Cytokine-dependent but acquired immunity-independent arthritis caused by DNA escaped from degradation (original) (raw)
Abstract
DNase II digests the chromosomal DNA in macrophages after apoptotic cells and nuclei from erythroid precursors are engulfed. The DNase II-null mice develop a polyarthritis that resembles rheumatoid arthritis. Here, we showed that when bone marrow cells from the DNase II-deficient mice were transferred to the wild-type mice, they developed arthritis. A deficiency of Rag2 or a lack of lymphocytes accelerated arthritis of the DNase II-null mice, suggesting that the DNase II−/− macrophages were responsible for triggering arthritis, and their lymphocytes worked protectively. A high level of TNFα, IL-1β, and IL-6 was found in the affected joints of the DNase II-null mice, suggesting an inflammatory-skewed cytokine storm was established in the joints. A lack of TNFα, IL-1β, or IL-6 gene blocked the expression of the other cytokine genes as well and inhibited the development of arthritis. Neutralization of TNFα, IL-1β, or IL-6 had a therapeutic effect on the developed arthritis of the DNase II-null mice, indicating that the cytokine storm was essential for the maintenance of arthritis in the DNase II-deficient mice. Methotrexate, an antimetabolite that is often used to treat patients with rheumatoid arthritis, had a therapeutic effect with the DNase II-null mice. These properties of arthritis in the DNase II-null mice were similar to those found in human systemic-onset juvenile idiopathic arthritis or Still's disease, indicating that the DNase II-null mice are a good animal model of this type of arthritis.
Keywords: inflammation, macrophages, apoptosis, DNase II, engulfment
Rheumatoid arthritis (RA), defined as a proliferative inflammation of the synovial membranes in multiple joints, is a chronic disease that afflicts up to 0.5–1% of the population (1). RA has been considered an autoimmune disease that involves T cells (2), B cells, or autoantibodies (3), macrophages (4), and inflammatory cytokines, such as TNFα, IL-1β, and IL-6 (5). However, the etiology of RA remains elusive, particularly owing to its complex heterogeneity.
Each mammalian cell carries about 6 pg of DNA, which is actively degraded by a group of enzymes (DNases) in physiological situations (6, 7). DNase II located in lysosomes (8) digests the DNA of apoptotic cells and of nuclei expelled from erythroid precursors (9, 10). DNase II−/− mice accumulate undigested DNA in the lysosomes of macrophages, which activates the macrophages to produce IFNβ. The elevated level of IFNβ causes lethal anemia in mouse embryos (11). DNase II−/− mice that also lack the type I IFN receptor (IFN-IR−/−) can live to adulthood, but develop chronic polyarthritis resembling RA (12). When the DNase II gene is inducibly deleted in adult mice, using the Mx1-Cre/loxP system, the mice (DNase IIΔ/−) also develop polyarthritis.
In both types of DNase II-null mice (DNase II−/−IFN-IR−/− and DNase IIΔ/−), the joints start to swell visibly around 2 mo of age, and the swelling gets worse over time. At this stage, the digits are deformed and difficult to bend at the joints. Histologically, cartilage destruction and bone erosion are evident in the affected joints. Proliferated synoviocytes, macrophages, and fibroblasts make up the pannus-like structure in the joints, where lymphocytes and neutrophils are also present. Genes for matrix metalloproteinase (MMP)-3 and inflammatory cytokines, including TNFα, IL-1β, and IL-6, are strongly activated in the affected joints.
Here we showed that the lack of the DNase II gene in bone marrow-derived cells is sufficient for the development of arthritis in the DNase II-null mice. The inhibition of TNFα, IL-1β, or IL-6 function blocked the gene expression for all of the inflammatory cytokines (TNFα, IL-1β, and IL-6) in the joints, and prevented arthritis. On the other hand, the lack of lymphocytes resulting from a Rag2-null mutation accelerated the development of arthritis. Methotrexate and anticytokine therapies, which are widely used to treat RA in humans, were also effective in the DNase II-null mice. The pathology of the RA-like arthritis in the DNase II-null mice and its response to cytokine inhibition are similar to systemic-onset juvenile idiopathic arthritis (soJIA). We propose that the activation of macrophages that leads to a cytokine storm in the joints can be one of the etiologies of RA.
Results
Deletion of the DNase II Gene in Bone Marrow-Derived Cells Causes Arthritis.
