The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2 (original) (raw)
. Author manuscript; available in PMC: 2012 Apr 6.
Published in final edited form as: Science. 2011 Feb 18;331(6019):912–916. doi: 10.1126/science.1197454
Abstract
During pregnancy, progesterone inhibits the growth-promoting actions of estrogen in the uterus. However, the mechanism for this is not clear. The attenuation of estrogen-mediated proliferation of the uterine epithelium by progesterone is a prerequisite for successful implantation. Our study reveals that progesterone-induced expression of the basic helix-loop-helix transcription factor Hand2 in the uterine stroma suppresses the production of several fibroblast growth factors (FGFs), which act as paracrine mediators of mitogenic effects of estrogen on the epithelium. In mouse uteri lacking Hand2, continued induction of these FGFs in the stroma maintains epithelial proliferation and stimulates estrogen-induced pathways, resulting in impaired implantation. Thus, Hand2 is a critical regulator of the uterine stromal-epithelial communication that directs proper steroid regulation conducive for the establishment of pregnancy.
A sequential and timely interplay of the steroid hormones 17β-estradiol (E) and progesterone (P) regulates critical uterine functions during the reproductive cycle and pregnancy (1–3). Whereas E drives uterine epithelial proliferation in cycling females, P counteracts E-induced endometrial hyperplasia. In mice, preovulatory ovarian E stimulates uterine epithelial growth and proliferation on days 1 and 2 of pregnancy (1). However, starting on day3, P produced by the corpora lutea terminates E-mediated epithelial proliferation. In response to P, epithelial cells exit from the cell cycle and enter a differentiation pathway to acquire the receptive state that supports embryo implantation on day4 of pregnancy (4–6). To identify the P-regulated pathways that underlie the implantation process, we had previously examined alterations in mouse uterine mRNA expression profiles in the peri-implantation period in response to RU486, a well-characterized progesterone receptor (PR) antagonist (7). Our results identified Hand2, a critical regulator of morphogenesis in a variety of tissues (8, 9), as a potential PR-regulated gene. Real-time PCR confirmed that the expression of Hand2 mRNA was greatly reduced in the uteri of RU486-treated mice (Fig. S1A). The expression of Hand2 protein, localized exclusively in the uterine stroma, was also abolished upon RU486 treatment (Fig. S1B), indicating that PR controls Hand2 expression in mouse uterus during early pregnancy.
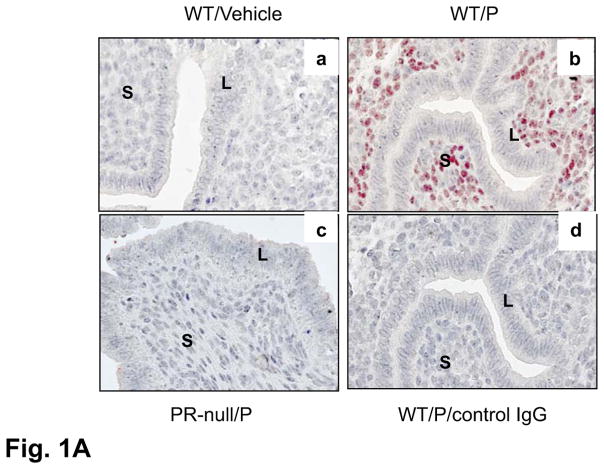
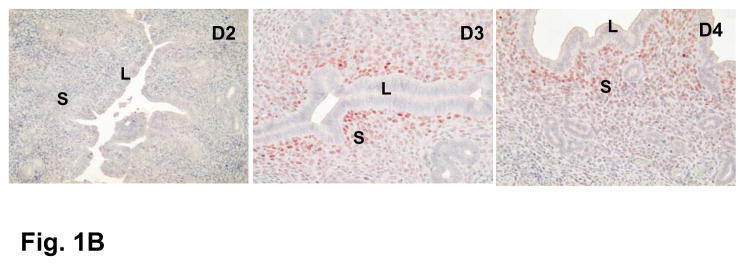
To further confirm P-regulation of Hand2, ovaries were removed from non-pregnant mice and then these animals were injected with either vehicle or P. We observed intense nuclear expression of Hand2 protein in uterine stromal cells upon P treatment. Similar treatment of PR-null females showed no induction of Hand2 protein (Fig. 1A). These results established that P induces Hand2 expression in the uterine stroma. Consistent with its regulation by P, Hand2 expression was observed in the stromal cells underlying the luminal epithelium on days 3 and 4 of pregnancy (Fig. 1B).
