Generation of mouse-induced pluripotent stem cells by transient expression of a single nonviral polycistronic vector (original) (raw)
Abstract
Induced pluripotent stem (iPS) cells have generated keen interest due to their potential use in regenerative medicine. They have been obtained from various cell types of both mice and humans by exogenous delivery of different combinations of Oct4, Sox2, Klf4, c-Myc, Nanog, and Lin28. The delivery of these transcription factors has mostly entailed the use of integrating viral vectors (retroviruses or lentiviruses), carrying the risk of both insertional mutagenesis and oncogenesis due to misexpression of these exogenous factors. Therefore, obtaining iPS cells that do not carry integrated transgene sequences is an important prerequisite for their eventual therapeutic use. Here we report the generation of iPS cell lines from mouse embryonic fibroblasts with no evidence of integration of the reprogramming vector in their genome, achieved by nucleofection of a polycistronic construct coexpressing Oct4, Sox2, Klf4, and c-Myc.
Keywords: iPS cells, nonintegrative technique, reprogramming, pluripotency
The reprogramming of somatic cells to induced pluripotent stem (iPS) cells by delivery of exogenous factors was first achieved in mouse embryonic fibroblasts (MEFs) by retroviral transduction of the transcription factors Oct4, Sox2, Klf4, and c-Myc (OSKM) (1). This result was confirmed and expanded in a series of reports which established that iPS cells are similar to ES cells in morphology, expression of pluripotency markers, and ability to differentiate into the 3 germ layers in vitro and in vivo, including the ability to produce germline-competent chimeras (2–4). The technique was quickly reproduced with human fibroblasts (5–7), providing a source of patient-specific pluripotent cells with potential for regenerative medicine. Reprogramming has been achieved in a range of cell types, including embryonic and adult fibroblasts, keratinocytes, stomach cells, liver cells, pancreatic β cells, lymphocytes, and neural progenitor cells (5–13). Moreover, reprogramming can be attained using different combinations of exogenous factors: human fibroblasts have been reprogrammed with Oct4, Sox2, Nanog, and Lin28 (14), and c-Myc is not strictly required for reprogramming, although it enhances its efficiency and speed (15, 16). Certain cell types have been reprogrammed with only 2 factors, like neural progenitors with Oct4 and c-Myc or Oct4 and Klf4 (10), and both mouse and human fibroblasts with Oct4 and either Sox2 or Klf4 in combination with small molecules (17, 18).
Although different reprogramming protocols have been reported, the delivery of the original OSKM transcription factor set remains the most commonly used method. Reprogramming requires the delivery of all of the factors to the cell and their adequate expression for a period of ≈8–12 days for fibroblasts and keratinocytes (13, 19, 20). The retroviral and (both constitutive and inducible) lentiviral (14, 19, 21, 22) vectors commonly used meet these requirements, but their permanent integration into the genome limits their use for eventual therapeutic applications because of the risk of both insertional mutagenesis and particularly the reactivation of the reprogramming factors leading to tumor formation (15). Therefore, reprogramming strategies avoiding genomic integration are needed. By using adenoviral delivery of the OSKM factors individually, Stadtfeld et al. (7) have recently obtained iPS cell lines with no indication of insertion of adenoviral or transgene sequences into the genome. However, the frequency of reprogramming was extremely low and a considerable percentage of clones were tetraploid. In a different way, by serial transfection and transient expression of 2 plasmids, one expressing c-Myc and a second construct expressing Oct4, Klf4, and Sox2 as a polycistronic unit, Okita et al. (23) have obtained mouse iPS lines from MEFs showing no evidence of integration. Here we report that iPS lines can be generated in the mouse by nucleofection of a single plasmid construct expressing OSKM as a single polycistronic unit. These iPS lines show no evidence of transgene insertion into their genome.
