Inactivation of neuronal forebrain A2A receptors protects dopaminergic neurons in a mouse model of Parkinson’s disease (original) (raw)
. Author manuscript; available in PMC: 2010 Dec 1.
Abstract
Adenosine A2A receptors antagonists produce neuroprotective effects in animal models of Parkinson’s disease (PD). Since neuroinflammation is involved in PD pathogenesis, both neuronal and glial A2A receptors might participate to neuroprotection. We employed complementary pharmacologic and genetic approaches to A2A receptor inactivation, in a multiple 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD, to investigate the cellular basis of neuroprotection by A2A antagonism. MPTP·HCl (20 mg/kg daily for 4 days) was administered in mice treated with the A2A antagonist SCH58261, or in conditional knockout mice lacking A2A receptors on forebrain neurons (fbnA2AKO mice). MPTP induced partial loss of dopamine neurons in substantia nigra pars-compacta (SNc) and striatum (Str), associated with increased astroglial and microglial immunoreactivity in these areas. Astroglia was similarly activated one, three and seven days after MPTP administration, whereas maximal microglial reactivity was detected on day one, returning to baseline seven days after MPTP administration. SCH58261 attenuated dopamine cell loss and gliosis in SNc and Str. Selective depletion of A2A receptors in fbnA2AKO mice completely prevented MPTP-induced dopamine neuron degeneration and gliosis in SNc, and partially counteracted gliosis in Str. Results provide evidence of a primary role played by neuronal A2A receptors in neuroprotective effects of A2A antagonists in a multiple MPTP injections model of PD. With the symptomatic antiparkinsonian potential of several A2A receptor antagonists being pursued in clinical trials, the present study adds to the rationale for broader clinical benefit and use of these drugs early in the treatment of PD.
Keywords: adenosine, MPTP, microglia, astroglia, tyrosine hydroxylase
Since the initial demonstration that caffeine can attenuate MPTP-induced toxicity, a neuroprotective role of A2A receptor blockade has been suggested in several experimental models of Parkinson’s disease (PD) (Chen et al., 2001; Schwarzschild et al., 2006). In rodents different A2A receptor antagonists have been shown to counteract the loss of dopaminergic neurons in the substantia nigra pars compacta (SNc), as well as dopamine depletion in the striatum (Str), induced by acute administration of systemic MPTP or acute infusion of 6-hydroxydopamine (6-OHDA) in the medial forebrain bundle (Chen et al., 2001 Chen et al., 2002; Ikeda et al., 2002; Pierri et al., 2005). Moreover, loss of striatal dopamine induced by acute MPTP was attenuated by the global genetic deletion of A2A receptors in A2A knockout (KO) mice (Chen et al., 2001). In contrast to acute models, neuroprotection by A2A antagonists in the neurotoxicity induced by multiple MPTP administration was never evaluated. On the other hand, previous studies have shown that acute or multiple injections MPTP delivery result in different histopathological features and different modes of cell death (Schmidt and Fergen, 2001). Interstingly, A2A receptor antagonism or gene KO has been found to be neuroprotective in different models of neurodegeneration, such as Alzheimer’s disease, Huntington’s disease and cerebral ischemia (Chen et al., 1999; Dall’Igna et al., 2003; Fink et al., 2004; Melani et al., 2003).
A2A receptors are enriched in the Str, where they are located either postsynaptically in striatopallidal neurons, or presynaptically in nerve terminals (Rebola et al., 2005; Rosin et al., 1998; Schiffman et al., 1991; Svenningsson et al., 1999). Moreover, A2A receptors are expressed at low level in other forebrain structures, such as cortex and hippocampus, whereas little or no A2A receptor immunoreactivity has been detected in dopaminergic neurons in the SNc (Rosin et al., 1998). Besides neurons, non-neuronal cell types such as microglia and astroglia also express A2A receptors (Fiebich et al., 1996; Saura et al., 2005).
The mechanism through which A2A receptor antagonists achieve neuroprotection in PD models has not been elucidated. Given the cellular distribution of A2A receptors in different cell types in brain, neuroprotection by A2A receptor blockade may be achieved through an action on receptors located either on neurons or on glial cells. Attenuation of gliosis by A2A receptor antagonists in the Str and SNc of mice acutely treated with MPTP, suggests that reduction of neuroinflammation may be involved (Ikeda et al., 2002; Pierri et al., 2005). Moreover, it was recently suggested that A2A receptors located on glial cells may play a role in neuroprotection mediated by A2A antagonists against an acute MPTP-induced striatal dopamine depletion (Yu et al, 2008).
In order to get insight into the mechanism by which A2A receptor antagonists induce neuroprotection, the present study evaluated 1) the neuroprotective activity of an A2A antagonist in a mouse model of PD obtained by a multiple injections MPTP delivery; 2) the role of neuronal A2A receptors on this effect and 3) whether neuronal A2A receptor blockade might affect glial response to MPTP. To this aim, we treated mice with MPTP plus the A2A antagonist SCH58261, or administered MPTP to genetically manipulated mice selectively lacking A2A receptors in forebrain neurons (fbnA2AKO mice) (Bastia et al., 2005; Shen et al., 2008). Neuronal damage was evaluated in the SNc and Str using tyrosine hydroxylase (TH) immunohistochemistry, whereas the inflammatory response in the SNc and Str was studied through analysis of glial fibrillary acidic protein (GFAP) and CD11b immunoreactivity as markers of astroglial and microglial cells, respectively.
The present study provides evidence of a primary role played by forebrain A2A receptors in the neuroprotection that A2A receptor antagonists confer against dopaminergic neuron degeneration and glial activation induced by repeated MPTP.
Methods
Animals and treatments
Male C57BL/6J mice (Charles River, Italy) were used for experiments involving pharmacological treatments with the A2A receptor antagonist. Mice were housed 5 per cage, with a 12:12 hrs light/dark cycle and with food and water ad libitum. Experiments were conducted in accordance with the guidelines for care and use of experimental animals of the European Communities Council Directive of 24 November 1986 (86/609/EEC) and the National Institutes of Health.
Pharmacological treatment: Male C57BL/6J mice (25–30 gm, 3 months old), received a multiple injections treatment with vehicle (N= 15), MPTP·HCl (20 mg/kg i.p.) once a day for 4 days (N = 18), or the A2A antagonist SCH58261 (0.5 mg/kg i.p.) twice a day plus MPTP (20 mg/kg i.p.) once a day for 4 days (N = 23). SCH58261 was injected half an hour before MPTP administration. After MPTP treatment discontinuation SCH58261 treatment continued once a day, until sacrifice, which occurred 1, 3 or 7 days after MPTP treatment. Injections were made at 8 a.m. and 8 p.m.
Postnatal forebrain neuron conditional A2AKO mice were generated using the Cre/loxP system based on the specificity of CaMKIIα promoter (Bastia et al, 2005, Yu et al, 2008; see also Additional Informations (A.I.) for online version). FbnA2AKO mice and littermate controls were treated with repeated MPTP (n=18 or n=15) or vehicle (n=12 or n=10) as described above. Experiments with the A2A antagonist (see below) showed that loss of TH-immunoreactive cells was stable 1, 3 and 7 days after MPTP treatment, and that CD11b immunoreactivity was greatest 1 and 3 days post-MPTP treatment. Based on these considerations, neuroprotection and glial reactivity parameters in transgenic mice were evaluated 3 days after MPTP treatment.
Drugs
MPTP·HCl (Sigma-Aldrich, Italy or USA) was dissolved in 0.9% saline in a volume of 0.1 ml/10 g; SCH58261 (kindly provided Prof Baraldi, Ferrara) was suspended in 0.5% of methylcellulose.
