Thymopoiesis in mice depends on a Foxn1-positive thymic epithelial cell lineage (original) (raw)
Abstract
The thymus is essential for T-cell development. Here, we focus on the role of the transcription factor Foxn1 in the development and function of thymic epithelial cells (TECs) of the mouse. TECs are of endodermal origin; they initially express Foxn1 and give rise to orthotopic (thoracic) and additional (cervical) thymi. Using _Foxn1_-directed cytoablation, we show that during embryogenesis, cervical thymi develop a few days after the thoracic lobes, and that bipotent epithelial progenitors of cortical and medullary compartments express Foxn1. We also show that following acute selective near-total ablation during embryogenesis, complete regeneration of TECs does not occur, providing an animal model for human thymic aplasia syndromes. Finally, we address the functional role of _Foxn1_-negative TECs that arise postnatally in the mouse. Lineage tracing shows that such _Foxn1_-negative TECs are descendants of _Foxn1_-positive progenitors; furthermore, _Foxn1_-directed subacute intoxication of TECs by polyglutamine-containing EGFP proteins indicates that a presumptive _Foxn1_-independent lineage does not contribute to thymopoietic function of the adult thymus. Our findings therefore support the notion that Foxn1 is the essential transcription factor regulating the differentiation of TECs and that its expression marks the major functional lineage of TECs in embryonic and adult thymic tissue.
Keywords: epithelium, progenitor cell, thymus, mouse
The thymus is a primary lymphoid organ required for normal T-cell development. Though much is known about the genetic underpinnings of differentiation and selection of the different T-cell lineages, important aspects of the biology of the thymic microenvironment are still unclear. Here, we focus on the role of the transcription factor Foxn1 in the development and function of the thymic epithelium. The experiments described in this report address the developmental origin of thymic epithelia, the biology of epithelial progenitor cells, and the functional role of _Foxn1_-negative epithelial cells.
The thymic anlage is of endodermal origin and emanates from the epithelium of the pharyngeal pouch, adjacent to the developing primordium of the parathyroid (1). The two organ anlagen are distinguished by the mutually exclusive expression of two transcription factor genes, Foxn1 and Gcm2 (2). The thymic anlage expresses Foxn1 (3), which is required for the differentiation of thymic epithelial cells and whose deficiency leads to failure of thymopoiesis (4). The parathyroid anlage is distinguished by the expression of Gcm2, whose activity is essential for the further development of this endocrine organ (5). Hence, Foxn1 and Gcm2 are considered to determine organ identities in the common anlage. During the course of further development, the common anlage of the thymus and parathyroid splits, and the two anlagen migrate to their final positions; whereas the parathyroid associates with the developing thyroid gland, the thymus moves in a ventrocaudal direction to a location above the heart. Recently, it has been found that a second thymus emerges in the neck of mouse embryos (6, 7), although the frequency with which cervical thymi arise varies among different mouse strains. In contrast to the invariant location of the orthotopic (or thoracic) thymus, the location of cervical thymi is variable; when two cervical thymi are found in a mouse, their positions are rarely symmetrical. Intriguingly, epithelial cells of both the orthotopic and the cervical thymi express Foxn1 (7), suggesting their close developmental and functional relationship. The emergence of the cervical thymus appears to be delayed as compared with that of the thoracic thymus, and it has been hypothesized that this is due to a second specification event (8) in a remnant of the unspecified common parathyroid/thymic anlage. Here, we test this notion by conditionally ablating _Foxn1_-expressing cells in early embryogenesis to determine whether the development of cervical thymi is affected by this early intervention.
The thymic epithelium comprises two main types, which, according to their position in the organ, are referred to as cortical and medullary epithelium (1). Although functionally distinct, they both arise by differentiation of a common epithelial progenitor (9, 10). It is known that the differentiation of thymic epithelial cells (TECs) depends on Foxn1 function, but it is not clear whether the epithelial progenitor itself expresses Foxn1. The _Foxn1_-directed cell-ablation strategy described herein lends itself to a direct test of this possibility.
Previous work using tetraparental chimeras derived from aggregation of wild-type and _Foxn1_−/− embryos has shown that the thymic anlage comprises a fixed number of epithelial progenitor cells (11). How many TEC progenitors are specified during embryogenesis probably depends on the activity of the Shh signaling pathway (12) and the function of Pax3 (13), as they control the size of the _Foxn1_-expressing domain in the third pharyngeal pouch epithelium. It is assumed that the number of epithelial progenitor cells is proportional to the number of functionally mature epithelial cells (11) and that the latter is proportional to the number of thymocytes developing in the organ (14). However, it is unclear whether the progenitor pool can be replenished when acute losses occur after the initial specification event. Here, we have used subtotal cell ablation to examine this question.
Recent findings have raised the possibility that once a functional thymic microenvironment is established, Foxn1 might no longer be required to sustain thymopoiesis in the postnatal period (15), although the observation that partial deletion of Foxn1 in the postnatal period causes a reduction in thymocyte numbers (16) argues against this possibility. To resolve this issue, we generated a model of subacute intoxication of TECs based on the _Foxn1_-directed expression of polyglutamine-containing EGFP.
Results
Two Temporally Distinct Specification Events of TECs.
