Naturally occurring aminoacyl-tRNA synthetases editing-domain mutations that cause mistranslation in Mycoplasma parasites (original) (raw)
Abstract
Mycoplasma parasites escape host immune responses via mechanisms that depend on remarkable phenotypic plasticity. Identification of these mechanisms is of great current interest. The aminoacyl-tRNA synthetases (AARSs) attach amino acids to their cognate tRNAs, but occasionally make errors that substitute closely similar amino acids. AARS editing pathways clear errors to avoid mistranslation during protein synthesis. We show here that AARSs in Mycoplasma parasites have point mutations and deletions in their respective editing domains. The deleterious effect on editing was confirmed with a specific example studied in vitro. In vivo mistranslation was determined by mass spectrometric analysis of proteins produced in the parasite. These mistranslations are uniform cases where the predicted closely similar amino acid replaced the correct one. Thus, natural AARS editing-domain mutations in Mycoplasma parasites cause mistranslation. We raise the possibility that these mutations evolved as a mechanism for antigen diversity to escape host defense systems.
Keywords: amino acid editing, fidelity, quality control, statistical proteins, host-pathogen interactions
M_ycoplasma_ are characterized by their lack of a cell wall and dependence on a vertebrate host (1). Their relationship with the host can be parasitic or they can coexist as an obligate commensal. The persistent survival of Mycoplasma within their host has been attributed to a phenotypic plasticity that allows these pathogens to facilely alter their antigenic properties (2). Paradoxically, this phenotypic plasticity is generated in spite of the _Mycoplasma_’s extremely small genomes, which have lost many of the components that typically comprise signaling pathways to adapt to changing environments (2). Remarkably, these wall-less bacteria have a highly dynamic surface architecture comprised of membrane proteins that confer antigenic and functional versatility (2).
As with other pathogenic and nonpathogenic organisms, Mycoplasma contain a complete set of AARSs (aminoacyl-tRNA synthetases), which are essential to translate the genetic code into functional proteins (3). Each AARS has evolved for specificity to a single standard amino acid to maintain the fidelity of the genetic code. The AARS enzyme family activates and transfers amino acids to their cognate tRNA isoacceptor. Once the tRNA is “charged” with an amino acid, it is shuttled to the ribosome for incorporation into nascent polypeptides.
About half of the AARSs are prone to mistakes by activating structurally similar amino acids and mischarging them to tRNA. To minimize the potential for creating statistical proteins that contain mistranslated amino acids, these AARSs have developed a second “sieve” (4); they have adapted to include hydrolytic editing domains that are distinct from their canonical aminoacylation core domains. In some cases, aminoacylation accuracy is also enhanced by independent editing domains that function as tRNA-specific deacylases (5, 6).
We have identified multiple AARSs with editing domains in Mycoplasma and closely related species that appeared to be degenerate based on substitutions at key sites in the hydrolytic active site. This was surprising because functional defects in AARS editing that decrease the fidelity of tRNA aminoacylation have been clearly shown to result in amino acid toxicities, cell death, as well as neurological disease in mammals (7–9). As such, in order to achieve the threshold levels of translational fidelity that are required for cell viability, these amino acid editing functions have been broadly conserved across all three domains of life.
In contrast, we determined that Mycoplasma mobile exhibits AARS-dependent translational infidelities. Editing-defective AARSs mischarge tRNA, which subsequently results in mistranslation in vivo. It is possible that this AARS-dependent mechanism could provide a unique pathway to introduce heterogeneity into the cell’s proteome that could confer phenotypic plasticity in Mycoplasma pathogens.
Results
Mycoplasma Have Evolved AARSs with Inactivated Editing Domains.
Using bioinformatic approaches to broadly scrutinize genomes across the three domains of life, we identified AARSs with unusual amino acid editing domains. In Mycoplasma and closely related species, we discovered AARSs with deletions and substitutions that we hypothesized would abolish their editing activities (Fig. 1). These Mycoplasma AARSs have substitutions at key residues in the hydrolytic active sites of the editing domains. In one extreme case, the editing domain was completely deleted from the M. mobile leucyl-tRNA synthetase (LeuRS).
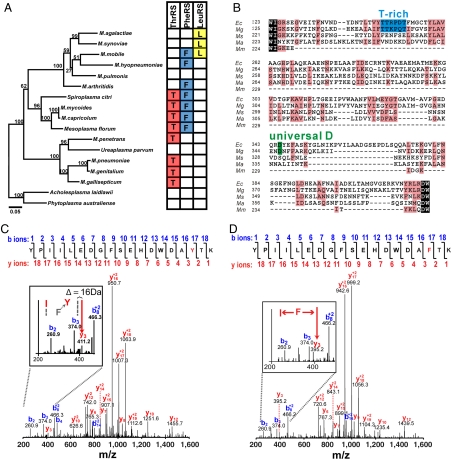
Fig. 1.
