The N-Ethyl-N-Nitrosourea-Induced Goldenticket Mouse Mutant Reveals an Essential Function of Sting in the In Vivo Interferon Response to Listeria monocytogenes and Cyclic Dinucleotides (original) (raw)
Abstract
Type I interferons (IFNs) are central regulators of the innate and adaptive immune responses to viral and bacterial infections. Type I IFNs are induced upon cytosolic detection of microbial nucleic acids, including DNA, RNA, and the bacterial second messenger cyclic-di-GMP (c-di-GMP). In addition, a recent study demonstrated that the intracellular bacterial pathogen Listeria monocytogenes stimulates a type I IFN response due to cytosolic detection of bacterially secreted c-di-AMP. The transmembrane signaling adaptor Sting (Tmem173, Mita, Mpys, Eris) has recently been implicated in the induction of type I IFNs in response to cytosolic DNA and/or RNA. However, the role of Sting in response to purified cyclic dinucleotides or during in vivo L. monocytogenes infection has not been addressed. In order to identify genes important in the innate immune response, we have been conducting a forward genetic mutagenesis screen in C57BL/6 mice using the mutagen _N_-ethyl-_N_-nitrosourea (ENU). Here we describe a novel mutant mouse strain, Goldenticket (Gt), that fails to produce type I IFNs upon L. monocytogenes infection. By genetic mapping and complementation experiments, we found that Gt mice harbor a single nucleotide variant (T596A) of Sting that functions as a null allele and fails to produce detectable protein. Analysis of macrophages isolated from Gt mice revealed that Sting is absolutely required for the type I interferon response to both c-di-GMP and c-di-AMP. Additionally, Sting is required for the response to c-di-GMP and L. monocytogenes in vivo. Our results provide new functions for Sting in the innate interferon response to pathogens.
Type I interferons (IFNs) comprise a small family of cytokines, including beta IFN (IFN-β), that signal through the type I IFN receptor (IFNAR) and exert pleiotropic effects on the immune system (27). In addition to their role in induction of an antiviral state (6), type I IFNs have many systemic effects, including stimulation of antigen presentation pathways (15) and NK and CD8+ T cell cytotoxicity (13, 21). Although predominantly thought to be critical in the response to viruses, type I IFNs are also made in response to bacterial infections, though their roles in this response appear to be complex (18).
The receptors and signaling cascades leading to induction of type I IFNs are almost as diverse as their activities. Signaling via multiple Toll-like receptors (TLRs) leads to type I IFN production, particularly in specialized cell types such as plasmacytoid dendritic cells (12). In addition, several cytosol-localized receptors recognize nucleic acids and induce type I IFNs. Retinoic acid inducible gene I (RIG-I) and melanoma differentiation associated gene 5 (MDA5) are part of the RIG-I-like helicase (RLH) family of sensors that recognize RNA in the cytoplasm and signal through the adaptor protein mitochondrial antiviral signaling (MAVS) (IPS1) to induce type I IFNs (32). Cytosolic DNA also induces an IFN response, but this response is less well characterized. The DNA binding protein DAI (Z-DNA-binding protein 1 [ZBP1]) has been implicated in the IFN response to cytosolic DNA (31). In addition, at least one unknown DNA sensor exists, as targeted deletion of DAI does not abrogate the IFN response to transfected DNA in most cell types (7, 16, 36). This sensor was recently proposed to be IFN-inducible protein 16 (IFI16), a member of the PYHIN family of DNA binding proteins (34). In addition to recognition of DNA and RNA, host cells also appear to be able to mount a type I IFN response to an unusual nucleic acid, called cyclic-di-GMP (c-di-GMP), which is produced only by bacteria (17). Since the DNA and c-di-GMP sensors remain unknown, it remains uncertain if they are identical or distinct from each other.
The signaling pathways downstream of the cytosolic nucleic acid sensors are increasingly well understood. Tank-binding kinase 1 (TBK1), as well as its substrates, the IFN regulatory factor 3 (IRF3) and IRF7 transcription factors, are signaling components downstream of all cytosolic sensors leading to type I IFN induction (3, 26). Sting (transmembrane protein 173 [Tmem173], Mita, MPYS, or ERIS) was recently found to be an essential adaptor downstream of the response to cytosolic DNA (8, 30, 38). Although Sting is reported to interact directly with MAVS, its precise role in the response to different stimulatory RNA species is unclear (9, 38).
Listeria monocytogenes is a Gram-positive pathogen that replicates in the cytosol of host cells and can cause serious disease in pregnant women and immunocompromised individuals (35). L. monocytogenes utilizes a pore-forming cholesterol-dependent cytolysin, listeriolysin O (encoded by the hly gene), to rupture the phagosome and access the host cell cytosol (25). Upon entry of the bacterium into the cytosol, host cells activate a type I IFN response (22, 29). Recently, a novel bacterial second messenger, c-di-AMP, was identified to be an IFN-stimulatory ligand secreted by L. monocytogenes into the host cell cytosol (37). The adaptor molecule Sting was recently reported to be required for the type I IFN response to L. monocyotogenes in vitro (9). However, it is unknown whether Sting is required for the type I IFN response to cyclic dinucleotides and/or for the response to L. monocytogenes in vivo.