Results and Discussion
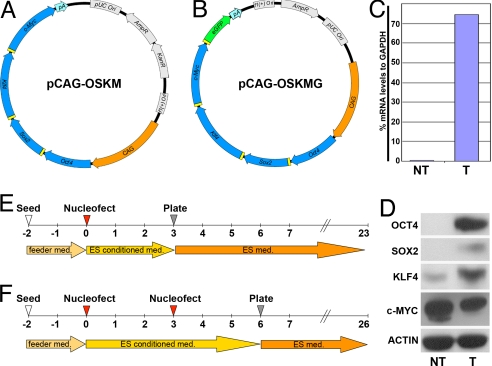
An initial obstacle in direct reprogramming is how to deliver all 4 factors to the same cell. We took advantage of the 2A peptide sequence originally reported in picornaviruses (24). This peptide allows efficient, near-stoichiometric production of up to 3 discrete protein products, when cloned between ORFs, via a ribosomal skipping mechanism. Such a system was shown to work efficiently in embryonic stem cells (25). Extending this strategy, we cloned a CAG-driven polycistronic plasmid expressing Oct4, Sox2, Klf4, and c-Myc (pCAG-OSKM, Fig. 1A). The functionality of the construct was confirmed by transient transfection into MEFs followed by real-time RT-PCR and Western blot analysis. A primer pair, specific to the transgene, spanning the 2A peptide sequence junction between Sox2 and Klf4 detected the polycistronic transgene mRNA (Fig. 1C). Consistently, Western blot analysis confirmed expression of the OCT4, SOX2, and KLF4 protein products. Overexpression of C-MYC was not detected, probably due to the high levels of this protein that were endogenously expressed in the transfected fibroblast line (Fig. 1D). We asked whether an ORF cloned into the fourth position of the polycistron could be expressed. When we replaced c-Myc with GFP (pCAG-OSKG, Fig. S1_A_), we could detect GFP-positive MEFs 1 day after nucleofection of the construct (Fig. S1_B_). Supporting our vector design, a recent report describes the generation of mouse and human iPS cells by lentiviral delivery of a similar OSKM, 2A peptide-based construct (26). We concluded that the polycistronic construct efficiently expressed the 4 reprogramming factors.
Fig. 1.
Generation of iPS cells by transfection of a single nonviral polycistronic construct. (A and B) Maps of the polycistronic constructs used in this study. (C) Expression of transgene mRNA relative to GAPDH in transfected (T) and nontransfected (NT) fibroblasts by real-time RT-PCR. (D) Western blot analysis of transgene factor expression in T and NT cells. (E and F) Timeline of iPS production with either 1 (E) or 2 (F) nucleofections.
We then sought to reprogram MEFs by nucleofection of pCAG-OSKM. MEFs were nucleofected once or twice following the timelines depicted in Fig. 1 E and F, respectively, and seeded onto irradiated MEFs (irMEFs). Approximately 12 days after the final seeding, colonies appeared in both treatments and were allowed to grow for 1 week, after which single clones were manually passaged onto fresh irMEFs. Although several colonies did not initially resemble mouse ES (mES) cells, they acquired typical mES cell morphology after 2 passages. A total of 75 colonies were observed and picked, of which we could successfully establish 46 iPS cell lines.