Immunohistochemistry
Animals were anesthetized with chloral hydrate (400 mg/kg i.p.) or Avertin (0.1 ml/10 g i.p.) prior to transcardial perfusion with 20 ml of saline and 60 ml of 4% paraformaldehyde. Brains were removed and post-fixed for 2 hour. Adjacent coronal sections (50 μm) from the Str and SNc were cut on a vibratome and stored at −20°C in an antifreeze medium until use (Schintu et al, 2009). For TH, GFAP and CD11b immunostaining, adjacent sections were processed as described (Schintu et al, 2009; See also A.I.).
Analysis and statistics
Images were digitized (videocamera Pixelink PL-A686) under constant light conditions to standardize the measurements. Immunostained sections containing left and right SNc, were captured at 10X magnification (the entire SNc, corresponding to three frames, was digitized for the analysis). Immunostained sections of bilateral striata were captured at 20X magnification. One portion from the dorsolateral Str and one from the ventromedial Str (520 μm × 380 μm) were analysed. For each animal, three sections corresponding to rostral (within −2.90/−3.20 mm from bregma), medial (−3.20/−3.50) and caudal (−3.50/−3.80) SNc levels, and three sections corresponding to rostral (within 1.20/0.90 mm from bregma), medial (0.90/0.60) and caudal (0.60/0.30) Str (accordingly to Mouse Brain Atlas, Paxinos and Franklin, 2001) were analysed for each protein marker evaluated in the study. TH and GFAP analyses: since the number of cells was different in the three SNc and Str levels analysed, for each mouse the number of TH- or GFAP-positive cells/level was first normalized with respect to the vehicle. Individual values from the 3 levels were then averaged to generate a mean. Adjacent SNc sections were Nissl-stained, in order to confirm cell loss in this area.
The SNc from three mice randomly selected from each experimental group were counterstained with cresyl violet for evaluation by unbiased stereological counting, and the mean of TH-ir and Nissl-stained neurons per mm3 ± SEM was calculated (see A.I).
CD11b analysis: images were digitized in a grey scale, and CD11b immunostaining was evaluated with the analysis program Scion Image. A threshold, the value of which was set above the mean value ± S.E.M. of the background, was applied for background-correction. Inside each frame, the area occupied by grey values above the threshold was automatically calculated. For each level of SNc or Str, the obtained value was first normalized with respect to vehicle; the individual values from the 3 levels were then averaged to generate a mean.
Results from mice treated with MPTP plus SCH58261, or from fbnA2AKO mice were statistically compared with a two-factors ANOVA, followed by Tukey’s post hoc test, for comparison between experimental groups.
Results
A2A receptor antagonist prevents dopaminergic cell loss in the SNc
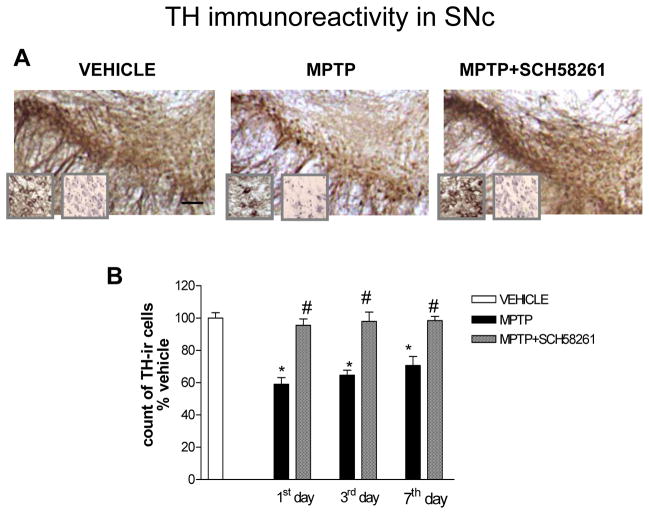
In C57BL/6J mice, MPTP treatment induced a partial dopaminergic neurons degeneration in the SNc (Fig 1A, B and Tab 1). Counting of Nissl-stained cells confirmed this result (Tab 1). Dopaminergic neurons loss was statistically significant 1 day after MPTP treatment (N= 5) and remained significant after 3 (N=5) and 7 days timepoints (N=8). Combined treatment with A2A antagonist SCH58261 plus MPTP, attenuated TH-positive neurons loss in the SNc at 1 (N= 5), 3 (N=5) and 7 days (N=13) (Fig 1A, B and Tab 1), as confirmed by Nissl-staining (Tab 1). Two-factors ANOVA showed a significant effect of treatment (see Suppl. Table 1 in A.I. for corresponding F and P values). In the Str, MPTP-induced decrease in TH-immunoreactivity was significantly attenuated by treatment with the A2AR antagonist, as measured three days after treatment (Fig 2).
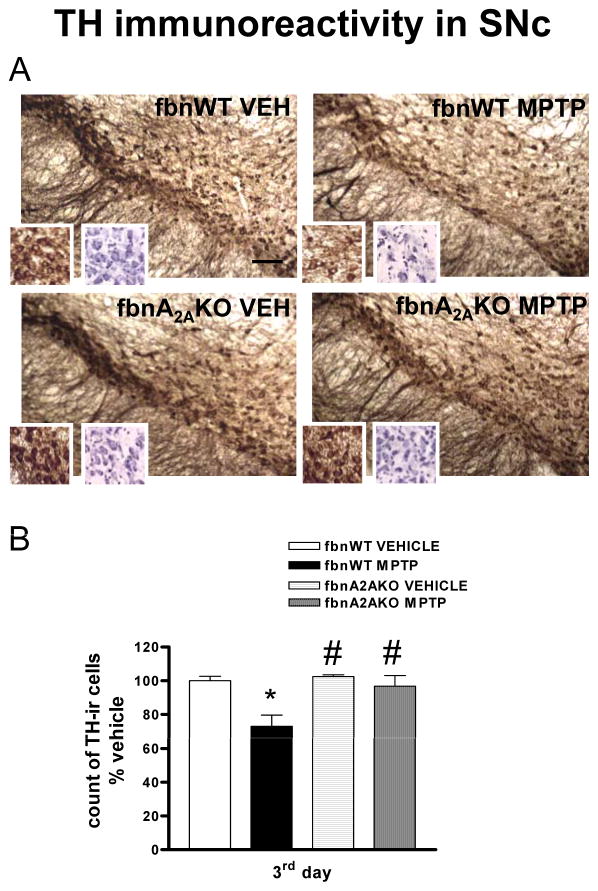
Fig 1. Adenosine A2A receptor antagonist SCH58261 prevents dopaminergic cell loss in the SNc.
(A) shows representative sections immunostained for TH from SNc of mice sacrificed 3 days after MPTP treatment. Left insert shows TH-positive cells at higher magnification, right insert shows cresyl violet-stained sections; scale bar: 50 μm. Mice were treated with MPTP-HCl (20 mg/kg once a day for 4 days), plus SCH58261 (0.5 mg/kg) or vehicle (twice a day during MPTP treatment and once daily thereafter until sacrifice), and sacrificed 1, 3, 7 days after MPTP treatment. (B) shows analysis of TH immunostaining at 1, 3, 7 days after MPTP, reported as a percentage of TH-positive cells as compared to vehicle-treated mice. * indicates p<0.001 versus vehicle; # indicates p<0.001 versus MPTP group, by Tukey’s post hoc test. Scale bar: 50 μm.
Table 1.
