Intrinsic antibody-dependent enhancement of microbial infection in macrophages: disease regulation by immune complexes (original) (raw)
Summary
A wide range of microorganisms can replicate in macrophages, and cell entry of these pathogens via non-neutralising IgG antibody complexes can result in increased intracellular infection through idiosyncratic Fcγ-receptor signalling. The activation of Fcγ receptors usually leads to phagocytosis. Paradoxically, the ligation of monocyte or macrophage Fcγ receptors by IgG immune complexes, rather than aiding host defences, can suppress innate immunity, increase production of interleukin 10, and bias T-helper-1 (Th1) responses to Th2 responses, leading to increased infectious output by infected cells. This intrinsic antibody-dependent enhancement (ADE) of infection modulates the severity of diseases as disparate as dengue haemorrhagic fever and leishmaniasis. Intrinsic ADE is distinct from extrinsic ADE, whereby complexes of infectious agents with non-neutralising antibodies lead to an increased number of infected cells. Intrinsic ADE might be involved in many protozoan, bacterial, and viral infections. We review insights into intracellular mechanisms and implications of enhanced pathogenesis after ligation of macrophage Fcγ receptors by infectious immune complexes.
Introduction
Over the past four decades, information from different lines of scientific inquiry has improved our understanding of antibody-mediated mechanisms that modulate severity of infections by diverse microorganisms. Independent studies of pathogenesis of cellular and host responses to acute and chronic human and animal infectious diseases have generated evidence that cross-linking of immune complexes with Fcγ receptors increases cellular infection, contributing to disease severity by a mechanism we call intrinsic antibody-dependent enhancement (ADE) of infection. Intrinsic ADE is distinct from extrinsic ADE, whereby complexes of infectious agents with non-neutralising antibodies lead to an increased number of infected cells. Hawkes1 made early observations in studies of the neutralisation of Murray Valley encephalitis virus by use of the serum-dilution, virus-constant method. Chick embryo fibroblast monolayers exposed to virus mixed with high dilutions of chicken antisera had more plaques than did those exposed to virus alone.1 In follow-up studies,2 this finding seemed to result from antibody stabilisation of infectivity by the Murray Valley encephalitis virus. A different explanation emerged when sequential infections with dengue viruses resulted in dengue haemorrhagic fever.3 When monocytes and macrophages were identified as the main hosts of dengue infection, ADE was implicated.4, 5 Infection with the Murray Valley encephalitis virus was increased by ADE in the 2% of functional chicken macrophages identified in chick embryo fibroblasts. Because of the conformational requirement that Fc receptors and Fcγ termini must be of the same phylogenetic class, ADE in chick embryo fibroblasts was reported only when Murray Valley encephalitis virus antibodies were raised in chickens, not in mammals.6, 7
Macrophage biology
Monocytes are produced in the bone marrow by haemopoietic stem-cell precursors and then circulate in the bloodstream for about 1–3 days; about half of these cells are stored in the spleen. Monocytes mature to replenish resident macrophages and dendritic cells. Macrophages are scavengers that remove worn-out cells and other debris. They also present antigens that initiate the immune response. Macrophages have receptors for lymphokines that enhance their function. Two signals are needed to produce macrophages activated by the classic pathway: interferon γ and tumour necrosis factor (TNF) or interferon γ and exogenous toll receptor ligands such as lipopolysaccharide, resulting in macrophages that are able to kill intracellular organisms.8 Macrophages activated by the classic pathway are essential components of the host defence system, but their activation must be tightly controlled because the cytokines and mediators that they produce can lead to host tissue damage and immunopathological disorders such as rheumatoid arthritis and inflammatory bowel disease.
Macrophages have great plasticity and can respond to various environmental cues by adopting many different states of activation.8 T-helper-2 (Th2) immune responses, commonly elicited by disturbances at mucosal surfaces, result in the production of interleukin 4 and interleukin 13, which stimulate macrophages to secrete components of the extracellular matrix, making the cells effective in wound healing.8 These activated or wound-healing macrophages produce minimum amounts of proinflammatory cytokines and are less efficient at killing intracellular microbes than are macrophages activated by the classic pathway.
Regulatory macrophages typically arise during late stages of adaptive immune responses with a primary role of reducing immune responses and restricting inflammation. These cells can occur in response to toll-like receptor stimulation in the presence of glucocorticoids, prostaglandin E2, interleukin 10, or immune complexes. Regulatory macrophages are programmed to produce high concentrations of interleukin 10 and to suppress production of interleukin 12.9 Many other unique populations of activated macrophages with distinct phenotypes are likely to arise in response to specific diseases.8
Protozoan parasitism of macrophages
Leishmaniasis is caused by protozoan parasites of the genus Leishmania that are transmitted by the bite of sandflies. About 21 of 30 Leishmania species that infect mammals can cause human infection. The disease exists as two major forms: cutaneous and visceral leishmaniasis. Cutaneous leishmaniasis is endemic to many parts of the world, and is closely linked to specific geographical regions—with villages as little as 15 miles apart having different infection rates. Visceral leishmaniasis, also known as kala-azar, is the most severe form of leishmaniasis and, after malaria, is the second-leading cause of death from parasitic infection, with an estimated 500 000 cases each year worldwide.10 Some Leishmania species are closely linked to human beings and are commonly reported in cities (eg, L chagasi, L tropica), whereas others are more traditionally associated with animal species and are classified as zoonoses (eg, L major).
Leishmania species are transmitted as promastigotes, motile forms that infect macrophages, and spread within hosts as amastigotes, the obligate parasites of macrophages. In human hosts, the responses to infection vary with Leishmania species and the patients' immune reactions. Patients whose lymphocytes produce high amounts of interferon γ from Th1-type T cells usually recover from cutaneous infections without treatment; after recovery, these patients are immune to reinfection.11 Patients infected with visceral forms of the parasite produce high titres of antibodies, which do not contribute to host defence. Without treatment, these patients are not likely to recover from visceral leishmaniasis.11
Mouse models exist for the study of cutaneous and visceral leishmaniasis. For example, BALB/c mice are susceptible to this disease and develop progressive non-healing lesions with many intracellular parasites within macrophages.12 Despite the presence of high concentrations of antiparasite antibodies, these parasites usually disseminate to the liver, spleen, and bone marrow, thus mimicking human visceral leishmaniasis. In-vitro models are also available as promastigote or amastigote infections in cultures of bone marrow or peritoneal macrophages from mice or in human differentiated peripheral blood mononuclear cells (PBMCs).13
During the 1980s and 1990s, several mammalian cytokines were discovered. Among these cytokines was mouse cytokine synthesis inhibitory factor produced by Th2 cells, later renamed interleukin 10.14, 15 Interleukin 10 is a type 2 cytokine and the first identified member of a family of cytokines that include interleukins 19, 20, 22, 24, 26, 28, and 29.16 All these cytokines have similar genomic organisation, bind to receptors with similar structures and, in some cases, shared components, and all activate Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signalling pathways. Interleukin 10 is capable of inhibiting synthesis of proinflammatory cytokines such as interferon γ, interleukins 2, 3, and 12, TNF, and granulocyte macrophage colony-stimulating factor. Interleukin 10 also has a potent ability to suppress the antigen-presentation capacity of antigen-presenting cells. However, interleukin 10 is not always immunosuppressive. The cytokine can promote survival of natural killer cells and B cells and production of B-cell antibodies.17 Interleukin 10 is made by macrophages and various T cells, including Th1, Th2, Th17, and T-regulatory cells.18