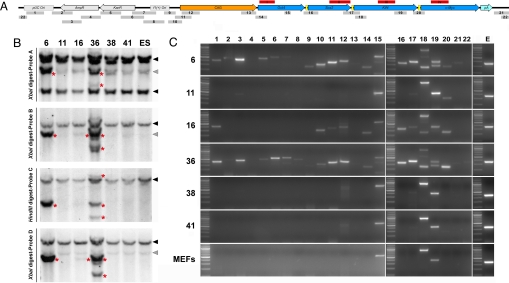
We first subjected all established cell lines to a Southern blot by using a probe against Sox2 (Fig. 2A, probe i) and found that 43 of the lines had integrated the transgene. The 3 remaining lines (clones 11, 38, and 41) were further analyzed together with 3 integrated lines (clones 6, 16, and 36) by using probes against Oct4, Klf4, and c-Myc (Fig. 2A, probes i–iv, shown in red bars). Clones 11, 38, and 41 were indistinguishable from WT control ES cells analyzed with the same probes (Fig. 2B). As expected, clones 6, 16, and 36 also showed additional bands for this other set of probes. To exclude integrations of small portions of pCAG-OSKM not revealed by Southern blot, we analyzed those lines by PCR using a set of 22 primer pairs spanning the entire vector (Fig. 2A, primer pairs 1–22). Consistently, clones 6 and 36—positive for probes i–iv by Southern blot—were positive for most PCR reactions, whereas line 16—only positive for probes ii and _iv_—was negative for a subset of backbone-specific primers. Clones 11, 38, and 41 resembled the pattern of control WT MEFs with bands appearing only for PCR conditions allowing the amplification of endogenous loci (PCR 15, 18, and 19). However, a careful examination of the gel revealed that PCR 3, 9, 12, and 20 were positive in clone 11 (Fig. 2C). Although we cannot strictly rule out the integration of small segments of the plasmid into the genome, these data strongly suggest that these 3 iPS clones do not contain the original transgene used to reprogram them.
Fig. 2.
Integration analysis of iPS cell lines. (A) Linear representation of pCAG-OSKM showing the location of the probes used for Southern blot (red bars, i–iv) and the approximate position and length of the amplicons generated by PCR (gray bars 1–22). (B) Southern blot analysis of clones 6, 11, 16, 36, 38, and 41, and mouse ES cells using probes against Oct4, Sox2, Klf4, and c-Myc. Black and gray arrowheads point respectively to specific and nonspecific endogenous bands present in the genomic DNA of control ES cells. Extra bands are highlighted by a red asterisk in clones 6, 16, and 36, indicating variable degrees of insertion of the transgene. (C) PCR analysis of clones 6, 11, 16, 36, 38, 41. Consistent with Southern blot, clones 6 and 36 are positive for almost the full set of primer pairs, whereas clone 16 is not positive for the backbone-specific primers 2–9. Clone 11 shows a weak signal for primer pairs 3, 9, 12, and 20, whereas clones 38 and 41 are negative for all primer pairs tested except faint bands for primer pairs 17 and 20.
Real-time RT-PCR on iPS clones 6, 11, 16, 36, 38, and 41 revealed that they all had reactivated a set of endogenous pluripotent-specific genes (Oct4, Sox2, Nanog, Utf1, and Zfp42) to levels similar to mES cells (Fig. 3A, Upper). Consistently, the fibroblast specific markers Thy1 and Col6a2 were down-regulated in all these clones, although line 16 seems to have only partially silenced those genes (Fig. 3A, Lower Left). Next, we tested the expression level of the transgene by using a set of primers spanning the junction between the coding sequences of Sox2 and Klf4 of pCAG-OSKM (Fig. 3A, Lower Right). As expected, clones 6, 16, and 36 (transgene-positives) showed significant expression of the polycistronic transcript, whereas the expression could not be detected for clones 11, 38, and 41.
Fig. 3.
Characterization of integrative and NiPS cell clones: comparison with MEFs and mouse ES cells. (A Upper) Real-time RT-PCR showing up-regulation of endogenous expression of the pluripotency markers Oct4, Sox2, Nanog, Utf1, and Zfp41 (Rex1). (Lower Left) Real-time RT-PCR showing down-regulation of expression of the fibroblast markers Col6a2, Grem2, and Thy1. (Lower Right) Real-time RT-PCR showing level of silencing of the transgene, if present. (B) Oct4 promoter methylation analysis. Percentages of methylation of the Oct4 promoter are indicated in red. (C) Immunofluorescence analysis showing expression of the ES cell markers NANOG, SOX2, OCT4, and SSEA1.