Figure 1.
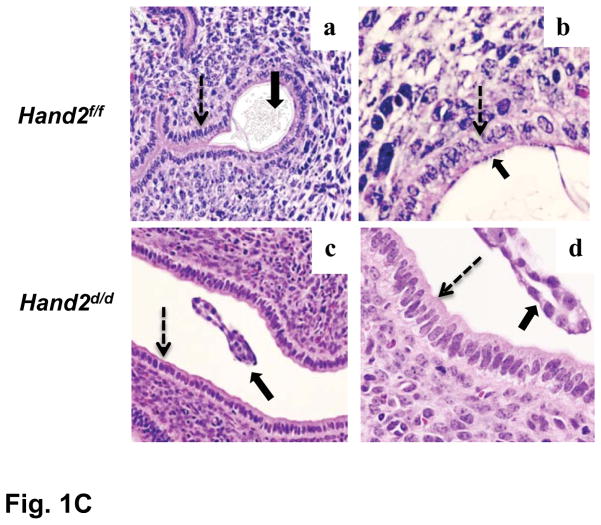
P-regulated expression of Hand2 in the uterus is critical for implantation. (A) IHC of Hand2 protein in the uterine sections of ovariectomized WT mice treated with vehicle (a) or P (b) and PR-null mice treated with P (c). Panel d represents sections treated with non-immune IgG. (B) Uterine sections obtained from mice on days 2 to 4 of pregnancy were subjected to IHC using Hand2 antibody. Magnification 20X. (C) H and E staining of uterine sections obtained from Hand2f/f (a, b) and Hand2d/d (c, d) mice on day 5 (n=6) of pregnancy. b and d represent magnified images of a and c, respectively. Solid and dotted arrows point to embryo and luminal epithelium. L and S represent luminal epithelium and stroma, respectively.
To investigate the function of Hand2 in the uterus, we created a conditional knockout of this gene in the adult uterine tissue. Crossing of mice harboring the “floxed” Hand2 gene (Hand2f/f) with PR-Cre mice generated Hand2d/d mice in which the Hand2 gene is deleted selectively in cells expressing PR. As shown in Fig. S2, Hand2 expression was successfully abrogated in uteri of Hand2d/d mice. A breeding study demonstrated that Hand2d/d females are infertile (Table S1). An analysis of the ovulation and fertilization in Hand2f/f and Hand2d/d females revealed no significant difference in either the number or the morphology of the embryos recovered from their uteri (Figs. S3A and 3B). The serum levels of P and E were comparable in Hand2f/f and Hand2d/d females on day4 of pregnancy, indicating normal ovarian function (Figs. S3C and S3D).
We next examined embryo attachment to the uterine epithelium by employing the blue dye assay, which assesses increased vascular permeability at implantation sites. Hand2f/f mice displayed distinct blue bands, indicative of implantation sites on day5 of pregnancy (Fig. S4). In contrast, none of the Hand2d/d females showed any sign of implantation. Implanted embryos with decidual swellings were also absent in Hand2d/d uteri on days 6 and 7 of pregnancy. Histological analysis of Hand2f/f females on day5 of pregnancy showed, as expected, a close contact of embryonic trophectoderm with uterine luminal epithelium (Fig. 1C, a and b). In contrast, in Hand2d/d uteri, blastocysts remained unattached in the lumen (c and d). These results suggested that in the absence of Hand2 expression in the stroma, the luminal epithelium fails to acquire competency for embryo implantation.
In mice, the window of uterine receptivity coincides with the P-mediated down-regulation of ER activity in uterine luminal epithelium (5, 6). As shown in Fig. S5, the levels of PR and ERα proteins in the luminal epithelium or stroma of Hand2d/d uteri were comparable to those of Hand2f/f controls. An examination of the phosphorylation of ERα at serine 118, indicative of its transcriptionally active state (10), revealed a sharp reduction of this modification in the luminal epithelial cells of Hand2f/f uteri on days 3 and 4 of pregnancy (Fig. S6, a–d). In contrast, an increase in ERα phosphorylation was evident on these days in luminal epithelium of Hand2d/d uteri (Fig. S6, e–h).