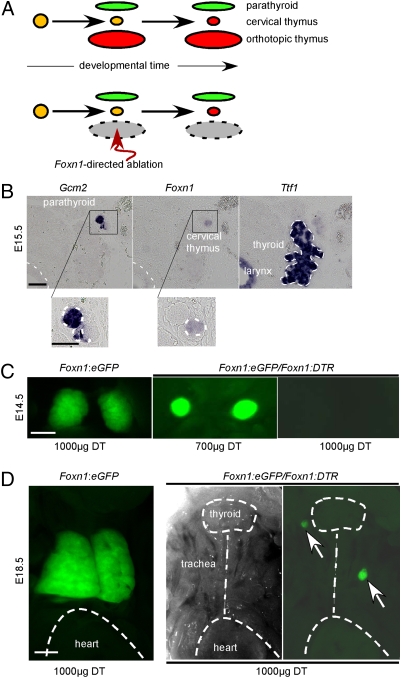
The expression of Foxn1 is considered to specify thymic identity of pharyngeal epithelial cells. Foxn1 expression can first be detected in the prospective thymus domain of the common thymus/parathyroid anlage at approximately embryonic day 10.5 (E10.5) (2, 3). It has been suggested that cervical thymi develop later than the orthotopic thymus (7), possibly as a result of a second specification event (8) (Fig. 1_A_). Indeed, rudiments of cervical thymi are first observed at E15.5 as small patches of _Foxn1_-expressing epithelial cells, most often located next to the developing parathyroid (Fig. 1_B_). At this time, the orthotopic thymus has already separated from the common parathyroid/thymic primordium and descended to a location above the heart. This observation suggests that the onset of Foxn1 expression in cervical thymi occurs ≈5 d later than in the thoracic thymus. However, it is possible that the rudiments of the cervical thymus are initially so small that they escape detection before E15.5. To examine this possibility, we attempted to ablate _Foxn1_-expressing cells during embryogenesis before the presumed second specification event. To this end, we created transgenic mice expressing the human diphtheria toxin receptor (DTR) gene (17) under the control of the Foxn1 promoter. The Foxn1 promoter faithfully directs expression of transgenes into TECs (8, 18) of both cervical and orthotopic thymi (7). Female mice transgenic for the Foxn1:eEGFP reporter (7) were mated with males transgenic for the Foxn1:DTR construct; in this mating scheme, the female is spared the cytotoxic effects of diphtheria toxin (DT), eliminating possible systemic effects on the developing offspring. DT was injected three times, at E10.5, E11.5, and E12.5 (i.e., beginning at the time of onset of Foxn1 expression in the thoracic thymus, but well before the first detectable signs of a developing cervical thymus). Foxn1:eGFP single-transgenic embryos and Foxn1:eGFP/Foxn1:DTR double-transgenic embryos were analyzed at various time points thereafter. As expected, the degree of TEC ablation was dose dependent (Fig. 1_C_); with higher doses, complete destruction of the thoracic thymic anlage (as determined by GFP fluorescence) was achieved. Siblings lacking the Foxn1:DTR transgene had a normal thymus, indicating that the observed phenotype was the result of a transgene-specific effect. The orthotopic thymus did not recover in treated double-transgenic embryos, suggesting that it was completely destroyed and that TECs of the embryonic thoracic thymus (including the presumptive progenitor cells) express Foxn1. However, when embryos treated between E10.5 and E12.5 were examined shortly before birth, at E18.5, many exhibited two (rarely only one) small thymic masses in the neck region (Fig. 1_D_), reminiscent of cervical thymi in both size and location (7). Given the effectiveness of _Foxn1_-directed cytoablation, this experiment therefore directly shows that the onset of Foxn1 expression (including the Foxn1 promoter-driven transgenes) in the anlagen of the cervical thymi indeed occurs later than in the orthotopic thymus.
Fig. 1.
Varying times of onset of Foxn1 expression in the epithelial cells of thoracic and cervical thymi. (A) Hypothetical scheme of the development of organ anlagen from the epithelium of the third pharyngeal pouch in mouse embryos. A common anlage (yellow) differentiates into separate parathyroid (green) and thymic (red) domains, but a small undifferentiated primordium remains; at a later stage, this gives rise to the anlage of the cervical thymus (Upper). _Foxn1_-directed cytoablation during early stages of embryogenesis destroys the primordium of the thoracic thymus but does not affect the subsequent development of the cervical thymus (Lower). (B) RNA in situ hybridization analysis of adjacent transverse sections of an E15.5 mouse embryo. The cervical thymus with _Foxn1_-positive epithelium is located directly adjacent to the parathyroid (see enlargements underneath). Hybridization with Ttf1, which is expressed in the primordium of the thyroid and the epithelium of the larynx, is shown for orientation (it is expressed at low levels in parathyroid and cervical thymus). Note that at this stage of development, the thoracic thymus has already descended to the mediastinum. This section is representative of a group of four embryos. (Scale bars: Upper, 100 μm; Lower, 50 μm.) (C) Macroscopic view of the mediastinal region of Foxn1:eGFP single- and Foxn1:eGFP, Foxn1:DTR double-transgenic embryos treated with the indicated doses of diphtheria toxin (DT). Pregnant female mice were injected with DT three times at E10.5, E11.5, and E12.5; photographs were taken under UV illumination at E14.5. The pictures shown are representative of groups of two, three, and five embryos dissected for the three treatment/genotype combinations, respectively. (Scale bar: 100 μm.) (D) Macroscopic view of thoracic and neck regions of Foxn1:eGFP single- and Foxn1:eGFP, Foxn1:DTR double-transgenic embryos treated with DT as in C. Photographs were taken under UV (Left and Right) or bright-light (Center) illumination. Anatomical landmarks are indicated, and the two cervical thymi are marked with arrows. The embryos shown are representative of groups of >100 (Left) and 20 (Center and Right). (Scale bar: 500 μm.)