Degenerated editing domains of Mycoplasma AARS. (A) Phylogenetic tree of Mycoplasma based on 16S rRNA. Bootstrap values are shown for each node and scale bar denotes substitutions per site. Predicted editing-defective AARS are indicated in boxes (Right) (see also Figs. S1–S3). (B) Alignment of LeuRS CP1 domain with key editing site residues indicated (see also Table S3). Shaded and black boxes represent conserved and homologous residues. (C) Tandem MS analysis of precursor peak m/z = 796.4 at z = 3 identified a mistranslated peptide in M. mobile. A fragment peak (m/z = 411.2; _y_3 ion of YPIILEDGFSEHDWDA(Y)TK; phosphopyruvate hydratase) was confirmed by the balance of the fragmentation spectrum. (Inset) Predicted _y_2 and _y_3 ion positions for the genome-encoded peptide (dotted bars) and observed mistranslated product (solid bars; F → Y). (D) Identification of faithfully translated peptide YPIILEDGFSEHDWDA(F)TK for phosphopyruvate hydratase (peak of m/z = 395.2 at the _y_3 ion position).
In six different Mycoplasma and also two closely related species, threonyl-tRNA synthetase (ThrRS) contained editing domains in which the editing site had amino acid substitutions at critical residues (Fig. 1A). In each of these cases, critical histidine, aspartic acid, and cysteine residues (Fig. S1) within two distal peptides that folded to form the editing active site were replaced (10–12). The editing domains of the editing-defective ThrRSs have a lower average sequence identity (24.4%) compared to the canonical cases (44.6%), while retaining similar levels of conservation for their aminoacylation domains (54.7% versus 56.1%). This suggested that the editing domain in these Mycoplasma species was preferentially prone to retaining substitutions (Table S1).
In some Mycoplasma species, conserved motifs in the editing site of phenylalanyl-tRNA synthetase (PheRS) (13, 14) have also acquired substitutions at key sites (Fig. S2), and the overall sequence identity for their editing domains (19.2%) is lower than the canonical counterparts (31.3%), although the two groups share similar identities in the aminoacylation domains (46.8% versus 44.1%; Table S1). In at least four organisms, PheRS and ThrRS proteins are simultaneously encoded to express editing domains that appear to be functionally defective (Fig. 1A). In another set of Mycoplasma species, the fidelity domains of either PheRS (Fig. S2) or LeuRS (Fig. 1B and Fig. S3) or both have been mutated suggesting that they are editing-deficient. Phylogenetic analysis indicates that in different Mycoplasma lineages, these mutational events in the three AARS genes have taken place independently multiple times (Fig. 1A).
M. mobile Has a Statistical Proteome.
We hypothesized that Mycoplasma with editing-defective AARSs would be prone to generating statistical substitutions during protein synthesis. The genome of M. mobile (15), a fish pathogen, encodes a PheRS with substitutions at key sites in the editing pocket. In addition, LeuRS is completely missing its editing domain that is called CP1 (16, 17). In order to hunt for statistical proteins, we bioinformatically rescreened data obtained for the complete proteome of M. mobile (15) by instructing the database search program to allow for potential mistranslations corresponding to suspected editing-defective AARSs. These Mycoplasma were grown in rich media under conditions that would not be expected to bias a bacteria to incorporate mutations (15). However, analysis of the proteome identified examples of statistical mutations including an F322Y substitution in phosphopyruvate hydratase (Fig. 1C) that would be indicative of an editing-defective PheRS, which generates mischarged Tyr-tRNAPhe (13). In parallel, a faithfully translated peptide from phosphopyruvate hydratase was also detected that did not contain the F322Y mutation via mass spectrometry analysis of the peptide pool (Fig. 1D), which supported the statistical nature of these mistakes.