Unbiased evaluation of TH-IR and Nissl-stained neurons by stereological analysis in the SNc of mice following pharmacological blockade of A2ARs with the antagonist SCH58261 or A2AR genetic depletion.
Stereological evaluation of TH-IR and Nissl-stained neurons in the substantia nigra pars compacta
| Treatment | N | Density of TH-IR neurons/mm3 | Density of Nissl-IR neurons/mm3 |
|---|---|---|---|
| pharmacological blockade of A2AR | |||
| Vehicle | 3 | 29725.89 ± 439.56 | 37702.70 ± 2520,89 |
| MPTP | 3 | 15211.79 ± 1300.06* | 24827.52 ± 1689.72* |
| SCH + MPTP | 3 | 23003.66 ± 495.76*^ | 32817.67 ± 1225.20^ |
| fbnA2AR depletion | |||
| WT vehicle | 3 | 27447.34 ± 1365.08 | 32198.28 ± 3341,77 |
| WT MPTP | 3 | 14278.85 ± 2493.30* | 21784.92 ± 2266.61* |
| KO vehicle | 3 | 23654.36 ± 2277.74 | 30903.52 ± 4264.73 |
| KO MPTP | 3 | 24267.96 ± 2277.83^ | 29832.48 ± 883.17^ |
Fig 2. Adenosine A2A receptor antagonist SCH58261 attenuates degeneration of dopaminergic terminals in the Str.
(A) representative sections immunostained for TH, from the Str of mice sacrificed 3 days after MPTP treatment. (B) Results from pharmacological blockade with SCH58261 or genetic A2AR depletion are presented. In the left column, − and + indicate the administration of vehicle or SCH58261to MPTP-treated mice. In the right column + and − indicate fbnA2AWT and fbnA2AKO mice, respectively.
A2A receptor antagonist inhibits astroglia and microglia activation
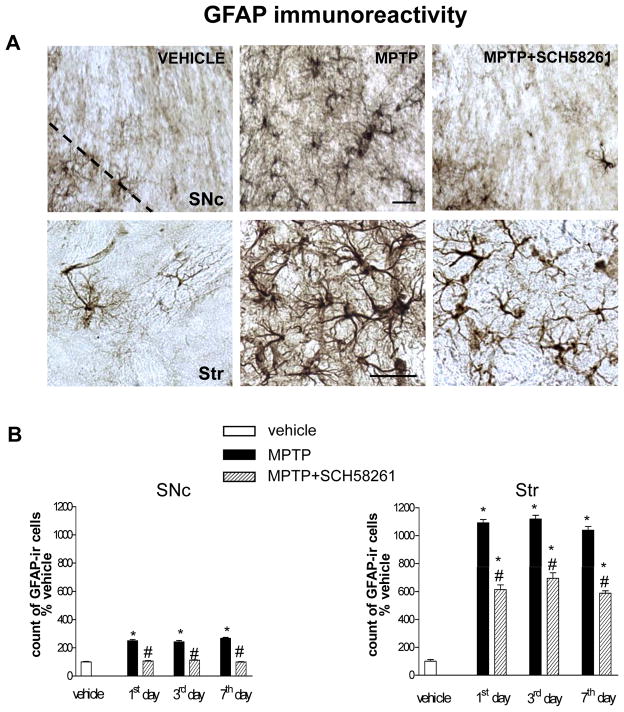
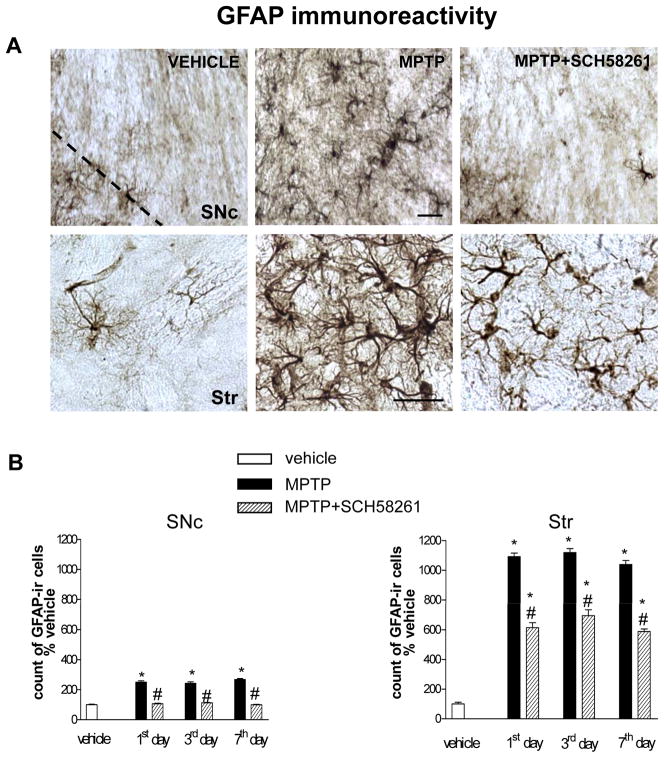
In brain sections from vehicle-treated mice, few GFAP-positive cells (Fig 3), and a low CD11b immunostaining (Figs 3, 4), were detected in the SNc and Str. Repeated MPTP treatment induced an increase in GFAP and CD11b-positive cells in both the SNc and Str of C57BL/6J mice.
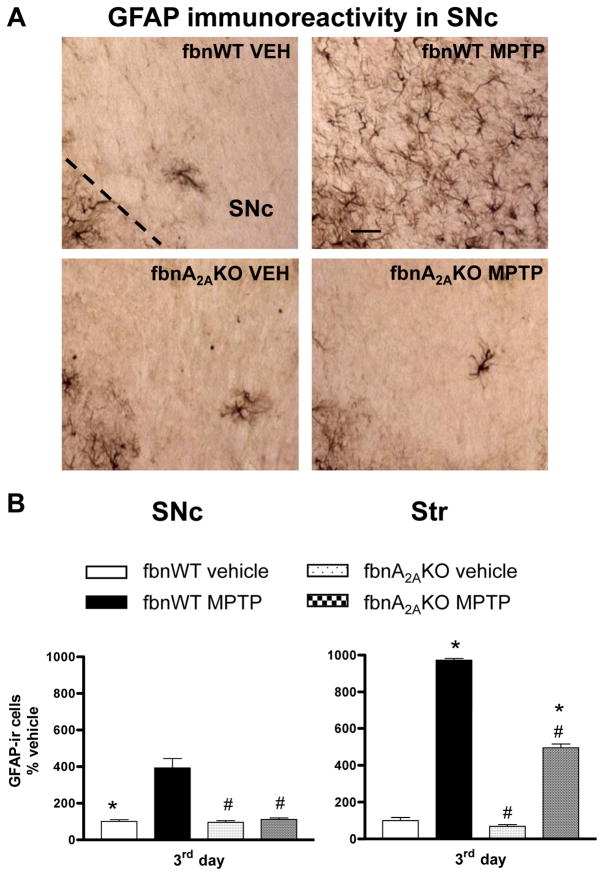
Fig 3. Adenosine A2A receptor antagonist SCH58261 counteracts astroglia activation in the SNc and Str.
(A) shows representative sections immunostained for GFAP, from SNc (upper images) and Str (lower images) of mice sacrificed 3 days after MPTP treatment. Mice were treated as described in Fig 1. (B) shows analysis of GFAP immunostaining 1, 3, 7 days after MPTP, reported as percentage of GFAP-positive cells as compared to vehicle-treated mice in the SNc (left graph) and in the Str (right graph). * indicates p<0.001 versus corresponding vehicle and MPTP+SCH58261 groups; # indicates p<0.001 versus corresponding MPTP group, by Tukey’s post hoc test. Scale bar: 50 μm.