Thymic Epithelial Progenitor Cells Express Foxn1.
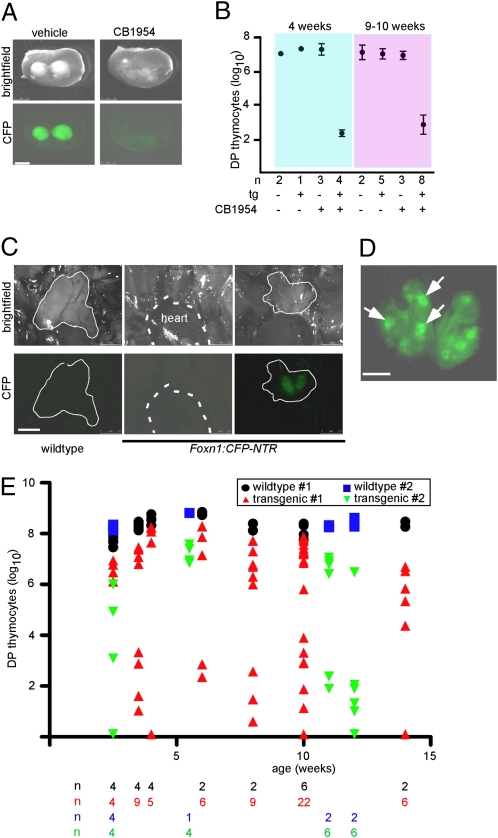
Our previous findings (9) and the above experiment indicated that the bipotent thymic epithelial progenitor expresses Foxn1. We verified this conclusion using cytoablation based on a different cytotoxic principle. To this end, we created mice transgenic for a fusion gene between the fluorescent protein CFP and the bacterial nitroreductase (NTR) (19), obviating the need to combine two transgenes in the same animal. Nitroreductase is capable of converting prodrugs (such as CB1954) into effective cytotoxic agents, leading to cell death. In the transgenic mouse strains developed here, treatment with either DT or CB1954 cannot be extended beyond the E15.5 time point, owing to the occurrence of severe defects in the skin of newborn mice, because Foxn1 is also expressed in this tissue (20). To be able to examine whether TEC progenitors in the postnatal thymus still express Foxn1, intact orthotopic (thoracic) thymi from transgenic and nontransgenic E15.5 embryos were transplanted under the kidney capsule of nude mice (lacking the Foxn1:CFP-NTR transgene). One week after transplantation, corresponding to postnatal day 4, mice were treated with CB1954. The transplants were analyzed either 4 or 9–10 wk after the last treatment, providing enough time for any remaining progenitors to give rise to a thymopoietic epithelial microenvironment. Thymic tissue of transgenic transplants was completely resorbed in CB1954-treated mice as indicated by the lack of fluorescent cells (Fig. 2_A_); correspondingly, few, if any, CD4/CD8 double-positive thymocytes (DPs) were recovered from the entire kidney tissue (Fig. 2_B_). Hence, it appears that the epithelial progenitor cells did not survive transplantation and subsequent treatment with CB1954, compatible with their expressing Foxn1. This finding suggests that, at least in embryonic and early postnatal periods, epithelial progenitors are Foxn1 positive; otherwise, thymopoiesis should have recovered after cytoablative treatment of _Foxn1_-expressing cells.
Fig. 2.
Thymic epithelial progenitors express Foxn1. (A) Thymic lobes from E15.5 Foxn1:CFP-NTR transgenic mice were transplanted under the kidney capsule of nude mice. After 1 wk, animals were treated with vehicle or vehicle plus CB1954 at 500 μg four times per week for 3 wk. After a recovery period, kidneys were removed and photographed under bright-light and UV illumination. (Scale bar: 2.5 mm.) (B) Number of CD4/CD8 double-positive T cells isolated from thymus transplants (such as those shown in A) 4 (Left) or 9–10 (Right) weeks after the last treatment. The number of thymic lobes (n) analyzed, the presence (+) or absence (−) of the Foxn1:CFP-NTR transgene (tg), and treatment with vehicle (−) or CB1954 (+) for the respective groups are noted. (C) Macroscopic views of thoracic regions of sibling wild-type or Foxn1:CFP-NTR transgenic mice at 2 mo of age photographed under bright-light (Upper) and UV illumination (Lower). During the embryonic period, pregnant wild-type mothers received i.p. injections of CB1954 at 500 μg per mouse at E12.5, E13.5, and E14.5. Thymic tissue is outlined with solid lines; the heart is indicated with broken lines. Note that no thymic tissue could be detected for the animal shown in the center panel. (Scale bar: 2.5 mm.) (D) Macroscopic appearance of a small thoracic thymus (about 1/8 the size of that in wild-type sibling) observed at the age of 2 wk in a Foxn1:CFP-NTR animal treated as in C. In the photograph taken under UV illumination, note several clusters of strongly fluorescent epithelial cells, corresponding to individual medullary islets. (Scale bar: 500 μm.) (E) Number of CD4/CD8 double-positive thymocytes recovered from adolescent and adult mice exposed in utero to CB1954 as in C. The number of animals in each group is shown underneath the graph. Animals from group 1 were exposed to CB1954 in utero at E12.5, E13.5, and E14.5; animals from group 2 at E13.5, E.14.5, and E15.5.