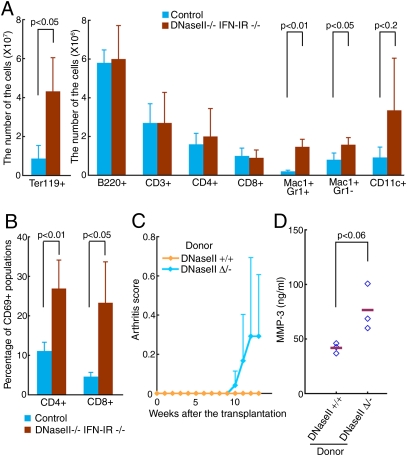
To investigate whether the immune system is activated in the DNaseII-null mice, we analyzed by FACS their splenocytes. At age 1–2.5 mo, around the onset of arthritis, DNase II−/−IFN-IR−/− mice developed splenomegaly, which was mainly caused by the expansion of Ter119+ erythroid population (Fig. 1_A_). The number of CD3+ T cells and B220+ B cells in the DNase II−/−IFN-IR−/− mice was comparable to that in control mice, but the populations of activated CD4+ and CD8+ T cells, estimated by the CD69 expression, were increased from 11 to 27%, and 5 to 23%, respectively, by the DNase II null mutation (Fig. 1_B_). The serum concentration of IgG and anti-double stranded DNA antibodies was elevated in DNase II−/−IFN-IR−/− mice (12), and the number of neutrophils, macrophages, and dendritic cells increased by seven-, two-, and threefold, respectively (Fig. 1_A_). To examine the involvement of hematopoietic cells in the arthritis of the DNase II-null mice, 1-mo-old WT mice were exposed to γ-rays, and bone marrow cells from DNase IIΔ/− (poly(I):poly(C)-injected DNaseIIflox/−Mx1-CreT mice) or WT mice were transplanted i.v. into the irradiated mice. The mice that received the DNase II-null, but not the WT, bone marrow cells started to develop arthritis 10 wk after the transplantation (Fig. 1_C_), accompanied by an increase in serum MMP-3 (Fig. 1_D_), a marker used to diagnose arthritis (13), indicating that the loss of the DNase II gene in the bone marrow-derived cells was sufficient to cause this arthritis.
Fig. 1.
Activation of lymphocytes in DNase II-null mice and bone marrow transfer. (A and B) A FACS analysis of splenocytes from 1- to 2.5-mo-old DNase II−/−IFN-IR−/− and DNase II+/− or +/+IFN-IR−/− littermate mice (control) (n = 4 for each group). The average cell number of the indicated cell type (A), and the average percentage of CD69+ cells in the CD4+ or CD8+ T cell population (B) are shown with SD. (C and D) Cells from the bone marrow of DNase IIΔ/− or WT mice were transplanted into irradiated 1-mo-old WT mice. Mean arthritis scores at the indicated time after the transplantation were plotted with SD (n = 3–4 for each condition) (C). Three months after the transplantation, the serum MMP-3 was quantified by ELISA. The mean values are indicated (bars) (D). In A, B, and D, P value is shown when it is <0.2.
No Requirement of Lymphocytes for the Arthritis in DNase II-Null Mice.
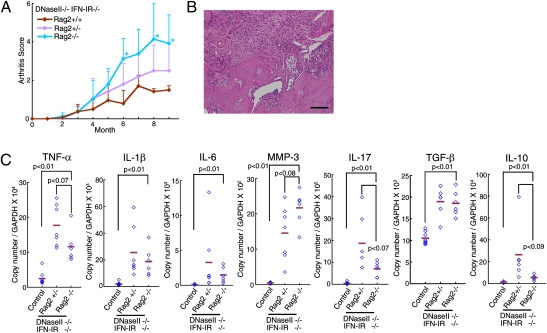
Lymphocytes are involved in various models of arthritis (2, 3). On the other hand, when a Rag2−/− mutation that blocks the development of lymphocytes was introduced into DNase II−/−IFN-IR−/− mice, the DNase II−/−IFN-IR−/−Rag2−/− mice developed arthritis with a more accelerated time frame than the DNase II−/−IFN-IR−/−Rag2+/+ mice (Fig. 2_A_). A histological analysis of the joints of the DNase II−/−IFN-IR−/−Rag2−/− mice revealed massive inflammation accompanied by pannus formation, cartilage destruction, and bone erosion (Fig. 2_B_). The MMP-3 gene was up-regulated by more than 40-fold in the joints of the DNase II−/−IFN-IR−/−Rag2−/− mice, which was significantly higher than in DNase II−/−IFN-IR−/−Rag2+/− mice (Fig. 2_C_). The genes for the inflammatory cytokines TNFα, IL-1β, and IL-6 were expressed in the joints of the DNase II−/−IFN-IR−/−Rag2−/− mice, although at a slightly lower level than in those of the Rag2-expressing mice. The genes for the antiinflammatory cytokines TGFβ and IL-10 are expressed in the joints of RA patients as a feedback response to the chronic inflammation (14). These genes were also up-regulated 1.6- and 20.0-fold, respectively, in the DNase II−/−IFN-IR−/−Rag2+/− joints compared with the WT joints (Fig. 2_C_). The TGFβ mRNA level was not significantly affected by the Rag2-null mutation, but the IL-10 mRNA level was reduced to 20% of that in the Rag2+/−DNase II-null mice. These results suggest that most of the IL-10 in the affected joints of the DNase II-null mice is produced by the lymphocytes recruited to the joints, and that IL-10 inhibits the development of the arthritis, which seems to be consistent with the previous report (15).
Fig. 2.
Development of arthritis in DNase II−/−IFN-IR−/−Rag2−/− mice. (A) Arthritis scores of DNase II−/−IFN-IR−/−Rag2+/+, DNase II−/−IFN-IR−/−Rag2+/−, and DNase II−/−IFN-IR−/−Rag2−/− mice were plotted with SD (n = 3–11 for each). P values were calculated between the DNase II−/−IFN-IR−/−Rag2+/+ and DNase II−/−IFN-IR−/−Rag2−/−, and when the value was <0.05, it is shown by *. (B) A section through a joint of a 12-mo-old DNase II−/−IFN-IR−/−Rag2−/− mouse stained with H&E. (Scale bar, 0.1 mm.) (C) The mRNA level of the indicated genes in the joints of 11–14-mo-old WT, DNase II−/−IFN-IR−/−Rag2+/−, and DNase II−/−IFN-IR−/−Rag2−/− mice was quantified by real-time PCR and is expressed relative to the GAPDH mRNA level. Bars indicate the mean value. P values are shown.