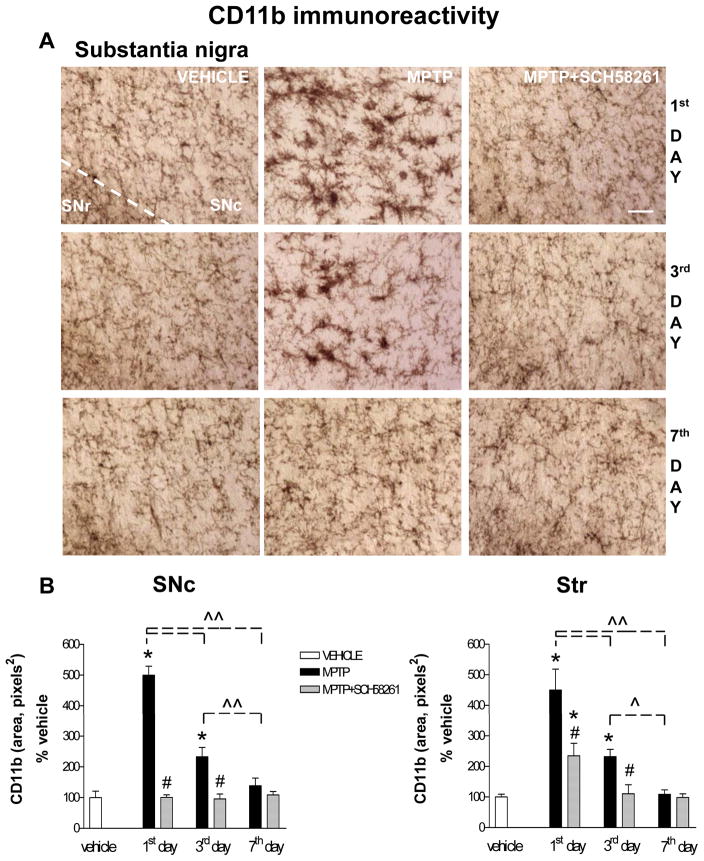
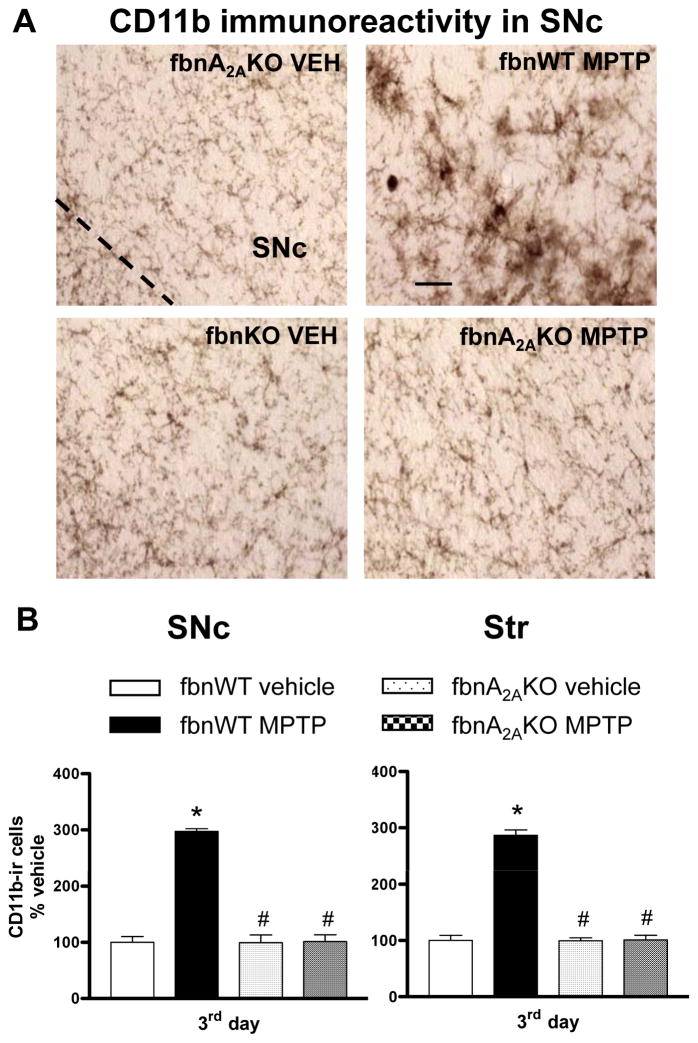
Fig 4. Adenosine A2A receptor antagonist SCH58261 counteracts microglia activation.
(A) Representative images from the SNc immunostained for CD11b as a marker of microglia activation. Mice were treated as described in Fig 1 and sacrificed 1, 3, 7 days after MPTP treatment. (B) CD11b analysis in SNc and Str was performed in grey-scale digitized images. The area occupied by grey values above a threshold was calculated and expressed as square pixels and as percentage of vehicle-treated mice. Tukey’s post hoc test: * p<0.001 versus vehicle and MPTP+SCH58261 group; # p<0.001 versus MPTP group; ^, ^^ p<0.05, p<0.001 versus the indicated time point. Scale bar: 50 μm.
GFAP-positive cells displayed a highly branched morphology with tiny processes and a small body in control sections, and became hypertrophic in response to MPTP treatment (Fig 3A); moreover, CD11b-positive cells were ramified at baseline, but took on an ameboid aspect after MPTP treatment (Fig 4A), indicative of astroglial and microglial activation. In both the SNc and Str, GFAP immunolabelling was of similar intensity 1, 3 and 7 days post-MPTP treatment (Fig 3B). Combined SCH58261 plus MPTP treatment, completely prevented the increase in GFAP immunoreactivity in the SNc and partially prevented it in the Str, at 1, 3 and 7 days after MPTP treatment (Fig 3B). Two-factors ANOVA for GFAP analysis showed a significant effect of the treatment at all time points analyzed in the SNc and in the Str (see Suppl. Table 1 in A.I.).
CD11b immunolabelling was highest 1 day after MPTP, gradually declining to basal levels after 7 days in both SNc and Str (Fig 4A, B). Combined SCH58261 plus MPTP treatment completely prevented the increase in CD11b immunoreactivity in the SNc 1 and 3 days after MPTP treatment (Fig 4A,B). In the Str, SCH58261 partially prevented MPTP-induced increase in CD11b at 1 day, and totally prevented it at 3 days after MPTP treatment. Two-factors ANOVA and post hoc analysis for CD11b revealed a significant effect of treatment, time, and a treatment/time interaction, 1 and 3 days after MPTP administration in the SNc and in the Str (se Suppl. Table 1 in A.I.).
Dopaminergic cell loss is prevented in fbnA2AKO mice
In fbnA2AWT mice, repeated MPTP treatment induced a significant loss of dopamine neurons in the SNc (Fig 5A, B and Tab 1). This result was confirmed by a reduction in Nissl-stained cells (Tab 1 and see right insert in fig 5A). In contrast, in fbnA2AKO mice, repeated MPTP treatment did not result in a decrease in TH-immunolabelling in the SNc (Fig 5A, B and Tab 1). Two-factors ANOVA revealed a significant effect of treatment and genotype (see Suppl. Table 1 in A.I. for corresponding F and P values). In the Str of fbnA2AKO mice repeated MPTP reduced TH immunolabelling to a lesser extent as compared with fbnA2AWT controls (Fig 2).
Fig 5. fbnA2A KO mice are protected against MPTP-induced loss of dopaminergic cells in the SNc.