The conclusion that embryonic thymic epithelial progenitor cells express Foxn1 was confirmed in an additional set of experiments. In this scheme, initial in utero treatment with CB1954 was done either between E12.5 and E14.5 or between E13.5 and E15.5, to achieve a selective near-total reduction of TECs, as suggested by prior dose-finding experiments. We then monitored thymopoietic activity of wild-type and Foxn1:CFP-NTR transgenic littermates at various time points after birth. We expected complete recovery of thymopoietic activity only in cases where TECs in the orthotopic thymus recovered to approximately normal numbers (note that cervical thymi are at least two orders of magnitude smaller than orthotopic thymi) (6, 7). As in the previous experiment, nontransgenic siblings were not affected by the treatment during the embryonic period; the size of the thymus and the number of thymocytes was indistinguishable from age-matched untreated control animals. By contrast, in all transgenic animals, the thymus was either smaller or entirely absent. In animals in which small thymic lobes were found (Fig. 2_C_), the characteristic pattern of densely packed medullae and less-populated cortical regions could be readily discerned by their distinctly different fluorescence (Fig. 2_D_). In some cases, the location of small masses of thymic tissue was again reminiscent of cervical thymi (as seen in the experiments with Foxn1:DTR transgene); in others, they clearly corresponded to small orthotopic thymi in the mediastinum. Correspondingly, when analyzed at various time points after birth, the number of DP thymocytes recovered per animal from all thymic tissues was highly variable, but always significantly reduced or nil in transgenic animals compared with wild-type controls (Fig. 2_E_). The low number of athymic mice in our cohort was expected, because the frequency of cervical thymi is relatively high in the FVB strain used here for the generation of transgenic mice. When residual thymic tissue was present, the relative distributions of thymocyte subsets were essentially normal, suggesting that T-cell development was quantitatively impaired but qualitatively normal. Importantly, at each time point, mice were identified that showed no evidence of ongoing thymopoiesis. Mice with low numbers of DPs also had fewer T cells in the periphery; these cells had the phenotype of activated peripheral T cells (CD44high, CD62Llow, CD25high), compatible with homeostatic proliferation in a lymphopenic host. Collectively, these experiments suggest that prenatal or early postnatal loss of TECs cannot be compensated for at later time points.
Foxn1 Expression in TECs During Ontogeny.
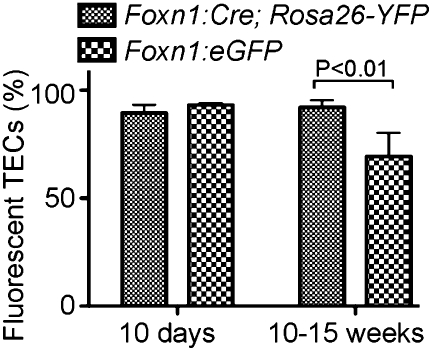
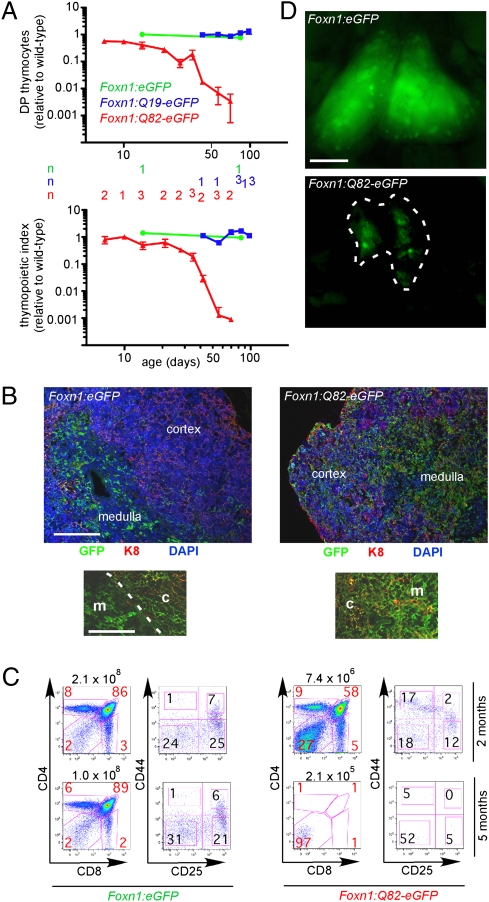
In contrast with embryonic and early postnatal thymi, the adult orthotopic thymus contains _Foxn1_-negative TECs, and it has been suggested that these cells correspond to a _Foxn1_-independent, but functionally competent, cell lineage (15). We addressed this possibility in two ways. First, we used an analytical approach to establish the developmental relationship between _Foxn1_-positive and _Foxn1_-negative TECs. To this end, we confirmed that _Foxn1_-negative TECs are readily detected in Foxn1:eGFP transgenic mice several weeks after birth (Fig. S1_A_). To determine whether these cells had previously expressed Foxn1, we marked them for their history of Foxn1 expression using a Foxn1:Cre transgene (21) on the Rosa26-YFP background, in which YFP is expressed only after Cre-mediated excision of a stop cassette (22). In these thymi, essentially all TECs express the fluorescent marker (Fig. S1_B_). Though this type of analysis does not rule out the presence of a small subset of _Foxn1_-negative TECs without a history of Foxn1 expression, it suggests that the majority of _Foxn1_-negative TECs arises from _Foxn1_-positive TECs (Fig. 3). We confirmed that the activity of the Foxn1:eGFP reporter transgene correlates with endogenous Foxn1 gene expression in TECs (Fig. S2). In a second experiment, our aim was to address the functional capacity of _Foxn1_-negative TECs. Initially, we attempted to ablate _Foxn1_-expressing cells in adult transgenic mice to create a thymic microenvironment solely composed of _Foxn1_-negative TECs. Unfortunately, as was the case for treatment in late stages of gestation, DT and CB1954 were toxic (most likely owing to the effects on the skin) and caused high mortality rates. Therefore, this strategy was abandoned and instead a model of subacute intoxication of TECs was developed. Polyglutamine stretches in various proteins are associated with neurodegenerative disorders (23), and it has been shown that such cellular pathology can also be achieved in other cell types (24). Proteins carrying only a short stretch of glutamines are considered nonpathogenic, whereas stretches longer than 50 residues are regarded as toxic (23). We reasoned that we could exploit this property to examine whether the postnatal TEC compartment contains a functionally relevant _Foxn1_-negative lineage, which would be unaffected by the polyglutamine-associated toxicity. To this end, we developed two additional transgenic strains of mice expressing variants of EGFP modified by the N-terminal addition of either 19 or 82 glutamine residues (24), under the control