To identify novel genes involved in the type I IFN response to L. monocytogenes, we screened thioglycolate-elicited peritoneal macrophages harvested from mice mutagenized with _N_-ethyl-_N_-nitrosourea (ENU), as pioneered by Beutler and colleagues (5). We identified a mutant mouse strain, Goldenticket (Gt), that harbors a point mutation (T596A) in Sting that results in an isoleucine-to-asparagine substitution (I199N) in the Sting protein. Here, we show by genetic mapping and complementation experiments that the Gt allele of Sting is a nonfunctional (null) allele that fails to produce detectable protein. Macrophages from Gt mutant mice were unable to produce type I IFNs in response to L. monocytogenes infection in vitro. In addition, we found that Sting was required for the type I IFN response to purified c-di-GMP and c-di-AMP in vitro. Finally, we also found that Sting is required for the type I IFN response to c-di-GMP and L. monocytogenes in vivo. Our results characterize a novel _Sting_-deficient mouse and describe new functions for Sting in the cytosolic response to pathogen-derived nucleic acids.
MATERIALS AND METHODS
ENU mutant mouse generation.
Five- to 6-week-old male C57BL/6 mice (The Jackson Laboratory) received weekly doses of 90 mg/kg of body weight _N_-ethyl-_N_-nitrosourea (Sigma) over a 3-week span. After a recovery period, each resulting G0 male mouse was mated to an untreated C57BL/6 female mouse to generate a maximum of 20 G1 offspring. Male G1 mice were crossed to C57BL/6 mice to create female G2 mice, which in turn were backcrossed to the G1 male mice to generate third-generation (G3) mice for screening.
Bacterial and cell culture.
Wild-type (10403S) and Δ_hly Listeria monocytogenes_ cultures were grown in brain heart infusion (BHI) broth overnight at 30°C for cell culture infections. For in vivo infections, bacterial cultures were grown overnight in BHI and backdiluted 1:5 in BHI at 37°C for 1.5 h until they reached an optical density at 600 nm of 0.5. To harvest peritoneal macrophages, G3 male mice were injected with 2 ml of aged 4% thioglycolate medium (BD Diagnostics Systems, Sparks, MD). Five days later, the mice were anesthetized with ketamine/xylazine, injected intraperitoneally with 8 ml of RPMI (Gibco, Grand Island, NY), and lavaged. Peritoneal exudates were plated for 3 h, at which time nonadherent cells were removed by washing, leaving the thioglycolate-elicited peritoneal macrophages used in these studies. Bone marrow-derived macrophages were prepared as previously described (11). Briefly, 6- to 8-week-old mice were killed and bone marrow was collected from the femur. Bone marrow cells were cultured in Dulbecco modified Eagle medium (DMEM) containing 10% 3T3-MCSF cell supernatant for 6 days, resulting in the differentiation into naïve bone marrow-derived macrophages. Wild-type C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). Sting_−/_− femurs were a kind gift from Glen Barber (University of Miami, Miami, FL). Sting Gt/Gt mice are described in this work.
Mutant macrophage IFN-β screening.
Peritoneal macrophages were harvested as described above and plated in 96-well dishes at 105 cells/well. At 36 h postharvest, macrophages were infected at an effective multiplicity of infection (MOI) of one bacterium per macrophage for 1 h. After 1 h, 50 μg/ml of gentamicin was added to the medium and infections were allowed to continue for five additional hours. After 6 h of infection, IFN-β in the infected macrophage supernatant was assayed using ISRE-L929 cells as previously described (17).
Sequencing.
The eight exons of Sting, including their splice donor and acceptor sites, were amplified by PCR from genomic DNA from mouse 1009, the Gt founder. PCR products were sequenced directly (ELIM Biopharm). The primers and their sequences were as follows: Exon 1-F, 5′-CACAGGGAGAACTGAACTGAGCG-3′; Exon1-R, 5′-CTTCCTACCAGCCTCCATAAACC-3′; Exon 2-F, 5′-AACACCTTCAGTTTGGGGGTTAG-3′; Exon 2-R, 5′-CACATAGCATCCTACAGCACCTGTC-3′; Exon 3-F, 5′-GACAGGTGCTGTAGGATGCTATGTG-3′; Exon 3-R, 5′-AGGAGTCAAGGGTGTGATACTTGC-3′; Exon 4-F, 5′-TACCTAGCACTTCACCTAGCCTCG-3′; Exon 4-R, 5′-TTAAACCCATCATCCCAAGCC-3′; Exon 5-F, 5′-TGGATGTTTGGCCTTCTGGTCC-3′; Exon 5-R, 5′-GGTGATTTTATGTACCCTGGGCTC-3′; Exon 6-F, 5′-TCACACTGAGAAGGCTAACGAGC-3′; Exon 6-R, 5′-CACCATAGAACAGGGATCACGC-3′; Exon 7-F, 5′-CAGTTACATCCCAGGGCTTTTG-3′; Exon 7-R, 5′-AGGGCAGGGTATAATCCCTCTGAC-3′; Exon 8-F, 5′-GTGGGCTTCCATGACTTTGATAAC-3′; and Exon 8-R, 5′-CAAGGAGTGCCTATTGAAGGGC-3′. Goldenticket mice were genotyped by amplification and sequencing of exon 6, which contains the T596A mutation.
