Identification of Akt Interaction Protein PHF20/TZP That Transcriptionally Regulates p53 (original) (raw)
Background: Akt exerts its cellular function through interaction/phosphorylation of downstream proteins.
Results: Akt interacts with and phosphorylates PHF20, which regulates p53 and inhibits cell proliferation and survival, resulting in loss of its function.
Conclusion: PHF20 up-regulates p53. Akt phosphorylates and abrogates PHF20 function.
Significance: We identified a novel interactor/substrate of Akt and an additional link between Akt and p53 cascades.
Keywords: Akt, Phosphorylation, Protein-Protein Interactions, Signal Transduction, Transcription Factors
Abstract
Akt regulates a diverse array of cellular functions, including cell survival, proliferation, differentiation, and metabolism. Although a number of molecules have been identified as upstream regulators and downstream targets of Akt, the mechanisms by which Akt regulates these cellular processes remain elusive. Here, we demonstrate that a novel transcription factor, PHF20/TZP (referring to Tudor and zinc finger domain containing protein), binds to Akt and induces p53 expression at the transcription level. Knockdown of PHF20 significantly reduces p53. PHF20 inhibits cell growth, DNA synthesis, and cell survival. Akt phosphorylates PHF20 at Ser291 in vitro and in vivo, which results in its translocation from the nucleus to the cytoplasm and attenuation of PHF20 function. These data indicate that PHF20 is a substrate of Akt and plays a role in Akt cell survival/growth signaling.
Introduction
Akt is also known as protein kinase B (1, 2). Viral Akt is highly activated and oncogenic because of the fact that viral Akt is constitutively associated with the cell membrane through a myristoylated Gag protein sequence fused to the N terminus of Akt (3). The important role of Akt in cellular transformation and tumorigenesis was shortly strengthened by the cloning of the human AKT2 gene (4) and by the discovery that AKT2 is frequently amplified and overexpressed in human cancers (4–6). Akt is activated by various stimuli in a phosphatidylinositol 3-kinase (PI3K)-dependent manner (7–10). Activation of the Akt kinase depends on the integrity of the pleckstrin homology domain, which mediates its membrane translocation, and on the phosphorylation of Thr308 in the activation loop and Ser473 (11–14). Phosphoinositides, phosphatidylinositol-3,4-P2 and phosphatidylinositol-3,4,5-P3, produced by PI3K bind directly to the pleckstrin homology domain of Akt, driving a conformational change in the molecule, which enables the activation loop of Akt to be phosphorylated by PDK1 at Thr308 (15). Full activation of Akt is also associated with phosphorylation of Ser473 (16) within a C-terminal hydrophobic motif characteristic of kinases in the AGC kinase family. Although the role of PDK1 in Thr308 phosphorylation is well established, the mechanism of Ser473 phosphorylation is controversial. A number of candidate enzymes responsible for this modification have been put forward, including integrin-linked kinase (17), Akt itself, through autophosphorylation (18), PKCα (19), PKCβII (20), DNA-dependent kinase (21), and the rictor-mTOR·mTORC2 complex (22). We and others have recently shown that IKBKE and TBK1 directly phosphorylate Thr308 and Ser473 of Akt (23–25) and that IKBKE activates Akt independent of PI3K/PDK1/mTORC2 as well as pleckstrin homology domain of Akt (23). Akt phosphorylates and/or interacts with a number of molecules to exert its normal cellular functions, which include roles in cell proliferation, survival, differentiation, and metabolism.
The p53 gene represents one of the most studied tumor suppressor genes. It is frequently mutated in a wide range of tumors and plays an essential role in maintaining genomic integrity (26–30). Exposure of a normal cell to genotoxic stress leads to an increase in p53 protein levels. The increase in p53 protein results in an increase in p53-dependent transcription of p53 target genes, which subsequently leads to cell cycle arrest or apoptosis (31–34). The practical implication of these facts is that when a cell undergoes alterations that predispose it to become cancerous, p53 is activated to trigger checkpoints that either mend the damage through its DNA repair function or eliminate the affected cells through induction of apoptosis, thereby preventing the development of tumors (30, 35). Therefore, regulation of p53 is critical to allow both normal cell growth and tumor suppression. The current dogma is that p53 regulation in DNA damage-activated cell cycle checkpoints occurs at the post-translational level. This includes regulation of p53 protein stability, post-translational modifications, protein-protein interactions, and subcellular localization. These mechanisms keep a strong check on p53 in normal circumstances but allow rapid activation in response to cellular stress that might be caused by or contribute to oncogenic progression (28, 30). However, little is known about the transcriptional regulation of the p53 gene and the contribution of this transcriptional control to DNA damage-induced cell cycle checkpoints. Previous studies have shown that p53 is transcriptionally up-regulated by the homeobox protein HOXA5 (36, 37), p53 itself (38), and death-promoting factor Btf (39). Recently, the Bcl6 oncoprotein was found to suppress p53 expression through binding to p53 and inhibiting p53 promoter activity (40). Several studies have raised the possibility that p53 may also be regulated at the transcriptional level in response to genotoxic stress (41, 42). However, the underlying mechanism and functional consequences remain unclear.
A link between Akt and p53 pathways was established by the identification of Akt phosphorylation of MDM2 (43). MDM2 is an E3 ubiquitin ligase that negatively regulates p53 transcriptional activity (44). Phosphorylation of MDM2 by Akt stimulates translocation of MDM2 to the nucleus, where it binds to p53 and targets it for degradation by the proteasome (45–47). Here, we have identified a novel transcription factor, PHF20, that interacts with Akt. PHF20 directly binds to the p53 promoter and up-regulates p53 at the mRNA level. As a result, PHF20 inhibits cell proliferation, DNA synthesis, and cell survival. Akt phosphorylates PHF20 on serine 291 within a PHF20 nuclear localization signal, which leads to PHF20 translocation from the nucleus to the cytoplasm and loss of its biochemical and cellular functions.
EXPERIMENTAL PROCEDURES
Yeast Two-hybrid Screening and Expression Constructs
Yeast two-hybrid system was employed to identify Akt interaction protein(s) using the C-terminal regulatory region of Akt as bait following the manufacturer's procedure (Clontech). A human fetal brain library (Clontech) was screened.
Full-length cDNA of PHF20, amplified from a human Marathon-ready skeletal muscle cDNA (Clontech) by PCR, was subcloned into 3× FLAG-pcDNA3, pEGFP-C1, and pTRE-tight vectors. PHF20 mutants were created with the QuikChange multiple site-directed mutagenesis kit (Stratagene). The cytomegalovirus-based expression constructs encoding Akt and p53 as well as pGL3-_p53_-Luc have previously been described (48, 49).
Cell Culture and Transfection
HEK293, MCF7, and HCT116 cells were obtained from ATCC and cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum. Lipofectamine Plus (Invitrogen) was used for transfection.
Glutathione S-transferase (GST) Fusion Protein and Generation of PHF20 Antibody
Different portions of PHF20, including AT-hook region, C2H2 zinc finger domain, C-terminal motif, and the regions containing each Akt phosphorylation site, were subcloned into pGEX-4T1. Expression and purification of the GST fusion protein were carried out as described previously (50). Polyclonal PHF20 antibody was raised in New Zealand White rabbits. Approximately 300 μg of GST fusion protein (GST-PHF20/AT-hook and GST-PHF20/C-terminal) was used to immunize rabbits every 2 weeks; rabbits were bled 10 days after each booster injection. The anti-PHF20 antibodies were affinity-purified with Affi-Gel protein A (Bio-Rad). The phospho-PHF20-Ser291 antibody was produced by New England Peptide.
Northern Blot, Immunoprecipitation, and Immunoblotting Analysis
Northern blot analysis of total cellular RNA was performed according to standard procedures (51). Cell lysate was prepared in lysis buffer and subjected to immunoprecipitation and immunoblot analysis as described previously (52). Briefly, lysates were precleared with protein A/protein G (2:1)-agarose beads. Following the removal of the beads by centrifugation, lysates were incubated with appropriate antibodies in the presence of protein A/protein G (2:1)-agarose beads for 2 h. After washing, the immunoprecipitates were subjected to immunoblotting. Protein expression was determined by probing Western blots of total cell lysates with the appropriate antibodies as noted in the figure legends.