We also measured the levels of demethylation of the endogenous Oct4 promoter by using sodium bisulfite mutagenesis on different integrative and nonintegrative iPS (NiPS) cell clones. Regardless of their integration status, the iPS clones analyzed were differentially demethylated, clustering either as ES-like (clones 1, 6, and 11), intermediate (clone 41), or MEF-like (clones 4 and 38). However, in all cases Oct4 promoter methylation was lower than in control MEFs, consistent with the endogenous reactivation of Oct4 observed in those clones by real-time RT-PCR (Fig. 3B). Furthermore, the clones were positive by immunofluorescence for OCT4, SOX2, SSEA1, and NANOG (Fig. 3C) and stained positive for alkaline phosphatase (Fig. S2). Taken together, these data show that, regardless of their integration status, our iPS clones display the molecular hallmarks of mES cells.
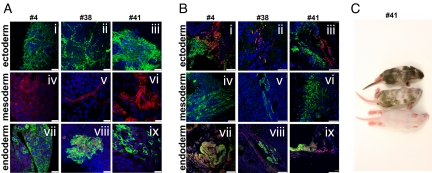
We then tested the differentiation potential of clone 4, a transgene-positive clone, by Southern blot, and clones 38 and 41 by generating embryoid bodies (EBs) and differentiating them to cell derivatives representative of the 3 germ layers. All iPS clones tested showed positive immunofluorescence staining for markers of ectoderm (TUJ1), mesoderm (a-SARCOMERIC-ACTIN), and endoderm (a-1-FETOPROTEIN and FOXA2) (Fig. 4A). Furthermore, clones 36, 38, and 41 were injected into severe combined immunodeficient (SCID) mice, resulting in the formation of teratomas containing tissues derived from the ectoderm, mesoderm, and endoderm (Fig. 4B). Finally, we injected clones 11, 38, and 41 in blastocysts, and chimeric pups were obtained from all of the clones injected (Fig. 4C, clone 41), confirming the pluripotency of the iPS cell lines generated with our nonintegrative approach.
Fig. 4.
Differentiation potential of iPS cell lines. (A) In vitro differentiation toward ectoderm (i–iii) (TuJ1-positive neuronal cells, green), mesoderm (iv–vi) (α-actinin-positive cardiac myocytes, red), and endoderm (vii–ix) (α-fetoprotein-positive, green; FOXA2-positive, red). Blue nuclear staining is DAPI. Scale bars, 25 μm. (B) In vivo differentiation (teratomas) toward ectoderm (i–iii) (TuJ1-positive neuronal cells, green; glial fibrillary acidic protein-positive cells, red), mesoderm (iv–vi) (α-actinin-positive cardiac myocytes, green), and endoderm (vii–ix) (α-fetoprotein-positive, green; FOXA2-positive, red). Scale bars: iv, 25 μm; all others, 50 μm; (C) In vivo differentiation (blastocyst injection) of chimeric (Top and Middle) and control (Bottom) pups obtained by injecting NiPS cell clone 41 in a ICR host blastocysts. Black hair is from iPS cells.
The low efficiency in recovering nonintegrative colonies requires the establishment and genotyping of a large number of colonies. To optimize the screening, we designed pCAG-OSKMG (Fig. 1B) by adding a GFP reporter gene linked by a P2A peptide to pCAG-OSKM. Transgene expression analysis suggested that the majority of integrated clones did not silence the transgene even after several passages (Fig. S3_A_). pCAG-OSKMG could therefore label the majority of integrated colonies in which the transgene is not silenced as GFP positives.
We first checked whether pCAG-OSKMG was able to express GFP by nucleofecting this construct once in MEFs and monitoring GFP expression by FACS over the time period required for reprogramming. At day 1 after nucleofection, 37% of the cells were GFP positives, and 6% were still labeled at 10 days (Fig. S3_B_). We nucleofected MEFs again with pCAG-OSKMG and plated them on feeder cells. This pilot experiment allowed us to generate 12 iPS-like colonies, all of them strongly expressing GFP (Fig. S3_C_).
