Monocytes from Patients with Type 1 Diabetes Spontaneously Secrete Pro-Inflammatory Cytokines Inducing Th17 Cells (original) (raw)
. Author manuscript; available in PMC: 2010 Oct 1.
Published in final edited form as: J Immunol. 2009 Sep 11;183(7):4432–4439. doi: 10.4049/jimmunol.0900576
Abstract
Autoimmune diseases including type 1 diabetes (T1D) are thought to have a Th1/Th17 bias. The underlying mechanisms driving the activation and differentiation of these pro-inflammatory T cells are unknown. We examined the monocytes isolated directly from the blood of T1D patients and found they spontaneously secreted the pro-inflammatory cytokines IL-1β and IL-6, which are known to induce and expand Th17 cells. Moreover, these in vivo activated monocytes from T1D subjects induced more IL-17 secreting cells from memory T cells as compared to monocytes from healthy control subjects. The induction of IL-17 secreting T cells by monocytes from T1D subjects was reduced in vitro with a combination of an IL-6 blocking antibody and IL-1 receptor antagonist. Here, we report a significant though modest increase in the frequency of IL-17 secreting cells in lymphocytes from long-term patients with T1D as compared to healthy controls. These data suggest that the innate immune system in T1D may drive the adaptive immune system by expanding the Th17 population of effector T cells.
Keywords: Human, diabetes, Th17, proinflammatory, monocytes
INTRODUCTION
A distinct and separate lineage of T helper cells secreting the pro-inflammatory cytokine IL-17 has been recently described (1). The discovery of these Th17 cells has had a major impact on our understanding of immune processes not readily explained by the Th1/Th2 paradigm. Th17 cells are intimately involved in promotion of autoimmunity (2), in particular rheumatoid arthritis (3), experimental autoimmune encephalomyelitis (4), and multiple sclerosis (5). Moreover, there is preliminary evidence that IL-17 is expressed in the pancreas in the course of T1D in the murine model of T1D (6), the non-obese diabetic mouse (NOD), and that reducing the number of Th17 cells with induction of IFNγ inhibited IL-17 production and restored normoglycemia at the prediabetic stage (7). Two recent papers found that transfer of islet-specific Th17 cells induced diabetes, but only after the cells converted to IFNγ producing cells (8, 9).
We and others recently demonstrated that while in humans, TGFβ and IL-21 can differentiate naïve CD4 cells into Th17 cells secreting IL-17, central memory CD4 cells are driven to secrete IL-17 with a combination of IL-1β and IL-6 (10-13). Monocytes stimulated with the TLR2 agonist peptidoglycan and to a lesser extent the TLR4 agonist lipopolysaccharide (LPS) secrete IL-6 and IL-1β, but not IL-12 and IL-23, and can induce naïve (13) and memory (14) T cells to secrete IL-17 and IFNγ.
Circulating monocyte-derived cytokines are known to be elevated in the sera of diabetic subjects. There is an increase in serum TNFα, IL-6, IL-1β and IL-1α in diabetic subjects compared to control subjects at onset of clinical disease (15, 16), as well as in healthy first degree relatives (17). In long standing diabetic subjects, monocytes have been investigated in the context of atherosclerosis or metabolic control. There is an increased secretion of IL-6, IL-1β and TNFα from monocytes with stimulation in diabetic subjects compared to controls (18-21). Recently it was shown that there are two monocyte gene expression clusters in T1D, one of which is defined by having increased pro-inflammatory cytokine expression, such as IL-6, IL-1β and TNFα (22).
Here, we investigated the activation state of monocytes in patients with T1D, hypothesizing that in vivo activation of pro-inflammatory, circulating monocytes were driving the differentiation/expansion of CD4 cells into Th17/Th1 cells. We found a striking activation of a subset of CD16- monocytes isolated ex vivo from patients with T1D that both spontaneously secreted and expressed mRNA transcripts for IL-1β/IL-6. These in vivo activated monocytes from T1D subjects induced IL-17 secreting cells from memory T cells as compared to monocytes from healthy control subjects and this in vitro induction was inhibited by a combination of an IL-6 blocking antibody and IL-1 receptor antagonist (IL-1Ra). These data suggest a mechanism by which an activated, pro-inflammatory innate immune system drives the expansion of Th17 cells in patients with T1D through spontaneous secretion of IL-6 and IL-1β.
METHODS AND MATERIALS
Patients
Peripheral venous blood was obtained from twenty-one recent-onset (<1 year from disease onset) T1D subjects (mean age ± SD: 21.3 ± 9.0 years; mean disease duration ± SD: 2.6 ± 3.4 months; 7 females and 14 males), 27 long-term (>1 year from disease onset) T1D subjects (mean age ± SD: 30.3 ± 9.3 years; mean disease duration ± SD: 181.4 ± 130.4 months; 13 females and 14 males), fifteen T2D subjects (mean age ± SD: 41.9 ± 9.2 years; mean disease duration ± SD: 8.6 ± 7.0 years; 8 females and 7 males) or forty-two healthy subjects (mean age ± SD: 31.8 ± 10.2 years; 27 females and 15 males) in compliance with institutional review board protocols. PBMCs were separated using density centrifugation on Ficoll-HyPaque (GE Healthcare). PBMCs were frozen at a concentration of 1-3×107/ml in 10% dimethylsulfoxide (Sigma-Aldrich)/90% fetal calf serum (Atlanta Biologicals). After thawing the PBMCs were washed in phosphate buffered saline (PBS).