In Vitro Kinase Assay
Akt kinase assay was performed as described previously (9, 48). Briefly, reactions were carried out in the presence of 10 μCi of [γ-32P]ATP and 3 μm cold ATP in 30 μl of buffer containing 20 mm Hepes, pH 7.4, 10 mm MgCl2, 2 mm MnCl2, and 1 mm dithiothreitol. GST-PHF20s were used as exogenous substrate. After incubation at room temperature for 30 min, the reactions were stopped by adding protein loading buffer and then separated in SDS-polyacrylamide gels. Each experiment was repeated three times. The relative amounts of incorporated radioactivity were determined by autoradiography.
In Vivo [32P]Orthophosphate Cell Labeling
HEK293 cells were co-transfected with FLAG-PHF20 and constitutively active Akt or pcDNA3 and labeled with [32P]orthophosphate (0.5 mCi/ml) in phosphate- and serum-free minimum essential medium for 4 h. Cell lysates were subjected to immunoprecipitation with FLAG antibody (Sigma). The immunoprecipitates were separated by SDS-PAGE and transferred to membrane. The phosphorylated PHF20 was examined by autoradiography (54).
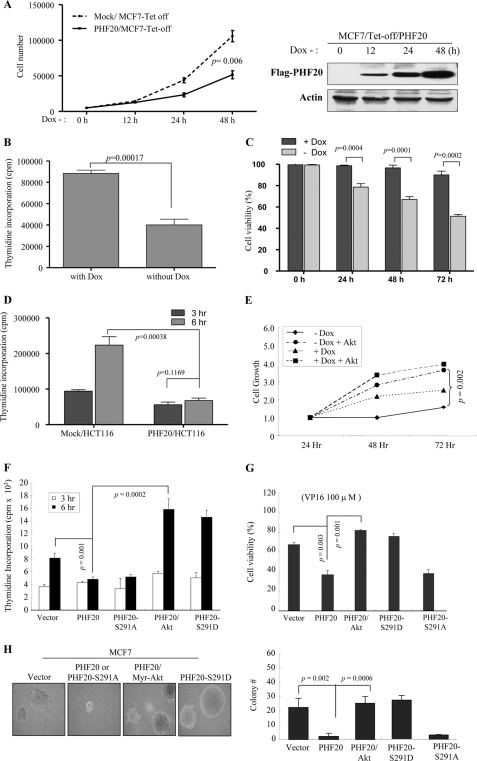
Cell Proliferation, Viability, and DNA Synthesis Assay
Cells were plated in 35-mm dishes at a density of 1.0 × 105 cells/dish. Cell number was measured with a Coulter Counter (Coulter Electronics, FL) daily for up to 3 days. MTS4 assays were performed according to the manufacturer's recommendation (Promega). The cells were plated in a 96-well plate at a density of 1.0 × 103 cells/well. The number of cells at 1–3 days was determined using cell counter and the colorimetric CellTiter96 Aqueous (MTS) assay. Results were depicted as absorbance at 490 nm as a function of time. Cell viability was examined by staining with trypan blue.
Thymidine incorporation was used to investigate the effect of PHF20 on DNA synthesis. The cells were grown to 80% confluence in 6-well plates, and during the last 16 h of growth, they were subjected to 5 μCi/ml of [3H] thymidine. After rinsing with ice-cold serum-free medium, the cells were incubated with 5 ml of 10% TCA for 10 min on ice and then lysed in 500 μl of 1% SDS in 0.3 n NaOH for 30 min at 37 °C. Incorporated radioactivity was quantified with a spectrometer.
Luciferase Reporter Assay
Cells were cultured in 12-well plates and were transiently transfected with pGL3/_p53_-Luc, PHF20, and/or Akt. The amount of DNA in each transfection was kept constant by the addition of empty vector. After transfection for 36 h, luciferase activity was measured using a luciferase assay reagent (Promega). Transfection efficiency was normalized by co-transfection with β-galactosidase expressing vector. The β-galactosidase activity was measured using Galato-Light (Tropix). Luciferase activity was expressed as relative luciferase activity.
Chromatin Association
Chromatin was isolated as described previously with small modifications (supplemental Fig. S1_A_) (55). Briefly, MCF7 cells stably transfected with FLAG-PHF20 were resuspended in buffer A (10 mm Hepes, pH 7.9, 10 mm KCl, 1.5 mm MgCl2, 0.34 m sucrose, 10% glycerol, 1 mm DTT, 5 μg/ml aprotinin, 5 μg/ml leupeptin, 0.1 mm phenylmethylsulfonyl fluoride). Nonidet P-40 (0.1%) was added, and the cells were incubated for 5 min on ice. Nuclei (P1) were collected by low speed centrifugation (5 min, 1,300 × g, 4 °C). The supernatant (S1) was further clarified by high speed centrifugation (15 min, 20,000 × g, 4 °C). Nuclei were washed once in buffer A and then lysed in buffer B (3 mm EDTA, 0.2 mm EGTA, 1 mm DTT, protease inhibitors as described above). Insoluble chromatin was collected by centrifugation (5 min, 1,700 × g, 4 °C) and washed once in buffer B. The final chromatin pellet (P3) was resuspended in sonication buffer (50 mm Tris, pH 8.1, 10 mm EDTA, 1% SDS) and sonicated in a Branson sonicator using a microtip at 10% amplitude. Chromatin was diluted 1:10 with dilution buffer (0.01% SDS, 1.1% Triton, 1.2 mm EDTA, 16.7 mm Tris-Cl, pH 8.2, 167 mm NaCl, 10 μg/ml PMSF, 10 μg/ml leupeptin, 10 μg/ml aprotinin), and FLAG-PHF20 was immunoblotted with FLAG antibody. To release chromatin-bound proteins by nuclease treatment, cell nuclei were resuspended in prewarmed buffer A plus 1 mm CaCl2 and 0.3 units of micrococcal nuclease (Sigma). After incubation at 37 °C for 1 min, the nuclease reaction was stopped by the addition of 1 mm EGTA. Nuclei were collected by low speed centrifugation and lysed according to the chromatin isolation protocol described above.
Cyclic Amplification and Selection of Targets (CASTing)
A 76-bp oligonucleotide containing 26 random nucleotides in the center flanked by sequences complementary to primers A and B was synthesized (Invitrogen). The sequences of the oligonucleotides are as follows: 76-base oligonucleotide, 5′-CAGGTCAGTTCAGCGGATCCTGTC(N)26GAGGCGAATTCAGTGCAACTGCGC-3′; primer A, 5′-GCTGCAGTTGCACTGAATTCGCCTC-3′; primer B, 5′-CAGGTCAGTTCAGCGGATCCTGTCG-3′. A random sequence library of double-stranded radiolabeled oligonucleotides was prepared by annealing the oligonucleotide to 5-fold excess of primer B followed by extension with Klenow enzyme. Electrophoretic mobility shift assay (EMSA) was performed by adding 5 μg of FLAG-PHF20 containing nuclear extract to radiolabeled DNA in DNA binding buffer (5% glycerol, 10 mm Hepes, pH 7.9, 75 mm KCl, 1 mm DTT, 2.5 mm MgCl2, 1 mm EDTA) in the presence of 0.5 μg of poly(dA·dT) and 1 μg of bovine serum albumin. The reaction was incubated at room temperature for 30 min, and subsequently the DNA·protein complexes were resolved by electrophoresis. The complexes formed specifically in the presence of FLAG-PHF20 proteins were detected by autoradiography, excised from gels, and eluted overnight at 37 °C in DNA elution buffer containing 0.3 m NaCl, 1 mm EDTA, and 0.1% SDS. The eluted DNA was extracted once in phenol/chloroform and then precipitated with ethanol. Purified DNA was subjected to re-amplification by PCR in the presence of [α-32P]dCTP. The amplified radiolabeled DNA was purified using G-50 Nick columns (Amersham Biosciences) and was used in subsequent EMSA experiments. After four cycles of CASTing, the final amplified DNA was cloned directly using pGEM-T cloning kit (Promega). Nucleotide sequences of 60 independent clones were determined (supplemental Fig. S1, B and C). The degenerated portion of the sequences was compiled and analyzed for shared sequence patterns by visual inspection and by weblogo software.