of the Foxn1 promoter. In specific pathogen-free conditions, mice expressing these transgenes do not have obvious skin defects, are fertile, and have a normal life span. As expected, thymopoiesis proceeded unimpaired in Foxn1:eGFP and Foxn1:Q19-eGFP mice (Fig. 4_A_). By contrast, thymopoiesis was severely compromised in the Foxn1:Q82-eGFP strain, as judged by the reduced numbers of DPs (Fig. 4_A_ Upper) and the decline of the thymopoietic index (ratio of DP thymocytes to TECs; Fig. 4_A_ Lower). These findings suggest that toxicity in TECs is subacute/chronic rather than acute, and that the phenotype is due to functional deterioration of TECs rather than to their acute loss. Hence, in Foxn1:Q82-eGFP transgenic mice, the thymic microenvironment is formed normally but deteriorates functionally after birth. Interestingly, CD4 and CD8 single-positive T-cell subsets were equally affected (as evidenced by the unchanged CD4/CD8 ratio), although we had originally expected that CD8 cells would be more affected owing to the presumed impairment of the proteasome-dependent protein degradation pathway and the resulting reduction of MHC class I ligands. Hence, the cytopathic effect of polyglutamine expression in TECs appears to affect general aspects of TEC physiology.
Fig. 3.
_Foxn1_-negative cells in the adult thymus are descendants of _Foxn1_-positive cells. Flow cytometric determination of the percentage of fluorescent TECs (Epcam+CD45−) for the indicated genotypes at two different time points. For representative flow cytometric profiles, see Fig. S1.
Fig. 4.
Expression of polyglutamine-containing proteins causes thymic malfunction. (A) Thymopoietic activity in age- and sex-matched mice transgenic for the indicated constructs, expressed relative to control. Values for CD4/CD8 double-positive thymocytes (Upper) and thymopoietic index [number of CD4/CD8 double-positive thymocytes divided by number of thymic epithelial cells (Lower)] are shown. The number of mice per data point (mean ± SD were applicable) is indicated and applies to both panels. (B) Abnormal architecture of thymic microenvironment in Foxn1:Q82-eGFP transgenic mice at 2 mo of age. Note the impaired separation of cortex (marked by K8 staining) and medulla (distinguished by strong GFP staining) (Upper). The altered shapes of medullary and cortical TECs are particularly evident in higher-power views of corticomedullary junctions (Lower). The sections are derived from the 2-mo-old animals also shown in C. The tissue architecture of Foxn1:Q19-eGFP transgenic mice is normal and comparable to Foxn1:eGFP mice. (Scale bars: Upper, 200 μm; Lower, 100 μm.) m, medulla; c, cortex. (C) Distribution of thymocyte subsets in sex-matched mice transgenic for the indicated constructs, analyzed at 2 and 5 mo of age. The numbers of total thymocytes are given above the panels; the relative proportions of thymocyte subsets are indicated in percentages (red numbers). For this experiment, flow cytometry profiles are representative of four animals in each group. Results from wild-type, Foxn1:eGFP, and Foxn1:Q19-eGFP transgenic mice are similar. (D) Macroscopic appearance of thymic tissue in aged mice. Note the size of the thymus and the widespread fluorescence of thymic epithelial cells in Foxn1:eGFP mice (50 wk of age) and the involuted thymus (outlined by dashed line) of a Foxn1:Q82-eGFP mouse that contains only few fluorescent cell clusters (42 wk of age). (Scale bar: 100 μm.)
The functional impairment correlates with structural abnormalities. Foxn1:eGFP mice exhibit a normal architecture with well-separated cortex and medulla, strong GFP staining in the medulla, and reticular GFP/K8 double-positive network in the cortex. In Foxn1:Q82-eGFP mice, the demarcation of cortex and medulla is less clear, and the reticular network in the cortex is much less elaborate (Fig. 4_B_). By 5 mo of age, the majority of Foxn1:Q82-eGFP mice lack appreciable thymopoietic activity, as evidenced by the number of CD4/CD8 double-positive thymocytes; the relative increase of DN thymocytes in Foxn1:Q82-eGFP mice suggests that T-cell development is affected at an early stage (Fig. 4_C_). At this age, _Foxn1_-negative cells comprise a substantial fraction of TECs in Foxn1:Q82-eGFP mice; for instance, at 5 mo of age, 25.1 ± 9.8% (n = 5) do not express the transgene. Lack of Foxn1:Q82-eGFP transgene expression correlates with diminished endogenous Foxn1 activity (Fig. S2). Nonetheless, the thymopoietic index is disproportionally (∼1,000×) lower than in wild-type or Foxn1:Q19-eGFP and Foxn1:eGFP mice. This strongly indicates that _Foxn1_-negative cells do not have any appreciable thymopoietic function. The most likely explanation for this finding is that, even after the cessation of transgene expression, the functional capacity of TECs does not recover. However, we observed that the loss of thymopoietic activity does not affect the entire cohort of Foxn1:Q82-eGFP mice, as some mice sustained minimal thymopoiesis up to the age of 2 y. These thymi contained _Foxn1_-positive TECs, albeit in much lower numbers than those of Foxn1:eGFP and Foxn1:Q19-eGFP strains (Fig. 4_D_). In contrast with peripheral T cells in Foxn1:eGFP and Foxn1:Q19-eGFP mice, Foxn1:Q82-eGFP mice had fewer peripheral T cells, exhibiting the phenotype of activated T cells, as expected for a lymphopenic environment.