Monocyte, dendritic cell, B cell and T cell isolation
Monocytes were isolated by negative selection using magnetic beads: Monocyte Isolation Kit, II (Miltenyi Biotech), with approximately 90% purity as defined by CD11b staining. The monocyte-depleted cells were FACS sorted into CD3+, CD19+, and CD3−CD19−CD11c+ populations. The monocyte CD16 subpopulations were FACS sorted using CD14, CD16 and CD56; the CD56 antibody was used to exclude NK cells in the CD14dim, CD16+ population. For the co-culture experiments the monocytes were FACS sorted after negative-isolation (FACS Aria, Becton Dickinson) based on CD11b expression. Naïve and memory CD4+ T cells were FACS sorted using the following markers; CD4+, CD25−, CD62L+, CD45RA+ and CD4+, CD25−, CD62L+, CD45RA− respectively (all antibodies are from BD BioSciences).
Real Time PCR
RNA from negatively isolated monocytes or FACS sorted populations was purified using the absolutely RNA microprep kit (Stratagene). cDNA was made using a Taqman kit with supplied random hexamers (Applied Biosystems). All primers and probes were obtained from Applied Biosystems and used accordingly to standard methodologies.
ELISpot
2.5×105 PBMCs/well were plated in HL-1 media supplemented with 2 mM L-glutamine, 5 mM HEPES, and 100 U/ml penicillin and 100 μg/ml streptomycin, 0.1 mM each non-essential amino acids, 1 mM sodium pyruvate (all from Lonza), and 1% heat-inactivated human male AB serum (Omega Scientific), in 96-well round-bottomed plates (Corning). For IL-17 ELISpot, the plates were first coated with anti-CD3 (OKT3, 1 μg/ml) in PBS. After 18 hours at 37°C/5% CO2, the cells were transferred to coated ELISpot plates, and left for an additional 16 hours at 37°C/5% CO2. For the isolated monocyte and monocyte depleted PBMC ELISpot, the cells were separated using magnetic beads; Monocyte Isolation Kit, II (Miltenyi Biotech), before being loaded on the ELISpot plate. Antibody capture and detection pairs are as follows: IL-6 (eBioscience), IL-1β (R&D) and IL-17 (eBioscience).
Monocyte-T cell co-cultures
Cells were cultured in complete HL-1 medium and 5% human serum. Memory T cells were cultured with monocytes (1:1) in the presence of plate bound anti-CD3 antibody (OKT3, 1μg/ml) for 5 days. For cytokine blocking experiments with memory T cells cultured with monocytes, IL-1Ra (125 ng/ml), anti-IL-6 (10 μg/ml), and anti-TNFα (10 μg/ml) (all from R&D Systems) were added to the initial incubation with anti-CD3. Then T cells were transferred to a new 96-well plate and incubated with recombinant IL-2 (20 U/ml) (The reagent was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: Human rIL-2 from Dr. Maurice Gately, Hoffmann-La Roche Inc.) (23) for an additional 7 days.
Intracellular staining
T cells from the co-culture experiments were stimulated with PMA (phorbol myristate acetate) (50 ng/ml) and ionomycin (250 ng/ml) (Sigma Aldrich) for 5 hours, and Golgistop® (BD Bioscience) was added for the final 3 hours. Cells were fixed with 4% paraformaldehyde and permeablized with reagents and protocols from BD Biosciences. T cells were then stained with APC (allophycocyanin)-IL-17 (eBioscience) and PE (phycoerythrin)-IFNγ (BD Bioscience). The data was acquired on a FACSCalibur (BD Bioscience) and analyzed with FlowJo software (Tree Star).
Statistical Analysis
Significant differences were calculated using Prism 4.0 software (GraphPad, San Diego, CA) using an unpaired two-tailed t-test, with the exception of the monocyte T cell co-culture experiments, which used a paired two-tailed t-test.
RESULTS
Ex vivo cytokine analysis of PBMCs from T1D subjects
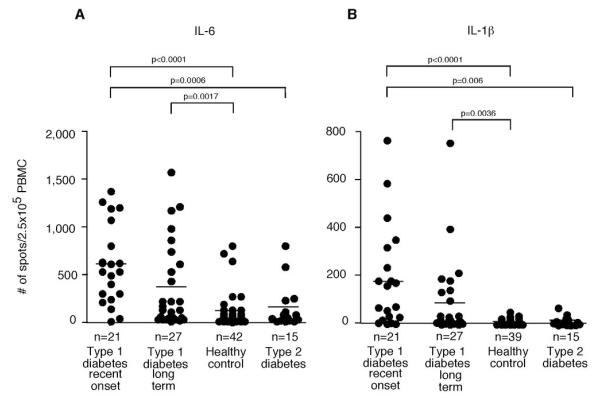
IL-6 and IL-1β have both been implicated in the differentiation and expansion of Th17 cells. Therefore, we examined the number of circulating IL-6 and IL-1β secreting cells ex vivo with no additional stimulus from T1D subjects as well as healthy controls and T2D subjects. ELISpot assays demonstrated that there was a marked increase of spontaneous IL-6 and IL-1β secreting cells in recent-onset T1D subjects (and to a lesser extent long-term T1D subjects) as compared to healthy, age matched controls and T2D subjects (Figure 1 and supplementary Figure 1A). The PBMCs derived from T2D subjects showed no difference in cytokine secretion as compared to those from healthy controls. There was no correlation between the number of cytokine secreting cells and age of the T1D patients or the age of disease onset (data not shown).