HEK293T cell overexpression.
HEK293T cells were cultured in DMEM supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 μM streptomycin, and 100 U/ml penicillin. The wild-type (WT) Sting pcDNA vector was a gift from Glen Barber. Constructs for expressing the Gt allele of Sting were made by altering the pcDNA-Sting construct using Stratagene's quick change site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA) using primers F (5′-GCAGGGAGCCGAAGACTGTACAACCTCTTTCCATTGGACTGTGGG-3′) and R (5′-CCCACAGTCCAATGGAAAGAGGTTGTACAGTCTTCGGCTCCCTGC-3′). Wild-type and mutant (Gt) Sting pcDNA constructs, IFN-β-firefly luciferase reporter plasmid, and thymidine kinase-Renilla luciferase plasmid were transfected into HEK293T cells with FuGENE 6 transfection reagent (Roche), according to the manufacturer's protocol. Plasmids were mixed with 0.5 μl FuGENE 6 in 50 μl Optimem medium per well of a 96-well plate, and the plates were incubated for 15 min. Total transfected DNA was normalized to 200 ng per well using an empty pcDNA3 plasmid. Firefly luciferase production was measured at 24 h posttransfection, and the level of production was normalized to that of the constitutively expressed Renilla luciferase signal.
Bone marrow-derived macrophage infections and stimulations.
Bone marrow-derived macrophages were harvested as described above and plated at 106 cells/well in six-well dishes. For L. monocytogenes infections, cells were infected with an effective MOI of one bacterium per cell for 30 min. Cells were washed, and at 1 h postinfection, gentamicin was added and infections were allowed to continue for three additional hours. poly(dAT:dTA) or poly(I:C) (500 μg/ml) was transfected into macrophages using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), according to the manufacturer's protocol. Macrophages were infected with Sendai virus (Charles River Laboratory, Wilmington, MA) at 150 hemagglutination units (HAU)/ml. Macrophages were treated with 100 ng/ml lipopolysaccharide (LPS). c-di-GMP (4 μg/ml) was transfected into macrophages using Lipofectamine 2000, according to the manufacturer's protocol. c-di-AMP (4 μg/ml) was delivered to the cytosol using digitonin permeabilization as previously described (37). Four hours following infection/treatment, RNA was harvested and quantitative reverse transcription-PCR was performed as previously described (14).
Western blotting.
Whole-cell lysates were collected in Laemmli buffer 4 h following stimulation, as indicated. Lysates were run on SDS-polyacrylamide gels, transferred to polyvinylidene difluoride membranes, and probed with anti-Sting antibody (1:250), a kind gift from Glen Barber, and anti-tubulin antibody (1:5,000; Sigma, St. Louis, MO). Secondary anti-rabbit IRdye680 (LI-COR Biosciences, Lincoln, NE) and anti-mouse IRdye800 were used to detect primary antibodies, and the blots were analyzed using Oddessy analysis software (LI-COR). Overexpressed wild-type or Gt Sting was detected 24 h posttransfection using anti-hemagglutinin (anti-HA) antibody (Roche, Indianapolis, IN), and β-actin was detected using an anti-β-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Proteins were detected using Pierce enhanced chemiluminescence Western blotting substrate (Thermo Scientific, Rockford, IL).
Immortalized macrophage complementation.
C57BL/6 and Sting G_t/Gt_ mouse bone marrow samples were infected with a v-myc/v-_raf_-expressing retrovirus (2), a gift from Kate Fitzgerald, followed by differentiation in 10% supernatant from 3T3-MCSF cells. After approximately 1 month in culture, the 3T3-MCSF cell supernatant was removed from the medium and the surviving macrophages established an immortalized cell line.
Complementation constructs were made by subcloning wild-type or Gt Sting from pcDNA into the BglII and NotI sites of the retroviral vector MSCV2.2. Immortalized macrophages were transduced by infecting them with vesicular stomatitis virus-G-pseudotyped MSCV2.2-based retrovirus that expressed wild-type Sting or Gt Sting, followed by an internal ribosome entry site-green fluorescent protein (GFP). Pseudotyped virus was generated using the GP2 packaging cell line. After 1 week in culture, the GFP-positive (GFP+) cells were sorted by fluorescence-activated cell sorter analysis to enrich to >90% GFP+ cells.
In vivo experiments.
Six- to 8-week-old mice were injected intraperitoneally with 200 μl of a 1 mM solution of c-di-GMP in 1% NaCl. At 12 h postinjection, retro-orbital bleeds were performed and serum was collected. For L. monocytogenes infections, mice were injected with 105 wild-type or Δ_hly_ bacteria intravenously. Retro-orbital bleeds were performed 4 or 24 h postinfection, and serum was collected. The IFN-β in the serum was analyzed using the ISRE-L929 cells. Anti-IFN-γ antibody (eBioscience, San Diego, CA) was used to block type II IFN in the serum, and blockade was confirmed by using recombinant IFN-γ (R&D Systems, Minneapolis, MN) as a control. The amount of tumor necrosis factor alpha (TNF-α) in the serum was measured using the ready-SET-go enzyme-linked immunosorbent assay (ELISA) from eBioscience, according to the manufacturer's protocol. For analysis of L. monocytogenes virulence, mice were infected with 1 × 104 wild-type bacteria intravenously. At 48 h postinfection, the spleens were harvested, homogenized, and plated for determination of the numbers of CFU as previously described (1).