Interleukin 10 has important roles in the regulation of immune responses and can also increase host susceptibility to intracellular infections. In a mouse model of cutaneous leishmaniasis, susceptibility to L major is associated with Th2 responses. CD4 T cells from susceptible BALB/c mice produce interleukins 4 and 10 when infected with L major, whereas CD4 T cells from resistant C57BL/6 mice express interferon γ and interleukin 2.19 The transient depletion of CD4 cells or the in-vivo neutralisation of interleukin 4 in BALB/c mice promotes death of intracellular parasites and recovery from leishmanial lesions. Interleukin 4 is not the only determinant of susceptibility, however, because susceptible BALB/c mice that do not express interleukin 10 are fully resistant to infection.20 Furthermore, in people in whom the Th1 to Th2 dichotomy is not as pronounced, interleukin 10 seems to be a major inducer of susceptibility. In people with visceral leishmaniasis, interleukin 10 titres in plasma directly correlate with disease severity.21 Interleukin 10 is found in lymph nodes taken from patients with visceral leishmaniasis.11 Furthermore, PBMCs from patients with acute visceral leishmaniasis respond to stimulation with leishmania lysate by producing interleukin 10 mRNA. When added to PBMCs, interleukin 10 suppresses production of interferon γ and interleukin 2, whereas, after treatment with anti-interleukin 10, PBMCs from patients with acute visceral leishmaniasis have a substantial increase in proliferative response to leishmania lysate. Results of in-vitro studies of peritoneal and bone marrow macrophages infected with Leishmania species show that the intracellular killing of this organism by macrophages activated by the classic pathway can be inhibited by treatment with exogenous interleukin 1022, 23 or by endogenous macrophage production of interleukin 10.22
Interleukin 12 has a complementary role to interleukin 10, acting directly on CD4 T cells to enhance priming for interferon-γ production and to reverse interleukin-4 priming.24 The most potent cytokine for the induction of leishmanicidal activity in macrophages is interferon γ.12 The sustained production of interferon γ in response to infection is commonly associated with the development of specific Th1 responses. In mice infected with L major, treatment with interleukin 12 increases interferon-γ production, reduces the severity of disease, potentiates vaccine-derived immunity, and suppresses interleukin-10 production.25 However, exposure of macrophages from susceptible mice to opsonised leishmania promastigotes suppresses the expression of interleukin 12.26
In bone-marrow-derived macrophages from BALB/c mice, the production of interleukin 12 in response to lipopolysaccharide is suppressed after ligation of Fc, complement, or scavenger receptors.27 Both mRNA synthesis and protein secretion are diminished to almost undetectable levels after receptor ligation. TNF production is not inhibited, so suppression is specific to interleukin 12. Additionally, the ligation of mouse Fcγ receptor with immune complexes increases the production of interleukin 10.28 Stimulation of mouse bone-marrow macrophages by lipopolysaccharide results in some interleukin-10 production, but the addition of red blood cells opsonised with IgG antibodies substantially increases interleukin-10 production. Immune complexes not only induce activated macrophages to produce interleukin 10, but they also induce both macrophages and dendritic cells to stop production of interleukin 12.27, 29
Interleukins 10 and 12 in macrophages are modulated by different mechanisms.30 The abrogation of interleukin-12 biosynthesis is a property shared by ligation of several macrophage receptors, the induction of interleukin 10 is specific to Fcγ receptors. Additional evidence for the role of interleukin 10 in promoting chronic infection is provided by the observation that normal BALB/c mice develop progressive non-healing lesions with many L major parasites, whereas BALB/c mice that do not express interleukin 10 control disease progression and have small lesions with 1000-times fewer parasites at the 5th week of infection.20 Furthermore, in established L donovani visceral infection in wild-type mice, treatment with monoclonal antibodies for either interleukin 10 or interleukin-10 receptor successfully induces intracellular parasite killing within liver macrophages.31
These findings and those from other studies32, 33 indicate that amastigotes of leishmania exploit an unusual and unexpected virulence factor: host IgG. When the surface of Leishmania amastigotes are coated with IgG, the resultant immune complexes allow them to ligate Fcγ receptors on inflammatory macrophages, preferentially inducing the production of high amounts of interleukin 10.16 This induction of interleukin 10 by the IgG-amastigotes complex did not occur in macrophages derived from mice that did not have the common γ chain that signals through Fcγ receptors 1, 3, and 4, indicating that one or all of these three receptors were involved.30 Results from subsequent studies that used defined immune complexes indicated that all three of the Fcγ receptors that signal through the gamma chain were capable of signalling for interleukin-10 production in macrophages.34 Therefore, in some settings, IgG itself seems to bias the immune response towards a Th2-type response. For some species of Leishmania, the persistence of infection depends on whether amastigotes are coated with IgG.23, 35, 36, 37
Production of interleukin 10 by ligation of Fcγ receptors is a generic process that does not require a non-microbial antigen.38 When exposed to ovalbumin alone, lipopolysaccharide-treated BALB/c mouse macrophages develop Th1-biased T-cell responses, characterised by the production of interferon γ.38 When the same antigen is complexed with IgG anti-ovalbumin, Th2 responses predominate with production of interleukin 4. This Th2 phenotype is stable and retained when T cells are subsequently stimulated again under non-biasing conditions. Mice vaccinated with IgG-opsonised ovalbumin produce high titres of IgG antibody of the IgG1 isotype.38 The T-cell biasing and its reversal via Fcγ receptor ligation is also seen in vivo.38 In macrophages from knockout mice, the production of interferon γ by T cells is controlled by the macrophage cytokine interleukin 12, and the production of interleukin 4 by interleukin 10. These findings show that the ligation of Fc receptors on activated macrophages reverses Th1 biasing that accompanies innate immune responses to microbial products.
In patients with visceral leishmaniasis, high titres of leishmanial antibodies correlate with peak parasitaemia and with negative delayed-type hypersensitivity responses.23 Successful treatment of leishmaniasis with amphotericin B results in decreased antibody titres and restoration of delayed-type hypersensitivity responses. Earlier observations identified polyclonal B-cell activation and high concentrations of immune complexes, as well as rheumatoid factor in patients with visceral leishmaniasis.39 In a small series, individuals infected with L donovani were more likely to develop rheumatoid arthritis than were non-infected controls.40 In experimental models of visceral leishmaniasis, infected hamsters develop immune complex glomerulonephritis.41 In addition to L donovoni and L major, humoral immune responses against L mexicana were not effective at killing organisms hiding in parasitophorous vacuoles because host IgG-coated amastigotes generated immunosuppressive interleukin-10 responses by infected macrophages.32, 33 Although perhaps caused by a different mechanism, a similar process might be involved in JhD mice infected with L amazonensis: in one study,42 infections were minimised in the absence of B cells or antibodies; when these immune elements were restored, lesions progressed by a process thought to involve CD4 T cells.
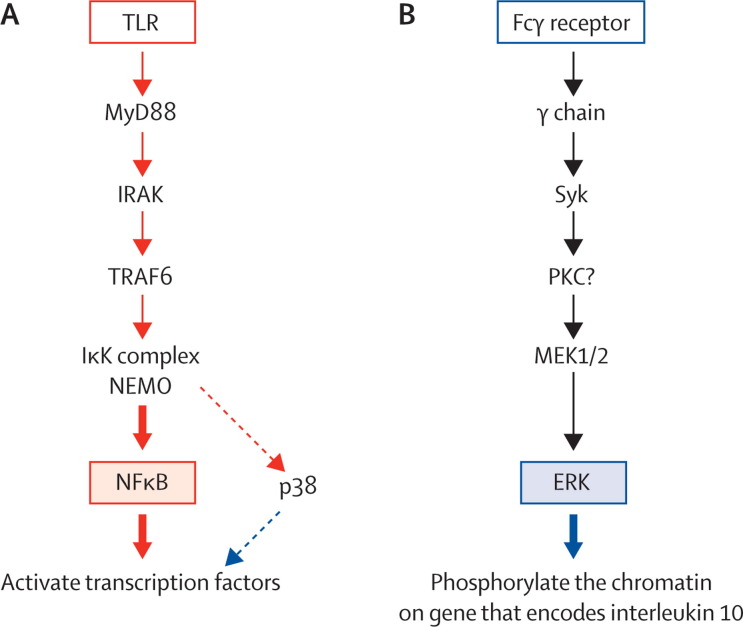
How do antibody-coated amastigotes result in the production of interleukin 10 by macrophages? Ligation of macrophage Fcγ receptors produces a rapid and enhanced activation of two mitogen-activated protein kinases (MAPKs): extracellular signal-regulated kinase (ERK) and p38.43 The activation of ERK leads to the phosphorylation of serine 10 on histone H3 on the gene encoding interleukin 10, increasing the accessibility of the promoter to transcription factors generated in response to p38 activation (figure 1). Activation of both MAPKs is needed for interleukin-10 synthesis. In addition to ERK activation, an inflammatory stimulus, such as low-molecular-weight hyaluronic acid from the extracellular matrix, is needed. The combination of these two signals results in the superinduction of interleukin 10.44 Macrophages that do not have Fcγ receptors, or macrophages treated with an inhibitor of spleen tyrosine kinase (which is activated after Fcγ-receptor ligation) do not activate ERK and fail to produce interleukin 10 after infection with Leishmania species amastigotes.44
Figure 1.
Pathways by which toll-like-receptor signals and ligation of Fcγ receptor lead to the production of interleukin 10
(A) Toll-like receptors (TLRs) signal through the adaptor molecule myeloid differentiation primary response gene (MyD88) to activate a kinase cascade, resulting in the activation of nuclear factor κB (NFκB) and p38. This pathway leads to the activation of transcription factors that have the potential to bind to the interleukin 10 promoter. In resting cells, this promoter is inaccessible to these transcription factors because it is tightly packed in chromatin. (B) The ligation of Fcγ receptors signals through a different protein kinase, Syk, to activate extracellular signal-regulated kinase (ERK). This pathway leads to the phosphorylation of chromatin, making the promoter accessible to transcription factors. IRAK= interleukin-1 receptor associated kinase. TRAF6=TNF receptor-associated factor 6. IκK=IκB kinase. NEMO=NFκB essential modulator. PKC=protein kinase C.