Figure 1. Type 1 diabetic subjects have a higher number of ex vivo IL-6 and IL-1β secreting PBMCs compared to control subjects.
Unstimulated PBMCs were incubated in HL-1 media with 1% human serum for 18 hours and then analyzed by ELISpot. Each circle represents the number of positive PBMCs derived from a single subject. 250,000 PBMCs were added per well. Horizontal bars indicate the mean. Significant differences in cytokine positive cells (p<0.05) between groups are shown in the figure. (A) The number of ex vivo IL-6 secreting cells from PBMCs from recent-onset T1D subjects was significantly greater than from healthy control subjects or T2D subjects. Long-term T1D subjects also had a greater number of IL-6 secreting cells compared to healthy control subjects. (B) The number of ex vivo IL-1β secreting cells from recent-onset and long-term T1D subjects was significantly greater than that from healthy controls. Recent-onset T1D subjects had a greater number of IL-1β secreting cells compared with T2D subjects.
Ex vivo cytokine analysis of circulating T1D monocytes
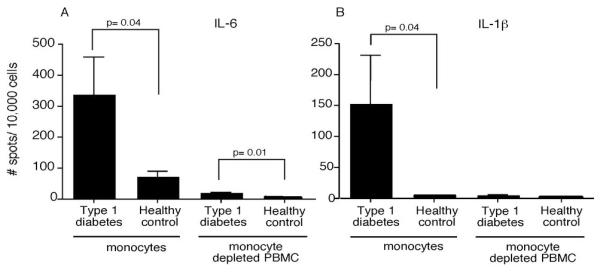
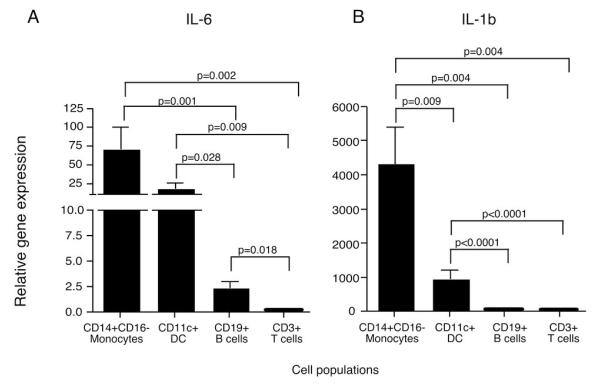
To determine which PBMC populations were secreting these pro-inflammatory cytokines, monocytes from recent-onset T1D and healthy control subjects were isolated by negative selection and analyzed by ELISpot assays with no additional stimulus. The corresponding monocyte depleted PBMCs were also examined. To avoid activation of monocytes with surface molecule ligation by antibody, monocytes were negatively isolated by magnetic bead exclusion of other cell types; CD3, CD7, CD16, CD19, CD56, CD123 and glycophorin A positive cells are removed; this isolation method purifies the classical CD14+CD16− monocyte population which account for approximately 90% of circulating monocytes. The majority of IL-6 and IL-1β secreting cells were observed in the monocyte population (Figure 2). The frequency of IL-6 and IL-1β secreting monocytes varied greatly, as would be predicted from the data in Figure 1. The frequency of IL-6 secreting monocytes from T1D subjects ranged from 0.3 to 10 percent with a mean of three percent, indicating that only a subset of the circulating monocytes were in the activated state. Interestingly, we observed an increased number of IL-6 secreting cells in the T1D monocyte-depleted PBMCs compared to those from healthy controls, suggesting that there may be other cell types with increased cytokine secretion. To address this, monocytes were negatively isolated, and the remaining cells were FACS sorted into CD11c, CD19 and CD3 positive populations. Quantitative RT-PCR was used to identify the relative gene expression of IL-6 and IL-1β (Figure 3). The negatively isolated monocytes had the highest expression, but the CD11c+ population also expressed significant amounts of IL-6 and IL-1β compared to the CD19+ and CD3+ cells. The CD11c population contains the myeloid DCs as well as the CD16+ monocytes that are removed in the negative isolation and are thought to be pro-inflammatory, more mature, and have a higher T cell stimulatory capacity than the CD16−, negatively-isolated monocytes (24). The CD16+ monocytes can be divided into two populations, CD14+CD16+ and CD14dimCD16+; both produce more TNFα than the classical monocytes when stimulated, but the CD14dimCD16+ do not produce IL-10 while the CD14+CD16+ do produce IL-10 upon stimulation (25). To examine the different populations of monocytes ex vivo in T1D subjects, we FACS sorted the CD14+CD16−, CD14+CD16+, and CD14dimCD16+ populations and analyzed their IL-6 relative gene expression. Both the CD14+CD16− and the CD14+CD16+ populations had increased IL-6 gene expression, while the CD14dimCD16+ did not (Figure 4).
Figure 2. Type 1 diabetic subjects have a higher number of ex vivo IL-6 and IL-1β secreting monocytes compared to control subjects.
Monocytes from T1D and healthy control subjects were negatively selected, and then both the monocytes and the monocyte-depleted PBMCs were analyzed by ELISpot. Significant differences in cytokine positive cells (p<0.05) between groups are shown in the figure. (A) The number of ex vivo IL-6 secreting cells from monocytes and monocyte-depleted PBMCs was significantly greater in the T1D subjects than from healthy control subjects, N=7. (B) The number of ex vivo IL-1β secreting cells from monocytes, but not monocyte-depleted PBMCs was significantly greater than that from healthy controls, N=8.