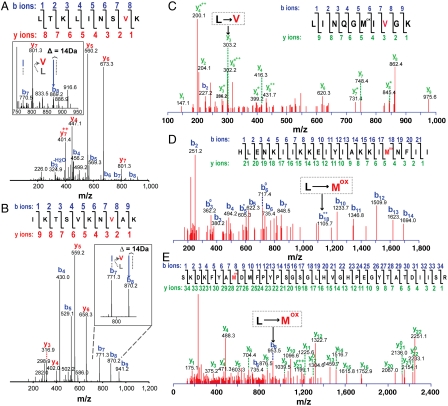
LeuRS fidelity is challenged by a broader scope of amino acids (9). Notably, the most predominant expected replacement for leucine would be isoleucine (9), but this change would not be detected by mass spectrometry because these isomers have identical molecular weights. However, examples where valine, which is only weakly misactivated by LeuRS (9) (Table S2), was substituted for leucine in phosphopentomutase (L318V; Fig. 2A) and phosphotransacetylase (L14V; Fig. 2B) provided additional evidence for statistical substitutions in the M. mobile proteome.
Fig. 2.
Tandem MS analysis of single peptides from M. mobile and E. coli that express M. mobile LeuRS demonstrate mistranslation of leucine codons. An L → V substitution of M. mobile peptides LTKLINS(L/V)K from phosphopentomutase (A) and IKTSVKN(L/V)AK from phosphotransacetylase (B) respectively generated peaks of m/z = 869.2 [confirmatory _b_8 ion of LTKLINS(V)K; precursor peak m/z = 508.5 at z = 2] and m/z = 870.2 [confirmatory _b_8 ion of IKTSVKN(V)AK; precursor peak m/z = 544.3 at z = 2]. (C) An L → V substitution of E. coli peptide LINQGMI(L)GK generated a peak of m/z = 303.2 corresponding to the confirmatory _y_3 ion of LINQGMI(V)GK. An L → M substitution of E. coli peptides HLENKIIKKEIYIAKKI(L)NFII (D) and SKDKFYA(L)D MFPYPSGSGLHVGHPEGYTATDIISR (E) respectively generated peaks of m/z = 1105.7 [confirmatory 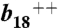 ion of HLENKIIKKEIY IAKKI(Mox)NFII] and m/z = 953.5 [confirmatory _b_8 ion of SKDKFYA(Mox)DMFPYPSGSGLHVGHPEGYTATDIISR]. Methionine was typically oxidized to methionine sulfoxide (see also Fig. S4).
ion of HLENKIIKKEIY IAKKI(Mox)NFII] and m/z = 953.5 [confirmatory _b_8 ion of SKDKFYA(Mox)DMFPYPSGSGLHVGHPEGYTATDIISR]. Methionine was typically oxidized to methionine sulfoxide (see also Fig. S4).
A typical rate for translational fidelity has been estimated to be 1/3,000 (18), and AARSs that cannot meet this threshold have evolved editing domains (19). In our peptide pool for the M. mobile proteome, we screened 138 phenylalanines and 518 leucines to determine an error of 1 and 2 substitutions, respectively. The probability that these statistical substitutions result from an editing-defective PheRS and LeuRS (Model E, Eqs. 1 and 2 below) is significant with values of 0.35 and 0.25, respectively, if we assume that the error rate of these aaRSs are approximately 1/200 (vide infra):
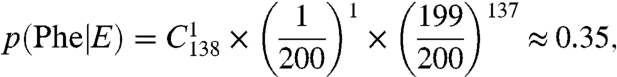 |
[1] |
|---|
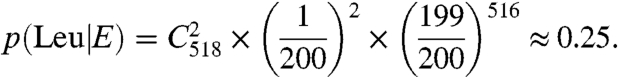 |
[2] |
|---|
This would compare to very low probabilities of less than 0.05 if these substitutions were based on normal mistranslation rates of 1/3,000. Thus, we propose that this high level of mistranslation is statistical for M. mobile and is caused by editing-defective PheRS and LeuRS that cannot clear their own mischarged tRNA products.
We also tested for M. mobile LeuRS-dependent mistranslations in Escherichia coli. Previously, we showed that LeuRS misactivates valine, isoleucine, and methionine (9). Because isoleucine cannot be distinguished from leucine via mass spectrometry, we focused on valine and methionine substitutions. To increase sensitivity, we induced expression of M. mobile LeuRS in E. coli BL21 cells (17 nmol/g cells) in the presence of increasing valine or methionine concentrations of up to 10 mM. Tandem mass spectrometry of purified M. mobile LeuRS that was trypsin-digested identified statistical substitutions for multiple sets of peptides, where valine or methionine was substituted for leucine (Fig. 2) as well as correlating examples where these specific sites had been faithfully translated (Fig. S4). In comparison, there was no mistranslation detected in BL21 cells that expressed E. coli LeuRS (22 nmol/g cells) under the same condition, even at high concentrations of noncognate amino acids. In addition, E. coli BL21 cells that expressed M. mobile LeuRS had a lengthy lag phase that slowed cell growth (Fig. S5).