Conclusions
We have designed and tested a construct expressing Oct4, Sox2, Klf4, and c-Myc from a single transcription unit, pCAG-OSKM. This construct allows expression of the 4 factors required for reprogramming in each transfected cell. The order of the genes was chosen arbitrarily, as 2A peptide-linked ORFs have been reported to be expressed in quasiequimolar amounts (24). The same order has been used to reprogram mouse and human cells using a lentiviral vector (26). Okita et al. (23) report differences of reprogramming efficiency depending on the cloning order of the genes. More exhaustive testing of this parameter is required to draw more definitive conclusions. We delivered pCAG-OSKM by nucleofection and succeeded in generating integration-free iPS lines. These NiPS cells are morphologically similar to mES cells, express endogenous markers of pluripotency, and are capable of differentiation into the 3 germ layers both in vitro and in vivo. However, the methylation levels of bivalent domain-containing genes such as Oct4 were variable in our iPS lines, regardless of their insertional status. If such epigenetic heterogeneity was observed in loci containing either tumor suppressor genes or oncogenes, the safety of NiPS cells should be further addressed. In this respect, further analysis of chimeras generated from NiPS cells will be extremely informative.
Southern blot analysis predicts that an integrative iPS line would have at least 1 inserted copy of the transgene per genome, resulting in a detectable band. Among the clones that had integrated the transgene, we found band numbers and signal intensities consistent with 1 to 3 copies. However, one of the 3 clones negative by Southern blot was positive for some transgene elements when genotyped by PCR (clone 11). We hypothesize that this clone, despite being picked from a single and isolated outgrowth, could be chimeric—that is, containing a small proportion of cells that incorporated transgene elements. However, iPS clones 38 and 41 showed no evidence of integration of the transgene into their genome.
We were unable to generate NiPS cells by using a single nucleofection protocol (0 of 10 clones established), but by using 2 nucleofections, 8% of the clones (3 of 36 clones established) were free of transgene integration. By using 4 serial transfections and 2 plasmids, Okita et al. (23) obtained 33% of NiPS cells (47 of 143 clones established). Because the final plating of the cells occurs at different time points in these 2 protocols, it is difficult to assess their absolute efficiencies. It is likely that the resulting colonies arise from the existence, prior to the final plating, of a reprogrammed progenitor pool. Our approach simplifies the method proposed by Okita et al., including all necessary factors in a single construct and delivering it by nucleofection. To truly assess the efficiency of NiPS cells induction, a single nucleofection of MEFs followed by direct plating on feeders would ensure the clonal status of the resulting iPS colonies. Such strategy implies the time-consuming establishment and screening of a large number of iPS lines to identify NiPS cells. To reduce this number, we designed the vector pCAG-OSKMG, which allows us to label (with GFP) the majority of iPS colonies in which the construct is integrated and not silenced.
Our results show that NiPS cell induction is possible by transient expression of a single polycistronic construct. We have generated NiPS cell lines with no evidence of transgene integration, thus avoiding the risk of transgene reactivation leading to tumor formation. This approach represents a step toward a safe derivation method of clinically relevant human iPS cells.
Materials and Methods
pCAG-OSKM Plasmid Construction.