ChIP Assay
ChIP assay was performed essentially as described previously (56). Solubilized chromatin was prepared from a total of 2 × 107 asynchronously dividing HCT116 cells. The chromatin solution was diluted 10-fold with ChIP dilution buffer (1.1% Triton X-100, 1.2 mm EDTA, 167 mm NaCl, 16.7 mm Tris-HCl, pH 8.1, 0.01% SDS, protease inhibitors), and precleared with protein-A beads blocked with 2 μg of sheared salmon sperm DNA and preimmune serum. The precleared chromatin solution was divided and utilized for immunoprecipitation assays with PHF20 antibody. Following washing, the antibody·protein·DNA complex was eluted from the beads by resuspension of the pellets in 1% SDS, 0.1 m NaHCO3 at room temperature for 20 min. After cross-linking, protein and RNA were removed by incubation with 10 μg of proteinase K and 10 μg of RNase A at 42 °C for 3 h. Purified DNA was subjected to PCR with primers spanning putative PHF20-binding sites of the p53 promoter. Amplified PCR products were resolved by 1.2% of agarose gel electrophoresis and visualized by BioImage. The sequences of oligonucleotides used for ChIP assays are 5′-CAATTCTGCCCTCACAGCTCTGGTTGC-3′ and 5′-CTCAAAACTTTTAGCGCCAGTCTTGAGC-3′.
RESULTS
Identification of a Novel Akt-binding Protein, PHF20/TZP
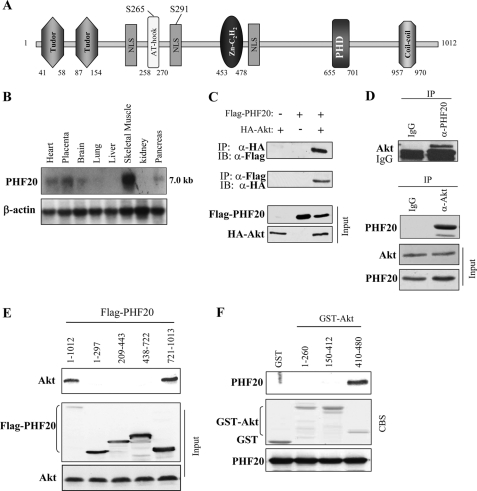
In an attempt to identify proteins that interact with Akt, the C-terminal regulatory domain of Akt (amino acids 410–480) was used as bait in a yeast two-hybrid screening. A human fetal brain cDNA library was used for this screen because Akt is highly expressed in brain (57, 58). Altogether, 32 clones that specifically interacted with the bait were identified. Sequence analysis revealed that three of the clones contained overlapping sequences of a cDNA. The largest clone contained a 262-amino acid open reading frame with a C-terminal region of PHF20/TZP. Additional cDNA clones were isolated from a human skeletal cDNA library by plaque hybridization using the largest clone as radiolabeled probe. Sequence analysis revealed that the full-length open reading frame of cDNA encodes a 1,012-amino acid protein composed of two Tudor domains, an A-T hook motif, a C2H2 zinc finger domain, and a PHD finger motif (Fig. 1A). Therefore, we initially named it as TZP (referring to Tudor and zinc finger domain containing protein; GenBankTM accession number AY027523). In addition, PHF20 contains three nuclear localization signals and two putative Akt phosphorylation consensus motifs (229KRGRPPSIA237 and 285LRRRKISKG293; Fig. 1A). The expression pattern of PHF20 is similar to that of Akt, being abundant in skeletal muscle, brain, pancreas, and heart (Fig. 1B). To confirm the association of Akt with PHF20 identified by yeast two-hybrid screening, HEK293 cells were co-transfected with FLAG-PHF20 and HA-Akt. Immunoprecipitation was performed with anti-FLAG and detected with anti-HA antibody or vice versa. As shown in Fig. 1C, HA-Akt was detected in the FLAG-PHF20 immunoprecipitates, and PHF20 was co-immunoprecipitated with HA-Akt. Their interaction at endogenous protein levels was confirmed in HCT116 cells (Fig. 1D). To define the binding region of PHF20 to Akt, we created deletion mutant constructs of FLAG-PHF20 and of GST-Akt. Immunoprecipitation and GST pulldown assays revealed that interaction of PHF20 and Akt occurs through their C-terminal regions (Fig. 1, E and F).
FIGURE 1.
PHF20 interacts with Akt. A, schematic structure of PHF20. PHF20 contains two Tudor, an AT-hook, a C2H2 zinc finger, and a PHD finger domain, three nuclear localization signals (NLS), as well as two putative Akt phosphorylation consensus sites. B, Northern blot analysis shows the expression of PHF20 in multiple human tissues. C, PHF20 binds to Akt. HEK293 cells were co-transfected with FLAG-PHF20 and HA-Akt. After incubation for 48 h, cells were lysed, immunoprecipitated (IP), and immunoblotted (IB) with the indicated antibodies. D, interaction of PHF20 with Akt at endogenous protein levels. Immunoprecipitation and immunoblotting analysis were performed with indicated antibodies in HCT116 cells. E, C-terminal region of PHF20 binds to Akt. MCF7 cells were transfected with the different truncation mutants of FLAG-PHF20, lysed, and immunoprecipitated with FLAG antibody. The immunoprecipitates were immunoblotted with Akt antibody (top). Middle and bottom panels show expression of FLAG-PHF20 and Akt, respectively. F, C-terminal domain of Akt interacts with PHF20. GST pulldown assay was performed by incubation of GST, GST-fused Akt proteins, with in vitro translated PHF20. The pulldown products were immunoblotted with PHF20 antibody (top). The middle panel is Coomassie Blue staining (CBS) and the bottom panel is immunoblotting of the in vitro translated products with PHF20 antibody.
Akt Phosphorylates PHF20-Serine 291 in Vitro and in Vivo
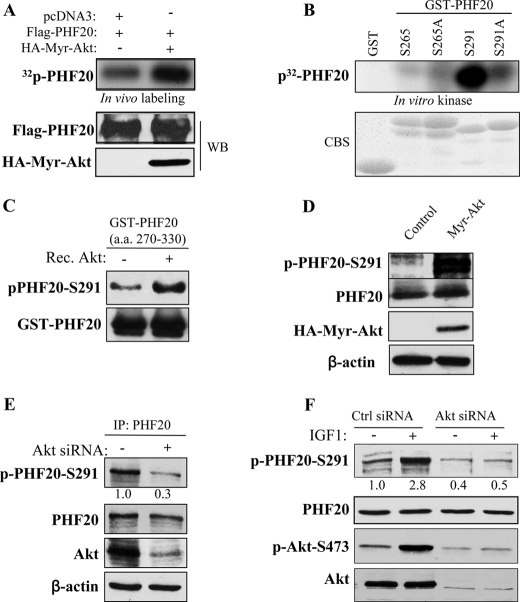
Because PHF20 contains two putative Akt phosphorylation consensus sites, serine 265 and serine 291 (Fig. 1A), we next determined whether PHF20 is phosphorylated by Akt. HEK293 cells were co-transfected with FLAG-PHF20 and constitutively active Akt or pcDNA3 vector. After labeling with [32P]orthophosphate, PHF20 was immunoprecipitated with FLAG antibody, separated, and blotted on a membrane. PhosphoImager quantification analysis revealed that the incorporation of 32P into PHF20 was 8-fold higher in cells co-transfected with Akt/PHF20 as compared with the cells transfected with pcDNA3/PHF20 (Fig. 2A). Furthermore, in vitro kinase assay revealed Akt phosphorylation of PHF20 on serine 291 but not serine 265 (Fig. 2B). We also generated phospho-PHF20-Ser291 antibody. Western blot analysis of in vitro kinase products using phospho-PHF20-Ser291 antibody showed that phospho-PHF20-Ser291 is elevated by incubation with Akt (Fig. 2C). Moreover, expression of constitutively active Akt significantly induced phospho-PHF20-Ser291 (Fig. 2D), whereas knockdown of Akt considerably decreased phospho-PHF20-Ser291 in MDA-MB-468 cells in which PTEN is mutated (Fig. 2E). We also observed that phospho-PHF20-Ser291 was induced by IGF1 and serum and that the IGF1-induced phospho-PHF20-Ser291 was inhibited by knockdown of Akt (Fig. 2F and supplemental Fig. S1). Taken collectively, we conclude that Akt phosphorylates PHF20-Ser291 in vitro and in vivo.
FIGURE 2.