IL-17 is produced by Th17 cells, and it seems to contribute to human RA (16). The mRNA encoding IL-17A, a representative cytokine in the IL-17 family, was about 50-fold higher in the inflamed joints of DNase II-null mice than in those of WT mice. The Rag2-mutation significantly reduced the IL17A mRNA level in the joints, but it was still 19 times greater than in control mice, indicating that IL-17 is produced not only by Th17 cells but also by nonlymphoid cells in the joints as reported previously (17).
TNFα-Dependent Arthritis.
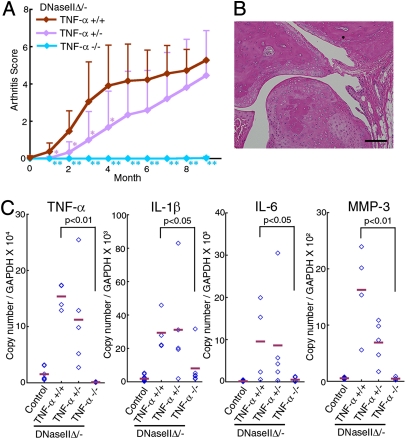
We previously showed that a neutralizing anti-TNFα mAb inhibits the development of arthritis in DNase II-null mice (12). To confirm the requirement for TNFα, DNase IIΔ/− mice were established on a background of the WT (TNFα+/+), heterozygous (TNFα+/−), or homozygous (TNFα −/−) null mutation of the TNFα gene. As shown in Fig. 3_A_, the DNase IIΔ/−TNFα+/− mice developed arthritis with delayed kinetics, whereas the DNase IIΔ/−TNFα−/− mice did not develop arthritis even 10 mo after poly(I):poly(C) injection. A histological analysis of the DNase IIΔ/−TNFα−/− mice showed that although heavy accumulation of DNA could be found in their bone marrow (Fig. S1_A_), the synovial tissues and joints were intact (Fig. 3_B_). The MMP-3 mRNA level in the joints was also normal (Fig. 3_C_). The deletion of the TNFα gene blocked the up-regulation of the IL-1β and IL-6 mRNA in the joints of the DNase II-null mice. That is, the IL-1β and IL-6 mRNA levels 9–12 mo after the poly(I):poly(C) injection were equivalent to their levels in the poly(I):poly(C)-injected control littermate mice (DNase IIflox/+Mx1-CreT) (Fig. 3_C_). In agreement with the delayed but eventually full-blown development of the arthritis in the DNase IIΔ/−TNFα+/− mice, the IL-1β and IL-6 mRNA levels in their affected joints 9–12 mo after the poly(I):poly(C) injection were similar to those observed in the DNase IIΔ/−TNFα+/+ mice. We thus concluded that TNFα plays an essential and rate-limiting role in the arthritis of DNase II-null mice, and its expression in the joints up-regulates the expression of other cytokine genes.
Fig. 3.
TNFα-dependent arthritis. (A) Arthritis scores of the DNase IIΔ/−TNFα+/+, DNase IIΔ/−TNFα+/−, and DNase IIΔ/−TNFα−/− mice (n = 6–9 for each) at the indicated time after poly(I):poly(C) injection, with SD. P values were calculated between the DNase IIΔ/−TNFα+/+ and DNase IIΔ/−TNFα−/−, and between the DNase IIΔ/−TNFα+/+ and DNase IIΔ/−TNFα+/−. *P = 0.01–0.05, **P < 0.01. (B) Section of a joint from a DNase IIΔ/−TNFα−/− mouse 11 mo after poly(I):poly(C) injection, stained with H&E. (Scale bar, 0.1 mm.) (C) RNA from the joints of WT, DNase IIΔ/−TNFα+/+, DNase IIΔ/−TNFα+/−, or DNase IIΔ/-TNFα−/− mice 9–12 mo after poly(I):poly(C) injection, was analyzed by real-time PCR for the indicated mRNA. The data are expressed relative to the GAPDH mRNA level with horizontal bars for the mean value. P values are shown.
IL-6 and IL-1β, but Not IL-18 Dependence of Arthritis.