RESULTS AND DISCUSSION
Goldenticket mice harbor a loss-of-function mutation in Sting.
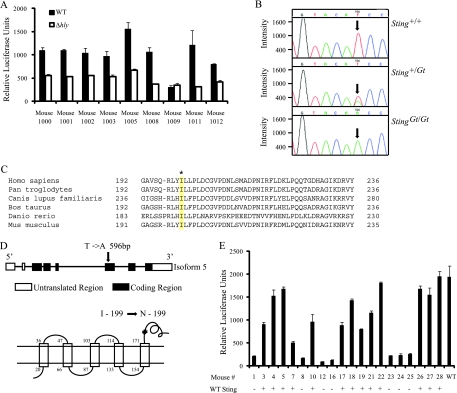
To identify the genes required for the type I IFN response to L. monocytogenes infection, we initiated a forward genetic screen in C57BL/6 mice using the potent germ line mutagen ENU. ENU-treated male mice were bred through three generations to produce G3 progeny harboring homozygous recessive mutations (5). Thioglycolate-elicited peritoneal macrophages collected from viable male G3 mice were screened by bioassay for their ability to produce type I IFNs in response to L. monocytogenes infection. Macrophages from a single G3 mouse (mouse 1009) were unable to produce type I IFNs in response to infection with wild-type L. monocytogenes (Fig. 1A). Mouse 1009 was used to establish a mutant stock, which we call Gt, on a pure C57BL/6 background. We sequenced multiple loci in the Gt mice that were predicted, on the basis of previous results, to be involved in the IFN response to L. monocytogenes, including Irf3 (29), Tbk1 (20), and Sting (8). Sequencing revealed a single base pair change, T596A, in exon 6 of Sting (Fig. 1B). The T596A missense mutation resulted in an isoleucine-to-asparagine change in amino acid 199, a conserved residue found in the C-terminal domain of the protein (Fig. 1C and D). Sequencing of the Sting allele in more than 20 F2 offspring of the Gt founder revealed a 100% correlation between the phenotype, the lack of IFN in response to L. monocytogenes infection, and the T596A mutation (Fig. 1E). These data suggest that the loss of the type I IFN response to L. monocytogenes infection in the Gt founder mouse is due to a single base pair change in Sting.
FIG. 1.
ENU mutagenesis identifies T596A mutation in Sting. (A) Thioglycolate-elicited peritoneal macrophages harvested from G3 ENU-mutagenized mice were infected with wild-type or Δ_hly L. monocytogenes_ at an MOI of 1. IFN-β was measured by bioassay at 6 h postinfection. (B) Sequencing of the Sting allele in wild-type, heterozygous, and homozygous mutant F2 Gt mice. (C) Sequence alignment showing the conserved amino acid mutated in the Goldenticket mouse. (D) Schematic showing the location of the mutation in the Sting locus and on a predicted topology diagram (http://proteinformatics.charite.de/rhythm/). (E) Bone marrow-derived macrophages harvested from F2 Gt mice were infected with wild-type L. monocytogenes at an MOI of 1 for 6 h. The amount of IFN-β in the culture supernatant was measured by bioassay.
The I199N mutation affects the stability and function of Sting.
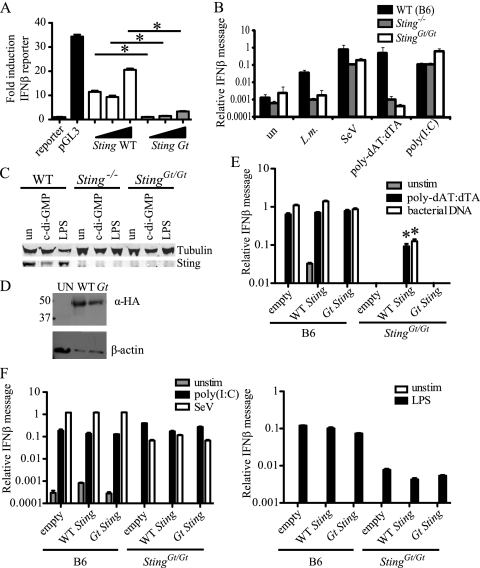
To further address the effect of the I199N mutation on Sting function, we overexpressed WT Sting or the Gt Sting allele in HEK293T cells and assayed the cells for activation of a cotransfected IFN-β promoter-luciferase reporter construct. As expected (8), overexpression of WT Sting resulted in ligand-independent activation of the IFN-β reporter; however, even large amounts of the Gt Sting expression construct were unable to significantly activate the IFN-β reporter (Fig. 2A). Therefore, the Goldenticket (I199N) mutation appears to strongly reduce the function of the Sting protein.
FIG. 2.