Other intracellular parasites and bacteria
Many bacteria replicate partly or solely in human macrophages. Many organisms that do infect macrophages produce chronic infections. One of the criteria of successful parasitism by microorganisms that produce systemic infections might be the ability to evade microbicidal mechanisms of macrophages. These defensive mechanisms have been much studied.45 Chronic infections themselves or successive infection by microorganisms with similar antigenic structures might contribute to the formation of pathogenic IgG immune complexes that control microbial survival. Infections that might be analogous to the leishmania model include: Mycobacterium tuberculosis, Mycobacterium leprae, Legionella pneumophila, Listeria monocytogenes, Brucella spp_, Salmonella_ spp_, Shigella_ spp_, Coxiella burnetii, Anaplasma phagocytophilum, Ehrlichia chaffeensis_, another protozoan, Toxoplasma gondii, and fungi (eg, Histoplasma capsulatum).
The role of Fc receptors or immune complexes has not been studied exhaustively for most of these organisms. Although studies on resistance to tuberculosis have focused on T-cell immunity, C57BL/6 mice deficient in inhibitory Fcγ receptor IIB have improved bacterial control and diminished pathological changes at 30 days but not 20 days after aerosol challenge with M tuberculosis.46 Mice deficient in Fcγ receptor IIB have increased production of the p40 subunit of interleukin 12. Interleukins 12 and 23 have the p40 subunit in common and both promote polarisation of naive CD4 T cells into Th1 effectors.47 Treatment of human macrophages with exogenous interleukin 12 combined with blockade of interleukin 27 reduced the burden of mycobacterial infection.48 These infections were characterised by large protective interferon-γ responses that coincided with increased activation of macrophages. In human beings, high concentrations of interleukin 10 are related to the suppression of host defence mechanisms and exacerbation of infection.49 In a model of reactivation tuberculosis, the presence of macrophage-derived interleukin 10 in the lungs of infected transgenic mice enables Th1 cells to efficiently express effector functions and secrete sufficient interferon γ to induce activation of macrophages by the classic pathway, characterised by the expression of inducible nitric oxide synthase (NOS2) and interferon-γ inducible protein member 1 (IRGM1; formerly LRG-47).50 However, mycobacteria survived and successfully proliferated within mouse macrophages with interleukin-10 production under control of a human CD68 promoter. Macrophage-derived interleukin 10 seems to override interferon-γ-dependent activation of macrophages by the classic pathway and other effector mechanisms against M tuberculosis by inducing an alternatively activated phenotype. So far, the specific contribution of _M tuberculosis_–IgG antibody complexes towards modulation of infections in model systems is unknown.
Viruses
The ADE mechanism has attracted wide interest in virology because many viruses replicate in macrophages in vivo and cause enhanced infections or disease.51 Not all severe viral infections have been irrefutably linked to antibodies. Enhanced infections need an initial immunological event, termed sensitisation, and viral infections can be categorised into two groups. Some viruses cause immune responses that sensitise hosts as a result of sequential infection by more than one antigenic type or as a result of the rapid evolution of antigenic diversity during the course of a chronic infection (table 1). Hosts can be sensitised by other viruses that are given as killed vaccines (table 2). Respiratory syncytial and measles viruses infect respiratory epithelial cells in vivo. and the enhanced infections observed in individuals given killed viral vaccines are not caused by ADE but have been attributed to poor antibody quality resulting from aberrant antigen presentation.71
Table 1.
Viruses that cause in-vitro antibody-dependent enhancement of infection or antibody-enhanced disease
| In-vitro ADE | In-vivo enhanced disease | |
|---|---|---|
| RNA virus group | ||
| Picornaviridae | ||
| Coxsackie B52 | + | + (animal model) |
| Flaviviridae | ||
| Dengue4 | + | + |
| Lactate dehydrogenase53 | + | − |
| Coronaviridae | ||
| Feline infectious peritonitis54, 55 | + | + |
| PRRSV56 | + | + |
| Simian haemorrhagic fever51 | + | + |
| Retroviridae | ||
| HIV57 | + | ? |
| Caprine arthritis58 | + | + |
| Equine infectious anaemia59 | + | + |
| DNA virus group | ||
| Parvoviridae | ||
| Aleutian disease of mink60, 61, 62 | + | + |
| Asfarviridae | ||
| African swine fever63 | .. | .. |
Table 2.
Viral diseases that are enhanced after sensitisation of hosts by vaccines
| In-vitro ADE | In-vivo enhanced disease | |
|---|---|---|
| RNA virus group | ||
| Orthomyxoviridae | ||
| Influenza A64 | + | + (mouse model) |
| Paramyxoviridae | ||
| Respiratory syncytial virus65 | + | + |
| Measles66 | + | + |
| Rhabdoviridae | ||
| Rabies67, 68 | + | + (accelerated disease onset) |
| Coronaviridae | ||
| Feline infectious peritonitis54 | + | + |
| PRRSV56 | + | + |
| Simian haemorrhagic fever69 | + | + |
| Retroviridae | ||
| HIV70 | + | ? |
| Equine infectious anaemia59 | + | + |
| Caprine arthritis58 | + | + |
| DNA virus group | ||
| Parvoviridae | ||
| Aleutian disease of mink60 | + | + |
During initial ADE research, increased yields of virus were assumed to result from higher numbers of cells infected in the presence of antibodies than in the absence of antibodies.51 This increased infection was shown to be mediated by Fcγ receptors.5, 72 For example, more West Nile virus particles were shown to be attached to the surface of mouse macrophages as immune complexes than as naked virus.73, 74 In feline infectious peritonitis virus, more peritoneal macrophages are infected in vitro in the presence of antibody than in the absence of antibody.75 Alternatively, immune complexes might be internalised more rapidly than virus alone. With HIV-1, viral replication is initiated and progeny viruses released sooner from cells pretreated with HIV-specific antibodies than in cells exposed to untreated virus.76 Protein and RNA synthesis are increased in cells infected with HIV-1 immune complexes than with virus alone.
Ross River virus
Acute infections with Ross River virus (RRV) often result in post-infection arthritis and arthralgia for many months. Synovial cells typically stain for RRV antigens, whereas synovial fluid often contains interferon γ and TNF. Attempts have been made to model this finding by establishing chronic RRV infections in mouse macrophage lines.77 Infection in these cells might be a model of how RRV persists in synovial tissues and produces arthritis and arthralgia.78 The incubation of RRV with diluted RRV antiserum results in increased infection in these cells.79 This finding has been confirmed in a human monocytic cell line, Mono mac 6, and in primary human monocytes and macrophages.79 RRV-infected RAW 264.7, continuous mouse macrophage cells, produced antiviral TNF when treated with lipopolysaccharide but, when these cells are infected via the ADE pathway, TNF production is abrogated and RRV infection greatly enhanced.79 Increased ADE production of virus was correlated with a downregulation of nitric oxide production and an inhibition of the transcription of genes for TNF, NOS2, interferon regulatory factor 1 (IRF-1), and nuclear factor κB (NFκB).