The Oct4 cDNA was amplified by using a reverse primer eliminating the Oct4 stop codon and adding a BspEI site; this fragment was cloned into pCRII (Invitrogen) to give pCRII-Oct4-Bsp (oriented NotI-5′cDNA3′-Acc65I). The Sox2 cDNA was amplified by using a forward primer containing an AgeI site followed by a P2A peptide sequence and a reverse primer eliminating the Sox2 stop codon and containing a BspEI site; this fragment was cloned in pCRII to give pCRII-Age-Sox2-Bsp (oriented NotI-5′cDNA3′-Acc65I). pCRII-Age-Sox2-Bsp was cut with AgeI and Acc65I and cloned into pCRII-Oct4-Bsp cut with BspEI-Acc65I, producing pCRII-Oct4-P2A-Sox2-BspEI. The strategy of cloning was repeated twice to incorporate Klf4 and c-Myc (producing pCRII-OSKM), or 3 times to incorporate Klf4, c-Myc, and GFP (producing pCRII-OSKMG). The last ORF was amplified to preserve the stop codon. These constructs were cut with EcoRI and cloned into an EcoRI site immediately downstream of the CAG promoter and upstream of the Bgh pA previously cloned into pCRII or pBSKII vectors, respectively, giving rise to pCAG-OSKM and pCAG-OSKMG. The plasmids were purified under endotoxin-free conditions (Qiagen) for subsequent nucleofection. For primer sequences, see Table S1.
Reprogramming with pCAG-OSKM.
P2 MEFs from embryonic day 13.5 C57BL/6 embryos were nucleofected by using the MEF2 Nucleofector Kit (Amaxa) and following the manufacturer's instructions. The embryos were treated as described in Fig. 1 D or E. For a detailed protocol, see Table S2.
Western Blot.
Cells were resuspended in radioimmunoprecipitation assay buffer containing 1× protein-inhibitor mixture (Roche), incubated on ice for 20 min, and cleared by centrifugation.
Protein Concentration.
Protein concentration was determined by Bradford assay (Pierce). Twenty-five micrograms of total protein were subjected to electrophoresis on 4–12% BIS-Tris denaturing gels (Invitrogen). Proteins were transferred to Immobilon-P membranes (Millipore). The membranes were blocked in PBS containing 10% nonfat powdered milk and 0.02% Tween 20. Primary antibodies were against OCT4 (Santa Cruz), SOX2 (Chemicon), C-MYC (Sigma), KLF4 (Santa Cruz), and ACTIN (Abcam).
Integration Analysis: Southern Blot.
Five micrograms of genomic DNA were cut with XbaI (for blots probed for Oct4, Klf4, and c-Myc) or HindIII (for blot probed with Sox2), separated on a 1% agarose gel, and transferred to a nylon membrane (Amersham). Digoxigenin-2′-deoxyuridine 5′-triphosphate (dUTP)-labeled probes were synthesized using a PCR DIG Probe Synthesis Kit (Roche) according to manufacturer's instructions (see Table S3). Blots were blocked, hybridized, washed, and then developed by using antidigoxigenin-AP Fab fragments (Roche). For PCR, 50 ng of genomic DNA were amplified with Taq polymerase (Roche) by using a set of 22 primer pairs spanning the pCAG-OSKM sequence (see Table S4 for primer sequences, amplification conditions, and expected product sizes).
RT-PCR Analysis.
Total mRNA was isolated by guanidinium thiocynate-phenol-chloroform extraction (TRIzol, Invitrogen) and treated with DNaseI. One microgram was used to synthesize cDNA by using the Cloned AMV First-Strand Synthesis Kit (Invitrogen). Quantitative PCR analysis was done in triplicate on 500 ng by using Platinum Syber Green qPCR Super Mix (Invitrogen) in an ABI Prism 7000 thermocycler (Applied Biosystems), and values were normalized to Gapdh. (For primer sequences, see Table S5.)
AP Staining.
iPS lines were seeded at low density on irMefs and allowed to grow for 3 days in ES-cells medium. Cells were washed with PBS, fixed 1 min in cold, 4% paraformaldehyde (PFA), and washed again with PBS. They were stained with Alkaline Phosphatase Blue Membrane Substrate Solution (Sigma) according to the manufacturer's guidelines.