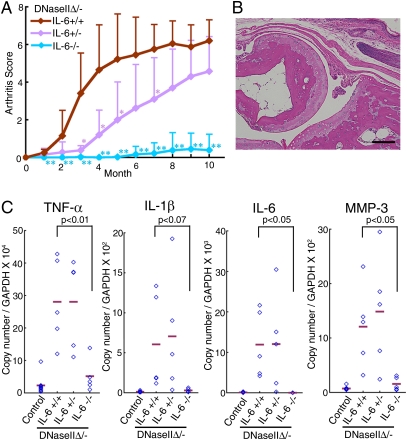
IL-6 plays an important pathological role in a number of inflammatory diseases (18). To examine its role in arthritis of the DNase II-null mice, the DNase IIflox/− Mx1-CreT locus was transferred to IL-6−/− mice, and the mice were treated with poly(I):poly(C). As shown in Fig. S1_A_, the loss of the IL-6 gene had no effect on the accumulation of DNA in the macrophages in the bone marrow. However, the development of arthritis in the DNaseIIΔ/−IL-6+/− mice was delayed, and a null mutation of the IL-6 gene completely blocked the development of arthritis (Fig. 4_A_). No pannus was observed in the joints, and the cartilage and bone were intact (Fig. 4_B_), and the MMP-3 gene was not up-regulated in the joints of the DNaseIIΔ/−IL-6−/− mice (Fig. 4_C_). The lack of IL-6 completely blocked the up-regulation of the TNFα and IL-1β gene expression in the joints of the DNase IIΔ/− mice, indicating that IL-6 transcriptionally regulates the expression of the TNFα and IL-1β genes in the DNase II-null mice.
Fig. 4.
IL-6–dependent arthritis. (A) Arthritis scores of the DNaseIIΔ/−IL-6+/+, DNase IIΔ/−IL-6+/−, and DNase IIΔ/−IL-6 −/− mice (n = 5–7 per genotype) after the injection of poly(I):poly(C), plotted with SD. P values were calculated between DNase IIΔ/−IL-6+/+ and DNase IIΔ/−IL-6−/− and between DNase IIΔ/−IL-6+/+ and DNase IIΔ/−IL-6 +/−. *P = 0.01–0.05; **P < 0.01. (B) A section of a joint from a DNase IIΔ/−IL-6−/− mouse 11 mo after the poly(I):poly(C) injection, stained with H&E. (Scale bar, 0.1 mm.) (C) Real-time PCR analysis of the joint RNA from WT, DNase IIΔ/−IL-6+/+, DNase IIΔ/−IL-6+/−, and DNase IIΔ/−IL-6−/− mice 11–12 mo after the poly(I):poly(C) injection for the indicated mRNA. The data are expressed relative to the GAPDH mRNA level with the mean value (bars). P values are shown.
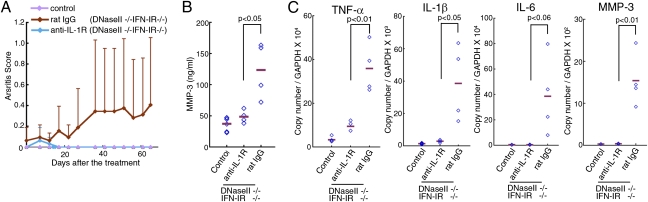
We next evaluated the contribution of IL-1β to the development of arthritis by treating the mice with an mAb (clone 35F5) against IL-1 receptor (19). Starting when they were 1 mo old, DNase II−/−IFN-IR−/− mice were given an i.p. injection every 5 d of rat control IgG or 35F5 mAb. By the age of 3.5 mo, the DNase II−/−IFN-IR−/− mice treated with the control IgG showed joint swelling and high serum MMP-3 (Fig. 5 A and B). In contrast, the joint swelling and high serum MMP-3 level were not observed in the mice that received the 35F5 mAb. The levels of TNFα, IL-1β, and IL-6 mRNA in the joints of the DNase II-null mice were reduced to a negligible level by blocking the IL-1 receptor signal (Fig. 5_C_). Thus, the IL-1 system is also required for the development of arthritis in the DNase II-null mice, and once the IL-1β gene is activated in the joints, it regulates the expression of the TNFα and IL-6 genes.
Fig. 5.
IL-1β–dependent arthritis. Rat anti-IL-1R mAb or control IgG was injected into 1-mo-old DNase II−/−IFN-IR−/− mice (n = 4 per group). (A) The arthritis scores were determined at the indicated time and are plotted with SD. (B) At 2.5 mo after the treatment, the serum MMP-3 level was determined. Horizontal bars indicate the mean value. The MMP-3 level of the age-matched WT littermate mice is also shown. (C) RNA from the joints of DNase II−/−IFN-IR−/− mice treated with rat IL-1R mAb or control IgG was analyzed by real-time PCR 2.5 mo after the treatment for the indicated mRNA. Values are expressed relative to the GADPH mRNA level with horizontal bars for the mean values. Values for the joints of the age-matched WT littermate mice are also shown. P values are shown for B and C.
We previously found that the DNase II-null mice have a high serum level of IL-18 (12), and IL-18 is implicated in various mouse models of RA (20). However, the DNaseIIΔ/−IL-18−/− mice developed arthritis with the same kinetics as the DNaseIIΔ/−IL-18+/+ or DNaseIIΔ/−IL-18+/− mice (Fig. S2_A_), and the pathological changes (destruction and erosion of the cartilage and bone) (Fig. S2_B_) and the expression of MMP-3 and the inflammatory cytokine genes (TNFα, IL-1β, and IL-6) in the affected joints were similar between the DNaseIIΔ/−IL-18−/− and the DNaseIIΔ/−IL-18+/+ mice (Fig. S2_C_).
Strain Independence of Arthritis in the DNase II-Null Mice.