Statistical Proteome Substitutions Correlate to AARS Amino Acid Editing Defect.
Preparation of pure (αβ)2 PheRS and dimeric ThrRS for in vitro analysis could be complicated by heterogeneity of their respective quaternary structures. Thus, we focused on the monomeric M. mobile LeuRS for enzymatic characterization of its putative editing defect to understand the molecular mechanism underlying error-prone translation in M. mobile. The gene was synthesized in order to convert TGA triplets (which are used to encode tryptophan in M. mobile) to TGG and also to optimize codon usage frequencies for expression in E. coli. The monomeric LeuRS was purified by affinity chromatography via an N-terminal six-histidine tag and the enzyme robustly aminoacylated in vitro transcribed M. mobile tRNALeu (Fig. S6) as well as E. coli tRNALeu (Fig. S7). Based on the _k_cat/K M for amino acid activation by M. mobile LeuRS (Table S2), the discrimination factors for noncognate amino acids versus leucine were well below the accepted threshold of 3,000. Indeed, the discrimination factor of 200 for isoleucine was consistent with our threshold calculations for misaminoacylation in Mycoplasma (vide supra). It also suggests that M. mobile LeuRS is even more prone to isoleucine misactivation than a typical LeuRS (Table 1).
Table 1.
Apparent kinetic parameters for amino acid activation by LeuRS
| K M, mM | _k_cat, s-1 | _k_cat/K m, mM-1 s-1 | ||||
|---|---|---|---|---|---|---|
| Enzyme | Leu | Ile | Leu | Ile | Leu | Ile |
| Ec LeuRS* | 0.018 ± 0.008 | 1.0 ± 0.3 | 71 ± 25 | 4.7 ± 1.3 | 3.9 × 103 | 4.7 |
| Ec LeuRS ΔCP1* | 0.021 ± 0.006 | 3.4 ± 0.9 | 0.23 ± 0.07 | 0.23 ± 0.07 | 11 | 0.65 |
| Mm LeuRS | 0.072 ± 0.025 | 6.0 ± 1.7 | 11.7 ± 4.2 | 4.8 ± 1.3 | 1.6 × 102 | 0.80 |
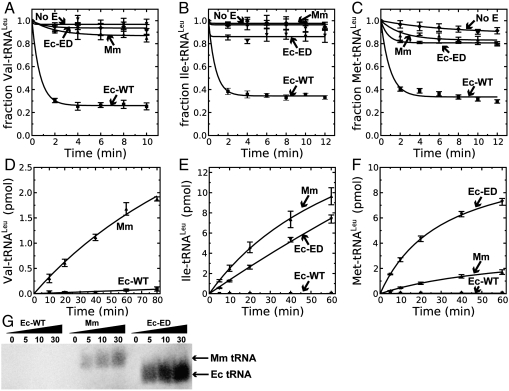
As would be expected, M. mobile LeuRS failed to deacylate mischarged Val-tRNALeu, Ile-tRNALeu, and Met-tRNALeu (Fig. 3), because it does not have a CP1 editing domain. These noncognate amino acids can be charged to M. mobile tRNA (Fig. 3). Consistent with the introduction of leucine to methionine or valine statistical substitutions in the E. coli proteome (Fig. 2), M. mobile LeuRS also mischarged E. coli tRNALeu with methionine or valine (Fig. S7).
Fig. 3.
Wild-type M. mobile LeuRS mischarges tRNALeu. Deacylation reactions contained approximately 20 μM [3H]-Val-tRNALeu (A), approximately 6.5 μM [3H]-Ile-tRNALeu (B) or 100 μM [35S]-Met-tRNALeu (C) and 100 nM M. mobile, E. coli wild-type or mutant LeuRS. Mischarging assays incorporated 160 μM [14C]-valine (50 μCi/mL; D), 21 μM [3H]-isoleucine (166 Ci/mmol; E) or 20 μM [35S]-methionine (20 μCi/mL; F) and 1 μM M. mobile or E. coli LeuRS. Editing-defective E. coli LeuRS (Ec-Ed) mutant is a positive control. (G) Acid gel of tRNALeu charged with [35S]-methionine. Abbreviations: (square), Ec-WT; (inverted triangle), Mm; (triangle), Ec-Ed; and (diamond), no enzyme control (No E). Error bars represent standard deviations from triplicated reactions.
