Human functional connectivity: New tools, unresolved questions (original) (raw)
Understanding how faulty neural circuits contribute to mental illness is hindered because there are no direct approaches to measuring connectivity in the living human brain. For this reason, there has been considerable recent effort to develop indirect measures. One approach, known as resting-state functional connectivity, measures intrinsic activity fluctuations that correlate between functionally connected regions (Fig. 1) (1). Resting-state functional connectivity has been used to characterize multiple brain systems as well as alterations associated with mental illness and neurodegenerative disease (for reviews, see refs. 2–5). In PNAS, Tomasi and Volkow (6) extend functional connectivity methods by demonstrating a computational strategy for assessing whether specific brain regions possess particularly high levels of coupling with adjacent regions—a property consistent with their role as connectivity hubs.
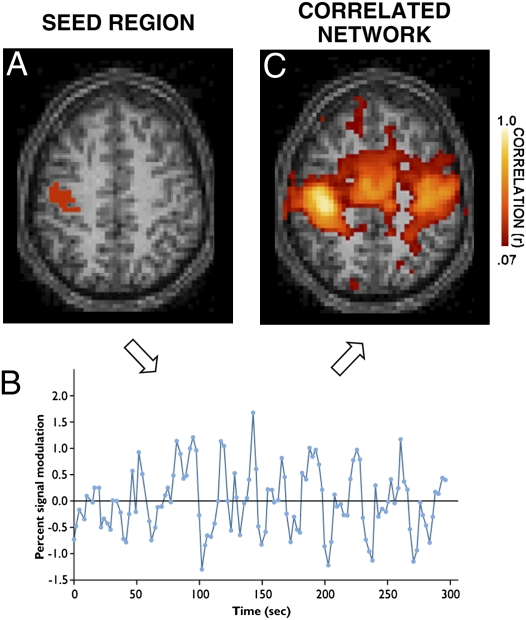
Fig. 1.
Tomasi and Volkow (6) use resting-state functional connectivity to rapidly estimate candidate “hubs” of connectivity in the human brain. The basis of functional connectivity is that spontaneous activity fluctuations measured at rest are correlated between regions. The presence and strength of correlations reflects a combination of anatomic connectivity and synaptic efficiency among other factors (Table 1). By measuring correlations between multiple regions, inferences can be made about the architecture of brain systems and whether differences exist between individuals. (A) Signal fluctuations are measured at rest from an example seed region within the motor cortex. Activity is measured indirectly through the blood oxygenation level–dependent MRI signal. (B) A representative time course of intrinsic activity fluctuations for the seed region is displayed for a period of 5 min. The general strategy of functional connectivity is to determine the network of brain regions that show correlated activity fluctuations over time with the seed region (1). (C) In this instance, the correlated network of regions reveals multiple cortical areas within the motor system, as well as regions within the cerebellum (not shown).
Their method, dubbed “functional connectivity density mapping” (FCDM), provides an extremely efficient approach for characterizing properties of connectivity in large numbers of subjects. Analysis of data from many thousands of subjects will be essential for certain genetic approaches and beneficial for studies of development and population variation. Furthermore, findings that functional connectivity estimates are stable within individuals for brief epochs of data collection (5), can be pooled across laboratories (7), and are heritable within the context of family studies (8), bolsters the potential of FCDM.
However, a better understanding of the possible signal sources that give rise to correlations between regions is required for the potential of functional connectivity methods to be fully realized. The frank reality is that, although functional connectivity provides a powerful strategy to measure brain organization in humans, it is a frustratingly indirect measure that reflects anatomic connectivity and synaptic efficiency, as well as several confounding factors (Table 1).
Table 1.
Possible sources that contribute to resting-state functional correlations
| Persistent sources |
|---|
| Monosynaptic and polysynaptic anatomic connectivity |
| Anatomic connectivity to a common source |
| Differences in synaptic efficiency across anatomically connected systems |
| Transient sources |
| Differences in synaptic efficiency reflecting recent experience |
| Confounding sources contributing to between-subject/between-group differences |
| Gross anatomic variability and atrophy |
| Residual variation in the location of brain areas in relation to gross anatomy |
| Motion, cardiac, and respiratory noise |
| Task engaged during measurement including compliance and strategy differences |
What Do Resting-State Functional Correlations Measure?
Anatomic connectivity is a major constraint on the observed patterns of resting-state functional correlations. Many well-characterized brain systems reveal strong correlations between regions as predicted by traditional anatomic approaches, including cerebrocerebellar circuits that are exclusively polysynaptic (2–5, 9). Studies contrasting functional correlations with measures of anatomic connectivity via diffusion-based methods suggest that the two measures are correlated (e.g., ref. 10), and severing commissural projections reduces functional correlation between homologous regions in the left and right hemispheres (11). However, other factors also affect functional correlations between regions beyond patterns of anatomic connectivity.
In a recent and striking observation, Fair et al. (12) found that certain long-distance functional correlation patterns develop in children after 8 y of age. Young children tend to show functional connectivity between anatomically close regions, whereas young adults show greater connectivity between spatially distant regions that constitute functional groups. Because axonal projections are largely formed by the first year of life, a combination of synaptic pruning, strengthening, and myelination is the likely explanation for differences emerging late in development (12).
The task engaged by the participant during measurement can also have a large effect, including on the local correlations targeted by the method of Tomasi and Volkow (13). Furthermore, learning can transiently modify the strength of intrinsic activity correlations measured during subsequent rest periods (14). These phenomena may reflect large-scale interactions that change dynamically to support task demands and processes associated with memory consolidation.