Figure 3. Negatively-isolated monocytes and CD11c+ cells from type 1 diabetic subjects have high gene expression for IL-6 and IL-1β.
Gene expression of IL-6 and IL-1β from negatively isolated monocytes, and FACS sorted CD11c+, CD19+ and CD3+ cells from five T1D subjects were analyzed by quantitative RT-PCR. The CD16- monocytes were removed by negative isolation, and the monocyte-depleted cells were FACS sorted into CD3+, CD19+, and CD3−CD19−CD11c+ populations. Significant differences are shown in the figure. Relative gene expression of (A) IL-6, and (B) IL-1β are increased in the classical monocyte and CD11c populations compared to the CD19 and CD3 populations. All gene expression is relative to β2-microglobulin.
Figure 4. Unstimulated CD14+CD16− and CD14+CD16+ monocytes from T1D subjects, but not CD14dimCD16+ monocytes have increased IL-6 gene expression compared to healthy control subjects.
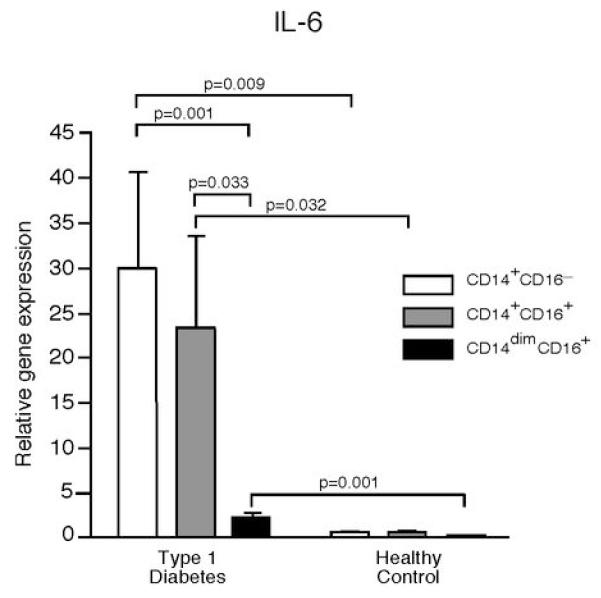
Gene expression of IL-6 from CD14+CD16−, CD14+CD16+ and CD14dimCD16+ FACS sorted monocytes from five T1D subjects and five healthy control subjects were analyzed by quantitative RT-PCR. Significant differences are shown in the figure. All gene expression is relative to b2-microglobulin.
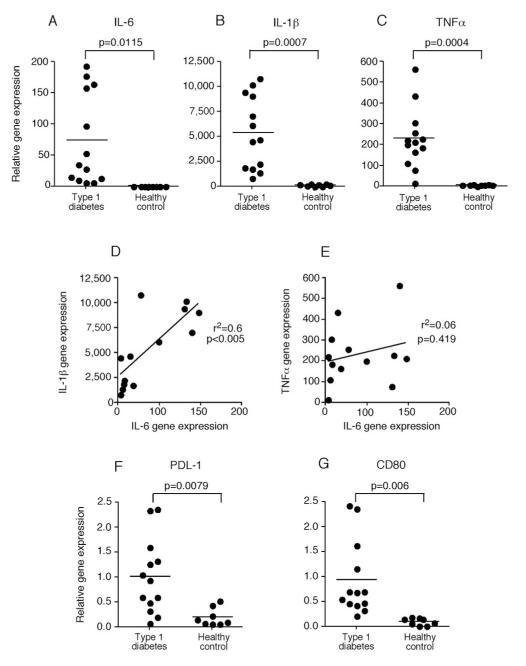
To directly examine the classical monocytes ex vivo for cytokine phenotype and avoid potential cell culture activation of this cell type, monocytes were isolated by negative selection and immediately deposited into RNA lysis buffer. Quantitative RT-PCR was used to evaluate mRNA levels of different monocyte-produced cytokines. Monocytes derived from recent-onset T1D subjects were found to have significantly increased gene expression of IL-6, IL-1β and TNFα as compared to those from healthy subjects (Figure 5A-C). Increased production of TNFα, IL-6 and IL-1β by circulating monocytes from T1D subjects is striking and suggests a systemic alteration in function in a subset of monocyte/macrophages. Moreover, the expression of IL-6 and IL-1β mRNA from T1D-derived monocytes was strongly correlated, while expression of IL-6 and TNFα was not (Figure 5D and E). There were no significant differences of IL-23 or TGFβ expression seen between monocytes derived from T1D subjects or healthy controls (data not shown). The monocytes derived from T1D subjects also had increased PDL-1 and CD80 gene expression as compared to those from healthy controls (Figure 5F and G); this is further evidence that the classical monocytes are in an activated state in T1D subjects.
Figure 5. Ex vivo monocytes from type 1 diabetic subjects have increased cytokine gene expression in comparison to healthy control subjects.
