified in 0.25 M EDTA, cleared in xylene and infiltrated with paraffin. Briefly, slides were deparaffinized in xylene, passed through a graded ethanol series, rinsed in water and briefly soaked in hematoxylin and then in eosin. Slides were dehydrated and mounted using EUKITT (GmbH).
Cartilage and bone staining for larvae and adults
5-day-old larvae were fixed overnight in 4% paraformaldehyde, rinsed in distilled water and stained overnight in Alcian Blue solution (1% HCl, 70% ethanol, 0.1% Alcian Blue). Larvae were then cleared by washing in 3% hydrogen peroxide, dehydrated through an ethanol series and equilibrated in 85% glycerol-PBS. Splanchnocranium and neurocranium were manually dissected from stained larvae and flat mounted in 85% glycerol-PBS.
Fixed juvenile/adult fish were eviscerated, skinned and dehydrated in 100% ethanol for 2 days. The carcasses were incubated in Alcian Blue solution for 12 hours and then in 100% ethanol overnight. Fish were then cleared in 1% KOH for 6 hours and stained in 0.05% Alizarin Red in 2% KOH for 3 hours. The fish were cleared in 2% KOH overnight and stored in 25% glycerol.
Morphometry
Measurements were taken using a dissecting microscope equipped with a micrometer lens.
Western blot analysis and Ras activation assay
Adult fish were killed by anesthetic overdose (0.04% MESAB, Sigma), which was ground to a fine powder in liquid nitrogen and resuspended in sample buffer (2% SDS, 10% glycerol, 60 mM Tris pH 6.8). Organs and larvae were lysed in sample buffer; 50 μg of total extracts were resolved by SDS-PAGE, transferred to nitrocellulose and tested with the following antibodies: Ras (1:500, BD Bioscience), GFP (1:2000, Torrey Pines Biolabs), phospho-p44/42 (1:1000, Cell Signaling), p44/42 (1:1000, Cell Signaling), phospho-Akt (1:1000, Cell Signaling), Akt (1:1000, Cell Signaling), phospho-Atm (1:600, Rockland), Atm (1:1000, Sigma).
We performed the StressXpress Ras Activation kit (Stressgen, Bioreagents) on protein extracts from larvae and juvenile fish according to the manufacturer’s instructions. Band intensities were quantified using ImageJ.
β-gal histochemistry
Acidic β-gal staining was performed on formalin-fixed zebrafish cryosections, as described previously (Dimri et al., 1995).
BrdU treatment, immunofluorescence and antisera
Embryos and adults were incubated in fish water containing 10 mM of BrdU for 2 hours (embryos and larvae) or overnight (juveniles and adults). Embryos and larvae were fixed in 4% paraformaldehyde, adult and juveniles were embedded unfixed in OCT, and sections were fixed in 2% paraformaldehyde for 2 minutes. For BrdU-phospho H3 (PH3) immunostaining, sections and larvae were treated with 2 N HCl for 20 minutes, followed by two rinses in 0.1 M sodium tetraborate. For γH2AX and pAtm immunostaining, unfixed cryostat sections were fixed in a 50:50 methanol:acetone mix for 2 minutes and then rinsed in PBS. In addition to the antisera used in the western blots, mouse monoclonal anti-BrdU (Sigma) and rabbit polyclonal anti-PH3 (Upstate) antisera were used. We used secondary antibodies conjugated with Alexa 488 or 568 (Molecular Probes).
Chromosome preparation and visualization
At 24 hpf, tg(Hsp70:GFP-H-RASV12)io3 embryos were incubated for 30 minutes at 39°C. At 32 hpf, 100 transgenic embryos, 100 irradiated embryos (20 Gy) and 100 untreated embryos, were incubated in colchicine (4 mg/ml) overnight and dechorionated by pronase treatment. Chromosome preparations were dropped onto slides and allowed to dry overnight at 37°C. Samples were mounted in Vectashield (Vector Laboratories) with DAPI. The images of chromosome spreads were acquired and elaborated with the Band View software system (Applied Spectral Imaging).