The strain background is known to affect the severity of arthritis in various mouse models (21, 22). However, when the DNase II−/−IFN-IR−/− B6 mice were backcrossed to BALB/c mice for five generations, they developed polyarthritis with the same kinetics as the DNase II−/−IFN-IR−/− B6 mice (Fig. S3 A and B). The MMP-3 mRNA as well as the inflammatory cytokine mRNA levels in the affected joints were similar to those in the DNase II-null B6 mice (Fig. S3_C_). The shared phenotypes of the DNase II-null mice despite the different B6 or BALB/c background indicated that heterogeneity of the MHC or other loci do not have a strong effect on the arthritis of the DNase II-null mice. The MHC-independent development of arthritis in these mice seems consistent with the dispensability of acquired immunity in this model. In other mouse models, the development of arthritis is blocked when the mice are kept in specific pathogen-free conditions (23). In contrast, DNase II-null mice develop arthritis spontaneously under the specific pathogen-free conditions, suggesting that a deficiency of the DNase II gene predicts a high risk for developing arthritis.
Therapeutic Model for Rheumatoid Arthritis.
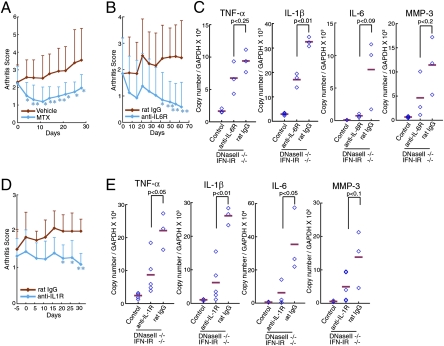
Human RA patients are treated with methotrexate (MTX) and biological agents targeted to inflammatory cytokines (TNFα, IL-1β, and IL-6) (5, 24). However, treatment with these reagents does not significantly improve established arthritis in animal models of RA such as collagen-induced arthritis (25, 26). Here, we examined the therapeutic effect of MTX, anti-IL–6 receptor, and anti-IL-1 receptor antibodies on their arthritis. When DNase II−/−IFN-IR−/− mice showing arthritis at the age of 3.5 mo were treated everyday per os (p.o.) with MTX, the joint swelling was clearly, but transiently, reduced for 10 d (Fig. 6_A_). In contrast, the joint swelling became progressively worse in the mice treated with vehicle. Similarly, the administration of the anti-IL–6 receptor mAb (MR16-1) (27) once a week or of the anti-IL-1 receptor mAb (35F5) every 5 d blocked the joint swelling (Fig. 6 B and D). The levels of TNFα, IL-1β, and IL-6 mRNAs, particularly of IL-6, were reduced by the 1 mo treatment with the anti-IL–6 receptor or anti-IL-1 receptor Ab (Fig. 6 C and E). These results confirm that a “cytokine storm” caused by several inflammatory cytokines is responsible for the development and maintenance of arthritis, in which each cytokine activates the other's gene expression and that blocking one cytokine can quell the storm and cure the disease.
Fig. 6.
Therapeutic effect of MTX, anti-IL–6R, and anti-IL-1R treatments. The DNase II−/−IFN-IR−/− mice (3.5–13 mo old) with affected joints were treated with MTX (n = 9–10 for each group) (A), anti-IL–6R (n = 9–10 for each group) (B and C), or anti-IL-1R mAb (n = 3–6 for each group) (D and E), and arthritis scores for the indicated mice are plotted with SD. After a 4-wk treatment with anti-IL–6R mAb (C) or anti-IL-1R mAb (E), the indicated mRNA level in the joints was quantified by real-time PCR and shown relative to the GAPDH mRNA. The mean value is indicated (bars). RNA from the age-matched littermate control mice was also analyzed. P values were calculated. In A, B, and D, *P = 0.01–0.05; **P < 0.01.
Discussion
In this report, we showed that the TNFα, IL-1β, and IL-6 genes were strongly expressed at the inflamed joints of DNase II-null mice, where they established a cytokine milieu that was highly skewed toward inflammation, a situation that is also called a cytokine storm. When the function of TNFα, IL-1β, or IL-6 was perturbed in mice with established arthritis, the levels of the TNFα, IL-1β, and IL-6 mRNAs dropped. These results indicated that the inflammatory-skewed cytokine milieu was sustained by the chronic expression of at least these three cytokines, and that each cytokine could activate the other's expression, creating a positive-feedback loop (Fig. S4). The fact that synovial cells could be activated in vitro by TNFα, IL-1β, and IL-6 to express these cytokines (Fig. S5) supports the above idea.
Lymphocytes, neutrophils, and macrophages were present in the affected joints of the DNase II-null mice. Accordingly, the expression of granulocyte colony-stimulating factor, and chemokines CCL-2, -3, -4, -7, and -20 were strongly up-regulated in the affected joints (Fig. S6, and data not shown), suggesting that these cytokines had recruited the lymphocytes, neutrophils, and macrophages. The expression of these cytokine and chemokine genes was completely prevented by blocking the action of TNFα, IL-1β, or IL-6. All of the pathological changes in the joints, such as the expression of MMP-3, pannus formation, and bone destruction, were also blocked when these cytokines were inhibited. These results indicate that these three cytokines play a critical role in the development of arthritis and that the inflammation-skewed cytokine milieu is fundamental for the establishment and maintenance of arthritis.