Consistent with this increase in ER’s transcriptional activity, expression of mRNAs corresponding to mucin 1 (Muc-1) and lactoferrin (Lf), well-characterized E-responsive genes in uterine epithelium (11), was significantly elevated in the _Hand2_-null uterus on day4 of pregnancy (Fig. 2A). In contrast, the expression of Ihh (12), Alox15 (7), and Irg1 (7), known P-responsive genes in uterine epithelium, remained unaltered in Hand2d/d uteri (Fig. S7). Additionally, the mRNA levels of Hoxa10, a P-regulated stromal factor (3), and Nr2f2, a downstream target of Ihh in the uterine stroma (12), were unaffected in the uteri of Hand2d/d mice. However the expression of leukemia inhibitory factor (Lif), a glandular factor that regulates uterine receptivity (2), was significantly reduced in Hand2d/d uteri (Fig. S8).
Figure 2.
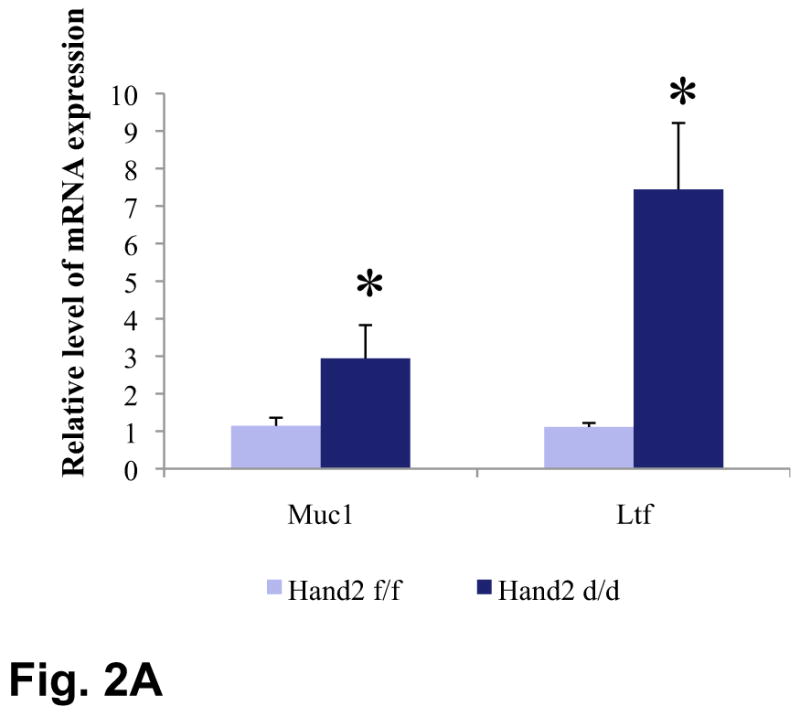
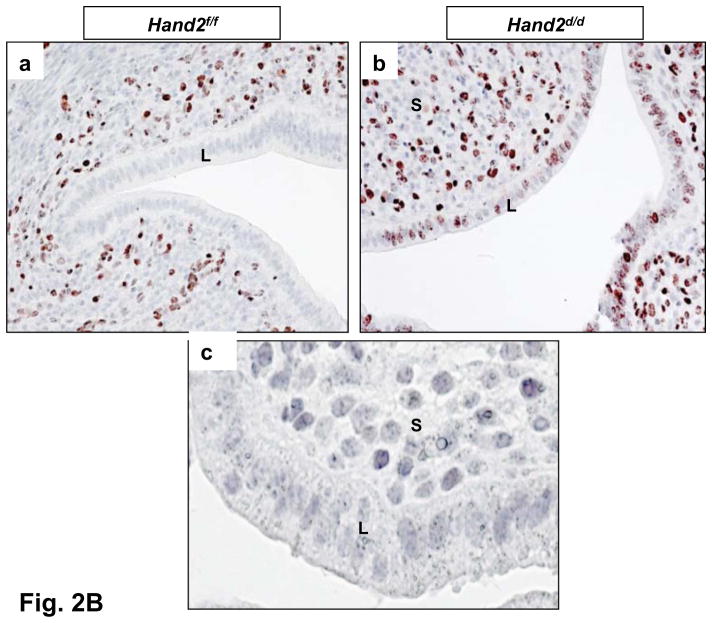
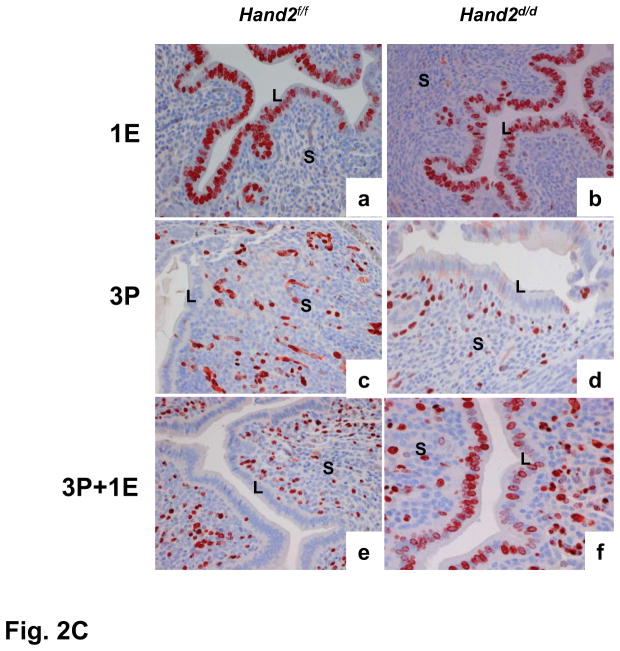
Enhanced ERα activity and proliferation in the luminal epithelium of Hand2d/d uteri. (A) Real-time PCR was performed to monitor the expression of Muc1 and Ltf in the uteri of day 4 pregnant Hand2f/f and Hand2d/d mice, *P<0.001. (B) IHC of Ki67 in Hand2f/f (a) and Hand2d/d (b) uteri on day 4 of pregnancy, 20X. Panel c shows uterine sections from Hand2d/d mice treated with non-immune IgG, 40X. (C) IHC of Ki67 in the uterine sections of ovariectomized Hand2f/f and Hand2d/d mice treated with E for one day (a and b), P for three days (c and d) or two days of P treatment followed by P and E (e and f).
Down-regulation of Muc-1 in the luminal epithelium is indicative of a receptive uterus (11). In contrast, persistent Muc-1 expression impairs acquisition of uterine receptivity and embryo implantation. On days 4 and 5 of gestation, a marked reduction in Muc-1 level was seen in