Counts of BrdU+ and TUNEL+ cells
The numbers of BrdU- or TUNEL-positive cells in whole-mount larvae were counted with a confocal microscope (Leica SP2 TCS). Counts were performed in the whole tail region (from the beginning of the yolk extension) or in the forebrain, where the count refers to a 100 μm thick section from the area between the olfactory bulb and the diencephalon. Section counts refer to an area of 2.4 mm2, from a serial section with a thickness of 20 μm, from the whole forebrain or whole heart. At least 5 sections, including the areas of interest (see Results), from 6 different specimens were analyzed.
Gene, constructs and morpholinos
The tbx20 (hRT) plasmid (Szeto et al., 2002) and the cdkn1a/p21 plasmid were used to transcribe antisense probes. We injected 10 ng of tp53 mo4 (www.zfin.org) into Tg (hsp70I: GFP-H-RASV12)io3 embryos at the one-cell stage. Larvae were heat shocked at 24 hpf for 30 minutes and sorted by fluorescence 4 hours later.
Supplementary Material
Supplementary Material
Acknowledgments
We especially thank Keith Cheng, Giuseppe Testa, Giorgio Scita and Bruno Amati for critically reading the manuscript and for the constructive comments. We thank O. Malazzi for help with the chromosomal spread and analysis, M. Fumagalli and R. di Micco for sharing reagents and protocols, A. Renna and C. Buratti for fish maintenance, P. M. Essomba for photography of adult fish and T. Schilling for consultation in bone staining. C.S. was supported by a 1-year fellowship from the Italian Association for Cancer Research (AIRC).
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
REFERENCES
- Adolf B., Chapouton P., Lam C. S., Topp S., Tannhauser B., Strahle U., Gotz M., Bally-Cuif L. (2006). Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev. Biol. 295, 278–293 [DOI] [PubMed] [Google Scholar]
- Aoki Y., Niihori T., Kawame H., Kurosawa K., Ohashi H., Tanaka Y., Filocamo M., Kato K., Suzuki Y., Kure S., et al. (2005). Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat. Genet. 37, 1038–1040 [DOI] [PubMed] [Google Scholar]
- Barbacid M. (1990). ras oncogenes: their role in neoplasia. Eur. J. Clin. Invest. 20, 225–235 [DOI] [PubMed] [Google Scholar]
- Bartkova J., Rezaei N., Liontos M., Karakaidos P., Kletsas D., Issaeva N., Vassiliou L. V., Kolettas E., Niforou K., Zoumpourlis V. C., et al. (2006). Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444, 633–637 [DOI] [PubMed] [Google Scholar]
- Becker T., Lieberoth B. C., Becker C. G., Schachner M. (2005). Differences in the regenerative response of neuronal cell populations and indications for plasticity in intraspinal neurons after spinal cord transection in adult zebrafish. Mol. Cell Neurosci. 30, 265–278 [DOI] [PubMed] [Google Scholar]
- Brown J. M. (1997). M. Stephen Meyn, ataxia telangiectasia and cellular responses to DNA damage. Cancer Res. 57, 2313–2315 [PubMed] [Google Scholar]
- Campisi J., d’Adda di Fagagna F. (2007). Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell. Biol. 8, 729–740 [DOI] [PubMed] [Google Scholar]
- Cichowski K., Jacks T. (2001). NF1 tumor suppressor gene function: narrowing the GAP. Cell 104, 593–604 [DOI] [PubMed] [Google Scholar]
- Collado M., Gil J., Efeyan A., Guerra C., Schuhmacher A. J., Barradas M., Benguria A., Zaballos A., Flores J. M., Barbacid M., et al. (2005). Tumour biology: senescence in premalignant tumours. Nature 436, 642. [DOI] [PubMed] [Google Scholar]