The propensity of M. mobile LeuRS to produce stably mischarged tRNALeu contrasts sharply with other examples of LeuRSs that were determined to rely on additional mechanisms to ensure fidelity. E. coli or yeast mitochondrial LeuRS maintained fidelity in the absence of their CP1 modules by activating an alternate pretransfer editing pathway (20). Also, M. mobile LeuRS has not evolved the higher threshold of amino acid discrimination that has been measured for human mitochondrial LeuRS, which lacks editing activity (21).
Discussion
In all three domains of life, LeuRS contains the CP1 editing domain as does isoleucyl (IleRS)- and valyl (ValRS)-tRNA synthetases (22). These homologous proteins have differentiated to accommodate different amino acid specificities for both aminoacylation and editing. They also can rely selectively or combinatorially on pre- and posttransfer editing mechanisms to respectively target the aminoacyl-adenylate intermediate or mischarged tRNA product to clear misactivated amino acids (23–25). To our knowledge, M. mobile LeuRS is the only known example for LeuRS, IleRS, or ValRS that is completely missing its CP1 editing module (Fig. 1B). Previously, we had shown that the fidelity of E. coli and yeast mitochondrial LeuRS were protected in the absence of its CP1 module (LeuRS-ΔCP1) by a latent pretransfer editing activity that cleared misactivated aminoacyl-adenylate intermediate (20). In this case, M. mobile LeuRS with its naturally missing CP1 editing module failed to recapture an alternate editing mechanism to posttransfer editing that was sufficient to completely protect the proteome.
Statistical proteins have been proposed to provide an advantage in primitive cells during the evolution of the modern protein synthesis machinery (26, 27). They have also been suggested to increase fitness in contemporary bacteria by providing a protein reservoir that is phenotypically diverse (28). In a transcription-dependent error-prone experimental model for Bacillus subtilis, proteome diversity caused by frame-shift or nonsense mutations enabled the organism to adapt to fluctuations in the environment, such as changes in temperature (28). In yeast, CUG codon ambiguity originating in Candida albacans was experimentally introduced to confer an adaptive advantage under stress conditions via induction of a unique set of stress proteins (29). Under specific stress conditions in mammalian systems, codon ambiguity increases significantly via activation of MetRS misacylation of noncognate tRNA isoacceptors (30). An artificial mutation in the ValRS editing domain also allowed E. coli to adapt for subsistence on the nonstandard amino acid α-aminobutyrate, rather than valine (31).
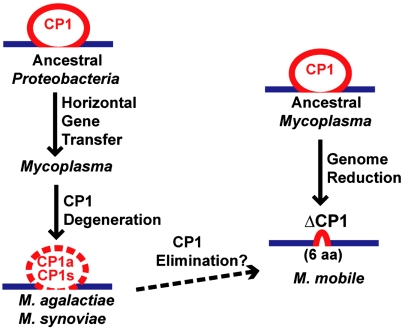
We have identified natural examples in Mycoplasma species and related species in which the gene encoding AARSs contains mutations that disrupt the amino acid editing function. The shedding of the editing domain of LeuRS in M. mobile was likely facilitated by existing mechanisms in Mycoplasma that have resulted in the dramatic evolutionary reduction of its genomes. Accordingly, the erosion of AARS-dependent translational fidelity may be a stepwise response based on the LeuRS CP1 domain’s massive sequence degeneration in Mycoplasma agalactiae and Mycoplasma synoviae, and then complete elimination of the entire module in M. mobile (Fig. 4).
Fig. 4.
Proposed evolutionary scheme for LeuRS CP1 editing module degeneration in Mycoplasma. M. synoviae and M. agalactiae LeuRS were acquired from Proteobacteria via horizontal gene transfer (Fig. S8). Primary structure degeneration of the CP1 domain resulted in M. agalactiae and M. synoviae LeuRS CP1a and CP1s (Left). Genome reduction in M. mobile completely eliminated the CP1 editing module in LeuRS (ΔCP1; Right).
Editing-defective AARSs in M. mobile introduce errors at the translational level of protein synthesis. Because of the strong selection pressure throughout the three domains of life to maintain translational fidelity, we hypothesize that the loss of AARS editing in Mycoplasma is likely due to idiosyncratic demands on the organism, which are benefited by the introduction of statistical proteomes (19). It is possible that this mechanism to introduce statistical proteins could confer antigenic diversity and phenotypic plasticity (2). This unique AARS-dependent mechanism at the translational level would be akin to the replication-dependent high rates of retroviral mutations that confer virus population heterogeneity and resistance (32) to escape host defense mechanisms.
Materials and Methods
Bioinformatic Analysis and M. mobile Proteome Mass Spectral Data.