ADE-mediated infections inhibit lipopolysaccharide-induced reactive nitrogen intermediates and TNF gene transcription and protein synthesis, but do not decrease general protein synthesis or increase transcription of non-antiviral control genes. In one study, ADE-mediated infections in lipopolysaccharide-treated RAW 264.7 cells resulted in a substantial increase in RRV-infected cells compared with virus-only controls at and after 12 h.80 Although 14·2% of ADE-infected cells stained for RRV antigen by immunofluorescence microscopy, a greater percentage of cells were positive by PCR and electron microscopy. RRV ADE infection suppresses signals for interferon-inducible protein 10 (IP-10), NOS2, IRF-1, TNF, and interferon β, but not for Sp1 (a human genetic transcription factor that regulates immune responses). A substantial increase in interleukin-10 gene transcription and protein production in these cells was reported. Crucially, the inhibition of antiviral factor transcription requires infectious virus in immune IgG complexes since the ligation of Fcγ receptors by zymosan–antibody complexes does not ablate antiviral transcription.81
Dengue virus
Dengue viruses are a group of four closely related members of the Flavivirus genus that share 60–70% genetic homology and are inoculated by the bite of infected Aedes aegypti. Initial infections raise cross-reactive non-neutralising antibodies that can enhance an infection with a different virus type.82 Data from in-vitro studies in Fc receptor-bearing cells indicate that any specificity of monoclonal dengue antibodies might form infectious immune complexes, the major requirement being antibody concentration below that needed for neutralisation.83, 84 In practice, antibodies directed at surface epitopes not involved in virus entry efficiently produce ADE.83, 85 The fact that infants might acquire severe dengue disease during a first dengue infection when placentally acquired maternal polyclonal dengue antibodies have diminished to below protective concentrations is a unique example of ADE.86
Results from studies of human tissues have established that monocytes, macrophages, and immature and mature dendritic cells are major targets for dengue virus infection.87, 88 In human beings, secondary dengue infections follow a stereotypical course with severe outcomes, such as shock or gastrointestinal haemorrhage, accompanying vascular collapse that results from capillary permeability around the time of defervescence.89 Indirect evidence suggests that cytokines mediate vascular permeability in patients with dengue infection. Much work has focused on the measurement of blood cytokine concentrations in patients late in the acute phase, just before onset of shock.90 Elevated viraemia and high concentrations of proinflammatory and immunomodulatory cytokines, including interleukin 10, are associated with severe disease.91
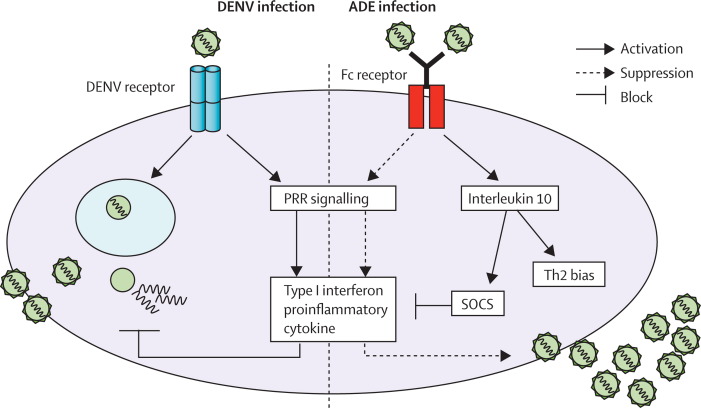
During in-vitro ADE infection of THP-1 cells (a human monocytic Fcγ receptor-bearing continuous cell line), intracellular dengue virus production is increased as a result of idiosyncratic Fcγ-receptor signalling.92 Infections with polyclonal antibodies plus dengue virus 2 result in a suppression of innate responses. After ligation of Fcγ receptor I and Fcγ receptor IIA, entry of infectious immune complexes activates expression of the negative regulators dihydroxyacetone kinase and the autophagy proteins Atg5–Atg12 of retinoic acid-inducible protein I/melanoma differentiation-associated gene 5 (RIG-I/MDA5), resulting in downregulation of the RIG-I/MDA5 signalling pathway and decreased production of type I interferon and interferon-activated antiviral molecules.93 After ligation of Fc receptors, dengue virus infection activates interleukin-10 production at an early phase of infection.92 The suppressive role of interleukin 10 during ADE infection has been confirmed and not only induces Th2 biasing but also inhibits a potent suppressor of the JAK–STAT signalling pathway via the suppressor of cytokine signalling (SOCS) system.93 As a result of these two suppression pathways, ADE-infected THP-1 cells secrete low concentrations of type I interferon, thus suppressing transcription and translation of interleukin 12, interferon γ, and TNF and facilitating expression and synthesis of anti-inflammatory cytokines. This pathway of infection also suppressed an innate dengue virus mediator, nitric oxide radicals, by disrupting the transcription of the NOS2 gene transcription factor, IRF-1. Thus, ADE infection not only facilitates virus entry (extrinsic ADE), but also modifies innate and adaptive intracellular antiviral mechanisms and enhances replication (intrinsic ADE; figure 2).
Figure 2.
Intrinsic ADE of dengue virus infection in THP-1 human monocytic cells
Attachment and entry of infectious virus–antibody complexes into Fc receptor-bearing cells results in increased production of virus by inhibition of type I interferon and production of proinflammatory cytokines. Additionally, interleukin-10 biosynthesis is activated, stimulating members of the suppressor of cytokine signalling (SOCS) family, which results in suppression of the Janus kinase–signal transducer and activator of transcription (JAK–STAT) signalling pathway and bias towards a Th2 response. By contrast, infection by naked virus via virus-specific receptor signals through pattern recognition receptor (PRRs) produces intracellular antiviral molecules. ADE=antibody-dependent enhancement. Th2=T helper 2. DENV=dengue virus.
Human in-vivo correlations can be made with these in-vitro responses. During the acute severe illness stage of secondary infections, increased production of interleukin 10 is accompanied by downregulation of several interferon regulatory genes.94, 95, 96 Genome-wide transcriptomes from PBMCs collected during the acute phase of mild dengue fever and dengue haemorrhagic fever in children were compared by use of microarray analysis.97 Compared with patients with milder illness, those with dengue haemorrhagic fever during secondary infections had low concentrations of nitric oxide, low interferon transcript in PBMCs, and high interleukin-10 blood concentrations. A cDNA array indicated that 47% of immune response genes strongly upregulated in PBMCs from children with mild illness were interferon-inducible and interferon-induced genes. The robust upregulation of type I interferon genes in PBMCs from children with mild illnesses was accompanied by increased plasma interferon-α concentrations. Interferon gene upregulation and production were substantially increased in patients with mild dengue illness compared with patients with severe dengue illness.
The notable abundance of interferon and interferon-inducible factors in patients with mild disease is consistent with a protective role of interferon in the control of dengue infection, as indicated in a mouse model and suggested for human beings with dengue fever.98, 99, 100 Patients who survive dengue haemorrhagic fever have higher concentrations of circulating interferon β than do those who die.94, 95 Patients with dengue fever have lower peak viraemia titres than do those with dengue haemorrhagic fever.101, 102
A role for another interferon-related mediator that reduces viral load in dengue fever has been suggested. Production of human IP-10 was upregulated in the PBMCs of patients with dengue fever.93 In a mouse model, IP-10 protected against dengue infection by serving as a chemoattractant for natural killer and T cells and by blocking interactions between dengue and its putative receptor, heparan sulphate.103, 104
Wound-healing macrophages readily support infection with all four dengue viruses in vitro.105 Human monocytes, activated macrophages, and mature dendritic cells express ADE, whereas immature dendritic cells do not (Marovich MA, unpublished). In macrophages, dengue virus 2 infection produced high concentrations of type I interferons (α/β) but these were downregulated under ADE conditions and replaced with the secretion of proinflammatory cytokines (interleukin 6 and TNFα) at peak enhancement titres. Mature dendritic cells secreted interferon β coincident with peak ADE. Immature dendritic cells are readily infected by dengue virus alone, a process efficiently mediated by the attachment molecule dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN).106 Monocytes secreted interleukin 10 during ADE infection at the same serum dilution that produced peak virus (Marovich MA, unpublished). Production of interleukin-10 protein was controlled by interleukin-10 promoter polymorphisms. Three single nucleotide polymorphisms at the 5′ flanking region of interleukin 10 (position −1082 A/G, −819 C/T, and −592 C/A) were studied. Dengue infection rates at enhancement titres were remarkably consistent in primary monocytes (10–15%) from 20 different donors; homozygous GCC haplotype was associated with high interleukin-10 secretion, whereas donors with the ACC haplotype produced an intermediate concentration of interleukin 10, and the ATA haplotype secreted the lowest interleukin-10 concentrations.
The precise role of interleukin-10 production induced from immune complexes on the clinical events of dengue infections is not known. The reported shift from Th1 response to Th2 response in severe secondary dengue illnesses could be attributed to interleukin 10.107 Use of a mouse model has indicated an ADE-related early vascular permeability that is mediated by TNF.108, 109 The contribution of Th1 or Th2 responses to vascular permeability in these mice is unknown.
In Asia and the Americas, preceding infection or immunisation with a non-dengue flavivirus—Japanese encephalitis in Asia and yellow fever or yellow fever vaccine in the Americas—does not predispose individuals to dengue haemorrhagic fever with subsequent dengue virus infection, although non-dengue flavivirus antibodies form infectious immune complexes with dengue viruses.110 In an in-vitro study with U937 cells, late convalescent sera from individuals who had had infections with Japanese encephalitis virus did not lead to ADE infections with dengue virus 2.111 Non-neutralising heterotypical flaviviral antibodies are generally non-neutralising and, therefore, attach to parts of the virion that are not crucial to neutralisation. Might the site of attachment of antibodies to virions control the outcome of intrinsic ADE?
Other viruses
ADE of virus infection might be a disease-enhancing factor for several human and animal viral diseases. Attributes of these diseases have been summarised in Table 1, Table 2. In this section, we briefly review three of these diseases in particular: feline infectious peritonitis, Aleutian disease of mink, and porcine reproductive and respiratory syndrome (PRRS).
Feline infectious peritonitis is caused by a coronavirus that replicates in macrophages and leads to peritonitis and occasionally a fatal pyogranulomatous disease in kittens and cats with ADE incriminated as a disease-enhancing factor.54, 112 Cats with active disease or who have acquired passive maternal antibodies develop a more rapid and fulminant disease after challenge with this virus than do seronegative cats. Disease enhancement has been reported in kittens that had vaccine-derived humoral immunity directed against the spike protein of feline infectious peritonitis virus.113 Similarly, kittens immunised with a recombinant vaccinia virus expressing the spike protein of this virus die earlier than do control animals. Feline infectious peritonitis virus derives from chronic infections with feline coronaviruses, during the course of which the virus mutates to present new antigens to the host.114 These kittens die earlier than do control animals.