Akt phosphorylates PHF20 in vitro and in vivo. A, PHF20 was phosphorylated by Akt in vivo. In vivo labeling was performed as described under “Experimental Procedures.” Briefly, HEK293 cells were co-transfected with FLAG-PHF20 and constitutively active HA-Myr-Akt or pcDNA3 and labeled with [32P]orthophosphate (0.5 mCi/ml) for 4 h. Cell lysates were immunoprecipitated with FLAG antibody and separated by SDS-PAGE and transferred to membrane. The phosphorylated PHF20 band was examined by autoradiography (top). Middle and bottom panels show the expression of transfected plasmids. B and C, Akt phosphorylates PHF20 in vitro. In vitro kinase assay was performed by incubation of recombinant active Akt with GST-fused PHF20 amino acids 200–280 (Ser265) and 270–330 (Ser291) and their mutant forms. The relative amount of incorporated radioactivity was determined by autoradiography (B) and phospho-PHF20-Ser291 antibody (C). D, Akt phosphorylates PHF20-Ser291 in vivo. HCT116 cells were transfected with constitutively active HA-Myr-Akt or pcDNA3. After incubation of 48 h, cell lysates were immunoblotted with indicated antibodies. E and F, knockdown of Akt reduces phospho-PHF20-Ser291. MDA-MB-468 (E) and MCF7 (F) cells were transfected with Akt siRNA or control siRNA. After 72 h, cell lysates were immunoblotted with indicated antibodies (E). F shows that Akt siRNA or control siRNA-treated MCF7 cells were serum-starved for 12 h, stimulated with IGF1 for 2 h and then immunoblotted with indicated antibodies. WB, Western blot; CBS, Coomassie Blue staining; IP, immunoprecipitation.
Akt Phosphorylation of PHF20 Results in PHF20 Translocation from the Nucleus into the Cytoplasm
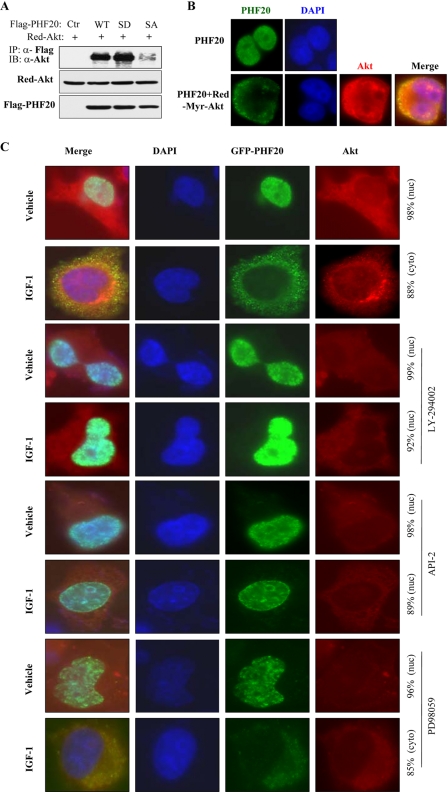
We further determined whether Akt phosphorylation of PHF20 affects their interaction. Phosphomimic and nonphosphorylatable PHF20 were created by mutation of serine 291 into aspartic acid (PHF20-S291D) and alanine (PHF20-S291A), respectively. Co-immunoprecipitation analysis revealed that wild-type PHF20 and phosphomimic PHF20-S291D bound to Akt, whereas nonphosphorylatable PHF20-S291A failed to interact with Akt (Fig. 3A), implying that phosphorylation of PHF20 by Akt is important for their interaction.
FIGURE 3.
Akt induces PHF20 translocation from the nucleus to the cytoplasm. A, phosphomimic PHF20-S291D but not nonphosphorylatable PHF20-S291A preferentially binds to Akt. MCF7 cells were transfected with indicated plasmids, immunoprecipitated (IP) with FLAG antibody, and detected with Akt antibody (top). Middle and bottom panels show the expression of the transfected plasmids. IB, immunoblot. B, PHF20 localizes in the nucleus, and Akt induces PHF20 nuclear-cytoplasm translocation. MCF7 cells were transfected with FLAG-PHF20 together with/without Red-myr-Akt. After 48 h, cells were stained with FITC-conjugated FLAG antibody and DAPI. C, PI3K or Akt inhibitors but not MEK inhibitor blocked IGF1-induced PHF20 nuclear-cytoplasm translocation. MCF7 cells were transfected with GFP-PHF20. After 36 h, cells were deprived from serum for 12 h and treated indicated drugs for 30 min prior to IGF1 stimulation for 1 h. Cells were stained with Akt antibody and Texas Red-conjugated secondary antibody.
Because the serine 291 of PHF20 locates at a nuclear localization signal (Fig. 1A), we next tested whether Akt phosphorylation of PHF20 affects its subcellular localization. MCF7 cells were transfected with FLAG-PHF20 and/or Red-Myr-Akt. PHF20 was localized exclusively in the nucleus in cells transfected with FLAG-PHF20 alone. However, co-transfection of constitutively active Red-Myr-Akt resulted in PHF20 redistribution from the nucleus to the cytoplasm (Fig. 3B). Furthermore, IGF1 stimulation also induced PHF20 translocation from the nucleus to the cytoplasm, which was blocked by pretreatment with PI3K inhibitor LY294002 and Akt inhibitor API-2/TCN (59) but not by MEK inhibitor PD98059 (Fig. 3C). Interestingly, Akt was primarily co-localized with PHF20 in the cytoplasm (Fig. 3, B and C). To further examine if PHF20 subcellular localization depends on Akt phosphorylation of PHF20-Ser291, MCF7 cells were transfected with FLAG-PHF20-S291A and FLAG-PHF20-S291D. Immunofluorescence staining revealed that PHF20-S291D was predominantly localized in the cytoplasm, and the majority of PHF20-S291A was in the nucleus even after treatment with IGF1 (supplemental Fig. S2). These results suggest that Akt phosphorylation of PHF20 leads to PHF20 translocation from the nucleus into the cytoplasm in a PHF20-Ser291 phosphorylation-dependent manner.
PHF20 Associates with Chromatin, Binds to a DNA Consensus Motif, and Transcriptionally Induces p53
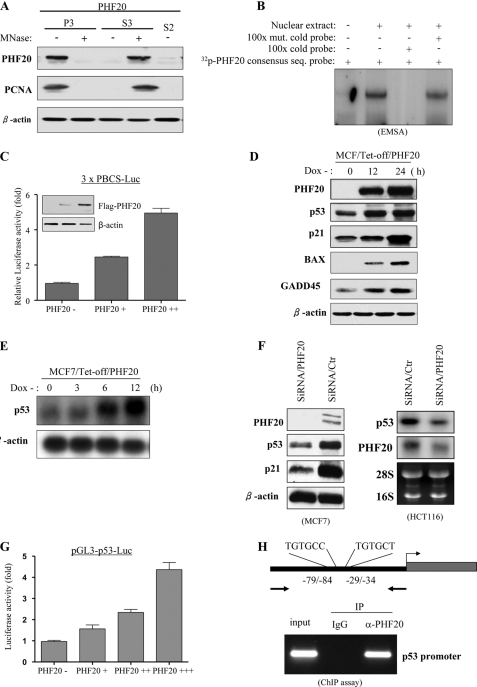
It has been demonstrated that AT-hooks bind to the A/T-rich DNA sequence and enhance accessibility of a promoter to a transcription factor (60). C2H2 zinc finger and PHD finger domains are known to involve DNA binding and protein-protein interaction (61). Because PHF20 has three putative DNA-binding motifs, i.e. an AT-hook, a C2H2 zinc finger, and a PHD finger domain, we examined whether PHF20 is associated with DNA in the form of chromatin. HEK293-FLAG-PHF20 cell lysates prepared with a nonionic detergent were divided by sequential centrifugation into soluble cytosolic components called S2 and nuclear fraction. The nuclear fraction was divided into two aliquots, treated with (+) or without (−) micrococcal nuclease, and then further fractionated into soluble nuclear components called S3 and insoluble nuclear pellets called P3 (supplemental Fig. S3_A_). Immunoblot analysis showed that PHF20 protein was not in the S2 cytoplasmic fraction and that the majority of the PHF20 in the P3 fraction becomes soluble (S3) after micrococcal nuclease treatment (Fig. 4A). We next examined the PHF20-DNA binding consensus sequence using cyclic amplification and selection of target (CASTing) assay (supplemental Fig. S3_B_). The oligonucleotide library selected after four rounds of PHF20 binding was cloned into pGEM-T, and the isolated clones were sequenced. Of 60 sequenced clones, all contained at least one R_GTG_NR (R = A or G and N = A, G, C, or T; supplemental Fig. S3_C_). To confirm this result, EMSA was carried out with the [32P]ATP-labeled PHF20 consensus sequence containing oligonucleotides. As shown in Fig. 4B, PHF20 bound to the oligonucleotides could be competed by excess of cold probe but not by its mutant oligonucleotides. Furthermore, we cloned three repeats of PHF20 binding consensus sequence (PBCS) into pGL3 luciferase vector (pGL3–3×PBCS-Luc). A reporter assay was performed in HEK293 cells that were transfected with pGL3–3×PBCS-Luc and increasing amounts of PHF20. Fig. 4C shows that promoter activity was induced by PHF20 in a dose-dependent manner. These data suggest that PHF20 associates with DNA in the form of chromatin and induces transcription by binding to a specific DNA element.