T596A mutation abrogates the function of Sting. (A) Luciferase production was measured 24 h after cotransfection of HEK293T cells with an IFN-β luciferase reporter and increasing concentrations (50 ng, 100 ng, 200 ng per well) of wild-type or Gt Sting. pGL3 is a positive-control luciferase expression vector. (B) Following infection with wild-type L. monocytogenes (L.m.; MOI, 1), infection with Sendai virus (SeV; 150 HAU/ml), or transfection of 500 μg/ml of poly(dAT:dTA) or poly(I:C) for 4 h, RNA was harvested from bone marrow-derived macrophages and the amounts of IFN-β transcripts were measured relative to those of β-actin transcripts. (C) Whole-cell lysates were collected and analyzed for Sting expression by Western blotting following 4 h of treatment with either 4 μg/ml of c-di-GMP or 100 ng/ml of LPS. (D) Whole-cell lysates were collected from HEK293T cells 24 h after transfection with 200 ng/ml of wild-type or Gt Sting. Sting was detected using anti-HA antibody, and β-actin was used as a loading control. (E and F) Sting +/+ or Sting Gt/Gt immortalized bone marrow-derived macrophages were transduced with either empty MSCV2.2 vector, WT Sting, or Gt Sting. (E) Transduced cells were transfected with 500 μg/ml poly(dAT:dTA) or 500 μg/ml purified bacterial (Legionella pneumophila) genomic DNA for 4 h, and then RNA was harvested and the amounts of IFN-β transcripts were measured relative to those of rps17 transcripts. (F) Transduced cells were transfected with 500 μg/ml poly(I:C), infected with Sendai virus at 150 HAU/ml, or treated with 100 ng/ml of LPS for 4 h; RNA was harvested; and the amounts of IFN-β transcripts were measured relative to those of rps17 transcripts. Data are representative of those from at least three independent experiments. *, P < 0.05 by Student's t test; un and Unstim, unstimulated; UN, untransfected.
To determine whether the Gt mutation in Sting phenocopies the previously constructed Sting knockout allele (8), bone marrow-derived macrophages from Sting +/+, Sting_−/_−, and Sting Gt/Gt mice were treated with a variety of IFN-inducing stimuli. Sting Gt/Gt bone marrow-derived macrophages were identical to Sting_−/_− macrophages in their IFN-β response to all tested stimuli, including a lack of a response to L. monocytogenes and transfected poly(dAT:dAT). The lack of IFN-β in response to L. monocytogenes infection was not due to differences in infection or bacterial burden, as there were very similar numbers of bacteria present in wild-type and Sting Gt/Gt macrophages at 2 h and 5 h postinfection (data not shown). Both Sting_−/_− and Sting Gt/Gt macrophages exhibited only a modest decrease in the response to Sendai virus, a virus that signals primarily via RIG-I/MAVS. In addition, as reported previously for Sting_−/_− cells (9), Sting Gt/Gt cells responded normally to transfection of poly(I:C) (Fig. 2B). Our results differ from those of Zhong et al., who reported that knockdown of Sting affected the response to poly(I:C) and Sendai virus (38). The different results of Zhong et al. may be due to the different cell types tested or perhaps to a nonspecific effect of RNA interference knockdown.
To determine if the I199N mutation results in a lack of expression or stability of the Sting protein, we performed Western blots on whole-cell lysates of Sting +/+, Sting_−/_−, and Sting Gt/Gt bone marrow-derived macrophages. Although there was detectable Sting protein in Sting +/+ cells, Sting protein was not detectable in Sting_−/_− or Sting Gt/Gt cells, indicating that the I199N mutation resulted in loss of stability or expression of Sting (Fig. 2C). Overexpression of the Gt allele of Sting in HEK293T cells resulted in some detectable protein (Fig. 2D); however, this mutant form of Sting was still inactive (Fig. 2A).
Complementation of the Goldenticket mutation with wild-type Sting.
In order to confirm that the phenotype of Goldenticket cells was due to the I199N mutation in Sting and not to another linked mutation, we developed a strategy to complement the Goldenticket mutants with wild-type Sting. To do this, we first generated immortalized bone marrow-derived macrophages from wild-type C57BL/6 and Goldenticket mutant mice. We then used retroviral transduction to stably express the wild-type and the Gt Sting alleles in the immortalized macrophage lines. Complementation of Sting Gt/Gt immortalized macrophages with WT Sting but not Gt Sting resulted in the rescue of IFN-β expression in response to poly(dAT:dTA) or bacterial DNA (Fig. 2E) The response to poly(I:C), Sendai virus, or LPS was unaffected by complementation with either WT or Gt Sting (Fig. 2F). The immortalized Sting Gt/Gt cells did appear to respond somewhat less well to LPS and Sendai virus than the immortalized wild-type cells (Fig. 2F), but unlike the response to DNA, this was not a phenotype that was complemented with wild-type Sting and, thus, is probably due to a defect in the immortalized cell line that is unrelated to the Goldenticket mutation. Taken together, these data indicate that the I199N mutation in Sting results in a loss of function of the protein and a substantial defect in the ability to induce type I IFN in response to L. monocytogenes or cytosolic DNA.