Monocytes from recent onset (<1 years) T1D subjects were negatively selected, RNA was immediately isolated, and the relative gene expression was measured using quantitative RT-PCR. Relative (A) IL-6 (B) IL-1β and (C) TNFα gene expression for T1D and healthy control subjects is shown. All gene expression is relative to β2-microglobulin. Each circle represents a subject; 13 recent-onset T1D subjects were compared to 8 healthy control subjects. Horizontal bars indicate the mean. Correlation of gene expression between (D) IL-6 and IL-1β and (E) IL-6 and TNFα for monocytes from recent-onset T1D is shown. The monocytes derived from T1D subjects are in a more activated state as shown by increased expression of (F) PDL1 and (G) CD80 as compared to those from healthy controls. Significant differences are shown in the figure.
Expansion and activation of memory Th17 cells by T1D monocytes
It has recently been demonstrated that IL-6 and IL-1β are involved in differentiation/expansion of Th17 cells (10-13). In this regard, we previously observed that IL-1β with or without IL-6 induced the secretion of IL-17 from memory T cells. It has also been shown that monocytes, stimulated with peptidoglycan or LPS, secreted IL-1β and IL-6 and were able to induce IL-17 secreting cells (13, 14). Thus, a major consequence of T1D-derived monocyte IL-6/IL-1β secretion could be the induction of IL-17 secreting cells. To test this hypothesis we examined whether unstimulated monocytes isolated ex vivo from patients with T1D induced naïve or memory T cells from healthy control subjects to secrete IL-17 as compared to monocytes isolated from control subjects. The CD4+CD25−CD62L+CD45RA+ naïve and CD4+CD25−CD62L+CD45RA− memory T cells from healthy controls were stimulated with anti-CD3 and classical monocytes from T1D or healthy control subjects for five days, and then further cultured in the presence of IL-2 for an additional seven days. While allogeneic responses result in cell proliferation and expansion, here the strong stimulus of immobilized anti-CD3 drove T cell proliferation (Supplementary Figure 2). Since the monocytes do not produce IL-21, a cytokine found to be critical to the differentiation of Th17 cells, we did not expect to see IL-17 secretion from the naïve T cells, and as predicted, we saw few IL-17 secreting cells in the co-cultures with the naïve T cells [% T cells expressing IL-17 cultured with monocytes from T1D: 0.89±0.34% (mean±SE); % T cells expressing IL-17 cultured with monocytes from healthy control: 0.16±0.06%; N=9]. We found that ex vivo, unstimulated T1D subject-derived monocytes preferentially induced IL-17 secretion from memory T cells as compared to monocytes from healthy controls; interestingly this IL-17 secreting population contains both IL-17+/IFNγ− and IL-17+/IFNγ+ populations as shown by intracellular staining (Table 1). The IL-17+/IFNγ+ subset is known to express CXCR3, a Th1 chemokine receptor (26), which has been implicated in T1D (27) and has also been implicated in the EAE model as the pathologic CD4 effector population (28). Few IL-17 secreting T cells were induced in the absence of monocytes from memory T cells under these conditions (Supplementary Figure 3).
Table I.
Monocytes from T1D subjects induce a higher percentage of IL-17 positive cells from healthy control memory CD4+ T cells than do monocytes from healthy control subjects1.
| Percent Cytokine PositiveT Cells | Monocytes from Type 1Diabetic Subjects | Monocytes from HealthyControl Subjects |
|---|---|---|
| % IL-17+ * | 24.16 ± 5.044 | 16.51 ± 3.750 |
| % IL-17+/IFNγ+* | 10.55 ± 2.072 | 6.690 ± 1.523 |
| % IL-17+/IFNγ−* | 13.49 ± 3.095 | 9.686 ± 2.276 |
| % IL-17−/IFNγ+ | 44.11 ± 3.251 | 39.84 ± 3.751 |
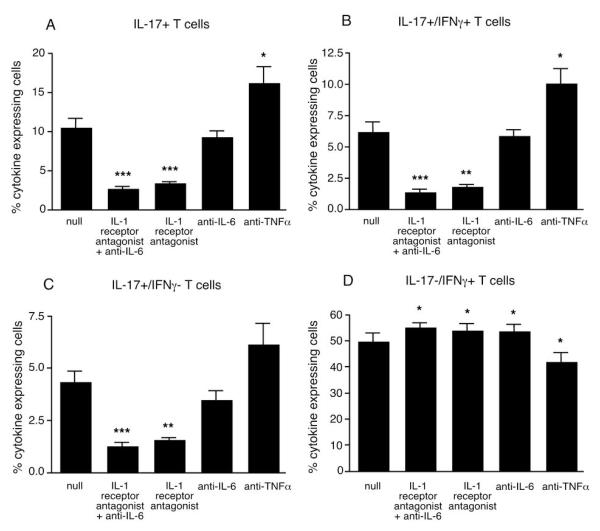
To determine which of the pro-inflammatory cytokines were driving IL-17 secretion, blocking antibodies against IL-6 or TNFα, and an IL-1 receptor antagonist (IL-1Ra) were added to co-cultures of monocytes from T1D patients and memory T cells from healthy subjects. Cultures were analyzed by intracellular staining for IL-17 and IFNγ-expressing T cells. Blocking IL-6 in combination with IL-1β or blocking IL-1β alone directly inhibited IL-17 secretion. The IL-1Ra reduced the number of IL-17 secreting cells by approximately 70%. Blocking IL-6 alone was not as effective as IL-1Ra, but did reduce by approximately 10% the number of IL-17-secreting cells, however this was not statistically significant. Blocking TNFα slightly increased the percentage of IL-17 secreting cells (Figure 6).
Figure 6. IL-1R antagonist reduced the number of IL-17 secreting memory T cells in monocyte-T cell co-cultures.