In Vitro Differentiation.
iPS cells were cultured on irMEFs in knockout DMEM (Gibco) supplemented with 15% FBS, 1,000 units/mL leukemia inhibitory factor (LIF), 2 mM l-glutamine, 0.1 mM nonessential amino acids (NEAA), 1,000 units/mL penicillin, streptomycin, and 100 mM β-mercaptoethanol. For the generation of EBs, the hanging-droplet culture method was used. Briefly, single cells were harvested by trypsinization and diluted to 40 cells per μL of ES cell media without LIF. Hanging droplets of 20 μL (800 cells per droplet) were suspended on the underside of 100 mm Petri dish lids and maintained at 37 °C, 5% CO2. To induce differentiation, EBs were collected after 3 days in hanging-droplet culture and transferred to differentiation medium. For neural differentiation, EBs were cultured in a solution containing DMEM F-12 (Invitrogen), N2 and B27 supplements (Invitrogen), 1 mM l-glutamine, 1% NEAA, and 0.1 mM β-mercaptoethanol for 3 days, after which they were seeded onto matrigel chamber slides with the same media, plus 10−6 M retinoic acid. Media was changed daily. For cardiomyocyte differentiation, EBs were replated onto gelatin-coated chamber slides. Cultures were maintained in ES cell media without LIF and supplemented with 100 μM ascorbic acid. Media was changed every 2 days. For endoderm differentiation, EBs were replated onto gelatin-coated chambers slides in ES cell media without LIF. Media changes were performed every 2 days.
Teratoma Formation and Blastocyst Injection.
For the teratoma assay, iPS cells (1 × 105 cells) were resuspended in 20–40 μL of mES medium and injected into the testis of SCID beige mice (Charles River Laboratories). Five weeks after cell injection, and tumors were processed and analyzed by immunofluorescence. For the chimera assay, NiPS cell clone 41 (passage 10) was injected into ICR host blastocysts, and embryos were transferred into 2.5-days-postcoitum, CD1, pseudopregnant recipient females. Chimerism was ascertained after birth by the appearance of black coat color (from the C57BL/6 iPS cells) over the white background from the host pups.
Immunofluorescent Staining.
For immunostainings, samples were fixed with 4% PFA for 15 min, washed with PBS, and blocked. Teratomas were fixed with 4% PFA, washed, dehydrated, included in paraffin, sectioned, and blocked. Primary antibodies used were anti-Oct3–4 (Santa Cruz SC-5279), anti-Nanog (Abcam ab21603), anti-Sox2 (M&D MAB 2018), anti-SSEA1 (Developmental Studies Hybridoma Bank, MC-480), anti-B-tubulin III (Tuj1) (Covance, MMS-435P-O), anti-GFAP (Advanced ICI, 31223), antisarcomeric-actin (Sigma, 2172), antialpha-1-fetoprotein (Dako, A0008), and FOXA2 (HNF3-B) (R&D Systems, AF2400). After overnight primary antibody incubation, samples were washed with PBS and incubated with secondary antibodies (Cyanine Series, all from Jackson Immunoresearch). Samples were also counterstained with DAPI (Invitrogen, 21490). Images were taken using Leica Acousto Optical Beam Splitter confocal microscope.
Oct4 Promoter Methylation Analysis.
Genomic DNA was extracted from 0.5 × 106 cells, and DNA mutagenesis was performed with EpiTect DNA mutagenesis kit (Qiagen) according to manufacturer's specifications. The Oct4 promoter was amplified by 2 subsequent PCRs using primers previously described (27). The resulting amplified products were cloned into PCR 2.1 or pGEM Easy plasmids, amplified in DH5a cells, purified and sequenced.
Flow Cytometry Analysis.
All analyses were performed on a MoFlo cell sorter (DakoCytomation, Dako) running Summit software. To measure the percentage of GFP-positive cells, cells were plated on gelatin and trypsinized at different time points after nucleofection, washed with PBS, and resuspended in PBA (phosphate-buffered salt solution with 0.1% BSA and 0.01% sodium azide) with 2 μg/mL propidium iodide. Flow cytometric analysis was done with an EPICS ELITE-ESP cytometer (Coulter).
Supplementary Material
Supporting Information
Acknowledgments.