Sequences were retrieved from the Swiss-Prot database (http://ca.expasy.org/), aligned and edited in MultiSeq/VMD (33) using CLUSTALW (34), and shaded by Biology Workbench (http://workbench.sdsc.edu). The phylogenetic tree of Mycoplasma and related species was constructed by a maximum-likelihood method based on 16S rRNA sequences using RAxML (35). The GTR model was used for nucleotide substitution and the Γ model for rate heterogeneity. The support values for the bipartitions were estimated from 1,000 nonparametric bootstrap runs.
Previous mass spectral data (15) were analyzed via SpectrumMill (Agilent) using the default parameters for ThermoFinnigan LCQ ion trap data. The homology mode substitutions F → Y and L → V were defined. The data were searched against all annotated trypsin-digested ORFs as described (15). Individual peptides with SpectrumMill scores > 13 and scored percent identity > 80% were further considered. These peptide spectra were searched against the M. mobile database in “no enzyme” mode as a test of specificity. Concordant results were verified by manual inspection.
Cloning and in Vitro Transcription of the M. mobile tRNALeu Gene.
The gene for M. mobile 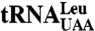 , the most abundant M. mobile tRNALeu isoacceptor, was cloned into pUC18 using BamHI and HindIII restriction sites to yield pUC18LiMmtRNALeu. The plasmid (450 μg) was digested overnight with 25 U of SacII and then used as a template for in vitro runoff transcription (36, 37). The SacII digestion cleaves the tRNA template after the penultimate nucleotide at the 3′ end to generate tRNALeu without the terminal A76 (
, the most abundant M. mobile tRNALeu isoacceptor, was cloned into pUC18 using BamHI and HindIII restriction sites to yield pUC18LiMmtRNALeu. The plasmid (450 μg) was digested overnight with 25 U of SacII and then used as a template for in vitro runoff transcription (36, 37). The SacII digestion cleaves the tRNA template after the penultimate nucleotide at the 3′ end to generate tRNALeu without the terminal A76 ( ). The
). The  transcript was purified by PAGE, and the terminal A76 was added as described previously (20).
transcript was purified by PAGE, and the terminal A76 was added as described previously (20).
Preparation and Characterization of M. mobile LeuRS.
The M. mobile LeuRS gene was amplified via PCR (30 cycles) from 50 ng M. mobile genomic DNA. The DNA sequence confirmed the originally reported DNA sequence (15). The gene sequence was then optimized (Geneart) for expression in E. coli, particularly with TGA codons changed to TGG, and synthesized with flanking NdeI and BamHI sites to subclone the DNA fragment into pET14b plasmid. The resulting p14LiMmoLeuRS plasmid was used to transform E. coli BL21 (DE3) strain, and LeuRS was expressed and purified (38). Enzymatic assays were carried out in vitro as described previously in detail (38). All biochemical results were plotted using Python and Matplotlib plotting library (39).
Mass Spectrometry Analysis of Mistranslation in E. coli Expressing M. mobile LeuRS.
E. coli BL21 (DE3) transformed with p14LiMmoLeuRS was grown in LB containing excess methionine or valine (0 mM, 1 mM, 5 mM, and 10 mM). The expressed protein was purified via its six-histidine tag (38) and then with Perfect-Focus (G-Biosciences). The LeuRS was digested with mass spectrometry grade trypsin (G-Biosciences) at 1∶50 wt/wt ratio in 25 mM ammonium bicarbonate. Digestion was performed using a CEM Discover Microwave Digestor (CEM Corporation) for 15 min at 75 W and 55 °C. The digested peptides were lyophilized to dryness and reconstituted in 5% acetonitrile with 0.1% formic acid at 200 μg/mL. A 10-μL aliquot was used for liquid chromatography/mass spectrometry (LC/MS) analysis.
LC/MS spectrometry was performed using a Waters Q-ToF connected to a nanoAcquity UPLC (Waters Corporation) and an Atlantis dC18 nanoAcquity UPLC column (75 μm × 150 mm; 3-μm particle size) with a flow rate of 250 nL/ min. The 60-min gradient was from 100% A to 60% B (A = water + 0.1% formic acid; B = acetonitrile + 0.1% formic acid). Data collection was performed with MassLynx 4.1 using Data Directed Analysis. The top four intensive precursor ions from each survey scan were subjected to MS/MS by collision-induced dissociation. The raw mass spectrometric data were processed using ProteinLynx Global Server 2.2.5 (Waters) for data filtering, smoothing, and deisotoping. The refined peak lists were analyzed with Mascot 2.2 (Matrix Science) using a tolerance of ± 0.4 Da for both the precursor ions and fragment ions. The searches were conducted against the National Center for Biotechnology Information nonredundant protein database. Specific modifications such as L → V or L → M (or its oxidized state) substitutions were analyzed as variable modifications in conjunction with the error-tolerant mode in Mascot.