Healthy control memory T cells were cultured with monocytes from T1D subjects for 5 days in the presence of plate-bound anti-CD3. T cells were then expanded for 7 days with the addition of IL-2. The cells were stimulated with PMA and ionomycin and analyzed by intracellular staining for IL-17/IFNγ expression. The number of (A) IL-17+, (B) IL-17+/IFNγ+ and (C) IL-17+/IFNγ− secreting cells, but not (D) IL-17−/IFNγ+ secreting cells, (N=8) could be reduced by IL-1R antagonist and anti-IL-6 or IL-1R antagonist alone, but were slightly increased by anti-TNFα. ***, p<0.0005; **, p<0.005; *, p<0.05.
Increased number of IL-17 secreting cells in T1D subjects
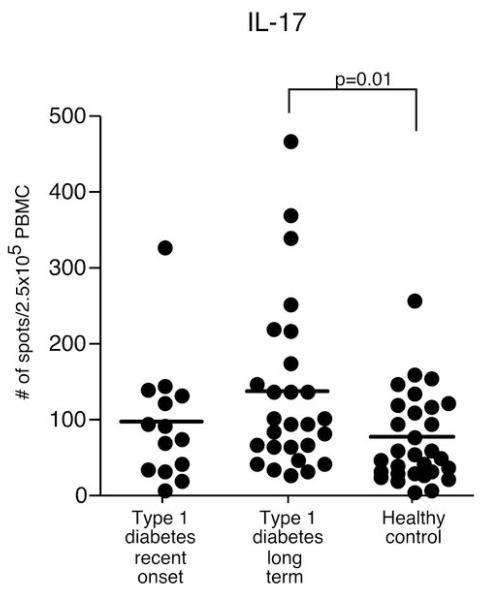
We directly measured the ex vivo frequency of IL-17 secreting CD4 cells. After 18 hours of T cell receptor crosslinking with anti-CD3, we found that the recent-onset T1D subjects do not have an increased frequency of IL-17 producers, but interestingly long-term T1D subjects have a moderate increase of IL-17 secreting cells as compared to healthy controls (Figure 7). To confirm that the positive cells in the ELISpot assay were derived from T cells, peripheral blood mononuclear cells (PBMC) were activated by PMA and ionomycin for 3 hours and then stained for CD3 and IL-17 expression, followed by FACS analysis. The percent of CD3+IL-17+ T cells corresponds to the number of positive spots determined by ELISpot from total PBMCs (Supplementary Figure 4).
Figure 7. IL-17 secreting cells from anti-CD3 stimulated PBMCs from long-term T1D subjects was greater as compared to those from healthy control subjects.
Anti-CD3 stimulated PBMCs were incubated in HL-1 media with 1% human serum for 18 hours and then analyzed by ELISpot. Each circle represents the number of positive PBMCs derived from a single subject. 250,000 PBMCs were added per well. Horizontal bars indicate the mean. Significant differences in cytokine positive cells between groups are shown in the figure.
DISCUSSION
Here, we examined a mechanism for the increased induction of Th17 cells observed in patients with autoimmune disease. While there have been several reports implicating Th17 cells in the NOD model, this is the first report to correlate increased IL-17 secreting T cells with human T1D. We also observed a marked increase in the frequency of a subset of circulating IL-1β and IL-6 secreting monocytes in recent-onset T1D patients as compared to age-matched healthy controls or patients with T2D. These results were confirmed by direct PCR examination of mRNA where increases in pro-inflammatory cytokines and costimulatory molecules were observed. These activated, IL-1β and IL-6 secreting monocytes from patients with T1D drove the in vitro induction of IL-17+ CD4 cells that may be associated with the increase in the frequency of IL-17 secreting CD4 cells in patients with the disease.
There is previous evidence that circulating monocytes in patients with T1D can be induced to secrete pro-inflammatory cytokines. While these previous studies demonstrate that monocytes from patients with T1D can be induced to secrete a more inflammatory cytokine milieu through activation with LPS or IFNγ, here we show that classical monocytes isolated by negative selection ex vivo spontaneously secrete pro-inflammatory cytokines that can drive the secretion of IL-17 from memory T cells. We demonstrated that not all of the monocytes are actively secreting cytokine; only a small subpopulation was actively secreting IL-6 and IL-1β. Interestingly, we found that both the CD14+CD16− and the CD14+CD16+ populations had increased IL-6 expression. It has been shown that CD14+CD16− monocytes can convert to CD14+CD16+ monocytes upon stimulation (29). The relationship between these two monocyte populations in type 1 diabetes requires further examination.
Other studies have compared monocytes and monocyte-derived cytokines from T1D and T2D subjects with differing results. Microarray analysis of PBMCs found that IL-1β was highly over-expressed in PBMCs derived from both T1D and T2D subjects (30). mRNA expression profiling has also been performed in monocytes from T2D patients as compared to healthy controls and T1D patients as measured by quantitative real-time RT-PCR (20). Stimulated monocytes from T2D patients showed significantly higher expression levels of cytokines including TNFα, IL-6 and IL-1 as compared to controls and T1D patients. In another investigation, TNFα and IL-6 levels were evaluated in LPS-stimulated monocytes obtained from T1D and T2D patients, and while TNFα secretion was elevated in monocytes derived from both T1D and T2D subjects, IL-6 was only elevated in T1D derived monocytes as compared to those from controls (19). A recent gene array study found an increase in pro-inflammatory cytokine secretion predominantly from adult-onset and latent autoimmune diabetes of the adult (LADA) T1D subjects, but not from juvenile onset T1D subjects (22). A fundamental difference between this study and ours is the separation of patients into groups based on disease onset (22) or by length of disease duration (< 1 year from disease onset or long-term T1D subjects, as described here). In the study presented here, recent-onset subjects were predominantly adult-onset while the long-term disease group (mean age of onset 15 years-old with 15 years of disease) was more similar to the juvenile-onset group in the referenced study (22). Larger studies will be needed to follow monocyte cytokine secretion as the disease progresses in each group of T1D subjects.