We are grateful to Meritxell Carrió and Vanesa Tobajas for expert assistance with cell culture techniques; to Mercé Gaudes Martí, Lola Mulero Pérez, Esther Melo, and Cristina Pardo for bioimaging assistance; to Silvia Gomez, Ainara Magdaleno, Jotaro Suzuki, Teruhisa Kawamura, and Yelena Dayn for help with chimeric mouse production; and to Angel Raya, Josipa Bilic, Vladimir Pekarik, and Cristina Eguizabal for helpful discussions. This work was partially supported by a fellowship from the Swiss National Science Foundation (F.G.), the Ramón y Cajal program (G.T.), the Juan de La Cierva program (N.M.P.), the G. Harold and Leila Y. Mathers Charitable Foundation, Marato, Terapia Celular (TERCEL), and Fundacion Cellex.
Footnotes
The authors declare no conflict of interest.
References
- 1.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Maherali N, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 4.Wernig M, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 5.Lowry WE, et al. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci USA. 2008;105:2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aoi T, et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702. doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]
- 7.Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stadtfeld M, Brennand K, Hochedlinger K. Reprogramming of pancreatic beta cells into induced pluripotent stem cells. Curr Biol. 2008;18:890–894. doi: 10.1016/j.cub.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanna J., et al. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133:250–264. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JB, et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008;454:646–650. doi: 10.1038/nature07061. [DOI] [PubMed] [Google Scholar]
- 11.Eminli S, Utikal J, Arnold K, Jaenisch R, Hochedlinger K. Reprogramming of neural progenitor cells into induced pluripotent stem cells in the absence of exogenous Sox2 expression. Stem Cells. 2008;26:2467–2474. doi: 10.1634/stemcells.2008-0317. [DOI] [PubMed] [Google Scholar]
- 12.Aasen T, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 13.Maherali N, et al. A high-efficiency system for the generation and study of human induced pluripotent stem cells. Cell Stem Cell. 2008;3:340–345. doi: 10.1016/j.stem.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa M, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 16.Wernig M, Meissner A, Cassady JP, Jaenisch R. c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell. 2008;2:10–12. doi: 10.1016/j.stem.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Huangfu D, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 18.Shi Y, et al. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell. 2008;3:568–574. doi: 10.1016/j.stem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Brambrink T, et al. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. 2008;2:151–159. doi: 10.1016/j.stem.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stadtfeld M, Maherali N, Breault DT, Hochedlinger K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell. 2008;2:230–240. doi: 10.1016/j.stem.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blelloch R, Venere M, Yen J, Ramalho-Santos M. Generation of induced pluripotent stem cells in the absence of drug selection. Cell Stem Cell. 2007;1:245–247. doi: 10.1016/j.stem.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hockemeyer D, et al. A drug-inducible system for direct reprogramming of human somatic cells to pluripotency. Cell Stem Cell. 2008;3:346–353. doi: 10.1016/j.stem.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 24.Ryan MD, Drew J. Foot-and-mouth disease virus 2A oligopeptide mediated cleavage of an artificial polyprotein. EMBO J. 1994;13:928–933. doi: 10.1002/j.1460-2075.1994.tb06337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasegawa K, Cowan AB, Nakatsuji N, Suemori H. Efficient multicistronic expression of a transgene in human embryonic stem cells. Stem Cells. 2007;25:1707–1712. doi: 10.1634/stemcells.2006-0813. [DOI] [PubMed] [Google Scholar]
- 26.Carey BW, et al. Reprogramming of murine and human somatic cells using a single polycistronic vector. Proc Natl Acad Sci USA. 2009;106:157–162. doi: 10.1073/pnas.0811426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blelloch R, et al. Reprogramming efficiency following somatic cell nuclear transfer is influenced by the differentiation and methylation state of the donor nucleus. Stem Cells. 2006;24:2007–2013. doi: 10.1634/stemcells.2006-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information