uterine epithelia of Hand2f/f mice, consistent with the attainment of receptive status (Fig. S9). However, Muc-1 expression persisted in uteri of Hand2d/d mice at the time of implantation. Thus, elevated epithelial ER signaling led to increased expression of Muc-1 and corresponded to disrupted uterine receptivity and implantation failure in Hand2d/d mice.
In normal pregnant uteri, the receptive state is also marked by a cessation in epithelial cell proliferation prior to implantation (1, 2). As expected, in Hand2f/f mice, Ki-67, a cell proliferation marker, was undetectable in the uterine epithelium as it attains receptive status on day4 of pregnancy (Fig. 2B, a). Hand2d/d uteri, on the other hand, exhibited robust Ki67 expression in the luminal epithelium, which indicated sustained epithelial cell proliferation in the absence of Hand2 (Fig. 2B, b).
The persistent proliferative state of uterine epithelium in the Hand2d/d mice raised the possibility that stromal expression of Hand2 mediates P action that opposes E-mediated epithelial proliferation. As shown in Fig. 2C, administration of E to ovariectomized Hand2f/f and Hand2d/d mice led to robust uterine epithelial proliferation (a and b). Treatment with P alone induced proliferation exclusively in the uterine stromal cells of both genotypes (c and d). In Hand2f/f mice pretreated with P, administration of E showed no proliferative activity in the epithelium, suggesting a complete blockade of E-dependent proliferation by P. Under similar treatment conditions, Hand2d/d uteri exhibited a marked E-induced epithelial proliferation, indicating an absence of antiproliferative effects of P on the uterine epithelium (e and f). These results established Hand2 as a critical mediator of the actions of P in the stroma that inhibit E-dependent epithelial proliferation.