The primary defect caused by the DNase II deficiency is the macrophages’ inability to digest DNA from apoptotic cells or erythroid precursor cells. In these mice, the macrophages carrying undigested DNA were present in the bone marrow and spleen and were observed even in the DNase II-null mice lacking TNFα or IL-6, which do not develop arthritis. Interestingly, the macrophages in the affected joints did not carry undigested DNA (Fig. S1_B_). Thus, a factor(s) released from the macrophages in the bone marrow, spleen, or other tissues seems to trigger the arthritis in the joint. IFNβ is expressed by macrophages carrying undigested DNA (11), but the arthritis in the DNase II-null mice develops in the IFN type I receptor-null background, indicating that type I-IFN is dispensable for the arthritis in our model. The TNFα mRNA is expressed by macrophages carrying undigested DNA in the presence or absence of lymphocytes or IL-6 (Fig. S7_A_), and a low but significant level of TNFα could be detected in the blood of the mice. Because the systemic and constitutive expression of a TNFα transgene causes arthritis in mice (28), it is likely that TNFα triggers arthritis in the DNase II-null mice. In addition, caspase 1 was activated in the macrophages carrying undigested DNA (Fig. S7_B_). Although we could not detect IL-1β or IL-6 in the serum of the DNase II-null mice, it is possible that an undetectable level of IL-1β or IL-6 produced by the macrophages triggers arthritis by stimulating synoviocytes in the joints.
There are two DNA-sensing systems in mammals that activate the innate immunity: the Toll-like receptor (TLR) system including TLR9, which recognizes bacterial DNA in lysosomes, and the TLR-independent system, which senses viral DNA in the cytosol (29). In fetal liver macrophages carrying undigested DNA, the IFNβ gene is activated in a TLR-independent manner (30). Similarly, TLR9 was dispensable for the development of arthritis in the DNase II-null mice (Fig. S8), and the TNFα gene was constitutively activated in their bone marrow (Fig. S7_A_), indicating that the undigested DNA activates the macrophages to produce TNFα in a TLR9-independent manner. Whether recently identified DNA sensors such as DAI, HMGB1, and AIM2 (31) function in the DNase II-null macrophages remains to be studied.
The DNase II-null bone marrow-derived cells were sufficient to cause arthritis, but the lymphocytes played a protective role. This finding concurs with the lymphocyte-independent arthritis reported in TNFα-transgenic mice (32), but does not explain the good therapeutic effect of lymphocyte-blocking agents in a significant population of RA patients (2, 3). RA is a heterogeneous syndrome (33). Activated CD4+ T cells in SKG mice and the B cells in K/BxN TCR transgenic mice cause lymphocyte-dependent chronic arthritis (34). In both cases, however, the TNFα, IL-1, and IL-6 genes are activated in the joints, and a null mutation in any of these genes blocks the development of arthritis (15, 35), indicating that the proinflammation-skewed cytokine milieu in the joints is a common event. Activated macrophages, activated helper T cells, and autoimmune serum can trigger the storm. The lymphocyte-triggered arthritis would be blocked by lymphocyte-blocking agents. However, our results indicated that arthritis triggered by activated macrophages can be aggravated by lymphocytes-blocking agents. This may be similar to the previous observation that the adaptive immune system attenuates the autoimmune disease caused by the α-mannosidase-II deficiency (36). In any case, the appropriate treatment of a particular RA patient could be chosen according to whether the disease is triggered by lymphocytes or macrophages.
soJIA, also called Still's disease, constitutes about 10% of juvenile idiopathic arthritis (JIA) and is distinguished clinically from other forms of JIA by the almost equal incidence in both sexes, generalized lymphadenopathy, lack of a strong MHC association or of the involvement of acquired immunity, and a good therapeutic response to IL-1β− or IL-6–blocking agents (37, 38). A high level of IL-18 in the serum and a strong association with macrophage activation syndrome (MAS) are also characteristic of soJIA (37). These properties are observed in DNase II-null mice, supporting the idea that arthritis in DNase II-null mice is a good model of soJIA. Vastert et al. (37) proposed that soJIA is an autoinflammatory syndrome rather than a classic autoimmune disease. The pathology of arthritis of the DNase II-null mice fully agrees with this idea. Arthritis in the DNase II-null mice is caused by the activation of macrophages, due to the defect in DNA degradation. It is possible that the activation of macrophages is also the first event in soJIA. Functional soJIA-specific SNPs have been reported in IL-6 and macrophage migration inhibitory factor (MIF) (39, 40). Our results indicate that the chronic activation of macrophages owing to the lack of lysosomal enzymes or by other causes can lead to autoinflammatory syndromes like soJIA, and it will be interesting to learn whether mutation in lysosomal enzymes can be found in the soJIA patients. In any case, DNase II-null mice will contribute to our understanding of RA, particularly soJIA.
Materials and Methods
Mice.
The mice are described in SI Materials and Methods. The pathological phenotypes were compared between different genotypes using littermates or mice kept in a closed colony. Mice were housed in a specific pathogen-free facility at Kyoto University Graduate School of Medicine, Oriental Bioservices, and Chugai Pharmaceutical. All animal experiments were carried out in accordance with protocols approved by the Animal Care and Use Committee at Kyoto University Graduate School of Medicine and Chugai Pharmaceutical.