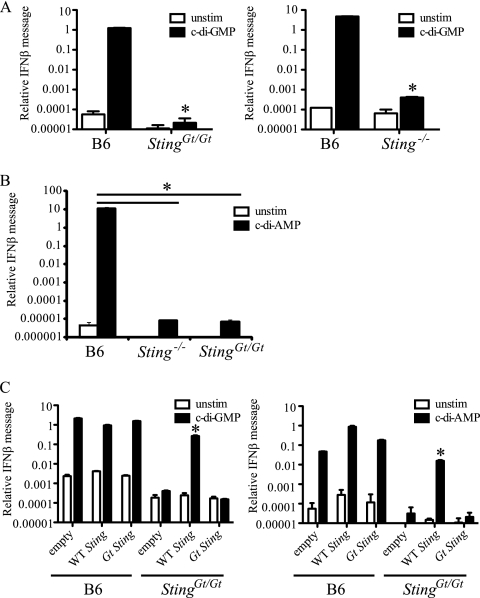
Sting is required for the in vitro response to purified cyclic dinucleotides.
It was recently proposed that the major bacterial ligand recognized during L. monocytogenes infections leading to IFN-β production is a unique bacterial signaling molecule called cyclic-di-AMP (37). Additionally, it was previously shown that macrophages make IFN-β in response to transfected c-di-GMP (17). Using microarray-based transcriptional profiling, McWhirter et al. found that the transcriptional response to c-di-GMP was almost identical to the response to cytosolic DNA (17). Since Sting has been implicated in the response to DNA and L. monocytogenes, we hypothesized that it might also be critical for the response to cyclic dinucleotides. To test this, we transfected Sting +/+, Sting Gt/Gt, and Sting_−/_− bone marrow-derived macrophages with purified c-di-GMP or c-di-AMP and assayed them for IFN-β expression. Wild-type Sting was absolutely required for the response to both c-di-GMP (Fig. 3A) and c-di-AMP (Fig. 3B). Complementation of Sting Gt/Gt macrophages with wild-type but not mutant (Gt) Sting resulted in a rescue of the IFN-β response to both c-di-GMP and c-di-AMP (Fig. 3C). Together these data indicate that Sting is required for the type I IFN response to cyclic dinucleotides. Although it is formally possible that DNA and cyclic dinucleotides are recognized by the same cytosolic receptor, the significant differences in the structure of the two ligands suggests that they may be sensed by separate receptors. Several DNA binding proteins, including DAI (31) and IFI16 (34), have been proposed to be involved in the interferon response to DNA. DAI is not required for the IFN response to c-di-GMP (17), but in future studies it will be interesting to test the potential involvement of IFI16. If separate sensors are required for the responses to DNA and cyclic dinucleotides, our data would be consistent with a model in which Sting is a common adaptor in multiple nucleic acid-sensing pathways that induce type I IFNs.
FIG. 3.
Sting is required for cyclic dinucleotide-induced IFN-β production. Sting +/+, Sting Gt/Gt, or Sting_−/_− bone marrow-derived macrophages were treated with 4 μg/ml c-di-GMP (A) or c-di-AMP (B) for 4 h. RNA was harvested, and the amounts of IFN-β transcripts were measured relative to those of rps17 (A) or β-actin (B) transcripts. (C) Sting +/+ or Sting Gt/Gt immortalized bone marrow-derived macrophages were transduced with either empty MSCV2.2 vector, WT Sting, or Gt Sting. Transduced cells were transfected with 4 μg/ml of c-di-GMP or c-di-AMP for 4 h. RNA was harvested, and the amounts of IFN-β transcripts were measured relative to those of rps17 transcripts. Data are representative of those from at least three independent experiments. *, P < 0.05 by Student's t test.
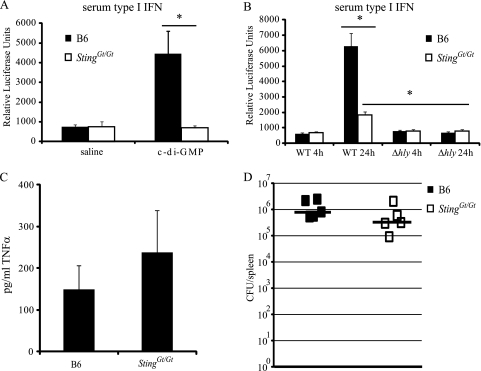
Sting is required for the in vivo response to both purified c-di-GMP and L. monocytogenes infection.
The macrophage type I IFN response to cyclic dinucleotides and L. monocytogenes infection in vitro required wild-type Sting. To address whether Sting is required for IFN-β induction in response to these stimuli in vivo, we injected wild-type or Sting Gt/Gt mice with purified c-di-GMP and 12 h later assayed their sera for IFN-β using an IFN bioassay (10). Although wild-type mice responded to c-di-GMP by producing IFN-β in the serum, there was no detectable induction of IFN-β in the serum of mice injected with Sting Gt/Gt (Fig. 4A). Furthermore, wild-type Sting was required for the IFN-β response to wild-type L. monocytogenes in vivo (Fig. 4B). This is consistent with our in vitro data, as well as with the observation that macrophages, in a TLR-independent manner, are the dominant producers of type I IFNs during L. monocytogenes infections in vivo (28). Importantly, Sting was involved only in the cytosolic response to L. monocytogenes infection, as TNF-α production, a MyD88-dependent response, was unaffected in Sting Gt/Gt mice (Fig. 4C). Interestingly, unlike the in vivo response to purified cyclic-di-GMP or the in vitro response of macrophages to L. monocytogenes, there was still a detectable in vivo IFN response to L. monocytogenes in the Sting Gt/Gt mice. This residual IFN may reflect non-_Sting_-dependent (e.g., TLR-dependent) pathways for IFN induction in vivo, perhaps mediated by cell types other than macrophages.