- Costagli A., Kapsimali M., Wilson S. W., Mione M. (2002). Conserved and divergent patterns of Reelin expression in the zebrafish central nervous system. J. Comp. Neurol. 450, 73–93 [DOI] [PubMed] [Google Scholar]
- Costello J. M. (1977). A new syndrome: mental subnormality and nasal papillomata. Aust. Paediatr. J. 13, 114–118 [DOI] [PubMed] [Google Scholar]
- Cox A. D., Der C. J. (1994). Biological assays for cellular transformation. Meth. Enzymol. 238, 277–294 [DOI] [PubMed] [Google Scholar]
- de Jong J. L., Zon L. I. (2005). Use of the zebrafish system to study primitive and definitive hematopoiesis. Annu. Rev. Genet. 39, 481–501 [DOI] [PubMed] [Google Scholar]
- Detrich H. W. (2004). The zebrafish: genetics, genomics, and informatics. San Diego, CA: Elsevier Academic Press [Google Scholar]
- Di Micco R., Fumagalli M., Cicalese A., Piccinin S., Gasparini P., Luise C., Schurra C., Garre M., Nuciforo P. G., Bensimon A., et al. (2006). Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 444, 638–642 [DOI] [PubMed] [Google Scholar]
- Dimri G. P., Campisi J. (1994). Molecular and cell biology of replicative senescence. Cold Spring Harb. Symp. Quant. Biol. 59, 67–73 [DOI] [PubMed] [Google Scholar]
- Dimri G. P., Lee X., Basile G., Acosta M., Scott G., Roskelley C., Medrano E. E., Linskens M., Rubelj I., Pereira-Smith O., et al. (1995). A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 92, 9363–9367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J. (1996). Control of ras activation. Cancer Surv. 27, 87–100 [PubMed] [Google Scholar]
- Elizondo M. R., Arduini B. L., Paulsen J., MacDonald E. L., Sabel J. L., Henion P. D., Cornell R. A., Parichy D. M. (2005). Defective skeletogenesis with kidney stone formation in dwarf zebrafish mutant for trpm7. Curr. Biol. 15, 667–671 [DOI] [PubMed] [Google Scholar]
- Gabellini D., D’Antona G., Moggio M., Prelle A., Zecca C., Adami R., Angeletti B., Ciscato P., Pellegrino M. A., Bottinelli R., et al. (2006). Facioscapulohumeral muscular dystrophy in mice overexpressing FRG1. Nature 439, 973–977 [DOI] [PubMed] [Google Scholar]
- Ghiselli G. (2006). SMC3 knockdown triggers genomic instability and p53-dependent apoptosis in human and zebrafish cells. Mol. Cancer 5, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandel H., Kaslin J., Ganz J., Wenzel I., Brand M. (2006). Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell fate. Dev. Biol. 295, 263–277 [DOI] [PubMed] [Google Scholar]
- Gripp K. W., Lin A. E., Stabley D. L., Nicholson L., Scott C. I., Jr, Doyle D., Aoki Y., Matsubara Y., Zackai E. H., Lapunzina P., et al. (2006). HRAS mutation analysis in Costello syndrome: genotype and phenotype correlation. Am. J. Med. Genet. A 140, 1–7 [DOI] [PubMed] [Google Scholar]
- Kaul A., Overmeyer J. H., Maltese W. A. (2007). Activated Ras induces cytoplasmic vacuolation and non-apoptotic death in glioblastoma cells via novel effector pathways. Cell Signal. 19, 1034–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K., Shima A., Kawakami N. (2000). Identification of a functional transposase of the Tol2 element, an Ac-like element from the Japanese medaka fish, and its transposition in the zebrafish germ lineage. Proc. Natl. Acad. Sci. USA 97, 11403–11408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster R. W., Fraser S. E. (2006). FGF signaling mediates regeneration of the differentiating cerebellum through repatterning of the anterior hindbrain and reinitiation of neuronal migration. J. Neurosci. 26, 7293–7304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus M. H., Yuasa Y., Aaronson S. A. (1984). A position 12-activated H-ras oncogene in all HS578T mammary carcinosarcoma cells but not normal mammary cells of the same patient. Proc. Natl. Acad. Sci. USA 81, 5384–5388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langheinrich U., Hennen E., Stott G., Vacun G. (2002). Zebrafish as a model organism for the identification and characterization of drugs and genes affecting p53 signaling. Curr. Biol. 12, 2023–2028 [DOI] [PubMed] [Google Scholar]