(A) shows representative sections from SNc immunostained for TH. Inserts show higher magnification of TH-labelled (left) and cresyl violet-labelled (right) cells. Mice were treated with MPTP (20 mg/kg once a day for 4 days)or vehicle. (B) shows analysis of TH immunostaining in fbnA2AKO mice, reported as a percentage of TH-positive cells as compared to vehicle-treated mice. Tukey’s post hoc test: * p<0.05 versus vehicle group; # p<0.05 versus WT MPTP group. Scale bar: 50 μm.
Astroglial and microglial activation is attenuated in fbnA2AKO mice
GFAP immunoreactivity in the SNc and Str was significantly enhanced in MPTP-treated fbnA2AWT mice (Fig 6A, B). In contrast, in fbnA2AKO mice, MPTP-induced increase in GFAP immunoreactivity was totally prevented in the SNc, and partially prevented in Str (Fig 6A, B). In both brain regions two-factors ANOVA revealed a significant effect of treatment, genotype and a significant treatment/genotype interaction (see Suppl. Table 1 in A.I.).
Fig 6. Astroglia activation is attenuated in SNc and Str of fbnA2AKO mice.
(A) representative images from the SNc immunostained for GFAP, as a marker of astroglial cells. (B) Graphs show the analysis of GFAP immunostaining in SNc and Str, in fbnWT and fbnA2AKO mice treated with vehicle or MPTP. Tukey’s post hoc test: * p<0.001 versus vehicle group; # p<0.001 versus WT MPTP group. Scale bar: 50 μm.
CD11b immunolabelling in the SNc and Str was significantly enhanced in MPTP-treated fbnA2AWT mice (Fig 7A, B). In fbnA2AKO mice CD11b activation was totally prevented in the SNc and Str (Fig 7A, B). Two-factors ANOVA revealed a significant effect of treatment, genotype and a treatment/genotype interaction (see Suppl. Table 1 in A.I.).
Fig 7. Microglia activation is prevented in SNc and Str of fbnA2AKO mice.
(A) representative images from the SNc immunostained for CD11b, as a marker of microglia activation. (B) Graphs show the analysis of CD11b immunostaining in SNc and Str, in fbnWT and fbnA2AKO mice treated with vehicle or MPTP. Tukey’s post hoc test: * p<0.001 versus vehicle group; # p<0.001 versus WT MPTP group. Scale bar: 50 μm.
Discussion
Antagonism of adenosine A2A receptors or their selective deletion in forebrain neurons produced similar protection of TH-positive nigral neurons in a multiple MPTP injections mouse model of PD. These complementary pharmacological and genetic means of A2A receptor disruption also attenuated the neurotoxin-triggered activations of astroglia and microglia along the nigrostriatal pathway. Together these data provide evidence that therapeutically accessible A2A receptors located on forebrain neurons play a critical role in nigral dopaminergic neuron degeneration and inflammatory processes in the multiple MPTP injections mouse model of PD.
Pharmacological blockade of A2A receptors prevents MPTP-induced dopaminergic neuron degeneration and glial activation
Systemic administration of the A2A receptor antagonist SCH58261 prevented the degeneration of nigrostriatal TH-positive neurons induced by repeated MPTP exposure in mice. Changes in number of TH-positive neurons correlated with changes in Nissl-stained (cresyl violet-positive) cells, indicating that MPTP treatment resulted in actual loss of dopaminergic neurons, which were rescued by SCH58261.
A neuroprotective effect of A2A receptor antagonists was previously observed upon acute administration of high MPTP doses in mice (Chen et al., 2001; Ikeda et al., 2002; Pierri et al., 2005; Yu et al, 2008). Here we report that neuroprotection with A2A antagonism can also be achieved upon multiple low-doses MPTP exposure. A number of studies have provided evidence that a repeated daily MPTP administration protocol similar to the one used here, presents histopathological features that more closely reproduce the human PD neuropathology, including apoptotic death of dopaminergic neurons (Jackson-Lewis et al., 1995; Tatton & Kish, 1997). Therefore, the present study further substantiates the neuroprotective potential of A2A antagonism in PD. To this regard, it is noteworthy that A2AR antagonists were shown to inhibit apoptotic neuronal death in hippocampal neurons (Silva et al, 2007).
Based on their differential location in the Str or other brain regions, A2A receptors may hold different levels of expression and intracellular signalling, reflecting A2A receptor multiple functions (Kull et al, 2000; Pedata et al, 2003; Rebola et al, 2005; Rosin et al, 2003; Shen et al, 2008). According to such varied roles of the A2A receptor, diverse effects have been attributed to A2A antagonists, ranging from symptomatic antiparkinsonian actions to neuroprotection in various neurodegenerative conditions (Alfinito et al., 2003; Blum et al., 2003; Chen et al., 1999; Melani et al., 2003; Monopoli et al., 1998; Popoli et al., 2002). Motor effects of A2A receptor antagonists are likely mediated by A2A receptors located on striatal neurons projecting to globus pallidus, whereas several mechanisms have been hypothesized for their neuroprotective effects, involving either neuronal or glial A2A receptors, though no single mechanism has yet been proven to prevail (Carta et al., 2003; Chen et al., 2001; Huang et al, 2006; Melani et al., 2003; Popoli et al., 1995; Pedata et al., 2003; Schwarzschild et al, 2006; Yu et al, 2008). Noticeably, in the present study neuroprotection by SCH58261 was achieved at doses similar to those effective in other neurodegenerative conditions, but several times lower than doses displaying a symptomatic efficacy in PD (Chen et al., 2001; Dall’Igna et al., 2003; Melani et al., 2003; Pinna et al., 2007), supporting the concept that different mechanisms might account for A2A-mediated neuroprotection or symptomatic effects.
SCH58261 fully prevented astroglia and microglia activation in the SNc, while only partially inhibiting astroglia and microglia reactivity in the striatum, in line with a partial protection of dopaminergic terminals. Noteworthy, A2A receptor antagonism prevented both astroglia and microglia activation in the SNc and Str at all time-points evaluated, in accordance with blockade of neurodegeneration.
Though the mechanism through which A2A receptor blockade produces neuroprotective effects in PD models is unclear, the modulation of neuroinflammation has been proposed as a likely target for neuroprotection (Hunot and Hirsch, 2003). Several findings have suggested that neuroinflammation may play an active role in the pathogenesis of neurodegeneration in PD, since focal inflammation has been described in in the SNc of PD patients and MPTP-treated primates (Barcia et al., 2004; McGeer et al., 1988). Intriguingly, blockade of microglia reactivity in mice rescued dopamine neurons from acute MPTP toxicity (Wu et al., 2002). Moreover, in mice acutely treated with MPTP, dopamine neuron neuroprotection by pre-treatment with an A2A antagonist was associated with an attenuation of astroglia and microglia activation in SNc and Str (Ikeda et al., 2002; Pierri et al., 2005), consistent with a causal relation between the two events.
Selective deletion of neuron specific forebrain (fbn) A2A receptors prevents MPTP-induced dopamine neuron degeneration and glial activation
Previous studies evaluating A2A receptor-mediated neuroprotection have hypothesised several mechanisms that might underlie this process. In order to determine the role of neuronal versus glial A2A receptors in neuroprotection of dopamine neurons, we exploited genetically modified mice with selective depletion of A2A receptors from forebrain neurons (Bastia et al., 2005; Shen et al, 2008). Importantly, in the fbnA2AKO mice, deletion of A2A receptor is not only spatially restricted (to forebrain A2A receptor) but is also temporally limited to postnatal A2A receptors (Bastia et al., 2005; Yu et al., 2008), thus avoiding potential confounds of compensatory responses to A2A receptor gene disruption during development as might occur in constitutive A2A knockout mice.