Aleutian disease of mink is caused by a parvovirus that is pathogenic for mink that replicates in macrophages and circulates in the blood mainly as fully infectious immune complexes, both in vivo and in vitro.115 Deposition of these soluble immune complexes on renal glomerular membranes or walls of capillary blood vessels leads to fatal glomerulonephritis. ADE might be an important contributing factor in the pathogenesis of this disease. Aleutian disease virus replicates in macrophages, and infected mink produce large amounts of non-neutralising antibodies.116 These antibodies subsequently produce infectious immune complexes, which lead to increased infection of macrophages. Passive transfer of virus-specific antibody at the peak of viral replication results in foci of necrosis around virus-infected cells, a reaction thought to be due to complement-mediated cytolysis.60 Infectious immune complexes result in increased infection of mink peritoneal macrophages.61 In one study, inoculation of an experimental killed Aleutian disease virus vaccine did not produce detectable neutralising antibodies, although eight of ten of vaccinated mink, but no control animals, developed the disease on challenge with live virus.60
PRRS is caused by a member of the Arterivirus genus of the coronavirus family that was first isolated in the Netherlands and classified in 1991. The syndrome was recognised in the USA in the mid-1980s and called mystery swine disease and has also been called blue ear disease. There are two prototype strains of PRRS virus (European and North American) that cause similar clinical symptoms but are distinct viral genotypes whose genomes diverge by about 40%.56, 117, 118 The genetic variation among the viruses varies geographically, increasing the difficulty of vaccine development.119 For reasons not understood, disease presentation varies between one herd and another. For every three herds exposed to PRRS, for the first exposure, one herd will show no recognisable disease, the second might show mild disease, and the third will show moderate to severe disease: the better the health status of the herd, the less severe the disease.117 Evidence exists that PRRS virus mutates as it multiplies, leading to some strains that are highly virulent and some that are not.117, 118 PRRS virus has a particular affinity for macrophages, particularly those in the lungs.56, 120, 121 The virus multiplies in macrophages and kills these cells. PRRS virus infections are often chronic and, once a herd is infected, the virus tends to remain present and active indefinitely.56
Conclusions
Most of the macrophage-tropic organisms discussed in this Review have evolved various offensive mechanisms. In some cases, these mechanisms are sufficient to reduce host defence mechanisms but, in other cases, Fcγ receptor-mediated intrinsic ADE might also result in either severe or sustained infections. In cases when host T-cell responses quickly predominate, effective control of infection with robust resistance to reinfection is achieved. Why there are these differential outcomes is not clear.
Complexity can characterise interactions of infectious pathogens with antibodies. For example, when Mahalingam and Lidbury80 added an irrelevant non-infectious immune complex to mouse macrophage cell line and then infected these cells with RRV, ADE did not occur—despite reports that Th2 responses after ligation of Fcγ receptors by IgG immune complexes were a generic process.38 ADE can lead to a Th2-biased proliferation of lymphocytes.
The specific outcome of ligation of each human Fcγ receptor by immune complexes needs careful study. Many years ago, an antibody-like molecule was recognised to attach to primary monocytes obtained from dengue-immune donors.5, 51, 72 From our present understanding of human Fcγ receptors, could cytophilic antibodies attach to high-affinity Fcγ receptor I?122 An interesting question is whether cytophilic or non-cytophilic immune complexes, or both, mediate enhanced and severe dengue disease. Furthermore, does intrinsic ADE result from interactions of immune complexes with Fcγ receptor IIb, the inhibitory Fc receptor?
Several lines of evidence suggest that monocyte/macrophage interactions with infectious virus or infectious virus–antibody complexes differ at an early stage of infection. The mutated form of the Fc portion of the polyvalent dengue virus monoclonal antibody E60-hIgG1-N297Q, which abolished Fc-receptor binding, had neutralising activity instead of enhancing activity.109 Moreover, not only the functional Fc portion is needed but the complete cytoplasmic tail of Fc receptor is essential for dengue virus–antibody complexes to promote enhancing activity, as indicated by the fact that disruption of the immune tyrosine activating motif (ITAM) or removal of the sequence between the two ITAM regions abolishes ADE activity.123 However, the innate immune responses cannot be suppressed by simply cross-linking Fc receptors with anti-CD32 or anti-CD64 antibodies (Ubol S, unpublished). Hence, the enhancing effect of virus–antibody complexes might start once the Fc portion is engaged with the Fc receptor, which in turn switches on the negative regulatory innate immune response. These events do not occur during the entry of naked virus.
Intrinsic ADE is linked to the immunobiology of interleukin 10, a complex type II cytokine that has a key role in many infectious processes.16 Results from in-vitro experiments suggest that early production of interleukin 10 in dengue infections might have an important role in promoting ADE, whereas interleukin 10 produced in the latter half of secondary dengue infections might be immunoregulatory. Careful studies that follow up individuals through the course of primary mild and secondary severe dengue infections are needed. Events that simply accompany secondary immune responses should be distinguished from those responses that might contribute to immunopathological changes.124
Several questions concern the essential and non-essential components of intrinsic ADE. Where, exactly, does interleukin-10 production fit into this process? Does interleukin-10 production lead to increased infection in the initial infected cell, or does interleukin 10 mainly function on bystander cells? Are there functional differences between interleukin 10 of T-cell and macrophage origin? Another profound effect of interleukin 10 is to inhibit the production of proinflammatory cytokines and mediators from macrophages and dendritic cells. The major inflammatory cytokines, interleukins 1, 6, and 12, and TNF, are all substantially repressed after exposure to interleukin 10.
Many, perhaps most, of the fundamental characteristics of intrinsic ADE are unknown. Studies into intrinsic ADE should expand our understanding of the mechanisms of pathogenesis for a broad range of infectious diseases and open many approaches for improvements to the treatment and prevention of such diseases.
Search strategy and selection criteria
References were identified from searches of PubMed from January, 1960, to August, 2010, for articles on in-vitro studies of all macrophage-tropic microorganisms. Searches included cross-references to “macrophages”, “macrophage biology”, “immune complexes”, “IgG gamma receptors”, “IL-10”, “interferon”, and “toll-like receptors”. Additional references from the authors' own files were also identified. Authors of several papers that were difficult to access were directly contacted.
Contributors
SBH was involved in the literature review, data interpretation, and writing and editing of the paper. SM was involved in writing the paper and providing intellectual input. MAM was involved with data analysis, writing the paper, and preparing the figures. SU was involved in writing the paper and preparing the figures. DMM was involved in data collection and interpretation and development of the figures.
Conflicts of interest
All authors declare that they have no conflicts of interest.
Contributors
SBH was involved in the literature review, data interpretation, and writing and editing of the paper. SM was involved in writing the paper and providing intellectual input. MAM was involved with data analysis, writing the paper, and preparing the figures. SU was involved in writing the paper and preparing the figures. DMM was involved in data collection and interpretation and development of the figures.
Conflicts of interest
All authors declare that they have no conflicts of interest.
References
- 1.Hawkes RA. Enhancement of the infectivity of arboviruses by specific antisera produced in domestic fowls. Aust J Exp Biol Med Sci. 1964;42:465–482. doi: 10.1038/icb.1964.44. [DOI] [PubMed] [Google Scholar]
- 2.Hawkes RA, Lafferty KJ. The enhancement of virus infectivity by antibody. Virology. 1967;33:250–261. doi: 10.1016/0042-6822(67)90144-4. [DOI] [PubMed] [Google Scholar]
- 3.Halstead SB, Nimmannitya S, Yamarat C, Russell PK. Hemorrhagic fever in Thailand; recent knowledge regarding etiology. Jpn J Med Sci Biol. 1967;20:96–103. [PubMed] [Google Scholar]
- 4.Halstead SB, Chow J, Marchette NJ. Immunologic enhancement of dengue virus replication. Nat New Biol. 1973;243:24–26. [PubMed] [Google Scholar]
- 5.Halstead SB, O'Rourke EJ. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977;146:201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kliks S, Halstead SB. An explanation for enhanced virus plaque formation in chick embryo cells. Nature. 1980;285:504–505. doi: 10.1038/285504a0. [DOI] [PubMed] [Google Scholar]
- 7.Kliks S, Halstead SB. Role of antibodies and host cells in plaque enhancement of Murray Valley encephalitis virus. J Virol. 1983;46:394–404. doi: 10.1128/jvi.46.2.394-404.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerber JS, Mosser DM. Reversing lipopolysaccharide toxicity by ligating the macrophage Fc gamma receptors. J Immunol. 2001;166:6861–6868. doi: 10.4049/jimmunol.166.11.6861. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Division of Parasitic Diseases Factsheet: Leishmania infection. http://www.cdc.gov/ncidod/dpd/parasites/leishmania/factsht_leishmania.htm (accessed Aug 31, 2010).