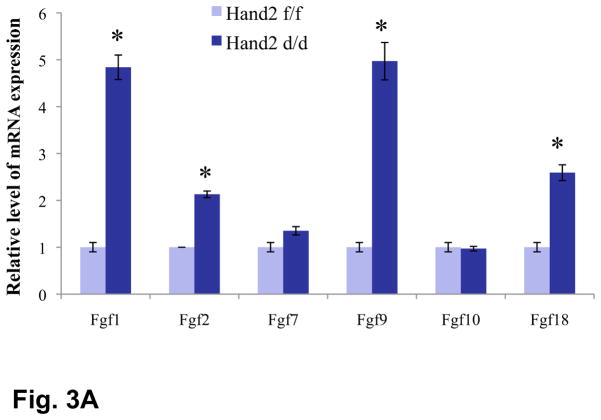
To identify downstream target(s) of Hand2 in the uterus, we performed gene expression profiling of uterine stromal cells isolated from Hand2f/f and Hand2d/d mice on day 4 of pregnancy. This study revealed elevated expression of mRNAs corresponding to several members of the fibroblast growth factor family (FGFs), namely Fgf1, Fgf2, Fgf9, and Fgf18, in uterine stroma of Hand2d/d mice. Real-time PCR confirmed the induction of Fgf1, Fgf9, Fgf2 and Fgf18 mRNAs in uterine stromal cells of Hand2d/d mice (Fig. 3A). The expression of Hbegf mRNA, encoding the heparin-binding epidermal growth factor (HB-EGF), was also increased in the mutant uteri (Fig. S10). In contrast, the expression of mRNAs of several other growth factor genes was either unaffected or slightly reduced in the _Hand2_-null uteri (Fig. S10). We also observed that the uterine expression of Fgf2, Fgf9, and Fgf18 progressively declined with the rise of Hand2 expression as pregnancy advanced from day 1 to day 4 (Fig. S11).
Figure 3.
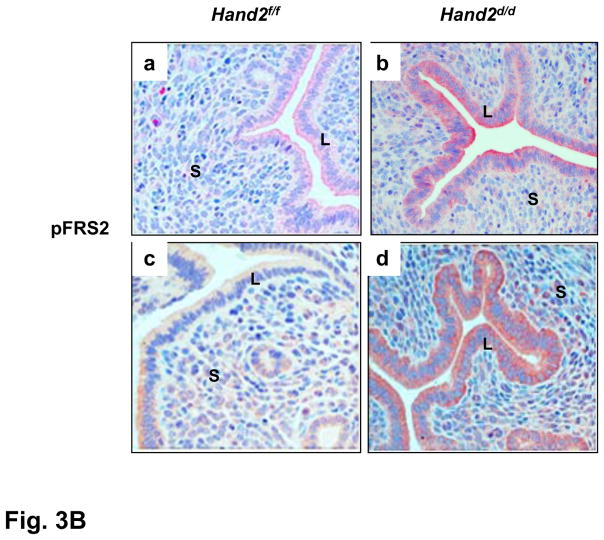
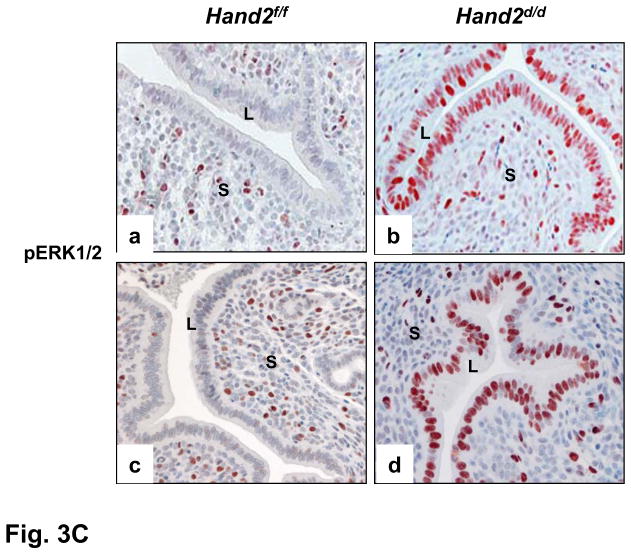
Enhanced FGFR signaling in the luminal epithelium of Hand2d/d uteri. (A) Relative level of expression of Fgf family of growth factors in the uterine stroma of Hand2f/f and Hand2d/d mice on day 4 of pregnancy, *P<0.001. The levels of p-FRS2 (B) and p-ERK1/2 (C) were examined in the uterine sections (n=3) of both genotypes by IHC. a & b represent uterine sections obtained from day 4 pregnant mice. c & d indicate uterine sections from ovariectomized mice treated with two days of P followed by P and E.