Previously, we and others have demonstrated that the combination of IL-6 and IL-1β resulted in secretion of both IL-17 and IFNγ from memory T cells (10, 14). Here, we show that monocytes from T1D subjects, which spontaneously secrete IL-6 and IL-1β, preferentially expanded memory T cells secreting IL-17, but did not expanded the IFNγ+ memory T cell population as compared to monocytes from healthy control subjects. The IL-17+/IFNγ+ subpopulation of inflammatory T cells is of particular interest as a similar population has been observed in the CNS of mice with EAE (28). In the NOD model, it has been shown that Th17 cells are required to convert to IFNγ secreting cells for the initiation of diabetes (8, 9). The mechanism of induction of IL-17 secretion in T1D was partially through the pro-inflammatory cytokines IL-6 and IL-1β as blocking their interaction with their receptor reduced the number of IL-17 secreting cells. These findings are of particular clinical interest as a Phase I clinical trial in newly diagnosed T1D subjects using the IL-1 antagonist, anakinra, is in progress (clinicaltrials.gov/NCT00645840).
There has been increasing attention regarding the role of the innate immune system in driving the activation of CD4 cells into a pathologic effector state. While there have been multiple reports on monocyte cell function after their ex vivo activation in human autoimmune diseases, we report here the presence of a strikingly activated innate immune system in patients with T1D. Moreover, we demonstrate these monocytes from diabetic subjects, partly by secretion of IL-1β and IL-6, can drive memory CD4 T cells to secrete IL-17. While it is possible that the hyperglycemic state of diabetic subjects could induce the secretion of these cytokines by monocytes, in these assays we do not observe the same amount of spontaneous cytokine secretion from PBMCs from patients with T2D, consistent with some previous reports examining cytokine secretion in these patients.
It has been demonstrated that islet-infiltrating macrophages and dendritic cells in patients with T1D secrete TNFα and IL-1β (31), suggesting that activated monocytes may recruit to the islets in T1D. However, the origin of these monocytes still needs to be determined. A significant proportion of the immune system resides in the gastrointestinal system in homeostasis with gut flora, and the pancreatic lymph nodes are linked not only to the pancreas, but also to the gastrointestinal tract. Recently it was shown that the innate immune system’s interaction with intestinal microbes is important in NOD mice developing diabetes (32). Interestingly, many similar allelic variants are being observed between inflammatory bowel disease and human autoimmune diseases including T1D. Future investigations will focus on elucidating the source of these circulating, pro-inflammatory monocytes.
In conclusion, we demonstrated a mechanism of Th17 expansion in T1D; a subset of monocytes isolated directly from the circulation of recent-onset T1D patients express pro-inflammatory mRNA and spontaneously secrete IL-1β and IL-6 that induce potentially pathogenic IL-17/IFN-γ secreting T cells. Blocking the monocyte-derived cytokines, IL-6 and IL-1β, but not TNFα, resulted in a reduced number of IL-17 secreting cells, confirming their role in Th17 expansion. These observations may allow a simple measure of innate immune system activation in patients with autoimmune diseases. Finally, elucidation of the mechanism and site of monocyte activation in patients with T1D may provide critical insight into disease pathogenesis.
Supplementary Material
Supplementary data
ACKNOWLEDGEMENTS
We would like to thank D. Kozoriz for flow cytometry assistance. We would like to thank B. Healy for help with statistical analysis.
ABBREVIATIONS
T1D
type 1 diabetes
T2D
type 2 diabetes
HC
healthy control
IL-1Ra
IL-1 receptor antagonist
Footnotes
1
These studies were supported by grants from the US National Institutes of Health to D.A.H. (P01 AI045757, U19 AI046130, U19 AI070352, P01 AI039671), E.M.B. (F32 AI651003) and W.E. (F32 NS059205). S.C.K. is supported by grants from the Juvenile Diabetes Research Foundation International. D.A.H. is also supported by a Jacob Javits Merit Award (NS2427) from the National Institute of Neurological Disorders and Stroke.
DISCLOSURES The authors have no financial conflict of interest.