Discussion
Our findings provide unique insight into several aspects of TEC development and function. We show that Foxn1 expression in cervical thymi begins after that in the thoracic thymus. Whether the induction of Foxn1 expression in these two regions is a result of similar or different mechanisms remains to be investigated. Cervical thymi typically arise in close proximity to the developing parathyroid, lending credibility to the idea that the common anlage of parathyroid and thymus retains small clusters of undifferentiated cells, perhaps at the boundary between the Gcm2 and Foxn1 expression domains that demarcate the two organ anlagen. Whether additional specification events also occur for the parathyroid is unknown; however, the high incidence of supernumerary parathyroid glands in humans suggests that this might indeed be the case (25). With respect to thymus biology, our strategy of chemical thymectomy provides a means of studying the immunological function of cervical thymi in the absence of thoracic thymic tissue without necessitating its transplantation into an immunocompromised host.
Our present experiments indicate that the epithelial progenitor cell expresses Foxn1, because it was possible to destroy the TEC compartment by _Foxn1_-directed cytoablation. Moreover, these ablation experiments suggest that early losses of _Foxn1_-expressing TECs cannot be compensated for, because treatment at embryonic stages invariably leads to persistently lower numbers of thymocytes after birth. Hence, the self-renewing capacity of TEC progenitors appears to be low. During early embryogenesis, the proper development of the epithelial compartment of the thymic rudiment depends on signals from the surrounding mesenchyme (26); whether this also true at later stages is unknown. Hence, it is possible that the lack of recovery of TEC progenitor cells is the result of an altered microenvironment. Given that the half-life of thymic epithelium is in the order of weeks, and that thymopoietic activity in mice is observed over a period of up to 2 y, it appears plausible that at a given time point thymopoietic activity is furnished by the differentiated descendants of only a subset of progenitor cells. Because self-renewal does not seem to be a significant characteristic of the progenitor pool, it eventually becomes exhausted. This is reflected in the slow decay of thymopoietic activity, commonly referred to as thymic involution; this effect is accelerated after the initial reduction of progenitor cell numbers. Though this qualitative model is compatible with the available data, quantitative treatment of this problem requires more refined methods and animal models. Nonetheless, our cytoablated mice represent an animal model of the thymus-specific aspects of diGeorge and other thymic aplasia syndromes, which are characterized by complete thymus agenesis or drastically reduced thymus size (27) as a result of aberrant formation of the epithelial thymic microenvironment. Because diGeorge patients, for instance, often present with an autoimmune phenotype in addition to their overt immunodeficiency (28), our transgenic mouse lines might prove useful in investigating the cellular basis of this phenomenon.
Our findings also address the functional importance of _Foxn1_-negative cells that are present in the adult thymus. Lineage tracing shows that the majority originates from _Foxn1_-expressing cells; thus, they do not represent a _Foxn1_-independent lineage of TECs. Rather, they might correspond to TECs that are at the end of their lifespan. Nonetheless, given the occurrence of successive specification of thymopoietic tissue in thoracic and cervical thymi, it is not inconceivable that a distinct _Foxn1_-negative TEC lineage might become established after birth from uncommitted precursors. Even if numerically small initially, the population of _Foxn1_-negative TECs might eventually contribute functionally to overall thymopoietic activity. Exposure of _Foxn1_-expressing TECs to the ill effects of polyglutamine toxicity was used to directly test this possibility. In this situation, we expected both _Foxn1_-positive TECs and their _Foxn1_-negative progeny to be functionally incapacitated, and cells derived from a _Foxn1_-negative lineage to be spared intoxication. The fact that thymopoiesis ceases prematurely in the majority of mice transgenic for a pathogenic Foxn1:Q82-eGFP construct argues against the presence of a functionally relevant _Foxn1_-negative TEC lineage, and also excludes the possibility that the _Foxn1_-positive-turned- _Foxn1_-negative fraction of TEC recovers from intoxication. In future studies, the phenotype of Foxn1:Q82-eGFP mice will be compared with that of mice carrying mutant Foxn1 alleles. For instance, in mice carrying a hypomorphic Foxn1 allele, from which an internal domain was deleted (29), T-cell differentiation appears to be affected at earlier stages than in Foxn1:Q82-eGFP mice.
In summary, our findings support not only the notion that Foxn1 is an essential transcription factor regulating the differentiation of thymic epithelial cells, but also that its expression marks the major functional lineage of the thymic microenvironment in young and adult mice.
Materials and Methods
Animals.