FIG. 4.
Sting is required for the response to c-di-GMP and L. monocytogenes in vivo. (A) A total of 200 nmol c-di-GMP was injected intraperitoneally into wild-type C57BL/6 (B6) or isogenic Sting Gt/Gt mice. Serum was collected at 12 h postinjection, and the amount of IFN-β was analyzed by bioassay. (B and C) A total of 1 × 105 wild-type or Δ_hly L. monocytogenes_ cells were injected intravenously into C57BL/6 or Sting Gt/Gt mice, and serum was collected at 4 and 24 h postinfection. The amount of IFN-β in the serum was measured by bioassay at 4 and 24 h postinfection (B), and the amount of TNF-α in the serum was measured by ELISA at 24 h postinfection (C). (D) Wild-type or Sting Gt/Gt mice were infected intravenously with 1 × 104 wild-type L. monocytogenes cells. At 48 h postinfection, spleens were harvested and the bacterial burden was quantified. Data are representative of those from at least two independent experiments. *, P < 0.05 by Mann-Whitney test.
It has previously been reported that Ifnar_−/_− and Irf3_−/_− mice are resistant to L. monocytogenes infection (1, 19). We therefore determined the numbers of CFU in the spleens of _L. monocytogenes_-infected wild-type and Sting Gt/Gt mice. Sting Gt/Gt mice were slightly more resistant to L. monocytogenes infection (Fig. 4D), but the slight resistance of Sting Gt/Gt mice was not statistically significant (P = 0.075). The lack of a significant effect on the numbers of CFU may be due to the residual IFN response that we observed in the Sting Gt/Gt mice. Taken together, these data indicate that wild-type Sting is involved in the in vivo IFN-β response to L. monocytogenes infection, likely due to its role in signaling downstream of cyclic dinucleotide detection.
Nucleotide second messengers are common signaling molecules across all kingdoms of life. However, cyclic dinucleotides are unique to bacteria and possibly archaea (23). c-di-GMP was first identified as a bacterial second messenger regulating cellulose production in Gluconacetobacter xylinus (24). GGDEF domains, the cyclase domain required for synthesis of c-di-GMP, have now been identified in most bacterial species, with some species encoding more than 60 GGDEF proteins (4). In addition to being widely produced, c-di-GMP is essential for regulation of virulence gene expression and other critical aspects of physiology in a number of pathogens (33). Further accentuating the importance of cyclic dinucleotides is the observation that in some organisms the cyclase required for synthesizing c-di-AMP, di-adenylate cyclase, appears to be essential (37). Therefore, cyclic dinucleotides serve as ideal pathogen-associated molecular patterns that are targets of immune recognition and are central to the survival/virulence of the pathogen. It is therefore not surprising that the host has evolved a system to detect and respond to cyclic dinucleotides.
In the future it will be important to understand how Sting selectively participates in some type I IFN signaling pathways but not others. This may lead to the identification of novel receptors upstream of Sting, including those responsible for the detection of cytosolic DNA and/or cyclic dinucleotides. We expect that the mutant Sting mice that we describe here will be useful for providing an understanding of the role of the type I IFN response during infections with diverse bacterial species.
Acknowledgments
We thank Glen Barber and Hiroki Ishikawa for bone marrow from _Sting_-deficient mice and anti-Sting antibody and Kate Fitzgerald for the J2 retrovirus used to immortalize bone marrow cells. We thank collaborators associated with the Bay Area Intracellular Pathogen Program Project.
This work was supported by NIH grants AI082357 (to R.E.V.), AI080749 (to R.E.V.), P01 A1063302 (to D.A.P.), and AI27655 (to D.A.P.). JD.-S. was supported by American Cancer Society grant PF-07-066-01-LIB. R.E.V. is supported by investigator awards from the Burroughs Wellcome Fund and the Cancer Research Institute.
Footnotes
▿
Published ahead of print on 22 November 2010.