FIGURE 4.
PHF20 binds to DNA and transcriptionally up-regulates p53. A, association of PHF20 with chromatin. Cells were resuspended in buffer A with Nonidet P-40 (0.1%). Nuclei were collected by low speed centrifugation. Insoluble chromatin pellet (P3) was collected by centrifugation and resuspended in SDS-loading buffer. Release of chromatin-bound PHF20 proteins was detected following treatment of the cell nuclei with micrococcal nuclease (MNase). B, electrophoretic mobility shift assay was performed as described under “Experimental Procedures.” C, PHF20 possesses transcription factor activity. Three repeats of PHF20-binding consensus binding sequence were cloned to pGL3-basic vector (3 × PBCS-Luc), which was co-transfected into MCF7 cells with indicated plasmids. Following incubation for 36 h, luciferase activity was measured and normalized to β-galactosidase. Results are the mean ± S.E. of three independent experiments performed in triplicate. D and E, PHF20 induces p53. MCF7/Tet-Off inducible PHF20 cells were cultured in the media without doxycycline (Dox) for the indicated time and then were immunoblotted with indicated antibodies (D) and were subjected to Northern blot analysis probed with p53 and β-actin (E). F, knockdown of PHF20 reduces p53 expression. MCF7 and HCT116 were transfected with PHF20 siRNA or control siRNA and subjected to immunoblotting (left) and Northern blot (right) analysis. G, PHF20 induces p53 promoter activity. Luciferase report assay was performed as described in C in MCF7 cells that were transfected with indicated plasmids. H, ChIP assay shows that PHF20 directly binds to p53 promoter in vivo. ChIP assay was performed with PHF20 antibody in HCT116 cells, and PCR was carried out using the primers spanning two PHF20-binding sites in the p53 promoter. IP, immunoprecipitation.
Because PHF20 is a putative transcriptional factor and inhibits cell proliferation and survival (see Fig. 6), we established a MCF7/Tet-off PHF20 cell line and examined the effects of PHF20 on expression of a dozen molecules that involve cell survival and cell proliferation. Immunoblot analysis revealed that PHF20 was induced upon withdrawal of doxycycline and that p53 as well as p53 downstream targets p21, Bax, and GADD45 were also elevated (Fig. 4D). The p53 mRNA level was also induced by PHF20 (Fig. 4E). Furthermore, knockdown of PHF20 resulted in significantly reduced p53 expression (Fig. 4F and supplemental Fig. S4_A_). In addition, sequence analysis revealed two putative PHF20-binding consensus sites within the −110-bp region of the p53 promoter, e.g. −29/−34 and −79/−84 bp (Fig. 4H and supplemental Fig. S5_A_). Luciferase reporter assay was performed in MCF7 cells that were transfected with pGL3-p53 (−110/+13) and increasing amounts of PHF20. As shown in Fig. 4G, PHF20 considerably induced the promoter activity of p53. Moreover, mutation of the two putative PHF20-binding sites by converting the core sequence GTG to AAA largely abrogated the pGL3-p53 promoter activity induced by PHF20 (supplemental Fig. S5_B_).
FIGURE 6.
PHF20 exhibited cell growth inhibitory function that was overridden by Akt. A–C, PHF20 inhibited cell proliferation, DNA synthesis, and cell survival. MCF7/Tet-Off PHF20 and MCF7/Tet-Off control cells were seeded in 24-well plate. After withdrawal of doxycycline (Dox) for indicated times, cell growth (A), DNA synthesis (B), and cell survival (C) were assayed with accounting cell number, thymidine incorporation, and MTS as described under “Experimental Procedures.” Right panel of A shows expression of PHF20. D, PHF20 reduced DNA synthesis in HCT116 cells. E–G, Akt inhibits PHF20 cellular function. MCF7/Tet-Off PHF20 and MCF7/Tet-Off control cells were transfected with myr-Akt and cultured in the absence and presence of doxycycline. Cell number was accounted at indicated times (E). F and G, MCF7 cells were transfected with indicated plasmids and then assayed for DNA synthesis (F) and cell viability (G). H, effects of PHF20 and Akt phosphorylation of PHF20 on cell anchorage-independent growth. MCF7 cells were transfected with indicated plasmids and then grown in soft agar for 3 weeks (left panel). The colony was accounted and quantified in three plates/transfectant (right panel).
To determine whether PHF20 directly binds to the PHF20-binding site of the p53 promoter in vivo, we carried out ChIP assay, which detects specific genomic DNA sequences that are associated with a particular transcription factor in intact cells. HEK293 cells were transfected with FLAG-PHF20 and immunoprecipitated with anti-FLAG antibody. The PHF20-bound chromatin was subjected to PCR using oligonucleotide primers that amplify the region spanning two PHF20-binding sites within the p53 promoter. As shown in Fig. 4H, the anti-FLAG antibody pulled down PHF20-binding sites of p53 (−110/−4). In contrast, immunoprecipitation with an irrelevant antibody (anti-IgG) resulted in the absence of a band. Furthermore, we demonstrated that endogenous PHF20 bound to the p53 promoter in MCF7 and MEF cells. The binding affinity is much higher in Akt1-null MEF than wild-type MEF (supplemental Fig. S6). Because the two PHF20-binding sites (−29/−34 and −79/−84) are very close to each other, ChIP assay is unable to distinguish which site directly binds to PHF20. Taken collectively, our data indicate that PHF20 transcriptionally regulates p53 by directly binding to and activating the p53 promoter.
Akt Inhibits PHF20 Transcriptional Activity and p53 Expression
Having demonstrated that Akt phosphorylates and induces PHF20 nuclear-cytoplasm translocation and that PHF20 transactivates p53, we next examined the effect of Akt on PHF20 transcriptional activity and on p53 expression at the mRNA level. Luciferase reporter assay revealed that PHF20-induced pGL3–3×PBCS-Luc activity was largely abrogated by expression of constitutively active Akt (Fig. 5A). Furthermore, the p53 promoter activity induced by PHF20 was also inhibited by Akt in a dose-dependent manner (Fig. 5B). Northern blot and semi-quantitative RT-PCR analysis showed that the p53 mRNA level was higher in Akt1−/− MEFs than wild-type MEFs. Reintroduction of Akt into Akt1−/− MEFs repressed p53 expression (Fig. 5C). In contrast, the p53 mRNA level was increased by expression of kinase-dead Akt or knockdown of Akt in MCF7 and HCT116 cells (Fig. 5, D and E). Moreover, expression of constitutively active Akt decreased PHF20-induced p53 expression (Fig. 5F).
FIGURE 5.
Akt inhibits PHF20-induced p53. A and B, constitutively active Akt inhibits PHF20 transcriptional activity. Luciferase reporter assay was performed in MCF7 cells that were transfected with 3×PBCS-Luc (A) or pLG3-p53-Luc (B) together with other indicated plasmids. C, expression of Akt repressed p53 mRNA level. Akt-null MEFs were transfected with/without Akt and subjected to Northern blot analysis (top). Bottom panel is loading control. D and E, inhibition of Akt increases p53 expression. MCF7 (D) and HCT116 (E) cells were transfected with dominant-negative Akt or Akt shRNA, respectively, and then subjected to semi-quantitative RT-PCR and Western blot (W.B.) analysis. F, Akt represses PHF20-induced p53 expression. HCT116 cells were transfected with indicated plasmids and then immunoblotted with indicated antibodies. G and H, inhibition of PHF20-induced p53 by Akt depends on phosphorylation of PHF20-Ser291. MCF7 cells were transfected with indicated plasmids and then subjected to ChIP (top panel of G) and immunoblotting (panels 2–4 of G and H) analyses. Cont, control.