Bone Marrow Transplantation.
Bone marrow cells (CD45.2+) were collected from a 2-mo-old DNase IIΔ/− or control mouse and injected i.v. into four 1-mo-old recipient mice (CD45.1+) treated with γ-ray (10 G). The mice received drinking water containing 1 mg/mL neomycin and ampicillin for 1 mo. The replacement of blood cells was confirmed by FACS analysis for CD45.1 or CD45.2.
Treatment with MTX and Monoclonal Antibodies.
MTX was administered p.o. daily at 3 mg/g body weight. Rat anti-IL-1 receptor mAb (35F5) (19) was administered i.v. at 27 μg/g body weight, followed by i.p. injection every 5 d at 13 μg/g body weight. Rat anti-IL–6R mAb (MR16-1) (27) was administered i.v. at 100 μg/g body weight, followed by i.p. injection at 25 μg/g body weight once a week. Normal rat IgG (Cappel) was administered as a control. Four days or more after the last injection, the blood and joints were collected.
Clinical Assessment and Histology.
Swelling of the fore and hind limb was manually inspected and scored: 0, no; 0.25, faint; 0.5, mild; 1, severe swelling or deformation. The digits and paw of each limb were assessed separately. The scores were summed, and a total score (maximum 8 per mouse) was assigned. To prepare paraffin sections, tissues were fixed with 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.2) containing 4% sucrose or with 8% formaldehyde and embedded in paraffin. Joints were incubated at room temperature for 24 h in Morse's solution (10% sodium citrate and 22.5% formic acid) for decalcification before the paraffinization. The blocks were sectioned at 2–4 μm, deparaffinized, and stained with H&E.
Statistical Analysis.
Two-tailed Student's t tests were used for statistical testing between two groups.
Supplementary Material
Supporting Information
Acknowledgments
We thank A. Hicks (Roche, Nutley, NJ) for 35F5 mAb, S. Akira (Osaka University, Osaka) for TLR9−/− mice, Y. Shinkai (Kyoto University, Kyoto) for Rag2−/− mice, K. Sekikawa (National Institute of Agrobiological Sciences, Nishinomiya, Japan) for TNFα−/− mice, K. Nakanishi and H. Tsutsui (Hyogo College of Medicine) for IL-18−/− mice, and M. Kopf (Eidgenössische Technische Hochschule, Zurich) for IL-6−/− mice. We thank K. Miwa and M. Ohtani for help at the initial stages of this work, M. Nishihara for help in MTX experiments, and T. Fujimoto and K. Ishihara (Kawasaki Medical School) for advice. We thank M. Fujii and M. Harayama for secretarial assistance. This work was supported in part by grants-in-aid from the Ministry of Education, Science, Sports, and Culture in Japan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.Cope AP. T cells in rheumatoid arthritis. Arthritis Res Ther. 2008;10(Suppl 1):S1. doi: 10.1186/ar2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tedder TF. CD19: A promising B cell target for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:572–577. doi: 10.1038/nrrheum.2009.184. [DOI] [PubMed] [Google Scholar]
- 4.Kinne RW, Bräuer R, Stuhlmüller B, Palombo-Kinne E, Burmester GR. Macrophages in rheumatoid arthritis. Arthritis Res. 2000;2:189–202. doi: 10.1186/ar86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldmann M, Maini SR. Role of cytokines in rheumatoid arthritis: An education in pathophysiology and therapeutics. Immunol Rev. 2008;223:7–19. doi: 10.1111/j.1600-065X.2008.00626.x. [DOI] [PubMed] [Google Scholar]
- 6.Nagata S. DNA degradation in development and programmed cell death. Annu Rev Immunol. 2005;23:853–875. doi: 10.1146/annurev.immunol.23.021704.115811. [DOI] [PubMed] [Google Scholar]
- 7.Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Evans CJ, Aguilera RJ. DNase II: Genes, enzymes and function. Gene. 2003;322:1–15. doi: 10.1016/j.gene.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 9.Kawane K, et al. Requirement of DNase II for definitive erythropoiesis in the mouse fetal liver. Science. 2001;292:1546–1549. doi: 10.1126/science.292.5521.1546. [DOI] [PubMed] [Google Scholar]
- 10.Kawane K, et al. Impaired thymic development in mouse embryos deficient in apoptotic DNA degradation. Nat Immunol. 2003;4:138–144. doi: 10.1038/ni881. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida H, Okabe Y, Kawane K, Fukuyama H, Nagata S. Lethal anemia caused by interferon-beta produced in mouse embryos carrying undigested DNA. Nat Immunol. 2005;6:49–56. doi: 10.1038/ni1146. [DOI] [PubMed] [Google Scholar]
- 12.Kawane K, et al. Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature. 2006;443:998–1002. doi: 10.1038/nature05245. [DOI] [PubMed] [Google Scholar]
- 13.Young-Min S, et al. Biomarkers predict radiographic progression in early rheumatoid arthritis and perform well compared with traditional markers. Arthritis Rheum. 2007;56:3236–3247. doi: 10.1002/art.22923. [DOI] [PubMed] [Google Scholar]
- 14.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy—review of a new approach. Pharmacol Rev. 2003;55:241–269. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 15.Hata H, et al. Distinct contribution of IL-6, TNF-alpha, IL-1, and IL-10 to T cell-mediated spontaneous autoimmune arthritis in mice. J Clin Invest. 2004;114:582–588. doi: 10.1172/JCI21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Berg WB, Miossec P. IL-17 as a future therapeutic target for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:549–553. doi: 10.1038/nrrheum.2009.179. [DOI] [PubMed] [Google Scholar]