A paradoxical possibility is that functional connectivity approaches may tell us something particularly relevant for understanding individual differences in brain function precisely because they reflect the combined influence of anatomic connectivity patterns and synaptic modifications arising from a person's prior experiences. Such a speculation needs further study in animal models that can directly measure anatomy and synaptic efficiency. Nonetheless, it is possible that measurement of intrinsic functional correlations may provide a view of neural circuit properties that is missed when the focus is exclusively on patterns of anatomic connectivity.
Estimating Properties of Brain Architecture from Connectivity Patterns
The complex matrices of correlations between regions also provide insight into global properties of connectivity, such as whether connection patterns maximize information flow (15). Among other contributions (e.g., ref. 12), this work has identified regions of the brain referred to as “hubs” (16, 17). Hubs are nexuses of connectivity hypothesized to allow efficient integration across widely distributed brain areas (15, 18). Classic studies of anatomy using tracer techniques in monkeys predicted the existence of hubs (for review, see ref. 19), but what is new is the possibility of measuring hubs in humans in ways that can estimate whether typical or aberrant patterns of connectivity are present in individuals.
Tomasi and Volkow propose FCDM as a method to rapidly estimate connectivity properties indicative of hubs. Their method is 1,000 times faster than traditional approaches. Such speed makes analysis of large datasets feasible and may allow individual patients to be assessed in real-time clinical settings. However, it is unclear whether FCDM estimates are fully comparable to prior estimates of cortical hubs. FCDM gains its efficiency by analyzing only local correlations. Graph theory metrics have traditionally defined hubs as nodes that allow efficient information flow through large numbers of other nodes within the graph. To possess this property requires that a node have the right combination of connections able to serve as bridges to widely distributed nodes throughout the graph (18). It is possible that brain areas with numerous local connections are also those that link the largest number of other areas, but this association is not obligated.
Several recent observations reveal potentially important differences between local and distant functional connectivity. Primary sensory and motor areas show preferentially local functional connectivity patterns when contrasted with association areas that are characterized by preferentially distributed interactions (13). FCDM may not capture these differences. Similarly, studies of development suggest a local-to-distributed progression of functional connectivity (12), such that regions falling within local connectivity networks in children emerge as having long-distance functional correlations in adults. Again, FCDM may miss this particularly valuable information for understanding atypical developmental trajectories.
Future studies are needed to understand when FCDM and other computationally efficient approaches can provide sufficient estimates of functional connectivity and under what contexts they can best be used.
Milestone for Human Brain Imaging
A final point to make is that Tomasi and Volkow's results demonstrate a milestone for the field of human neuroimaging that surrounds data sharing. Hundreds of laboratories presently conduct functional neuroimaging studies on both normal control subjects and clinical patients. Unlike other fields, the neuroimaging community has been slow to develop useful approaches to data sharing. In fact, despite the strong pressure to increase sample sizes to explore individual differences and links to genetic mechanisms, samples of more than 100 subjects are exceedingly rare in functional neuroimaging studies.
Tomasi and Volkow identified candidate cortical hubs in a sample of 979 subjects—among the largest studies to date. The study was based on an openly available repository of more than 1,200 subjects that combined data from 35 international sites (7). Thus, in addition to developing a unique method, their results demonstrate by example the viability of mass data aggregation for human functional brain imaging. This proof of concept that functional neuroimaging studies can scale up bodes well for upcoming phases of research that will require large, open collaborations (e.g., ref. 20).
Footnotes
The author declares no conflict of interest.
See companion article on page 9885 in issue 21 of volume 107.
References
- 1.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 2.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 3.Greicius MD. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 4.Bassett DS, Bullmore ET. Human brain networks in health and disease. Curr Opin Neurol. 2009;22:340–347. doi: 10.1097/WCO.0b013e32832d93dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Dijk KR, et al. Intrinsic functional connectivity as a tool for human connectomics: Theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomasi D, Volkow ND. Functional connectivity density mapping. Proc Natl Acad Sci USA. 2010;107:9885–9890. doi: 10.1073/pnas.1001414107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biswal BB, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glahn DC, et al. Genetic control over the resting brain. Proc Natl Acad Sci USA. 2010;107:1223–1228. doi: 10.1073/pnas.0909969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Margulies DS, et al. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci USA. 2009;106:20069–20074. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honey CJ, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci USA. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnston JM, et al. Loss of resting interhemispheric functional connectivity after complete section of the corpus callosum. J Neurosci. 2008;28:6453–6458. doi: 10.1523/JNEUROSCI.0573-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fair DA, et al. Functional brain networks develop from a “local to distributed” organization. PLOS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sepulcre J, et al. The organization of local and distant functional connectivity in the human brain. PLoS Comput Biol. 2010 doi: 10.1371/journal.pcbi.1000808. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albert NB, Robertson EM, Miall RC. The resting human brain and motor learning. Curr Biol. 2009;19:1023–1027. doi: 10.1016/j.cub.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bullmore E, Sporns O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 16.Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci. 2006;26:63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckner RL, et al. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.10.003. 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- 20.Akil H, et al. The future of psychiatric research: Genomes and neural circuits. Science. 2010;327:1580–1581. doi: 10.1126/science.1188654. [DOI] [PMC free article] [PubMed] [Google Scholar]