FGFs exert their paracrine responses through the cell surface FGF receptors (FGFRs) and a docking protein complex (13). FGF-stimulation of FGFRs leads to phosphorylation of specific tyrosine residues in a critical docking protein, FGFR substrate2 (FRS2), which guides the assembly of distinct multi-protein complexes, leading to activation of either ERK1/2 or PI3K/AKT signaling cascades (13). We determined the expression of FGFRs 1 and 2 in uteri on days 1 and 4 of pregnancy and found these receptor localized to the epithelium (Fig. S12A). The levels of mRNAs corresponding to Fgfr 1–3 were comparable in uteri of Hand2f/f and Hand2d/d mice on day4 of pregnancy (Fig. S12B).
The activation of the FGFR signaling pathway in uteri of Hand2f/f and Hand2d/d mice was monitored by examining the tyrosine phosphorylation status of FRS2. Whereas only low levels of phospho-FRS2 were seen in the uterine epithelium of Hand2f/f mice on day4 of pregnancy (Fig. 3B, a), a marked increase in its level was observed in the epithelium of _Hand2_-null uteri, indicating that FGF signaling is activated in the absence of Hand2 (b). Phospho-FRS2 was undetectable in uterine epithelium of ovariectomized Hand2f/f mice in which P effectively blocks E-mediated proliferation (c). However, the epithelial cells of _Hand2_-null uteri, which showed proliferative activity under similar hormone treatment conditions, exhibited a marked elevation in the level of phospho-FRS2, reflecting the activation of FGFR signaling in these cells (d). We also monitored the activated phosphorylated states of ErbB1 and ErbB4, the primary receptors mediating the actions of Hbegf (14). The activated forms of these receptors were undetectable in uterine epithelia of Hand2f/f and Hand2d/d mice on day4 of gestation (Fig. S13), indicating that the increased HB-EGF produced by the stroma of _Hand2_-null uteri did not act directly on the epithelial cells.
We next investigated whether the ERK1/2 and/or PI3K/AKT signaling pathways were activated downstream of FGFR in the epithelia of _Hand2_-ablated uteri. As shown in Fig. 3C, an increase in the level of phospho-ERK1/2 was seen in uterine epithelium of _Hand2_-null mice on day4 of pregnancy (b) and also in response to E treatment in the presence of P (d). In contrast, the phospho-AKT levels were undetectable or low and remained unaltered in the uterine epithelia of these mice (Fig. S14), suggesting that the ERK1/2 pathway, but not the PI3K/AKT pathway, is the key downstream mediator of enhanced FGF signaling in _Hand2_-null uteri.