- 17.Ishigame H, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Kishimoto T. Interleukin-6: From basic science to medicine—40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 19.Chizzonite R, et al. Two high-affinity interleukin 1 receptors represent separate gene products. Proc Natl Acad Sci USA. 1989;86:8029–8033. doi: 10.1073/pnas.86.20.8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei XQ, Leung BP, Arthur HM, McInnes IB, Liew FY. Reduced incidence and severity of collagen-induced arthritis in mice lacking IL-18. J Immunol. 2001;166:517–521. doi: 10.4049/jimmunol.166.1.517. [DOI] [PubMed] [Google Scholar]
- 21.Horai R, et al. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J Exp Med. 2000;191:313–320. doi: 10.1084/jem.191.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips K, Kedersha N, Shen L, Blackshear PJ, Anderson P. Arthritis suppressor genes TIA-1 and TTP dampen the expression of tumor necrosis factor alpha, cyclooxygenase 2, and inflammatory arthritis. Proc Natl Acad Sci USA. 2004;101:2011–2016. doi: 10.1073/pnas.0400148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshitomi H, et al. A role for fungal beta-glucans and their receptor Dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice. J Exp Med. 2005;201:949–960. doi: 10.1084/jem.20041758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Vollenhoven RF. Treatment of rheumatoid arthritis: State of the art 2009. Nat Rev Rheumatol. 2009;5:531–541. doi: 10.1038/nrrheum.2009.182. [DOI] [PubMed] [Google Scholar]
- 25.Takagi N, et al. Blockage of interleukin-6 receptor ameliorates joint disease in murine collagen-induced arthritis. Arthritis Rheum. 1998;41:2117–2121. doi: 10.1002/1529-0131(199812)41:12<2117::AID-ART6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 26.Fiehn C, Kratz F, Sass G, Müller-Ladner U, Neumann E. Targeted drug delivery by in vivo coupling to endogenous albumin: An albumin-binding prodrug of methotrexate (MTX) is better than MTX in the treatment of murine collagen-induced arthritis. Ann Rheum Dis. 2008;67:1188–1191. doi: 10.1136/ard.2007.086843. [DOI] [PubMed] [Google Scholar]
- 27.Okazaki M, Yamada Y, Nishimoto N, Yoshizaki K, Mihara M. Characterization of anti-mouse interleukin-6 receptor antibody. Immunol Lett. 2002;84:231–240. doi: 10.1016/s0165-2478(02)00202-x. [DOI] [PubMed] [Google Scholar]
- 28.Keffer J, et al. Transgenic mice expressing human tumour necrosis factor: A predictive genetic model of arthritis. EMBO J. 1991;10:4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 30.Okabe Y, Kawane K, Akira S, Taniguchi T, Nagata S. Toll-like receptor-independent gene induction program activated by mammalian DNA escaped from apoptotic DNA degradation. J Exp Med. 2005;202:1333–1339. doi: 10.1084/jem.20051654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hornung V, Latz E. Intracellular DNA recognition. Nat Rev Immunol. 2010;10:123–130. doi: 10.1038/nri2690. [DOI] [PubMed] [Google Scholar]
- 32.Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: Implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–398. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 33.Kochi Y, Suzuki A, Yamada R, Yamamoto K. Genetics of rheumatoid arthritis: Underlying evidence of ethnic differences. J Autoimmun. 2009;32:158–162. doi: 10.1016/j.jaut.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 34.Benoist C, Mathis D. A revival of the B cell paradigm for rheumatoid arthritis pathogenesis? Arthritis Res. 2000;2:90–94. doi: 10.1186/ar73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ji H, et al. Critical roles for interleukin 1 and tumor necrosis factor alpha in antibody-induced arthritis. J Exp Med. 2002;196:77–85. doi: 10.1084/jem.20020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green RS, et al. Mammalian N-glycan branching protects against innate immune self-recognition and inflammation in autoimmune disease pathogenesis. Immunity. 2007;27:308–320. doi: 10.1016/j.immuni.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Vastert SJ, Kuis W, Grom AA. Systemic JIA: New developments in the understanding of the pathophysiology and therapy. Best Pract Res Clin Rheumatol. 2009;23:655–664. doi: 10.1016/j.berh.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokota S, et al. Therapeutic efficacy of humanized recombinant anti-interleukin-6 receptor antibody in children with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2005;52:818–825. doi: 10.1002/art.20944. [DOI] [PubMed] [Google Scholar]
- 39.Fishman D, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102:1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donn R, et al. Mutation screening of the macrophage migration inhibitory factor gene: Positive association of a functional polymorphism of macrophage migration inhibitory factor with juvenile idiopathic arthritis. Arthritis Rheum. 2002;46:2402–2409. doi: 10.1002/art.10492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information