Our results revealed that selective deletion of A2A receptors from forebrain neurons totally prevented dopaminergic neuron loss in the SNc following multiple MPTP injections, while partially preventing damage to striatal dopaminergic terminals. A2AR deletion provided a greater protection of SNc neurons than the A2AR antagonist, as expected from a permanent as compared to a temporal pharmacological blockade of the receptor, in line with the reported half-life of SCH58261 of 2–3 h.
A2A receptors are located both pre- and post-synaptically in striatal and cortical neurons, and are expressed in microglial as well as astroglial cells (Cunha, 2001; Kust et al., 1999; Nishizaki et al, 2004; Rebola et al., 2005; Rosin et al., 2003). Positive modulation of postsynaptic signalling as well as of pre-synaptic release of neurotransmitters as glutamate and acetylcholine by A2A receptors have been described (Fredholm et al., 2003; Fuxe et al., 2003; Marchi et al., 2002; Popoli et al., 1995; Schiffmann et al., 2007; Schwarzschild et al, 2006). In addition, A2A receptors interfere with glia-mediated synthesis and release of neurotoxic factors such as COX-2, prostaglandins, nitric oxide and glutamate, which have been hypothesised to play central roles in inflammatory processes and neuronal damage (Fiebich et al., 1996; Li et al., 2001; Saura et al., 2005).
The present study, by showing that selective deletion of A2A receptors from forebrain neurons protects dopaminergic neurons from MPTP toxicity, endorses a primary role of neuronal receptors in mediating neuroprotection in this multiple injections MPTP model of PD. Since very low levels of A2A receptors are expressed by dopaminergic neurons in the SNc, it is unlikely that a direct action at this level might mediate neuroprotection from MPTP toxicity in the SNc. Rather, an indirect effect at the pre-synaptic level, through an inhibition of A2A-mediated glutamate release, which contribute to neuronal damage, could be envisaged (Aguirre et al., 2005; Battaglia et al., 2004; Monopoli et al., 1998; Popoli et al., 2002). Interestingly, recent studies have reported a tight cross-talk between adenosine and GDNF receptors, resulting in a fine modulation of glutamate and dopamine release (Gomes et al, 2006; 2009). In the Str, A2AR blockade would impair GDNF-stimulated increase of corticostriatal glutamate release, thus providing a beneficial effect on neurodegeneration. In addition, A2A receptor antagonism on striatopallidal or subthalamic (STN) neurons might be protective from MPTP toxicity by modulating excessive activation of subthalamic nucleus, thereby reducing excitotoxic glutamate efflux to SNc neurons (Wallace et al., 2007).
A study by Yu and colleagues reported that fbnA2AKO mice from the same line as used here, were not protected from striatal dopamine loss in response to acute MPTP exposure (in a single dose or multiple doses over 4 hr; Yu et al., 2008). These results open to several compelling interpretations. First, the cellular basis of A2A receptor-dependence of MPTP toxicity might vary depending on the duration of toxin exposure, in line with the different type of neurotoxicity produced by acute as compared to subchronic MPTP. Moreover, it should be taken into account the different parameters used to evaluate nigrostriatal neurons damage. The drop of striatal dopamine levels assessed by Yu et al. may reflect functional injury to dopaminergic terminals, whereas the measure of dopaminergic nigral neuron employed in our study may reflect an underlying neurodegenerative process in this area. All together results support the concept that A2A receptors display complex actions related to the duration of insult, cellular elements and brain areas targeted by neurodegenerative processes.
Lack of a glial reaction in fbnA2AKO mice, as compared to the robust astroglia and microglia activation in MPTP-treated control mice, indicates that deletion of neuronal A2A receptors may indirectly inhibit the inflammatory response. Glutamate is a main contributor to the complex neuron-glia cross-talk engaged by pathological events, which trigger both microglia and astroglia activation. For instance, by an action on NMDA receptors, glutamate release stimulates mitogen-activated protein kinases (MAPKs). Neuronal as well as glial p38 MAPK activation has been involved in cell suffering and apoptotic death, being activated and inducing several inflammatory mediators (Gianfriddo et al., 2004; Irving et al., 2000; Kawasaki et al., 1997; Piao et al., 2003). Therefore, A2A antagonists indirectly through a reduction of glutamate release might counteract glial reactivity and neuroinflammation in both SNc and Str (Melani et al., 2003, 2006). Activated glial cells, by the release of several toxic species as cytokines, free radicals, glutamate, is known to contribute to neuronal damage, and has been suggested to sustain a self-amplifying cycle which perpetuates MPTP toxicity (Hunot and Hirsh, 2003). Hence, interruption of such a detrimental vicious cycle might indirectly contribute to A2A receptor-dependent neuroprotection. Accordingly results suggest that in our model, operational glial A2A receptors did not contribute to MPTP-toxicity when A2A forebrain neuronal receptors where deleted. All together, our results suggest that A2A antagonists, by blocking neuronal A2A receptors, might act upstream in the cascade of toxic events and lead to an attenuation of dopamine neuron degeneration in PD.
Supplementary Material
Supplement. Data
Acknowledgments
This study was supported by Ministero dell’Università e della Ricerca Scientifica e Tecnologica, project FIRB [grant number RBNE03YA3L-2005] and National Institute of Health [grant numbers ES010804, S54978] and U.S. Army Medical Research Acquisition Activity [grant number W81XWH-04-1-0881].
The authors thank Prof P.G. Baraldi and Dr M.A. Tabrizi, Department of Pharmaceutical Sciences, University of Ferrara, for providing SCH58261, David H. Gutmann and M. Livia Bajenaru for providing GFAP-cre mice. Yuehang Xu for expert technical assistance.
Contributor Information
Anna R Carta, Email: acarta@unica.it.
Anil Kachroo, Email: AKACHROO@PARTNERS.ORG.
Nicoletta Schintu, Email: nicoletta.schintu@gmail.com.
Kui Xu, Email: xu@helix.mgh.harvard.edu.
Michael A. Schwarzschild, Email: MichaelS@helix.mgh.harvard.edu.
Jadwiga Wardas, Email: wardas@if-pan.krakow.pl.
Micaela Morelli, Email: morelli@unica.it.