Supplementary Material
Supporting Information
Acknowledgments.
We thank Ke Chen (University of Illinois, Urbana, IL) for providing aligned 16S rRNA sequences and Dr. Makoto Miyata (Osaka City University, Osaka, Japan) for his gift of M. mobile genomic DNA. This work was supported by grants from the National Science Foundation (MCB-0843611 and MCB-0844670) and the National Institutes of Health (GM063789 and P41RR05964).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Maniloff J. In: Molecular Biology and Pathogenicity of Mycoplasmas. Razin S, Herrmann R, editors. New York: Kluwer Academic; 2002. pp. 31–44. [Google Scholar]
- 2.Rottem S. Interaction of Mycoplasmas with host cells. Physiol Rev. 2003;83:417–432. doi: 10.1152/physrev.00030.2002. [DOI] [PubMed] [Google Scholar]
- 3.Ibba M, Söll D. Quality control mechanisms during translation. Science. 1999;286:1893–1897. doi: 10.1126/science.286.5446.1893. [DOI] [PubMed] [Google Scholar]
- 4.Fersht AR. Sieves in sequence. Science. 1998;280:541. doi: 10.1126/science.280.5363.541. [DOI] [PubMed] [Google Scholar]
- 5.An S, Musier-Forsyth K. Trans-editing of Cys-tRNAPro by Haemophilus influenzae YbaK protein. J Biol Chem. 2004;279:42359–42362. doi: 10.1074/jbc.C400304200. [DOI] [PubMed] [Google Scholar]
- 6.Ruan B, Söll D. The bacterial YbaK protein is a Cys-tRNAPro and Cys-tRNACys deacylase. J Biol Chem. 2005;280:25887–25891. doi: 10.1074/jbc.M502174200. [DOI] [PubMed] [Google Scholar]
- 7.Nangle LA, Motta CM, Schimmel P. Global effects of mistranslation from an editing defect in mammalian cells. Chem Biol. 2006;13:1091–1100. doi: 10.1016/j.chembiol.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Lee JW, et al. Editing-defective tRNA synthetase causes protein misfolding and neurodegeneration. Nature. 2006;443:50–55. doi: 10.1038/nature05096. [DOI] [PubMed] [Google Scholar]
- 9.Karkhanis VA, Mascarenhas AP, Martinis SA. Amino acid toxicities of Escherichia coli that are prevented by leucyl-tRNA synthetase amino acid editing. J Bacteriol. 2007;189:8765–8768. doi: 10.1128/JB.01215-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beebe K, Ribas De Pouplana L, Schimmel P. Elucidation of tRNA-dependent editing by a class II tRNA synthetase and significance for cell viability. EMBO J. 2003;22:668–675. doi: 10.1093/emboj/cdg065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dock-Bregeon AC, et al. Achieving error-free translation; the mechanism of proofreading of threonyl-tRNA synthetase at atomic resolution. Mol Cell. 2004;16:375–386. doi: 10.1016/j.molcel.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Beebe K, Merriman E, Ribas De Pouplana L, Schimmel P. A domain for editing by an archaebacterial tRNA synthetase. Proc Natl Acad Sci USA. 2004;101:5958–5963. doi: 10.1073/pnas.0401530101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ling J, Roy H, Ibba M. Mechanism of tRNA-dependent editing in translational quality control. Proc Natl Acad Sci USA. 2007;104:72–77. doi: 10.1073/pnas.0606272104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy H, Ling J, Irnov M, Ibba M. Post-transfer editing in vitro and in vivo by the β-subunit of phenylalanyl-tRNA synthetase. EMBO J. 2004;23:4639–4648. doi: 10.1038/sj.emboj.7600474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaffe JD, et al. The complete genome and proteome of Mycoplasma mobile. Genome Res. 2004;14:1447–1461. doi: 10.1101/gr.2674004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starzyk RM, Webster TA, Schimmel P. Evidence for dispensable sequences inserted into a nucleotide fold. Science. 1987;237:1614–1618. doi: 10.1126/science.3306924. [DOI] [PubMed] [Google Scholar]