- 11.Ghalib HW, Piuvezam MR, Skeiky YA. Interleukin 10 production correlates with pathology in human Leishmania donovani infections. J Clin Invest. 1993;92:324–329. doi: 10.1172/JCI116570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray HW, Masur H, Keithly JS. Cell-mediated immune response in experimental visceral leishmaniasis. I. Correlation between resistance to Leishmania donovani and lymphokine-generating capacity. J Immunol. 1982;129:344–350. [PubMed] [Google Scholar]
- 13.Pearson RD, Romito R, Symes PH, Harcus JL. Interaction of Leishmania donovani promastigotes with human monocyte-derived macrophages: parasite entry, intracellular survival, and multiplication. Infect Immun. 1981;32:1249–1253. doi: 10.1128/iai.32.3.1249-1253.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore KW, Vieira P, Fiorentino DF, Trounstine ML, Khan TA, Mosmann TR. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990;248:1230–1234. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- 16.Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rousset F, Garcia E, Defrance T. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci USA. 1992;89:1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Garra A, Vieira P. T(H)1 cells control themselves by producing interleukin-10. Nat Rev Immunol. 2007;7:425–428. doi: 10.1038/nri2097. [DOI] [PubMed] [Google Scholar]
- 19.Heinzel FP, Sadick MD, Mutha SS, Locksley RM. Production of interferon gamma, interleukin 2, interleukin 4, and interleukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc Natl Acad Sci USA. 1991;88:7011–7015. doi: 10.1073/pnas.88.16.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kane MM, Mosser DM. The role of IL-10 in promoting disease progression in leishmaniasis. J Immunol. 2001;166:1141–1147. doi: 10.4049/jimmunol.166.2.1141. [DOI] [PubMed] [Google Scholar]
- 21.Karp CL, el-Safi SH, Wynn TA. In vivo cytokine profiles in patients with kala-azar. Marked elevation of both interleukin-10 and interferon-gamma. J Clin Invest. 1993;91:1644–1648. doi: 10.1172/JCI116372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vieth M, Will A, Schroppel K, Rollinghoff M, Gessner A. Interleukin-10 inhibits antimicrobial activity against Leishmania major in murine macrophages. Scand J Immunol. 1994;40:403–409. doi: 10.1111/j.1365-3083.1994.tb03481.x. [DOI] [PubMed] [Google Scholar]
- 23.Miles SA, Conrad SM, Alves RG, Jeronimo SM, Mosser DM. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. J Exp Med. 2005;201:747–754. doi: 10.1084/jem.20041470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seder RA, Gazzinelli R, Sher A, Paul WE. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci USA. 1993;90:10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinzel FP, Schoenhaut DS, Rerko RM, Rosser LE, Gately MK. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993;177:1505–1509. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carrera L, Gazzinelli RT, Badolato R. Leishmania promastigotes selectively inhibit interleukin 12 induction in bone marrow-derived macrophages from susceptible and resistant mice. J Exp Med. 1996;183:515–526. doi: 10.1084/jem.183.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutterwala FS, Noel GJ, Clynes R, Mosser DM. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J Exp Med. 1997;185:1977–1985. doi: 10.1084/jem.185.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutterwala FS, Noel GJ, Salgame P, Mosser DM. Reversal of proinflammatory responses by ligating the macrophage Fcgamma receptor type I. J Exp Med. 1998;188:217–222. doi: 10.1084/jem.188.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson CF, Lucas M, Gutierrez-Kobeh L, Field AE, Mosser DM. T cell biasing by activated dendritic cells. J Immunol. 2004;173:955–961. doi: 10.4049/jimmunol.173.2.955. [DOI] [PubMed] [Google Scholar]
- 30.Gerber JS, Mosser DM. Stimulatory and inhibitory signals originating from the macrophage Fcgamma receptors. Microbes Infect. 2001;3:131–139. doi: 10.1016/s1286-4579(00)01360-5. [DOI] [PubMed] [Google Scholar]
- 31.Murray HW, Moreira AL, Lu CM. Determinants of response to interleukin-10 receptor blockade immunotherapy in experimental visceral leishmaniasis. J Infect Dis. 2003;188:458–464. doi: 10.1086/376510. [DOI] [PubMed] [Google Scholar]
- 32.Buxbaum LU. A detrimental role for IgG and FcgammaR in Leishmania mexicana infection. Immunol Res. 2008;42:197–209. doi: 10.1007/s12026-008-8074-5. [DOI] [PubMed] [Google Scholar]
- 33.Buxbaum LU, Scott P. Interleukin 10- and Fcgamma receptor-deficient mice resolve Leishmania mexicana lesions. Infect Immun. 2005;73:2101–2108. doi: 10.1128/IAI.73.4.2101-2108.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ioan-Facsinay A, de Kimpe SJ, Hellwig SM. FcgammaRI (CD64) contributes substantially to severity of arthritis, hypersensitivity responses, and protection from bacterial infection. Immunity. 2002;16:391–402. doi: 10.1016/s1074-7613(02)00294-7. [DOI] [PubMed] [Google Scholar]
- 35.Kima PE, Constant SL, Hannum L. Internalization of Leishmania mexicana complex amastigotes via the Fc receptor is required to sustain infection in murine cutaneous leishmaniasis. J Exp Med. 2000;191:1063–1068. doi: 10.1084/jem.191.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Padigel UM, Farrell JP. Control of infection with Leishmania major in susceptible BALB/c mice lacking the common gamma-chain for FcR is associated with reduced production of IL-10 and TGF-beta by parasitized cells. J Immunol. 2005;174:6340–6345. doi: 10.4049/jimmunol.174.10.6340. [DOI] [PubMed] [Google Scholar]
- 37.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 38.Anderson CF, Gerber JS, Mosser DM. Modulating macrophage function with IgG immune complexes. J Endotoxin Res. 2002;8:477–481. doi: 10.1179/096805102125001118. [DOI] [PubMed] [Google Scholar]
- 39.Pearson RD, Naidu TG, Young AC, de Alencar JE, Romito R, Davis JS. Circulating immune complexes and rheumatoid factors in visceral leishmaniasis. J Infect Dis. 1983;147:1102. doi: 10.1093/infdis/147.6.1102. [DOI] [PubMed] [Google Scholar]
- 40.Salama MM. Rheumatoid factor among several cases of visceral leishmaniasis. J Egypt Soc Parasitol. 1990;20:837–839. [PubMed] [Google Scholar]
- 41.Oliveira AV, Rossi MA, Rogue-Barreira MC. The potential role of Leishmania antigens and immunoglobulins in the pathogenesis of glomerular lesions of hamsters infected with Leishmania donovani. Ann Trop Med Parasitol. 1985;79:539–543. doi: 10.1080/00034983.1985.11811960. [DOI] [PubMed] [Google Scholar]
- 42.Wanasen N, Xin L, Soong L. Pathogenic role of B cells and antibodies in murine Leishmania amazonensis infection. Int J Parasitol. 2008;38:417–429. doi: 10.1016/j.ijpara.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lucas M, Zhang X, Prasanna V, Mosser DM. ERK activation following macrophage FcgammaR ligation leads to chromatin modifications at the IL-10 locus. J Immunol. 2005;175:469–477. doi: 10.4049/jimmunol.175.1.469. [DOI] [PubMed] [Google Scholar]
- 44.Yang Z, Mosser DM, Zhang X. Activation of the MAPK, ERK, following Leishmania amazonensis infection of macrophages. J Immunol. 2007;178:1077–1085. doi: 10.4049/jimmunol.178.2.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flannagan RS, Cosio G, Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol. 2009;7:355–366. doi: 10.1038/nrmicro2128. [DOI] [PubMed] [Google Scholar]
- 46.Maglione PJ, Xu J, Casadevall A, Chan J. Fc gamma receptors regulate immune activation and susceptibility during Mycobacterium tuberculosis infection. J Immunol. 2008;180:3329–3338. doi: 10.4049/jimmunol.180.5.3329. [DOI] [PubMed] [Google Scholar]
- 47.Watford WT, Moriguchi M, Morinobu A, O'Shea JJ. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003;14:361–368. doi: 10.1016/s1359-6101(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 48.Robinson CM, Nau GJ. Interleukin-12 and interleukin-27 regulate macrophage control of Mycobacterium tuberculosis. J Infect Dis. 2008;198:359–366. doi: 10.1086/589774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Almeida AS, Lago PM, Boechat N. Tuberculosis is associated with a down-modulatory lung immune response that impairs Th1-type immunity. J Immunol. 2009;183:718–731. doi: 10.4049/jimmunol.0801212. [DOI] [PubMed] [Google Scholar]
- 50.Schreiber T, Ehlers S, Heitmann L. Autocrine IL-10 induces hallmarks of alternative activation in macrophages and suppresses antituberculosis effector mechanisms without compromising T cell immunity. J Immunol. 2009;183:1301–1312. doi: 10.4049/jimmunol.0803567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Halstead SB. Immune enhancement of viral infection. Prog Allergy. 1982;31:301–364. [PubMed] [Google Scholar]