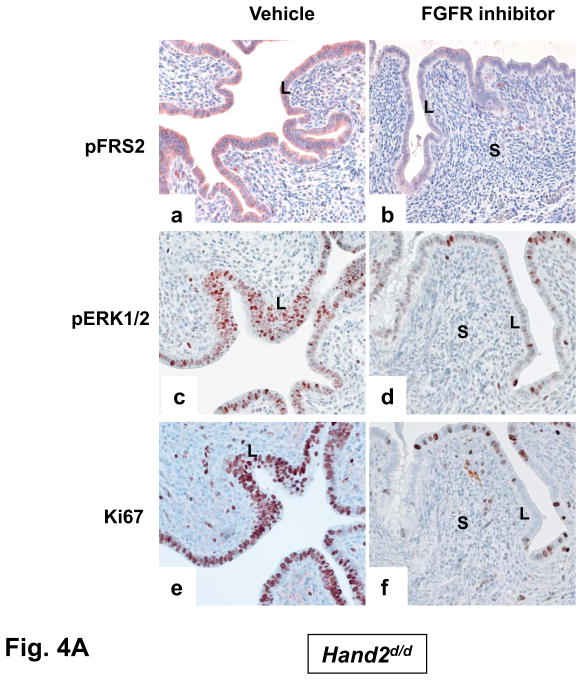
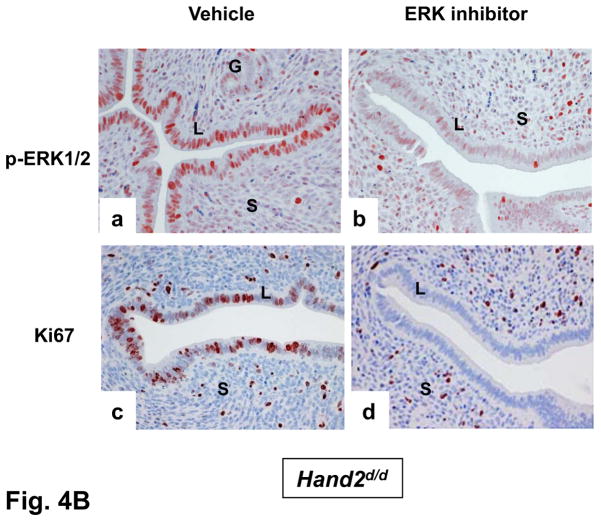
To examine whether the elevated mitogenic activity in the luminal epithelium of Hand2d/d uteri is induced by FGF and ERK1/2 signaling, we administered PD173074, a FGFR-specific inhibitor (15), or vehicle into uterine horns of Hand2d/d mice at the time of implantation. The epithelia of vehicle-treated horn showed prominent expression of p-FRS2 and p-ERK1/2 (Fig. 4A, a and c). However, the levels of both p-FRS2 and p-ERK1/2 were reduced in the epithelia of PD173074-treated horn on day4 of pregnancy (Fig. 4A, b and d). We also observed a marked decline in the proliferative activity of _Hand2_-null uterine epithelia as indicated by decreased Ki67 staining (Fig. 4A, e and f). In parallel experiments, administration of PD184352, an ERK1/2-specific inhibitor (16), to uterine horns of Hand2d/d mice suppressed the level of pERK1/2 (Fig. 4B, a and b) as well as luminal epithelial proliferation (Fig. 4B, c and d). Collectively these results are consistent with the hypothesis that increased FGF production by the _Hand2_-null uterine stroma stimulates epithelial proliferation by activating the FGFR-ERK1/2 pathway.
Figure 4.
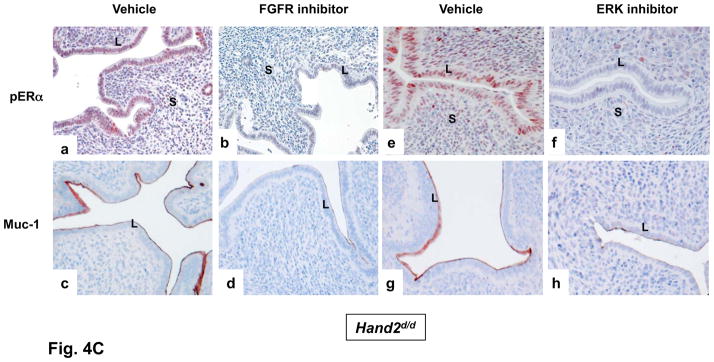
The inhibitor PD173074 (A) or PD184352 (B) was administered to one uterine horn of Hand2_d/d_ mice on day 3 of pregnancy (n=5). The other horn served as vehicle-treated control. Uterine horns were collected on day 4 morning and sections were subjected to IHC to detect p-FRS2, p-ERK1/2, and Ki67 (C) IHC of pERα and Muc-1 in uterine sections of Hand2_d/d_ mice treated with PD173074 or PD184352.