The Foxn1:eGFP (7), Foxn1:Cre (21), and the Rosa26-YFP (22) mouse strains have been described previously. All other strains used here were generated using a vector containing the genomic Foxn1 promoter fragment (pAHB14) described by Bleul and Boehm (18). The inserts for this vector were as follows. The Foxn1:DTR transgene contains a NotI fragment corresponding to nucleotides 59–1,178 in Genbank accession no. NM_001945. The fragment contained in the Foxn1:CFP-NTR strain was amplified with 5′-ATCTAGGCGGCCGCATGGTGAGCAAGGGCGAGG and 5′-ATCTAGGCGGCCGCTTACACTTCGGTTAAGGTGAT (NotI sites underlined) from plasmid tol2_MCS-CFP-NTR (19) (a kind gift of S. Curado and D. Stainier, University of California, San Francisco) and cloned into the NotI site of pAHB14. The Foxn1:Q19-EGFP strain contains the blunted EcoRI–NotI fragment from plasmid pEGFP-N1-Q19 (24) cloned into the PmeI site of pAHB14; the Foxn1:Q82-EGFP strain contains the blunted EcoRI–NotI fragment from plasmid pEGFP-N1-Q82 (24) cloned into the PmeI site of pAHB14. The parental plasmids carrying the modified EGFP genes were a kind gift of R. Morimoto (Rice Institute for Biomedical Research, Northwestern University, Evanston, IL).
Genotyping.
Genotyping of transgenic mice was performed as follows. For Rosa26_-Y_FP, with primers 5′-aaagtcgctctgagttgttat and 5′-gcgaagagtttgtcctcaacc (amplicon size, 350 bp). For Foxn1:eGFP, Foxn1:Q19-eGFP, and Foxn1:Q82-eGFP strains, with primers 5′-gtccctaatccgatggctagctc and 5′-gtgcagatgaacttcagggtc, resulting in amplicons of about 400, 460, and 650 bp, respectively. For the Foxn1:Cre transgene, primers 5′-gcgcggtctggcagtaaaaac and 5′-gcagatggcgcggcaacac were used (amplicon size, 520 bp). The presence of the Foxn1:CFP-NTR transgene was detected with primers 5′-gtccctaatccgatggctagctc and 5′-gccagacatcgtccatcgcgg (amplicon size, 1,000 bp). The presence of the Foxn1:DTR transgene was detected with 5′-gtccctaatccgatggctagctc and 5′-cagtgccgagagaactgcagc (amplicon size, 500 bp).
Pharmacological Treatments.
Treatment of mice with DT and CB1954 was done as follows. Pregnant mice were injected i.p. with different doses (from 100 μg to 1.5 mg) of DT (D0564; Sigma) dissolved in PBS as indicated; cytoablation in embryos was complete at doses of 1 mg or greater per pregnant mother. CB1954 (C2235; Sigma) was dissolved at 50 mg/mL in acetone mixed with 9 vol of peanut oil (P2144; Sigma); one dose consisted of 100 μL (=0.5 mg).
Flow Cytometry.
The number of thymic epithelial cells, thymocytes, and peripheral leukocytes was determined as described (9).
Immunohistochemistry.
Tissue sections were evaluated for protein expression as described previously (18).
RNA in Situ Hybridization.
Tissue sections were hybridized with haptenized RNA probes as described (18). The _Gcm2_- and _Foxn1_-specific probes were described earlier (18); the Ttf-1 probe corresponds to nucleotides 636–1,246 in Genbank accession no. BC057607.
RT-PCR.
RNA from purified TECs was reverse transcribed using random hexamers as primers (9), and the resulting cDNA amplified with primers specific for Foxn1 (either 5′-ctgacctggatgctatcaacc [nucleotides 1,673–1,693 in accession no. NM_008238.1] and 5′-cgcagctgatgttgggcatagc [nucleotides 2,041–2,062] or 5′-tgacggagcaccttcccttac [nucleotides 995–1,014] and 5′-gacaggttatggcgaacagaa [nucleotides 1,044–1,064]) and Hprt (5′-tcctcctcagaccgctttt [nucleotides 104–122 in accession no. NM_013556] and 5′-cctggttcatcatcgctaatc [nucleotides 173–193]).
Thymus Transplantation.
Thymic lobes were transplanted under the kidney capsule as described (9).
Supplementary Material
Supporting Information
Acknowledgments
We thank Drs. Morimoto, Curado, and Stainier for plasmids, Dr. Costantini (Department of Genetics and Development, Columbia University, New York) for mice, and B. Hammerschmidt, C. Happe, D. Diekhoff, E. Lopez, and P. Kellmann for help during various stages of this project. Financial support for these studies was provided by the Max-Planck Society.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Rodewald H-R. Thymus organogenesis. Annu Rev Immunol. 2008;26:355–388. doi: 10.1146/annurev.immunol.26.021607.090408. [DOI] [PubMed] [Google Scholar]
- 2.Gordon J, Bennett AR, Blackburn CC, Manley NR. Gcm2 and Foxn1 mark early parathyroid- and thymus-specific domains in the developing third pharyngeal pouch. Mech Dev. 2001;103:141–143. doi: 10.1016/s0925-4773(01)00333-1. [DOI] [PubMed] [Google Scholar]
- 3.Nehls M, et al. Two genetically separable steps in the differentiation of thymic epithelium. Science. 1996;272:886–889. doi: 10.1126/science.272.5263.886. [DOI] [PubMed] [Google Scholar]
- 4.Nehls M, Pfeifer D, Schorpp M, Hedrich H, Boehm T. New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature. 1994;372:103–107. doi: 10.1038/372103a0. [DOI] [PubMed] [Google Scholar]