- Lee J. S., Gil J. E., Kim J. H., Kim T. K., Jin X., Oh S. Y., Sohn Y. W., Jeon H. M., Park H. J., Park J. W., et al. (2008). Brain cancer stem-like cell genesis from p53-deficient mouse astrocytes by oncogenic Ras. Biochem. Biophys. Res. Commun. 365, 496–502 [DOI] [PubMed] [Google Scholar]
- Malumbres M., Barbacid M. (2003). RAS oncogenes: the first 30 years. Nat. Rev. Cancer 3, 459–465 [DOI] [PubMed] [Google Scholar]
- Poss K. D. (2007). Getting to the heart of regeneration in zebrafish. Semin. Cell Dev. Biol. 18, 36–45 [DOI] [PubMed] [Google Scholar]
- Poss K. D., Wilson L. G., Keating M. T. (2002). Heart regeneration in zebrafish. Science 298, 2188–2190 [DOI] [PubMed] [Google Scholar]
- Rauen K. A. (2007). HRAS and the Costello syndrome. Clin. Genet. 71, 101–108 [DOI] [PubMed] [Google Scholar]
- Rotchell J. M., Lee J. S., Chipman J. K., Ostrander G. K. (2001). Structure, expression and activation of fish ras genes. Aquat. Toxicol. 55, 1–21 [DOI] [PubMed] [Google Scholar]
- Sarkisian C. J., Keister B. A., Stairs D. B., Boxer R. B., Moody S. E., Chodosh L. A. (2007). Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat. Cell Biol. 9, 493–505 [DOI] [PubMed] [Google Scholar]
- Schmitt C. A., Fridman J. S., Yang M., Lee S., Baranov E., Hoffman R. M., Lowe S. W. (2002). A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 109, 335–346 [DOI] [PubMed] [Google Scholar]
- Schuhmacher A. J., Guerra C., Sauzeau V., Canamero M., Bustelo X. R., Barbacid M. (2008). A mouse model for Costello syndrome reveals an Ang II-mediated hypertensive condition. J. Clin. Invest. 118, 2169–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M., Lin A. W., McCurrach M. E., Beach D., Lowe S. W. (1997). Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 [DOI] [PubMed] [Google Scholar]
- Sun P., Yoshizuka N., New L., Moser B. A., Li Y., Liao R., Xie C., Chen J., Deng Q., Yamout M., et al. (2007). PRAK is essential for ras-induced senescence and tumor suppression. Cell 128, 295–308 [DOI] [PubMed] [Google Scholar]
- Szeto D. P., Griffin K. J., Kimelman D. (2002). HrT is required for cardiovascular development in zebrafish. Development 129, 5093–5101 [DOI] [PubMed] [Google Scholar]
- Tartaglia M., Gelb B. D. (2005). Noonan syndrome and related disorders: genetics and pathogenesis. Annu. Rev. Genomics Hum. Genet. 6, 45–68 [DOI] [PubMed] [Google Scholar]
- Vallone D., Santoriello C., Gondi S. B., Foulkes N. S. (2007). Basic protocols for zebrafish cell lines: maintenance and transfection. Methods Mol. Biol. 362, 429–441 [DOI] [PubMed] [Google Scholar]
- Westerfield M. (2000). The Zebrafish Book: A Guide For The Laboratory Use of Zebrafish (Danio rerio). Eugene: Institute of Neuroscience University of Oregon [Google Scholar]
- Zampino G., Pantaleoni F., Carta C., Cobellis G., Vasta I., Neri C., Pogna E. A., De Feo E., Delogu A., Sarkozy A., et al. (2007). Diversity, parental germline origin, and phenotypic spectrum of de novo HRAS missense changes in Costello syndrome. Hum. Mutat. 28, 265–272 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material