The ERK1/2-dependent phosphorylation of epithelial ERα at serine-118 is critical for the transcriptional activation of ERα (10). Interestingly, administration of either PD173074 (Fig. 4C, a–d) or PD184352 (Fig. 4C, e–h) to _Hand2_-null uterine horns blocked the phosphorylation of epithelial ERα at Ser 118 as well as Muc-1 expression. This result supported our view that elevated signaling by FGFR-ERK1/2 pathway in Hand2d/d uteri is responsible for phosphorylation and activation of ERα in epithelial cells, thereby promoting persistent expression of Muc-1, which creates a barrier, preventing embryo attachment.
Earlier studies, employing tissue recombinants prepared with uterine epithelium and stroma isolated from neonatal wild-type and PR-null mice, indicated that the stromal PR plays an obligatory role in mediating the inhibitory actions of P on E-induced epithelial cell proliferation (17). However, the mechanism of this stromal-epithelial communication remained unknown. Our study has delineated a unique pathway in which Hand2 operates downstream of P to regulate the production of FGFs, mitogenic paracrine signals that originate in the stroma and act on the FGFR(s) in epithelium to control its E-responsiveness (Fig. S15). The antiproliferative action of P in uterine epithelium is of clinical significance since breakdown of this action underpins E-dependent endometrial cancer (18). Hand2, therefore, is an important factor to be considered for the hormone therapy to block the proliferative actions of E in the endometrium.
Supplementary Material
Acknowledgments
We thank M. Laws for genotyping and Y. Li for IHC. This work was supported by the Eunice Kennedy Shriver NICHD/NIH through U54HD055787 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research. The GEO accession number for microarray is GSE25881.
References and Notes
- 1.Finn CA, Martin L. J Reprod Fert. 1974;39:195. doi: 10.1530/jrf.0.0390195. [DOI] [PubMed] [Google Scholar]
- 2.Carson DD, et al. Dev Biol. 2000;223:217. doi: 10.1006/dbio.2000.9767. [DOI] [PubMed] [Google Scholar]
- 3.Ramathal CY, et al. Semin Reprod Med. 2010;28:17. doi: 10.1055/s-0029-1242989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin L, Das RM. J Endocrinol. 1973;57:549. doi: 10.1677/joe.0.0570549. [DOI] [PubMed] [Google Scholar]
- 5.Bagchi IC, et al. Front Biosci. 2003;8:s852. doi: 10.2741/1148. [DOI] [PubMed] [Google Scholar]
- 6.Pan H, Deng Y, Pollard JW. Proc Natl Acad Sci. 2006;103:14021. doi: 10.1073/pnas.0601271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagchi IC, et al. Semin Reprod Med. 2005;23:38. doi: 10.1055/s-2005-864032. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava D, et al. Nat Genet. 1997;16:154. doi: 10.1038/ng0697-154. [DOI] [PubMed] [Google Scholar]
- 9.Firulli AB. Gene. 2003;312:27. doi: 10.1016/s0378-1119(03)00669-3. [DOI] [PubMed] [Google Scholar]
- 10.Kato S, et al. Science. 1995;270:1491. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 11.Surveyor GA, et al. Endocrinology. 1995;136:3639. doi: 10.1210/endo.136.8.7628404. [DOI] [PubMed] [Google Scholar]
- 12.Lee KY, et al. Nat Genet. 2006;38:1204. doi: 10.1038/ng1874. [DOI] [PubMed] [Google Scholar]
- 13.Eswarakumar VP, Lax I. Cytokine Growth Factor Rev. 2005;16:139. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Iwamoto R, Mekada E. Cytokine Growth Factor Rev. 2000;11:335. doi: 10.1016/s1359-6101(00)00013-7. [DOI] [PubMed] [Google Scholar]
- 15.Koziczak M, Holbro T. Oncogene. 2004;23:3501. doi: 10.1038/sj.onc.1207331. [DOI] [PubMed] [Google Scholar]
- 16.Solit DB, et al. Nature. 2006;439:358. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurita T, et al. Endocrinology. 1998;139:4708. doi: 10.1210/endo.139.11.6317. [DOI] [PubMed] [Google Scholar]
- 18.Kim JJ, Chapman-Davis E. Semin Reprod Med. 2010;28:81. doi: 10.1055/s-0029-1242998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.