To investigate if Akt inhibits PHF20 binding to the p53 promoter and if Akt regulation of p53 depends on phosphorylation of PHF20-Ser291, ChIP assay was performed and showed that Akt-nonphosphorylatable PHF20-S291A increased binding activity to the p53 promoter compared with wild-type PHF20, whereas phosphomimic PHF20-S219D did not bind to p53. Moreover, expression of constitutively active Akt abrogated the DNA binding activity of wild-type PHF20 toward p53 (Fig. 5G). Accordingly, expression of PHF20-S291A, but not PHF20-S291D, resulted in increased expression of p53, and Akt had no significant effect on p53 expression in both PHF20-S291A- and PHF20-S291D-transfected cells (Fig. 5H). These results indicate that Akt inhibits PHF20 transcriptional activity and regulates p53 transcription through phosphorylation of PHF20.
PHF20 Inhibits Cell Proliferation and Survival, and Akt Abrogates PHF20 Function
To examine the cellular function of PHF20, cell proliferation, DNA synthesis, and cell survival were evaluated in MCF7-PHF20-Tet-Off cells. As shown in Fig. 6A, cell proliferation was significantly inhibited by induction of PHF20 expression. Furthermore, [3H]thymidine incorporation experiments revealed that PHF20 represses DNA synthesis (Fig. 6, B and D). In addition, expression of PHF20 significantly decreased cell viability (Fig. 6C). To further demonstrate PHF20 biological function, we knocked down PHF20 in MCF7 cells. Cells treated with control shRNA were used as control. The increase in cell growth was observed in the cells with depletion of PHF20. Furthermore, knockdown of PHF20 reduced cell death induced by VP16 (supplemental Fig. S4, B and C). These data indicate that PHF20 acts as a growth inhibition gene and that its protein product regulates cell proliferation and cell survival.
We also investigated the effects of Akt on PHF20 cellular function. Expression of constitutively active Akt largely abrogated PHF20-inhibited cell growth (Fig. 6E), DNA synthesis (Fig. 6F), and cell survival (Fig. 6G). Moreover, PHF20-S291A exhibits inhibitory function, whereas PHF20-S291D failed to inhibit DNA synthesis and cell survival (Fig. 6, F and G). In addition, anchorage-independent growth in soft agar was used to assess the effects of PHF20 and Akt on transforming activity. As shown in Fig. 6H, expression of PHF20 significantly reduced colony formation of MCF7 cells, and this action was overridden by co-transfection of Myr-Akt. Phosphomimic PHF20-S291D lost and nonphosphorylatable PHF20-S291A retained inhibitory activity. These data suggest that Akt inhibits PHF20 cellular function and that phosphorylation of PHF20 by Akt represents a key regulation of PHF20 function.
DISCUSSION
Akt was shown to regulate p53 through phosphorylation of Mdm2. The phosphorylation of Mdm2 by Akt leads to its retention to the nucleus where Mdm2 inhibits p53 transactivation function and targets p53 protein for degradation (43, 45). In this study, we have identified PHF20 as a transcription factor that interacts with Akt. PHF20 transactivates the p53 promoter, resulting in induction of p53 mRNA. Akt phosphorylates PHF20 leading to its translocation from the nucleus to the cytoplasm and consequent abrogation of PHF20 transactivation of p53. Moreover, we have shown that knockdown of PHF20 significantly reduces the basal level of p53 mRNA and protein. These findings suggest a pivotal role of PHF20 in both Akt and p53 signaling and thus establish an additional link between Akt and p53 cascades.
PHF20 contains multiple functional domains, including two Tudor motifs, an AT-hook region, a C2H2 zinc finger, and a PHD finger domain. The Tudor domain was thought to act as an adaptor mediating intramolecular as well as intermolecular protein-protein interactions and protein-nucleic acid associations (60). AT-hooks bind to A/T-rich DNA sequences and enhance accessibility of a promoter to a transcription factor (61). C2H2 zinc finger and PHD finger domains are known to be involved in DNA binding and protein-protein interactions (62). In this study, we have demonstrated that PHF20 directly associates with chromatin and transactivates the p53 promoter. Recent reports suggest that Tudor and PHD finger domains bind to methylated lysine residue of histones (63, 64). Further studies are required to determine whether PHF20 binds to methylated histones and is involved in chromatin modification.
In addition to p53 gene mutation, frequent down-regulation of p53 mRNA has been detected in human malignancy (65, 66). However, little is known about the transcription factors that regulate p53 mRNA levels. HOXA5 was shown to be co-down-regulated with p53 in some breast tumors and to transcriptionally activate p53 (67). Our data reveal that expression of PHF20 inhibits cell proliferation and induces cell death. The PHF20 gene resides in chromosome band 20q11, a region frequently deleted in certain human malignancies (53), suggesting that PHF20 could be a putative tumor suppressor gene, which needs to be further investigated. Furthermore, activation of Akt largely abrogated PHF20 cellular function. We also showed that knockdown of PHF20 did not increase constitutively active Akt function (supplemental Fig. S4_D_). Notably, Akt nonphosphorylatable PHF20-S291A retains, whereas phosphomimic PHF20-S291D loses, its cellular function, indicating the phosphorylation of PHF20-Ser291 by Akt plays a critical role in regulating PHF20 function.
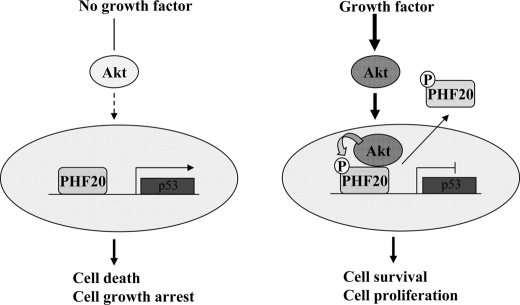
In summary, PHF20 is a novel transcriptional factor and both a binding partner and substrate of Akt. It exhibits putative tumor suppressor activity and plays a role in maintaining basal levels of p53. Based on our findings, we propose the following model of Akt regulation of PHF20. In the absence of cell growth and survival signals, PHF20 transactivates its target genes, including p53, which lead to cell growth arrest and cell death. Upon Akt activation, a fraction of the activated Akt translocates to the nucleus and phosphorylates PHF20, resulting in attenuation of PHF20 transcriptional activity and translocation into the cytoplasm where it co-localizes with Akt (Fig. 7). Further investigations are required to characterize PHF20 tumor suppressor function and its possible involvement in human malignancy. In addition, the genome-wide PHF20 target genes need to be identified to fully understand the normal cellular function of PHF20.
FIGURE 7.
Diagram depicting the regulation of PHF20 by Akt. In the absence of cell growth and survival signals, PHF20 locates in the nucleus where it binds to DNA and regulates p53 and other gene expression leading to cell growth arrest and cell death (left panel). Once Akt is activated, Akt phosphorylates PHF20Ser291 in the nucleus, which results in PHF20 nuclear-cytoplasmic translocation (right panel), which results in cell survival and proliferation.
Supplementary Material
Supplemental Data
*
This work was supported, in whole or in part, by National Institutes of Health Grants CA137041 (to J. Q. C.) and CA77429 (to J. R. T.). This work was also supported by Florida James and Esther King Biomedical Research Program Grant 1KG02 (to J. Q. C.).
The nucleotide sequence(s) reported in this paper has been submitted to the GenBankTM/EBI Data Bank with accession number(s) AY027523.
4
The abbreviations used are:
MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2_H_-tetrazolium
PHD
plant homeodomain.