REFERENCES
- 1.Auerbuch, V., D. G. Brockstedt, N. Meyer-Morse, M. O'Riordan, and D. A. Portnoy. 2004. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J. Exp. Med. 200**:**527-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blasi, E., et al. 1985. Selective immortalization of murine macrophages from fresh bone marrow by a raf/myc recombinant murine retrovirus. Nature 318**:**667-670. [DOI] [PubMed] [Google Scholar]
- 3.Fitzgerald, K. A., et al. 2003. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4**:**491-496. [DOI] [PubMed] [Google Scholar]
- 4.Galperin, M. Y. 2005. A census of membrane-bound and intracellular signal transduction proteins in bacteria: bacterial IQ, extroverts and introverts. BMC Microbiol. 5**:**35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoebe, K., et al. 2003. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 424**:**743-748. [DOI] [PubMed] [Google Scholar]
- 6.Isaacs, A., and J. Lindenmann. 1957. Virus interference. I. The interferon. Proc. R. Soc. Lond. B Biol. Sci. 147**:**258-267. [PubMed] [Google Scholar]
- 7.Ishii, K. J., et al. 2008. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature 451**:**725-729. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa, H., and G. N. Barber. 2008. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455**:**674-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishikawa, H., Z. Ma, and G. N. Barber. 2009. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461**:**788-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang, Z., et al. 2005. CD14 is required for MyD88-independent LPS signaling. Nat. Immunol. 6**:**565-570. [DOI] [PubMed] [Google Scholar]
- 11.Jones, S., and D. A. Portnoy. 1994. Characterization of Listeria monocytogenes pathogenesis in a strain expressing perfringolysin O in place of listeriolysin O. Infect. Immun. 62**:**5608-5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawai, T., and S. Akira. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11**:**373-384. [DOI] [PubMed] [Google Scholar]
- 13.Kolumam, G. A., S. Thomas, L. J. Thompson, J. Sprent, and K. Murali-Krishna. 2005. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202**:**637-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leber, J. H., et al. 2008. Distinct TLR- and NLR-mediated transcriptional responses to an intracellular pathogen. PLoS Pathog. 4**:**e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Bon, A., et al. 2003. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4**:**1009-1015. [DOI] [PubMed] [Google Scholar]
- 16.Lippmann, J., et al. 2008. IFNbeta responses induced by intracellular bacteria or cytosolic DNA in different human cells do not require ZBP1 (DLM-1/DAI). Cell. Microbiol. 10**:**2579-2588. [DOI] [PubMed] [Google Scholar]
- 17.McWhirter, S. M., et al. 2009. A host type I interferon response is induced by cytosolic sensing of the bacterial second messenger cyclic-di-GMP. J. Exp. Med. 206**:**1899-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monroe, K. M., S. M. McWhirter, and R. E. Vance. 2010. Induction of type I interferons by bacteria. Cell. Microbiol. 12**:**881-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Connell, R. M., et al. 2004. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J. Exp. Med. 200**:**437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Connell, R. M., et al. 2005. Immune activation of type I IFNs by Listeria monocytogenes occurs independently of TLR4, TLR2, and receptor interacting protein 2 but involves TNFR-associated NF kappa B kinase-binding kinase 1. J. Immunol. 174**:**1602-1607. [DOI] [PubMed] [Google Scholar]
- 21.Orange, J. S., and C. A. Biron. 1996. Characterization of early IL-12, IFN-alphabeta, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J. Immunol. 156**:**4746-4756. [PubMed] [Google Scholar]
- 22.O'Riordan, M., C. H. Yi, R. Gonzales, K. D. Lee, and D. A. Portnoy. 2002. Innate recognition of bacteria by a macrophage cytosolic surveillance pathway. Proc. Natl. Acad. Sci. U. S. A. 99**:**13861-13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romling, U. 2008. Great times for small molecules: c-di-AMP, a second messenger candidate in Bacteria and Archaea. Sci. Signal. 1**:**pe39. [DOI] [PubMed] [Google Scholar]
- 24.Ross, P., et al. 1987. Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature 325**:**279-281. [DOI] [PubMed] [Google Scholar]
- 25.Schnupf, P., and D. A. Portnoy. 2007. Listeriolysin O: a phagosome-specific lysin. Microbes Infect. 9**:**1176-1187. [DOI] [PubMed] [Google Scholar]
- 26.Sharma, S., B. R. tenOever, N. Grandvaux, G. P. Zhou, R. Lin, and J. Hiscott. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science 300**:**1148-1151. [DOI] [PubMed] [Google Scholar]
- 27.Stetson, D. B., and R. Medzhitov. 2006. Type I interferons in host defense. Immunity 25**:**373-381. [DOI] [PubMed] [Google Scholar]
- 28.Stockinger, S., et al. 2009. Characterization of the interferon-producing cell in mice infected with Listeria monocytogenes. PLoS Pathog. 5**:**e1000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stockinger, S., et al. 2004. IFN regulatory factor 3-dependent induction of type I IFNs by intracellular bacteria is mediated by a TLR- and Nod2-independent mechanism. J. Immunol. 173**:**7416-7425. [DOI] [PubMed] [Google Scholar]
- 30.Sun, W., et al. 2009. ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization. Proc. Natl. Acad. Sci. U. S. A. 106**:**8653-8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takaoka, A., et al. 2007. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448**:**501-505. [DOI] [PubMed] [Google Scholar]
- 32.Takeuchi, O., and S. Akira. 2008. MDA5/RIG-I and virus recognition. Curr. Opin. Immunol. 20**:**17-22. [DOI] [PubMed] [Google Scholar]
- 33.Tamayo, R., J. T. Pratt, and A. Camilli. 2007. Roles of cyclic diguanylate in the regulation of bacterial pathogenesis. Annu. Rev. Microbiol. 61**:**131-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unterholzner, L., et al. 2010. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 11**:**997-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vazquez-Boland, J. A., et al. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14**:**584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang, Z., et al. 2008. Regulation of innate immune responses by DAI (DLM-1/ZBP1) and other DNA-sensing molecules. Proc. Natl. Acad. Sci. U. S. A. 105**:**5477-5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woodward, J. J., A. T. Iavarone, and D. A. Portnoy. 2010. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 328**:**1703-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhong, B., et al. 2008. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 29**:**538-550. [DOI] [PubMed] [Google Scholar]