- 17.Cusack S, Yaremchuk A, Tukalo M. The 2 Å crystal structure of leucyl-tRNA synthetase and its complex with a leucyl-adenylate analogue. EMBO J. 2000;19:2351–2361. doi: 10.1093/emboj/19.10.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loftfield RB. The frequency of errors in protein biosynthesis. Biochem J. 1963;89:82–92. doi: 10.1042/bj0890082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reynolds NM, et al. Cell-specific differences in the requirements for translation quality control. Proc Natl Acad Sci USA. 2010;107:4063–4068. doi: 10.1073/pnas.0909640107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boniecki MT, Vu MT, Betha AK, Martinis SA. CP1-dependent partitioning of pretransfer and posttransfer editing in leucyl-tRNA synthetase. Proc Natl Acad Sci USA. 2008;105:19223–19228. doi: 10.1073/pnas.0809336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lue SW, Kelley SO. An aminoacyl-tRNA synthetase with a defunct editing site. Biochemistry. 2005;44:3010–3016. doi: 10.1021/bi047901v. [DOI] [PubMed] [Google Scholar]
- 22.Lin L, Hale SP, Schimmel P. Aminoacylation error correction. Nature. 1996;384:33–34. doi: 10.1038/384033b0. [DOI] [PubMed] [Google Scholar]
- 23.Martinis SA, Boniecki MT. The balance between pre- and post-transfer editing in tRNA synthetases. FEBS Lett. 2010;584:455–459. doi: 10.1016/j.febslet.2009.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schimmel P, Schmidt E. Making connections: RNA-dependent amino acid recognition. Trends Biochem Sci. 1995;20:1–2. doi: 10.1016/s0968-0004(00)88937-9. [DOI] [PubMed] [Google Scholar]
- 25.Dulic M, Cvetesic N, Perona JJ, Gruic-Sovulj I. Partitioning of tRNA-dependent editing between pre- and post-transfer pathways in class I aminoacyl-tRNA synthetases. J Biol Chem. 2010;285:23799–23809. doi: 10.1074/jbc.M110.133553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woese CR. On the evolution of the genetic code. Proc Natl Acad Sci USA. 1965;54:1546–1552. doi: 10.1073/pnas.54.6.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woese CR. On the evolution of cells. Proc Natl Acad Sci USA. 2002;99:8742–8747. doi: 10.1073/pnas.132266999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyerovich M, Mamou G, Ben-Yehuda S. Visualizing high error levels during gene expression in living bacterial cells. Proc Natl Acad Sci USA. 2010;107:11543–11548. doi: 10.1073/pnas.0912989107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santos MA, Cheesman C, Costa V, Moradas-Ferreira P, Tuite MF. Selective advantages created by codon ambiguity allowed for the evolution of an alternative genetic code in Candida spp. Mol Microbiol. 1999;31:937–947. doi: 10.1046/j.1365-2958.1999.01233.x. [DOI] [PubMed] [Google Scholar]
- 30.Netzer N, et al. Innate immune and chemically triggered oxidative stress modifies translational fidelity. Nature. 2009;462:522–6. doi: 10.1038/nature08576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doring V, et al. Enlarging the amino acid set of Escherichia coli by infiltration of the valine coding pathway. Science. 2001;292:501–504. [Google Scholar]
- 32.Preston BD, Dougherty JP. Mechanisms of retroviral mutation. Trends Microbiol. 1996;4:16–21. doi: 10.1016/0966-842x(96)81500-9. [DOI] [PubMed] [Google Scholar]
- 33.Roberts E, Eargle J, Wright D, Luthey-Schulten Z. MultiSeq: Unifying sequence and structure data for evolutionary analysis. BMC Bioinformatics. 2006;7:382. doi: 10.1186/1471-2105-7-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 36.Sampson JR, Uhlenbeck OC. Biochemical and physical characterization of an unmodified yeast phenylalanine transfer RNA transcribed in vitro. Proc Natl Acad Sci USA. 1988;85:1033–1037. doi: 10.1073/pnas.85.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhai Y, Martinis SA. Two conserved threonines collaborate in the Escherichia coli leucyl-tRNA synthetase amino acid editing mechanism. Biochemistry. 2005;44:15437–15443. doi: 10.1021/bi0514461. [DOI] [PubMed] [Google Scholar]
- 38.Karkhanis VA, Boniecki MT, Poruri K, Martinis SA. A viable amino acid editing activity in the leucyl-tRNA synthetase CP1-splicing domain is not required in the yeast mitochondria. J Biol Chem. 2006;281:33217–33225. doi: 10.1074/jbc.M607406200. [DOI] [PubMed] [Google Scholar]
- 39.Hunter JD. Matplotlib: A 2D graphics environment. Comput Sci Eng. 2007;9:90–95. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information