REFERENCES
- 1.Coffer P. J., Woodgett J. R. (1991) Molecular cloning and characterization of a novel putative protein-serine kinase related to the cAMP-dependent and protein kinase C families. Eur. J. Biochem. 201, 475–481 [DOI] [PubMed] [Google Scholar]
- 2.Jones P. F., Jakubowicz T., Pitossi F. J., Maurer F., Hemmings B. A. (1991) Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc. Natl. Acad. Sci. U.S.A. 88, 4171–4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellacosa A., Testa J. R., Staal S. P., Tsichlis P. N. (1991) A retroviral oncogene, akt, encoding a serine threonine kinase containing an SH2-like region. Science 254, 274–277 [DOI] [PubMed] [Google Scholar]
- 4.Cheng J. Q., Godwin A. K., Bellacosa A., Taguchi T., Franke T. F., Hamilton T. C., Tsichlis P. N., Testa J. R. (1992) AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc. Natl. Acad. Sci. U.S.A. 89, 9267–9271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng J. Q., Ruggeri B., Klein W. M., Sonoda G., Altomare D. A., Watson D. K., Testa J. R. (1996) Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc. Natl. Acad. Sci. U.S.A. 93, 3636–3641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellacosa A., de Feo D., Godwin A. K., Bell D. W., Cheng J. Q., Altomare D. A., Wan M., Dubeau L., Scambia G., Masciullo V., Ferrandina G., Benedetti Panici P., Mancuso S., Neri G., Testa J. R. (1995) Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int. J. Cancer 64, 280–285 [DOI] [PubMed] [Google Scholar]
- 7.Burgering B. M., Coffer P. J. (1995) Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376, 599–602 [DOI] [PubMed] [Google Scholar]
- 8.Franke T. F., Yang S. I., Chan T. O., Datta K., Kazlauskas A., Morrison D. K., Kaplan D. R., Tsichlis P. N. (1995) The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81, 727–736 [DOI] [PubMed] [Google Scholar]
- 9.Liu A. X., Testa J. R., Hamilton T. C., Jove R., Nicosia S. V., Cheng J. Q. (1998) AKT2, a member of the protein kinase B family, is activated by growth factors, v-Ha-ras, and v-src through phosphatidylinositol 3-kinase in human ovarian epithelial cancer cells. Cancer Res. 58, 2973–2977 [PubMed] [Google Scholar]
- 10.Shaw M., Cohen P., Alessi D. R. (1998) The activation of protein kinase B by H2O2 or heat shock is mediated by phosphoinositide 3-kinase and not by mitogen-activated protein kinase-activated protein kinase-2. Biochem. J. 336, 241–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan T. O., Rittenhouse S. E., Tsichlis P. N. (1999) AKT/PKB and other D3 phosphoinositide-regulated kinases. Kinase activation by phosphoinositide-dependent phosphorylation. Annu. Rev. Biochem. 68, 965–1014 [DOI] [PubMed] [Google Scholar]
- 12.Datta S. R., Brunet A., Greenberg M. E. (1999) Cellular survival. A play in three Akts. Genes Dev. 13, 2905–2927 [DOI] [PubMed] [Google Scholar]
- 13.Testa J. R., Bellacosa A. (2001) AKT plays a central role in tumorigenesis. Proc. Natl. Acad. Sci. U.S.A. 98, 10983–10985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brazil D. P., Park J., Hemmings B. A. (2002) PKB-binding proteins. Getting in on the Akt. Cell 111, 203–303 [DOI] [PubMed] [Google Scholar]
- 15.Alessi D. R., James S. R., Downes C. P., Holmes A. B., Gaffney P. R., Reese C. B., Cohen P. (1997) Characterization of a 3-phosphoinositide-dependent protein kinase that phosphorylates and activates protein kinase Bα. Curr. Biol. 7, 261–269 [DOI] [PubMed] [Google Scholar]
- 16.Alessi D. R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P., Hemmings B. A. (1996) Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15, 6541–6551 [PMC free article] [PubMed] [Google Scholar]
- 17.Persad S., Attwell S., Gray V., Mawji N., Deng J. T., Leung D., Yan J., Sanghera J., Walsh M. P., Dedhar S. (2001) Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase. Critical roles for kinase activity and amino acids arginine 211 and serine 343. J. Biol. Chem. 276, 27462–27469 [DOI] [PubMed] [Google Scholar]
- 18.Toker A., Newton A. C. (2000) Akt/protein kinase B is regulated by autophosphorylation at the hypothetical PDK-2 site. J. Biol. Chem. 275, 8271–8274 [DOI] [PubMed] [Google Scholar]
- 19.Partovian C., Simons M. (2004) Regulation of protein kinase B/Akt activity and Ser-473 phosphorylation by protein kinase Cα in endothelial cells. Cell. Signal. 16, 951–957 [DOI] [PubMed] [Google Scholar]
- 20.Kawakami Y., Nishimoto H., Kitaura J., Maeda-Yamamoto M., Kato R. M., Littman D. R., Leitges M., Rawlings D. J., Kawakami T. (2004) Protein kinase CβII regulates Akt phosphorylation on Ser-473 in a cell type- and stimulus-specific fashion. J. Biol. Chem. 279, 47720–47725 [DOI] [PubMed] [Google Scholar]
- 21.Feng J., Park J., Cron P., Hess D., Hemmings B. A. (2004) Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J. Biol. Chem. 279, 41189–41196 [DOI] [PubMed] [Google Scholar]
- 22.Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 [DOI] [PubMed] [Google Scholar]
- 23.Guo J. P., Coppola D., Cheng J. Q. (2011) IKBKE protein activates Akt independent of phosphatidylinositol 3-kinase/PDK1/mTORC2 and the pleckstrin homology domain to sustain malignant transformation. J. Biol. Chem. 286, 37389–37398 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Xie X., Zhang D., Zhao B., Lu M. K., You M., Condorelli G., Wang C. Y., Guan K. L. (2011) IκB kinase ϵ and TANK-binding kinase 1 activate AKT by direct phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 108, 6474–6479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ou Y. H., Torres M., Ram R., Formstecher E., Roland C., Cheng T., Brekken R., Wurz R., Tasker A., Polverino T., Tan S. L., White M. A. (2011) TBK1 directly engages Akt/PKB survival signaling to support oncogenic transformation. Mol. Cell 41, 458–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finlay C. A., Hinds P. W., Levine A. J. (1989) The p53 proto-oncogene can act as a suppressor of transformation. Cell 57, 1083–1093 [DOI] [PubMed] [Google Scholar]
- 27.Baker S. J., Markowitz S., Fearon E. R., Willson J. K., Vogelstein B. (1990) Suppression of human colorectal carcinoma cell growth by wild-type p53. Science 249, 912–915 [DOI] [PubMed] [Google Scholar]
- 28.Vogelstein B., Lane D., Levine A. J. (2000) Surfing the p53 network. Nature 408, 307–310 [DOI] [PubMed] [Google Scholar]
- 29.Vogelstein B., Kinzler K. W. (2004) Cancer genes and the pathways they control. Nat. Med. 10, 789–799 [DOI] [PubMed] [Google Scholar]
- 30.Sherr C. J. (2004) Principles of tumor suppression. Cell 116, 235–246 [DOI] [PubMed] [Google Scholar]
- 31.Bourdon J. C., Laurenzi V. D., Melino G., Lane D. (2003) p53. 25 years of research and more questions to answer. Cell Death Differ. 10, 397–399 [DOI] [PubMed] [Google Scholar]
- 32.El-Deiry W. S. (2003) The role of p53 in chemosensitivity and radiosensitivity. Oncogene 22, 7486–7495 [DOI] [PubMed] [Google Scholar]
- 33.Oren M. (2003) Decision making by p53. Life, death, and cancer. Cell Death Differ. 10, 431–442 [DOI] [PubMed] [Google Scholar]
- 34.Okada H., Mak T. W. (2004) Pathways of apoptotic and nonapoptotic death in tumor cells. Nat. Rev. Cancer 4, 592–603 [DOI] [PubMed] [Google Scholar]
- 35.Vousden K. H., Lu X. (2002) Live or let die. The cell's response to p53. Nat. Rev. Cancer 2, 594–604 [DOI] [PubMed] [Google Scholar]
- 36.Raman V., Tamori A., Vali M., Zeller K., Korz D., Sukumar S. (2000) HOXA5 regulates expression of the progesterone receptor. J. Biol. Chem. 275, 26551–26555 [DOI] [PubMed] [Google Scholar]
- 37.Stasinopoulos I. A., Mironchik Y., Raman A., Wildes F., Winnard P., Jr., Raman V. (2005) HOXA5-twist interaction alters p53 homeostasis in breast cancer cells. J. Biol. Chem. 280, 2294–2299 [DOI] [PubMed] [Google Scholar]