References
- Aguirre JA, Kehr J, Yoshitake T, Liu FL, Rivera A, Fernandez-Espinola S, Andbjer B, Leo G, Medhurst AD, Agnati LF, Fuxe K. Protection but maintained dysfunction of nigral dopaminergic nerve cell bodies and striatal dopaminergic terminals in MPTP-lesioned mice after acute treatment with the mGluR5 antagonist MPEP. Brain Res. 2005;1033:216–20. doi: 10.1016/j.brainres.2004.11.040. [DOI] [PubMed] [Google Scholar]
- Alfinito PD, Wang SP, Manzino L, Rijhsinghani S, Zeevalk GD, Sonsalla PK. Adenosinergic protection of dopaminergic and GABAergic neurons against mitochondrial inhibition through receptors located in the substantia nigra and striatum, respectively. J Neurosci. 2003;23:10982–87. doi: 10.1523/JNEUROSCI.23-34-10982.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcia C, Sánchez Bahillo A, Fernández-Villalba E, Bautista V, Poza Y, Poza M, Fernández-Barreiro A, Hirsch EC, Herrero MT. Glia. Vol. 46. 2004. Evidence of active microglia in substantia nigra pars compacta of parkinsonian monkeys 1 year after MPTP exposure; pp. 402–09. [DOI] [PubMed] [Google Scholar]
- Bastia E, Xu YH, Scibelli AC, Day YJ, Linden J, Chen JF, Schwarzschild MA. A crucial role for forebrain adenosine A(2A) receptors in amphetamine sensitization. Neuropsychopharmacology. 2005;30:891–900. doi: 10.1038/sj.npp.1300630. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Busceti CL, Molinaro G, Biagioni F, Storto M, Fornai F, Nicoletti F, Bruno V. Endogenous activation of mGlu5 metabotropic glutamate receptors contributes to the development of nigro-striatal damage induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. J Neurosci. 2004;24:828–35. doi: 10.1523/JNEUROSCI.3831-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum D, Galas MC, Pintor A, Brouillet E, Ledent C, Muller CE, Bantubungi K, Galluzzo M, Gall D, Cuvelier L, Rolland AS, Popoli P, Schiffmann SN. A dual role of adenosine A2A receptors in 3-nitropropionic acid-induced striatal lesions: implications for the neuroprotective potential of A2A antagonists. J Neurosci. 2003;23:5361–69. doi: 10.1523/JNEUROSCI.23-12-05361.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta AR, Pinna A, Tronci E, Morelli M. Adenosine A2A and dopamine receptor interactions in basal ganglia of dopamine denervated rats. Neurology. 2003;61:S39–43. doi: 10.1212/01.wnl.0000095210.55600.9c. [DOI] [PubMed] [Google Scholar]
- Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19:9192–200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Xu K, Petzer JP, Staal R, Xu YH, Beilstein M, Sonsalla PK, Castagnoli K, Castagnoli N, Jr, Schwarzschild MA. Neuroprotection by caffeine and A(2A) adenosine receptor inactivation in a model of Parkinson’s disease. J Neurosci. 2001;21:RC143, 1–6. doi: 10.1523/JNEUROSCI.21-10-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int. 2001;38:107–25. doi: 10.1016/s0197-0186(00)00034-6. [DOI] [PubMed] [Google Scholar]
- Dall’Igna OP, Porciúncula LO, Souza DO, Cunha RA, Lara DR. Neuroprotection by caffeine and adenosine A2A receptor blockade of beta-amyloid neurotoxicity. Br J Pharmacol. 2003;138:1207–09. doi: 10.1038/sj.bjp.0705185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebich BL, Biber K, Lieb K, van Calker D, Berger M, Bauer J, Gebicke-Haerter PJ. Cyclooxygenase-2 expression in rat microglia is induced by adenosine A2a-receptors. Glia. 1996;18:152–60. doi: 10.1002/(SICI)1098-1136(199610)18:2<152::AID-GLIA7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Fink JS, Kalda A, Ryu H, Stack EC, Schwarzschild MA, Chen JF, Ferrante RJ. Genetic and pharmacological inactivation of the adenosine A2A receptor attenuates 3-nitropropionic acid-induced striatal damage. J Neurochem. 2004;88:538–44. doi: 10.1046/j.1471-4159.2003.02145.x. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Cunha RA, Svenningsson P. Pharmacology of adenosine A2A receptors and therapeutic applications. Curr Top Med Chem. 2003;3:413–26. doi: 10.2174/1568026033392200. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Agnati LF, Jacobsen K, Hillion J, Canals M, Torvinen M, Tinner-Staines B, Staines W, Rosin D, Terasmaa A, Popoli P, Leo G, Vergoni V, Lluis C, Ciruela F, Franco R, Ferré S. Receptor heteromerization in adenosine A2A receptor signaling: relevance for striatal function and Parkinson’s disease. Neurology. 2003;61:S19–23. doi: 10.1212/01.wnl.0000095206.44418.5c. [DOI] [PubMed] [Google Scholar]
- Gianfriddo M, Melani A, Turchi D, Giovannini MG, Pedata F. Adenosine and glutamate extracellular concentrations and mitogen-activated protein kinases in the striatum of Huntington transgenic mice. Selective antagonism of adenosine A2A receptors reduces transmitter outflow. Neurobiol Dis. 2004;17:77–88. doi: 10.1016/j.nbd.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Gomes CA, Vaz SH, Ribeiro JA, Sebastião AM. Glial cell line-derived neurotrophic factor (GDNF) enhances dopamine release from striatal nerve endings in an adenosine A2A receptor-dependent manner. Brain Res. 2006;1113:129–36. doi: 10.1016/j.brainres.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Gomes CA, Simões PF, Canas PM, Quiroz C, Sebastião AM, Ferré S, Cunha RA, Ribeiro JA. GDNF control of the glutamatergic cortico-striatal pathway requires tonic activation of adenosine A receptors. J Neurochem. 2009;108:1208–19. doi: 10.1111/j.1471-4159.2009.05876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang QY, Wei C, Yu L, Coelho JE, Shen HY, Kalda A, Linden J, Chen JF. Adenosine A2A receptors in bone marrow-derived cells but not in forebrain neurons are important contributors to 3-nitropropionic acid-induced striatal damage as revealed by cell-type-selective inactivation. J Neurosci. 2006;26:11371–8. doi: 10.1523/JNEUROSCI.1907-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunot S, Hirsch EC. Neuroinflammatory processes in Parkinson’s disease. Ann Neurol. 2003;53:S49–58. doi: 10.1002/ana.10481. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Kurokawa M, Aoyama S, Kuwana Y. Neuroprotection by adenosine A2A receptor blockade in experimental models of Parkinson’s disease. J Neurochem. 2002;80:262–70. doi: 10.1046/j.0022-3042.2001.00694.x. [DOI] [PubMed] [Google Scholar]
- Irving EA, Barone FC, Reith AD, Hadingham SJ, Parsons AA. Differential activation of MAPK/ERK and p38/SAPK in neurones and glia following focal cerebral ischaemia in the rat. Brain Res Mol Brain Res. 2000;77:65–75. doi: 10.1016/s0169-328x(00)00043-7. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration. 1995;4:257–69. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Morooka T, Shimohama S, Kimura J, Hirano T, Gotoh Y, Nishida E. Activation and involvement of p38 mitogen-activated protein kinase in glutamate-induced apoptosis in rat cerebellar granule cells. J Biol Chem. 1997;272:18518–21. doi: 10.1074/jbc.272.30.18518. [DOI] [PubMed] [Google Scholar]
- Kull B, Svenningsson P, Fredholm BB. Adenosine A(2A) receptors are colocalized with and activate g(olf) in rat striatum. Mol Pharmacol. 2000;58:771–7. doi: 10.1124/mol.58.4.771. [DOI] [PubMed] [Google Scholar]
- Küst BM, Biber K, van Calker D, Gebicke-Haerter PJ. Regulation of K+ channel mRNA expression by stimulation of adenosine A2a-receptors in cultured rat microglia. Glia. 1999;25:120–30. [PubMed] [Google Scholar]