- 52.Sauter P, Hober D. Mechanisms and results of the antibody dependent enhancement of viral infections and the role in the pathogenesis of coxsackie virus B-induced diseases. Microbes Infect. 2009;11:443–451. doi: 10.1016/j.micinf.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 53.Stueckemann JA, Holth M, Swart WJ. Replication of lactate dehydrogenase-elevating virus in macrophages. 2. Mechanism of persistent infection in mice and cell culture. J Gen Virol. 1982;59:263–272. doi: 10.1099/0022-1317-59-2-263. [DOI] [PubMed] [Google Scholar]
- 54.Weiss RC, Scott FW. Antibody-mediated enhancement of disease in feline infectious peritonitis: comparisons with dengue hemorrhagic fever. Comp Immune Microbiol Infect Dis. 1981;4:175–188. doi: 10.1016/0147-9571(81)90003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olsen CW. A review of feline infectious peritonitis virus: molecular biology, immunopathogenesis, clinical aspects, and vaccination. Vet Microbiol. 1993;36:1–37. doi: 10.1016/0378-1135(93)90126-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoon KJ, Wu LL, Zimmerman JJ, Hill HT, Platt KB. Antibody-dependent enhancement (ADE) of porcine reproductive and respiratory syndrome virus (PRRSV) infection in pigs. Viral Immunol. 1996;9:51–63. doi: 10.1089/vim.1996.9.51. [DOI] [PubMed] [Google Scholar]
- 57.Burke DS. Human HIV vaccine trials: does antibody-dependent enhancement pose a genuine risk? Perspect Biol Med. 1992;35:511–530. doi: 10.1353/pbm.1992.0048. [DOI] [PubMed] [Google Scholar]
- 58.Mdurvwa EG, Ogunbiyi PO, Gakou HS, Reddy PG. Pathogenic mechanisms of caprine arthritis-encephalitis virus. Vet Res Commun. 1994;18:483–490. doi: 10.1007/BF01839425. [DOI] [PubMed] [Google Scholar]
- 59.Mealey RH, Leib SR, Littke MH, Wagner B, Horohov DW, McGuire TC. Viral load and clinical disease enhancement associated with a lentivirus cytotoxic T lymphocyte vaccine regimen. Vaccine. 2009;27:2453–2468. doi: 10.1016/j.vaccine.2009.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Porter AD, Larsen AE, Porter HG. The pathogenesis of Aleutian disease of mink. II Enhancement of tissue lesions following the administration of a killed virus vaccine or passive antibody. J Immunol. 1972;109:1–7. [PubMed] [Google Scholar]
- 61.Kanno H, Wohlinbarger JB, Bloom ME. Aleutian mink disease parvovirus infection of mink macrophages and human macrophage cell line *937: demonstration of antibody-dependent enhancement of infection. J Virol. 1993;67:7017–7024. doi: 10.1128/jvi.67.12.7017-7024.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Best SM, Bloom ME. Aleutian mink disease parvovirus. In: Kerr JR, Cotmore SF, Bloom ME, Linden RM, Parrish CR, editors. The parvoviruses. Hodder Arnold; London: 2006. pp. 457–471. [Google Scholar]
- 63.Gomez-Villamandos JC, Carrasco L, Bautista MJ. African swine fever and classical swine fever: a review of the pathogenesis. Dtsch Tierarztl Wochenschr. 2003;110:165–169. [PubMed] [Google Scholar]
- 64.Webster RG, Askonas BA. Cross-protection and cross reactive cytotoxic T cells induced by influenza virus vaccines in mice. Eur J Immunol. 1980;10:396–401. doi: 10.1002/eji.1830100515. [DOI] [PubMed] [Google Scholar]
- 65.Chanock RM, Parrott RH, Kapakian AZ. Possible role of immunological factors in pathogenesis of RS virus in lower respiratory tract disease. Perspec Virol. 1968;6:125–135. [Google Scholar]
- 66.Fulginiti FA, Eller JJ, Downie AW, Kempe CH. Altered reactivity to measles virus. Atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA. 1967;202:1075–1080. doi: 10.1001/jama.202.12.1075. [DOI] [PubMed] [Google Scholar]
- 67.King AA, Sands JJ, Porterfield JS. Antibody-mediated enhancement of rabies virus infection in a mouse macrophage cell line (P388D1) J Gen Virol. 1984;65:1091–1093. doi: 10.1099/0022-1317-65-6-1091. [DOI] [PubMed] [Google Scholar]
- 68.Prabhakar BS, Nathanson N. Acute rabies deaths mediated by antibody. Nature. 1981;290:590–5991. doi: 10.1038/290590a0. [DOI] [PubMed] [Google Scholar]
- 69.Tirado SM, Yoon KJ. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003;16:69–86. doi: 10.1089/088282403763635465. [DOI] [PubMed] [Google Scholar]
- 70.Burke DS. Human HIV vaccine trials: does antibody-dependent enhancement pose a genuine risk? Perspect Biol Med. 1992;35:511–530. doi: 10.1353/pbm.1992.0048. [DOI] [PubMed] [Google Scholar]
- 71.Delgado MF, Coviello S, Monsalvo AC. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Halstead SB, O'Rourke EJ, Allison AC. Dengue viruses and mononuclear phagocytes: II. Identity of blood and tissue leukocytes supporting in vitro infection. J Exp Med. 1977;146:218–228. doi: 10.1084/jem.146.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gollins SW, Porterfield JS. Flavivirus infection enhancement in macrophages: radioactive and biological studies on the effect of antibody on virus fate. J Gen Virol. 1984;65:1261–1272. doi: 10.1099/0022-1317-65-8-1261. [DOI] [PubMed] [Google Scholar]
- 74.Gollins SW, Porterfield JS. Flavivirus infection enhancement in macrophages: an electron microscopic study of viral cellular entry. J Gen Virol. 1985;66:1969–1982. doi: 10.1099/0022-1317-66-9-1969. [DOI] [PubMed] [Google Scholar]
- 75.Olsen C, Scott F. Evaluation of antibody-dependent enhancement of feline infectious peritonitis virus infectivity using in situ hybridization. Microb Pathog. 1993;14:275–285. doi: 10.1006/mpat.1993.1027. [DOI] [PubMed] [Google Scholar]
- 76.Robinson WE, Montefiori DC, Gillespie DH, Mitchell WM. Complement-mediated, antibody-dependent enhancement of HIV-1 infection in vitro is characterized by increased protein and RNA syntheses and infectious virus release. J Acquir Immune Defic Syndr. 1989;2:33–42. [PubMed] [Google Scholar]
- 77.Linn ML, Aaskov JG, Suhrbier A. Antibody-dependent enhancement and persistence in macrophages of an arbovirus associated with arthritis. J Gen Virol. 1996;77:407–411. doi: 10.1099/0022-1317-77-3-407. [DOI] [PubMed] [Google Scholar]
- 78.Suhrbier A, Mahalingam S. The immunobiology of viral arthritides. Pharmacol Ther. 2009;124:301–308. doi: 10.1016/j.pharmthera.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 79.Lidbury BA, Mahalingam S. Specific ablation of antiviral gene expression in macrophages by antibody-dependent enhancement of Ross River virus infection. J Virol. 2000;74:8376–8381. doi: 10.1128/jvi.74.18.8376-8381.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mahalingam S, Lidbury BA. Suppression of lipopolysaccharide-induced antiviral transcription factor (STAT-1 and NF-kB) complexes by antibody-dependent enhancement of macrophage infection by Ross River virus. Proc Natl Acad Sci USA. 2002;99:13819–13824. doi: 10.1073/pnas.202415999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suhrbier A, La Linn M. Suppression of antiviral responses by antibody-dependent enhancement of macrophage infection. Trends Immunol. 2003;24:165–168. doi: 10.1016/s1471-4906(03)00065-6. [DOI] [PubMed] [Google Scholar]
- 82.Halstead SB, O'Rourke EJ. Antibody-enhanced dengue virus infection in primate leukocytes. Nature. 1977;265:739–741. doi: 10.1038/265739a0. [DOI] [PubMed] [Google Scholar]
- 83.Halstead SB. Neutralization and antibody dependent enhancement of dengue viruses. Adv Virus Research. 2003;60:421–467. doi: 10.1016/s0065-3527(03)60011-4. [DOI] [PubMed] [Google Scholar]
- 84.Halstead SB, Venkateshan CN, Gentry MK, Larsen LK. Heterogeneity of infection enhancement of dengue 2 strains by monoclonal antibodies. J Immunol. 1984;132:1529–1532. [PubMed] [Google Scholar]
- 85.Dejnirattisai W, Jumnainsong A, Onsirisakul N. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 2010;328:745–748. doi: 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kliks SC, Nimmannitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg. 1988;38:411–419. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- 87.Jessie K, Fong MY, Devi S, Lam SK, Wong KT. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in site hybridization. J Infect Dis. 2004;189:1411–1418. doi: 10.1086/383043. [DOI] [PubMed] [Google Scholar]
- 88.Balsitis SJ, Coloma J, Castro G. Tropism of dengue virus in mice and humans defined by viral nonstructural protein 3-specific immunostaining. Am J Trop Med Hyg. 2009;80:416–424. [PubMed] [Google Scholar]
- 89.Cohen SN, Halstead SB. Shock associated with dengue infection. I. Clinical and physiologic manifestations of dengue hemorrhagic fever in Thailand, 1964. J Pediatrics. 1966;68:448–456. doi: 10.1016/s0022-3476(66)80249-4. [DOI] [PubMed] [Google Scholar]