- 5.Liu Z, Yu S, Manley NR. Gcm2 is required for the differentiation and survival of parathyroid precursor cells in the parathyroid/thymus primordia. Dev Biol. 2007;305:333–346. doi: 10.1016/j.ydbio.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dooley J, Erickson M, Gillard GO, Farr AG. Cervical thymus in the mouse. J Immunol. 2006;176:6484–6490. doi: 10.4049/jimmunol.176.11.6484. [DOI] [PubMed] [Google Scholar]
- 7.Terszowski G, et al. Evidence for a functional second thymus in mice. Science. 2006;312:284–287. doi: 10.1126/science.1123497. [DOI] [PubMed] [Google Scholar]
- 8.Boehm T. Thymus development and function. Curr Opin Immunol. 2008;20:178–184. doi: 10.1016/j.coi.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Bleul CC, et al. Formation of a functional thymus initiated by a postnatal epithelial progenitor cell. Nature. 2006;441:992–996. doi: 10.1038/nature04850. [DOI] [PubMed] [Google Scholar]
- 10.Rossi SW, Jenkinson WE, Anderson G, Jenkinson EJ. Clonal analysis reveals a common progenitor for thymic cortical and medullary epithelium. Nature. 2006;441:988–991. doi: 10.1038/nature04813. [DOI] [PubMed] [Google Scholar]
- 11.Jenkinson WE, Bacon A, White AJ, Anderson G, Jenkinson EJ. An epithelial progenitor pool regulates thymus growth. J Immunol. 2008;181:6101–6108. doi: 10.4049/jimmunol.181.9.6101. [DOI] [PubMed] [Google Scholar]
- 12.Moore-Scott BA, Manley NR. Differential expression of Sonic hedgehog along the anterior-posterior axis regulates patterning of pharyngeal pouch endoderm and pharyngeal endoderm-derived organs. Dev Biol. 2005;278:323–335. doi: 10.1016/j.ydbio.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Griffith AV, et al. Increased thymus- and decreased parathyroid-fated organ domains in Splotch mutant embryos. Dev Biol. 2009;327:216–227. doi: 10.1016/j.ydbio.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray DH, et al. Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood. 2006;108:3777–3785. doi: 10.1182/blood-2006-02-004531. [DOI] [PubMed] [Google Scholar]
- 15.Itoi M, Tsukamoto N, Amagai T. Expression of Dll4 and CCL25 in Foxn1-negative epithelial cells in the post-natal thymus. Int Immunol. 2007;19:127–132. doi: 10.1093/intimm/dxl129. [DOI] [PubMed] [Google Scholar]
- 16.Cheng L, et al. Postnatal tissue-specific disruption of transcription factor FoxN1 triggers acute thymic atrophy. J Biol Chem. 2010;285:5836–5847. doi: 10.1074/jbc.M109.072124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saito M, et al. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol. 2001;19:746–750. doi: 10.1038/90795. [DOI] [PubMed] [Google Scholar]
- 18.Bleul CC, Boehm T. BMP signaling is required for normal thymus development. J Immunol. 2005;175:5213–5221. doi: 10.4049/jimmunol.175.8.5213. [DOI] [PubMed] [Google Scholar]
- 19.Curado S, Stainier DY, Anderson RM. Nitroreductase-mediated cell/tissue ablation in zebrafish: A spatially and temporally controlled ablation method with applications in developmental and regeneration studies. Nat Protoc. 2008;3:948–954. doi: 10.1038/nprot.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meier N, Dear TN, Boehm T. Whn and mHa3 are components of the genetic hierarchy controlling hair follicle differentiation. Mech Dev. 1999;89:215–221. doi: 10.1016/s0925-4773(99)00218-x. [DOI] [PubMed] [Google Scholar]
- 21.Soza-Ried C, Bleul CC, Schorpp M, Boehm T. Maintenance of thymic epithelial phenotype requires extrinsic signals in mouse and zebrafish. J Immunol. 2008;181:5272–5277. doi: 10.4049/jimmunol.181.8.5272. [DOI] [PubMed] [Google Scholar]
- 22.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams AJ, Paulson HL. Polyglutamine neurodegeneration: Protein misfolding revisited. Trends Neurosci. 2008;31:521–528. doi: 10.1016/j.tins.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holmberg CI, Staniszewski KE, Mensah KN, Matouschek A, Morimoto RI. Inefficient degradation of truncated polyglutamine proteins by the proteasome. EMBO J. 2004;23:4307–4318. doi: 10.1038/sj.emboj.7600426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pattou FN, et al. Supernumerary parathyroid glands: Frequency and surgical significance in treatment of renal hyperparathyroidism. World J Surg. 2000;24:1330–1334. doi: 10.1007/s002680010220. [DOI] [PubMed] [Google Scholar]
- 26.Anderson G, Jenkinson EJ, Moore NC, Owen JJ. MHC class II-positive epithelium and mesenchyme cells are both required for T-cell development in the thymus. Nature. 1993;362:70–73. doi: 10.1038/362070a0. [DOI] [PubMed] [Google Scholar]
- 27.Nezelof C. Thymic pathology in primary and secondary immunodeficiencies. Histopathology. 1992;21:499–511. doi: 10.1111/j.1365-2559.1992.tb00437.x. [DOI] [PubMed] [Google Scholar]
- 28.McLean-Tooke A, Spickett GP, Gennery AR. Immunodeficiency and autoimmunity in 22q11.2 deletion syndrome. Scand J Immunol. 2007;66:1–7. doi: 10.1111/j.1365-3083.2007.01949.x. [DOI] [PubMed] [Google Scholar]
- 29.Su DM, Navarre S, Oh WJ, Condie BG, Manley NR. A domain of Foxn1 required for crosstalk-dependent thymic epithelial cell differentiation. Nat Immunol. 2003;4:1128–1135. doi: 10.1038/ni983. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information