REFERENCES
- 1.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature Immunology. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 2.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nature Medicine. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 3.Murphy CA, Langrish CL, Chen Y, Blumenschein WM, McClanahan TK, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. Journal of Experimental Medicine. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cua D, Sherlock J, Chen Y, Murphy C, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 5.Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vukkadapu SS, Belli JM, Ishii K, Jegga AG, Hutton JJ, Aronow BJ, Katz JD. Dynamic interaction between T cell-mediated beta-cell damage and beta-cell repair in the run up to autoimmune diabetes of the NOD mouse. Physiol. Genomics. 2005;14:201–211. doi: 10.1152/physiolgenomics.00173.2004. [DOI] [PubMed] [Google Scholar]
- 7.Jain R, Tartar DM, Gregg RK, Divekar RD, Bell JJ, Lee HH, Yu P, Ellis JS, Hoeman CM, Franklin CL, Zaghouani H. Innocuous IFNgamma induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J Exp Med. 2008;205:207–218. doi: 10.1084/jem.20071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39:216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bending D, De La Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, Cooke A. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009 doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 13.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 14.Evans HG, Suddason T, Jackson I, Taams LS, Lord GM. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc Natl Acad Sci U S A. 2007;104:17034–17039. doi: 10.1073/pnas.0708426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dogan Y, Akarsu S, Ustundag B, Yilmaz E, Gurgoze MK. Serum IL-1beta, IL-2, and IL-6 in insulin-dependent diabetic children. Mediators Inflamm. 2006;2006:59206. doi: 10.1155/MI/2006/59206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussain MJ, Peakman M, Gallati H, Lo SSS, Hawa M, Viberti GC, Watkins PJ, Leslie RDG, Vergani D. Elevated serum levels of macrophage-derived cytokines precede and accompany the onset of IDDM. Diabetologia. 1996;39:60–69. doi: 10.1007/BF00400414. [DOI] [PubMed] [Google Scholar]
- 17.Hussain MJ, Maher J, Warnock T, Vats A, Peakman M, Vergani D. Cytokine overproduction in healthy first degree relatives of patients with IDDM. Diabetologia. 1998;41:343–349. doi: 10.1007/s001250050913. [DOI] [PubMed] [Google Scholar]
- 18.Devaraj S, Dasu MR, Rockwood J, Winter W, Griffen SC, Jialal I. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab. 2008;93:578–583. doi: 10.1210/jc.2007-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foss-Freitas MC, Foss NT, Donadi EA, Foss MC. In vitro TNF-alpha and IL-6 production by adherent peripheral blood mononuclear cells obtained from type 1 and type 2 diabetic patients evaluated according to the metabolic control. Ann N Y Acad Sci. 2006;1079:177–180. doi: 10.1196/annals.1375.027. [DOI] [PubMed] [Google Scholar]
- 20.Giulietti A, Stoffels K, Decallonne B, Overbergh L, Mathieu C. Monocytic expression behavior of cytokines in diabetic patients upon inflammatory stimulation. Ann N Y Acad Sci. 2004;1037:74–78. doi: 10.1196/annals.1337.011. [DOI] [PubMed] [Google Scholar]
- 21.Devaraj S, Glaser N, Griffen S, Wang-Polagruto J, Miguelino E, Jialal I. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes. 2006;55:774–779. doi: 10.2337/diabetes.55.03.06.db05-1417. [DOI] [PubMed] [Google Scholar]
- 22.Padmos RC, Schloot NC, Beyan H, Ruwhof C, Staal FJ, de Ridder D, Aanstoot HJ, Lam-Tse WK, de Wit H, de Herder C, Drexhage RC, Menart B, Leslie RD, Drexhage HA. Distinct monocyte gene-expression profiles in autoimmune diabetes. Diabetes. 2008;57:2768–2773. doi: 10.2337/db08-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lahm HW, Stein S. Characterization of recombinant human interleukin-2 with micromethods. J Chromatogr. 1985;326:357–361. doi: 10.1016/s0021-9673(01)87461-6. [DOI] [PubMed] [Google Scholar]
- 24.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 25.Skrzeczynska-Moncznik J, Bzowska M, Loseke S, Grage-Griebenow E, Zembala M, Pryjma J. Peripheral blood CD14high CD16+ monocytes are main producers of IL-10. Scand J Immunol. 2008;67:152–159. doi: 10.1111/j.1365-3083.2007.02051.x. [DOI] [PubMed] [Google Scholar]
- 26.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 27.Frigerio S, Junt T, Lu B, Gerard C, Zumsteg U, Hollander GA, Piali L. Beta cells are responsible for CXCR3-mediated T-cell infiltration in insulitis. Nature medicine. 2002;8:1414–1420. doi: 10.1038/nm1202-792. [DOI] [PubMed] [Google Scholar]
- 28.Dardalhon V, Korn T, Kuchroo VK, Anderson AC. Role of Th1 and Th17 cells in organ-specific autoimmunity. Journal of autoimmunity. 2008;31:252–256. doi: 10.1016/j.jaut.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skinner NA, MacIsaac CM, Hamilton JA, Visvanathan K. Regulation of Toll-like receptor (TLR)2 and TLR4 on CD14dimCD16+ monocytes in response to sepsis-related antigens. Clin Exp Immunol. 2005;141:270–278. doi: 10.1111/j.1365-2249.2005.02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaizer EC, Glaser CL, Chaussabel D, Banchereau J, Pascual V, White PC. Gene expression in peripheral blood mononuclear cells from children with diabetes. J Clin Endocrinol Metab. 2007;92:3705–3711. doi: 10.1210/jc.2007-0979. [DOI] [PubMed] [Google Scholar]
- 31.Uno S, Imagawa A, Okita K, Sayama K, Moriwaki M, Iwahashi H, Yamagata K, Tamura S, Matsuzawa Y, Hanafusa T, Miyagawa J, Shimomura I. Macrophages and dendritic cells infiltrating islets with or without beta cells produce tumour necrosis factor-alpha in patients with recent-onset type 1 diabetes. Diabetologia. 2007;50:596–601. doi: 10.1007/s00125-006-0569-9. [DOI] [PubMed] [Google Scholar]
- 32.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data