- 90.Bethell DB, Flobbe K, Cao XT. Pathophysiologic and prognostic role of cytokines in dengue hemorrhagic fever. J Infect Dis. 1998;177:778–782. doi: 10.1086/517807. [DOI] [PubMed] [Google Scholar]
- 91.Green S, Vaughn DW, Kalayanarooj S. Elevated plasma interleukin-10 levels in acute dengue correlate with disease severity. J Med Virol. 1999;59:329–334. [PubMed] [Google Scholar]
- 92.Chareonsirisuthigul T, Kalayanarooj S, Ubol S. Dengue virus (DENV) antibody-dependent enhancement of infection upregulates the production of anti-inflammatory cytokines, but suppresses anti-DENV free radical and pro-inflammatory cytokine production, in THP-1 cells. J Gen Virol. 2007;88:365–375. doi: 10.1099/vir.0.82537-0. [DOI] [PubMed] [Google Scholar]
- 93.Ubol S, Phuklia W, Kalayanarooj S, Modhiran N. Mechanisms of immune evasion induced by a complex of dengue virus and preexisting enhancing antibodies. J Infect Dis. 2010;201:923–935. doi: 10.1086/651018. [DOI] [PubMed] [Google Scholar]
- 94.Chen LC, Lei HY, Liu CC. Correlation of serum levels of macrophage migration inhibitory factor with disease severity and clinical outcome in dengue patients. Am J Trop Med Hyg. 2006;74:142–147. [PubMed] [Google Scholar]
- 95.Simmons CP, Popper S, Dolocek C. Patterns of host genome-wide gene transcript abundance in the peripheral blood of patients with acute dengue hemorrhagic fever. J Infect Dis. 2007;195:1097–1107. doi: 10.1086/512162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nguyen TH, Lei HY, Nguyen TL. Dengue hemorrhagic fever in infants: a study of clinical and cytokine profiles. J Infect Dis. 2004;189:221–232. doi: 10.1086/380762. [DOI] [PubMed] [Google Scholar]
- 97.Ubol S, Masrinoul P, Chaijaruwanich J, Kalayanarooj S, Charoensirisuthikul T, Kasisith J. Differences in global gene expression in peripheral blood mononuclear cells indicate a significant role of the innate responses in progression of dengue fever but not dengue hemorrhagic fever. J Infect Dis. 2008;197:1459–1467. doi: 10.1086/587699. [DOI] [PubMed] [Google Scholar]
- 98.Shresta S, Kyle JL, Snider HM, Basavapatna M, Beatty PR, Harris E. Interferon-dependent immunity is essential for resistance to primary dengue virus infection in mice, whereas T- and B-cell-dependent immunity are less critical. J Virol. 2004;78:2701–2710. doi: 10.1128/JVI.78.6.2701-2710.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shresta S, Sharar KL, Prigozhin DM, Snider HM, Beatty PR, Harris E. Critical roles for both STAT1-dependent and STAT1-independent pathways in the control of primary dengue virus infection in mice. J Immunol. 2005;175:3946–3954. doi: 10.4049/jimmunol.175.6.3946. [DOI] [PubMed] [Google Scholar]
- 100.Navarro-Sanchez E, Despres P, Cedillo-Barron L. Innate immune responses to dengue virus. Arch Med Res. 2005;36:425–435. doi: 10.1016/j.arcmed.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 101.Vaughn DW, Green S, Kalayanarooj S. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 102.Libraty DH, Endy TP, Houng HS. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J Infect Dis. 2002;185:1213–1221. doi: 10.1086/340365. [DOI] [PubMed] [Google Scholar]
- 103.Hsieh MF, Lai SL, Chen JP. Both CXCR3 and CXCL10/IFN-inducible protein 10 are required for resistance to primary infection by dengue virus. J Immunol. 2006;177:1855–1863. doi: 10.4049/jimmunol.177.3.1855. [DOI] [PubMed] [Google Scholar]
- 104.Chen JP, Lu HL, Lai SL. Dengue virus induces expression of CXC chemokine ligand 10/IFN-gamma-inducible protein 10, which competitively inhibits viral binding to cell surface heparan sulfate. J Immunol. 2006;177:3185–3192. doi: 10.4049/jimmunol.177.5.3185. [DOI] [PubMed] [Google Scholar]
- 105.Miller JL, deWet BJ, Martinez-Pomares L. The mannose receptor mediates dengue virus infection of macrophages. PLoS Pathog. 2008;4:e17. doi: 10.1371/journal.ppat.0040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Boonnak K, Slike BM, Burgess TH. Role of dendritic cells in antibody-dependent enhancement of dengue virus infection. J Virol. 2008;82:3939–3951. doi: 10.1128/JVI.02484-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chaturvedi UC, Raghupathy R, Pasca AS. Shift from a Th1-type response to Th1-type in dengue haemorrhagic fever. Curr Sci. 1999;76:63–69. [Google Scholar]
- 108.Zellweger RM, Prestwood TR, Shresta S. Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell Host Microbe. 2010;7:128–139. doi: 10.1016/j.chom.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Balsitis SJ, Williams KL, Lachica R. Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS Pathog. 2010;6:e1000790. doi: 10.1371/journal.ppat.1000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Halstead SB, Porterfield JS, O'Rourke EJ. Enhancement of dengue virus infection in monocytes by flavivirus antisera. Am J Trop Med Hyg. 1980;29:638–642. doi: 10.4269/ajtmh.1980.29.638. [DOI] [PubMed] [Google Scholar]
- 111.Putvatana R, Yoksan S, Chayayodhin T, Bhamarapravati N, Halstead SB. Absence of dengue 2 infection enhancement in human sera containing Japanese encephalitis antibodies. Am J Trop Med Hyg. 1984;33:288–294. doi: 10.4269/ajtmh.1984.33.288. [DOI] [PubMed] [Google Scholar]
- 112.Petersen NC, Boyle JF. Immunologic phenomena in the effusive form of feline infectious peritonitis. Am J Vet Res. 1980;41:868–876. [PubMed] [Google Scholar]
- 113.Vennema H, DeGroot RJ, Harbour DA. Early death after feline infectious peritonitis challenge due to recombinant vaccinia virus immunization. J Virol. 1990;64:1407–1409. doi: 10.1128/jvi.64.3.1407-1409.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vennema H, Poland A, Foley J, Pedersen NC. Feline infectious peritonitis viruses arise by mutation from endemic feline enteric coronaviruses. Virology. 1998;243:150–157. doi: 10.1006/viro.1998.9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Porter DDL, Larsen AE, Porter HG. Aleutian disease of mink. Adv Immunol. 1980;29:261–286. doi: 10.1016/s0065-2776(08)60046-2. [DOI] [PubMed] [Google Scholar]
- 116.Porter DD, Larsen AE, Porter HG. The pathogenesis of Aleutian disease of mink. I. In vivo viral replication and the host antibody response to viral antigen. J Exp Med. 1969;130:575–593. doi: 10.1084/jem.130.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cancel-Tirado SM, Evans RB, Yoon KJ. Monoclonal antibody analysis of porcine reproductive and respiratory syndrome virus epitopes associated with antibody-dependent enhancement and neutralization of virus infection. Vet Immunol Immunopathol. 2004;102:249–262. doi: 10.1016/j.vetimm.2004.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vanhee M, Costers S, Van Breedam W, Geldhof MF, Van Doorsselaere J, Nauwynck HJ. A variable region in GP4 of European-type porcine reproductive and respiratory syndrome virus induces neutralizing antibodies against homologous but not heterologous virus strains. Viral Immunol. 2010;23:403–413. doi: 10.1089/vim.2010.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kimman TG, Cornelissen LA, Moormann RJ, Rebel JM, Stockhofe-Zurwieden N. Challenges for porcine reproductive and respiratory syndrome virus (PRRSV) vaccinology. Vaccine. 2009;27:3704–3718. doi: 10.1016/j.vaccine.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 120.Van Breedam W, Van Gorp H, Zhang JQ, Crocker PR, Delputte PL, Nauwynck HJ. The M/GP(5) glycoprotein complex of porcine reproductive and respiratory syndrome virus binds the sialoadhesin receptor in a sialic acid-dependent manner. PLoS Pathog. 2010;6:e1000730. doi: 10.1371/journal.ppat.1000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jung K, Renukaradhya GJ, Alekseev KP, Fang Y, Tang Y, Saif LJ. Porcine reproductive and respiratory syndrome virus modifies innate immunity and alters disease outcome in pigs subsequently infected with porcine respiratory coronavirus: implications for respiratory viral co-infections. J Gen Virol. 2009;90:2713–2723. doi: 10.1099/vir.0.014001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 123.Moi ML, Lim CK, Takasaki T, Kurane I. Involvement of the Fc{gamma} receptor IIA cytoplasmic domain in antibody-dependent enhancement of dengue virus infection. J Gen Virol. 2010;91:103–111. doi: 10.1099/vir.0.014829-0. [DOI] [PubMed] [Google Scholar]
- 124.Fink J, Gu F, Vasudevan SG. Role of T cells, cytokines and antibody in dengue fever and dengue haemorrhagic fever. Rev Med Virol. 2006;16:263–275. doi: 10.1002/rmv.507. [DOI] [PubMed] [Google Scholar]