- Kwon CH, Zhu X, Zhang J, Knoop LL, Tharp R, Smeyne RJ, Eberhart CG, Burger PC, Baker SJ. Pten regulates neuronal soma size: a mouse model of Lhermitte-Duclos disease. Nat Genet. 2001;29:404–411. doi: 10.1038/ng781. [DOI] [PubMed] [Google Scholar]
- Li XX, Nomura T, Aihara H, Nishizaki T. Adenosine enhances glial glutamate efflux via A2a adenosine receptors. Life Sci. 2001;68:1343–50. doi: 10.1016/s0024-3205(00)01036-5. [DOI] [PubMed] [Google Scholar]
- Marchi M, Raiteri L, Risso F, Vallarino A, Bonfanti A, Monopoli A, Ongini E, Raiteri M. Effects of adenosine A1 and A2A receptor activation on the evoked release of glutamate from rat cerebrocortical synaptosomes. Br J Pharmacol. 2002;136:434–40. doi: 10.1038/sj.bjp.0704712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–91. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- Melani A, Pantoni L, Bordoni F, Gianfriddo M, Bianchi L, Vannucchi MG, Bertorelli R, Monopoli A, Pedata F. The selective A2A receptor antagonist SCH 58261 reduces striatal transmitter outflow, turning behavior and ischemic brain damage induced by permanent focal ischemia in the rat. Brain Res. 2003;959:243–50. doi: 10.1016/s0006-8993(02)03753-8. [DOI] [PubMed] [Google Scholar]
- Melani A, Gianfriddo M, Vannucchi MG, Cipriani S, Baraldi PG, Giovannini MG, Pedata F. The selective A2A receptor antagonist SCH 58261 protects from neurological deficit, brain damage and activation of p38 MAPK in rat focal cerebral ischemia. Brain Res. 2006;1073–1074:470–80. doi: 10.1016/j.brainres.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Nishizaki T. ATP- and adenosine-mediated signaling in the central nervous system: adenosine stimulates glutamate release from astrocytes via A2a adenosine receptors. J Pharmacol Sci. 2004;94:100–2. doi: 10.1254/jphs.94.100. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2. Academic Press; San Diego: 2001. [Google Scholar]
- Pedata F, Pugliese AM, Melani A, Gianfriddo M. A2A receptors in neuroprotection of dopaminergic neurons. Neurology. 2003;61:S49–50. doi: 10.1212/01.wnl.0000095212.19029.04. [DOI] [PubMed] [Google Scholar]
- Piao CS, Yu YM, Han PL, Lee JK. Dynamic expression of p38beta MAPK in neurons and astrocytes after transient focal ischemia. Brain Res. 2003;976:120–24. doi: 10.1016/s0006-8993(03)02579-4. [DOI] [PubMed] [Google Scholar]
- Pierri M, Vaudano E, Sager T, Englund U. KW-6002 protects from MPTP induced dopaminergic toxicity in the mouse. Neuropharmacology. 2005;48:517–24. doi: 10.1016/j.neuropharm.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Pinna A, Pontis S, Borsini F, Morelli M. Adenosine A2A receptor antagonists improve deficits in initiation of movement and sensory motor integration in the unilateral 6-hydroxydopamine rat model of Parkinson’s disease. Synapse. 2007;61:606–14. doi: 10.1002/syn.20410. [DOI] [PubMed] [Google Scholar]
- Popoli P, Pintor A, Domenici MR, Frank C, Tebano MT, Pèzzola A, Scarchilli L, Quarta D, Reggio R, Malchiodi-Albedi F, Falchi M, Massotti M. Blockade of striatal adenosine A2A receptor reduces, through a presynaptic mechanism, quinolinic acid-induced excitotoxicity: possible relevance to neuroprotective interventions in neurodegenerative diseases of the striatum. J Neurosci. 2002;22:1967–75. doi: 10.1523/JNEUROSCI.22-05-01967.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli P, Betto P, Reggio R, Ricciarello G. Adenosine A2A receptor stimulation enhances striatal extracellular glutamate levels in rats. Eur J Pharmacol. 1995;287:215–17. doi: 10.1016/0014-2999(95)00679-6. [DOI] [PubMed] [Google Scholar]
- Rebola N, Canas PM, Oliveira CR, Cunha RA. Different synaptic and subsynaptic localization of adenosine A2A receptors in the hippocampus and striatum of the rat. Neuroscience. 2005;132:893–903. doi: 10.1016/j.neuroscience.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Hettinger BD, Lee A, Linden J. Anatomy of adenosine A2A receptors in brain: morphological substrates for integration of striatal function. Neurology. 2003;61:S12–18. doi: 10.1212/01.wnl.0000095205.33940.99. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Robeva A, Woodard RL, Guyenet PG, Linden J. Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. J Comp Neurol. 1998;401:163–86. [PubMed] [Google Scholar]
- Saura J, Angulo E, Ejarque A, Casadó V, Tusell JM, Moratalla R, Chen JF, Schwarzschild MA, Lluis C, Franco R, Serratosa J. Adenosine A2A receptor stimulation potentiates nitric oxide release by activated microglia. J Neurochem. 2005;95:919–29. doi: 10.1111/j.1471-4159.2005.03395.x. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferré S. Adenosine A2A receptors and basal ganglia physiology. Prog Neurobiol. 2007;83:277–92. doi: 10.1016/j.pneurobio.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann SN, Jacobs O, Vanderhaeghen JJ. Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not by substance P neurons: an in situ hybridization histochemistry study. J Neurochem. 1991;57:1062–7. doi: 10.1111/j.1471-4159.1991.tb08257.x. [DOI] [PubMed] [Google Scholar]
- Schmidt N, Ferger B. Neurochemical findings in the MPTP model of Parkinson’s disease. J Neural Transm. 2001;108:1263–1282. doi: 10.1007/s007020100004. [DOI] [PubMed] [Google Scholar]
- Schintu N, Frau L, Ibba M, Garau A, Carboni E, Carta AR. Progressive dopaminergic degeneration in the chronic MPTPp mouse model of Parkinson’s disease. Neurotox Res. 2009;16:127–39. doi: 10.1007/s12640-009-9061-x. [DOI] [PubMed] [Google Scholar]
- Schwarzschild MA, Agnati L, Fuxe K, Chen JF, Morelli M. Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci. 2006;29:647–54. doi: 10.1016/j.tins.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Shen HY, Coelho JE, Ohtsuka N, Canas PM, Day YJ, Huang QY, Rebola N, Yu L, Boison D, Cunha RA, Linden J, Tsien JZ, Chen JF. A critical role of the adenosine A2A receptor in extrastriatal neurons in modulating psychomotor activity as revealed by opposite phenotypes of striatum and forebrain A2A receptor knock-outs. J Neurosci. 2008;28:2970–5. doi: 10.1523/JNEUROSCI.5255-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva CG, Porciúncula LO, Canas PM, Oliveira CR, Cunha RA. Blockade of adenosine A(2A) receptors prevents staurosporine-induced apoptosis of rat hippocampal neurons. Neurobiol Dis. 2007;27:182–89. doi: 10.1016/j.nbd.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–96. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Tatton NA, Kish SJ. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience. 1997;77:1037–48. doi: 10.1016/s0306-4522(96)00545-3. [DOI] [PubMed] [Google Scholar]
- Wallace BA, Ashkan K, Heise CE, Foote KD, Torres N, Mitrofanis J, Benabid AL. Survival of midbrain dopaminergic cells after lesion or deep brain stimulation of the subthalamic nucleus in MPTP-treated monkeys. Brain. 2007;130:2129–45. doi: 10.1093/brain/awm137. [DOI] [PubMed] [Google Scholar]
- Yu L, Shen HY, Coelho JE, Araújo IM, Huang QY, Day YJ, Rebola N, Canas PM, Rapp EK, Ferrara J, Taylor D, Müller CE, Linden J, Cunha RA, Chen JF. Adenosine A2A receptor antagonists exert motor and neuroprotective effects by distinct cellular mechanisms. Ann Neurol. 2008;63:338–46. doi: 10.1002/ana.21313. [DOI] [PubMed] [Google Scholar]
- Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22:1763–71. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement. Data