- 38.Deffie A., Wu H., Reinke V., Lozano G. (1993) The tumor suppressor p53 regulates its own transcription. Mol. Cell. Biol. 13, 3415–3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu H., Lu Z. G., Miki Y., Yoshida K. (2007) Protein kinase Cδ induces transcription of the TP53 tumor suppressor gene by controlling death-promoting factor Btf in the apoptotic response to DNA damage. Mol. Cell. Biol. 27, 8480–8491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phan R. T., Dalla-Favera R. (2004) The BCL6 proto-oncogene suppresses p53 expression in germinal-centre B cells. Nature 432, 635–639 [DOI] [PubMed] [Google Scholar]
- 41.Sun X., Shimizu H., Yamamoto K. (1995) Identification of a novel p53 promoter element involved in genotoxic stress-inducible p53 gene expression. Mol. Cell. Biol. 15, 4489–4496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hellin A. C., Calmant P., Gielen J., Bours V., Merville M. P. (1998) Nuclear factor κB-dependent regulation of p53 gene expression induced by daunomycin genotoxic drug. Oncogene 16, 1187–1195 [DOI] [PubMed] [Google Scholar]
- 43.Mayo L. D., Donner D. B. (2001) A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc. Natl. Acad. Sci. U.S.A. 98, 11598–11603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yin Y., Stephen C. W., Luciani M. G., Fåhraeus R. (2002) p53 stability and activity is regulated by Mdm2-mediated induction of alternative p53 translation products. Nat. Cell Biol. 4, 462–467 [DOI] [PubMed] [Google Scholar]
- 45.Zhou B. P., Liao Y., Xia W., Zou Y., Spohn B., Hung M. C. (2001) HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat. Cell Biol. 3, 973–982 [DOI] [PubMed] [Google Scholar]
- 46.Ogawara Y., Kishishita S., Obata T., Isazawa Y., Suzuki T., Tanaka K., Masuyama N., Gotoh Y. (2002) Akt enhances Mdm2-mediated ubiquitination and degradation of p53. J. Biol. Chem. 277, 21843–21850 [DOI] [PubMed] [Google Scholar]
- 47.Oren M., Damalas A., Gottlieb T., Michael D., Taplick J., Leal J. F., Maya R., Moas M., Seger R., Taya Y., Ben-Ze'Ev A. (2002) Regulation of p53. Intricate loops and delicate balances. Ann. N.Y. Acad. Sci. 973, 374–383 [DOI] [PubMed] [Google Scholar]
- 48.Sun M., Wang G., Paciga J. E., Feldman R. I., Yuan Z. Q., Ma X. L., Shelley S. A., Jove R., Tsichlis P. N., Nicosia S. V., Cheng J. Q. (2001) AKT1/PKBα kinase is frequently elevated in human cancers and its constitutive activation is required for oncogenic transformation in NIH3T3 cells. Am. J. Pathol. 159, 431–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Q., Kaneko S., Yang L., Feldman R. I., Nicosia S. V., Chen J., Cheng J. Q. (2004) Aurora-A abrogation of p53 DNA binding and transactivation activity by phosphorylation of serine 215. J. Biol. Chem. 279, 52175–52182 [DOI] [PubMed] [Google Scholar]
- 50.Dan H. C., Sun M., Kaneko S., Feldman R. I., Nicosia S. V., Wang H. G., Tsang B. K., Cheng J. Q. (2004) Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP). J. Biol. Chem. 279, 5405–5412 [DOI] [PubMed] [Google Scholar]
- 51.Lee H., Kim D., Dan H. C., Wu E. L., Gritsko T. M., Cao C., Nicosia S. V., Golemis E. A., Liu W., Coppola D., Brem S. S., Testa J. R., Cheng J. Q. (2007) Identification and characterization of putative tumor suppressor NGB, a GTP-binding protein that interacts with the neurofibromatosis 2 protein. Mol. Cell. Biol. 27, 2103–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang B., Golemis E. A., Kruh G. D. (1997) ArgBP2, a multiple Src homology 3 domain-containing, Arg/Abl-interacting protein, is phosphorylated in v-Abl-transformed cells and localized in stress fibers and cardiocyte Z-disks. J. Biol. Chem. 272, 17542–17550 [DOI] [PubMed] [Google Scholar]
- 53.Vauhkonen H., Vauhkonen M., Sajantila A., Sipponen P., Knuutila S. (2006) DNA copy number aberrations in intestinal-type gastric cancer revealed by array-based comparative genomic hybridization. Cancer Genet. Cytogenet. 167, 150–154 [DOI] [PubMed] [Google Scholar]
- 54.Dan H. C., Sun M., Yang L., Feldman R. I., Sui X. M., Ou C. C., Nellist M., Yeung R. S., Halley D. J., Nicosia S. V., Pledger W. J., Cheng J. Q. (2002) Phosphatidylinositol 3-kinase/Akt pathway regulates tuberous sclerosis tumor suppressor complex by phosphorylation of tuberin. J. Biol. Chem. 277, 35364–35370 [DOI] [PubMed] [Google Scholar]
- 55.Méndez J., Stillman B. (2000) Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle. Assembly of prereplication complexes in late mitosis. Mol. Cell. Biol. 20, 8602–8612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lassus P., Opitz-Araya X., Lazebnik Y. (2002) Requirement for caspase-2 in stress-induced apoptosis before mitochondrial permeabilization. Science 297, 1352–1354 [DOI] [PubMed] [Google Scholar]
- 57.Dudek H., Datta S. R., Franke T. F., Birnbaum M. J., Yao R., Cooper G. M., Segal R. A., Kaplan D. R., Greenberg M. E. (1997) Regulation of neuronal survival by the serine threonine protein kinase Akt. Science 275, 661–665 [DOI] [PubMed] [Google Scholar]
- 58.Owada Y., Utsunomiya A., Yoshimoto T., Kondo H. (1997) Expression of mRNA for Akt, serine threonine protein kinase, in the brain during development and its transient enhancement following axotomy of hypoglossal nerve. J. Mol. Neurosci. 9, 27–33 [DOI] [PubMed] [Google Scholar]
- 59.Yang L., Dan H. C., Sun M., Liu Q., Sun X. M., Feldman R. I., Hamilton A. D., Polokoff M., Nicosia S. V., Herlyn M., Sebti S. M., Cheng J. Q. (2004) Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 64, 4394–4399 [DOI] [PubMed] [Google Scholar]
- 60.Lee J. K., Moon K. Y., Jiang Y., Hurwitz J. (2001) The Schizosaccharomyces pombe origin recognition complex interacts with multiple AT-rich regions of the replication origin DNA by means of the AT-hook domains of the spOrc4 protein. Proc. Natl. Acad. Sci. U.S.A. 98, 13589–13594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moosmann P., Georgiev O., Le Douarin B., Bourquin J. P., Schaffner W. (1996) Transcriptional repression by RING finger protein TIF1β that interacts with the KRAB repressor domain of KOX1. Nucleic Acids Res. 24, 4859–4867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huyen Y., Zgheib O., Ditullio R. A., Jr., Gorgoulis V. G., Zacharatos P., Petty T. J., Sheston E. A., Mellert H. S., Stavridi E. S., Halazonetis T. D. (2004) Methylated lysine 79 of histone H3 targets 53BP1 to DNA double strand breaks. Nature 432, 406–411 [DOI] [PubMed] [Google Scholar]
- 63.Kim J., Daniel J., Espejo A., Lake A., Krishna M., Xia L., Zhang Y., Bedford M. T. (2006) Tudor, MBT and chromo domains gauge the degree of lysine methylation. EMBO Rep. 7, 397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takahashi T., Nau M. M., Chiba I., Birrer M. J., Rosenberg R. K., Vinocour M., Levitt M., Pass H., Gazdar A. F., Minna J. D. (1989) p53. A frequent target for genetic abnormalities in lung cancer. Science 246, 491–494 [DOI] [PubMed] [Google Scholar]
- 65.Lee K., Jeon K., Kim J. M., Kim V. N., Choi D. H., Kim S. U., Kim S. (2005) Down-regulation of GFAP, TSP-1, and p53 in human glioblastoma cell line, U373MG, by IE1 protein from human cytomegalovirus. Glia 51, 1–12 [DOI] [PubMed] [Google Scholar]
- 66.Raman V., Martensen S. A., Reisman D., Evron E., Odenwald W. F., Jaffee E., Marks J., Sukumar S. (2000) Compromised HOXA5 function can limit p53 expression in human breast tumors. Nature 405, 974–978 [DOI] [PubMed] [Google Scholar]
- 67.Peng Z., Zhou C., Zhang F., Ling Y., Tang H., Bai S., Liu W., Qiu G., He L. (2002) Loss of heterozygosity of chromosome 20 in sporadic colorectal cancer. Chin. Med. J. 115, 1529–1532 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data