Detection of an autoreactive T-cell population within the polyclonal repertoire that undergoes distinct autoimmune regulator (Aire)-mediated selection (original) (raw)
Abstract
The autoimmune regulator (Aire) plays a critical role in central tolerance by promoting the display of tissue-specific antigens in the thymus. To study the influence of Aire on thymic selection in a physiological setting, we used tetramer reagents to detect autoreactive T cells specific for the Aire-dependent tissue-specific antigen interphotoreceptor retinoid-binding protein (IRBP), in the polyclonal repertoire. Two class II tetramer reagents were designed to identify T cells specific for two different peptide epitopes of IRBP. Analyses of the polyclonal T-cell repertoire showed a high frequency of activated T cells specific for both IRBP tetramers in Aire−/− mice, but not in Aire+/+ mice. Surprisingly, although one tetramer-binding T-cell population was efficiently deleted in the thymus in an Aire-dependent manner, the second tetramer-binding population was not deleted and could be detected in both the Aire−/− and Aire+/+ T-cell repertoires. We found that Aire-dependent thymic deletion of IRBP-specific T cells relies on intercellular transfer of IRBP between thymic stroma and bone marrow-derived antigen-presenting cells. Furthermore, our data suggest that Aire-mediated deletion relies not only on thymic expression of IRBP, but also on proper antigen processing and presentation of IRBP by thymic antigen-presenting cells.
Keywords: autoimmunity, uveitis
Thymic tolerance mechanisms play an important role in preventing autoimmunity. A key mediator in thymic tolerance is the Autoimmune Regulator (Aire), a transcriptional regulator that is highly expressed in medullary thymic epithelial cells (mTECs) (1). Aire promotes the expression of peripheral tissue-specific self-antigens (TSAs) in mTECs for the purpose of tolerizing self-reactive T cells to these TSAs. Both patients and mice with defects in Aire develop multiorgan autoimmunity, reinforcing the importance of this process in controlling immune tolerance (2). Several organ-specific autoimmune diseases in the Aire-deficient model can be linked to a failure in the appropriate thymic expression of a given TSA under the control of Aire (3–6). Autoimmunity in the eyes of Aire-deficient mice arises as a response against the retina-specific protein interphotoreceptor retinoid binding protein (IRBP) (3). IRBP is expressed in the thymus in an Aire-dependent fashion, and thymic transfer of IRBP-deficient thymi into athymic (nude) WT hosts is sufficient to induce posterior uveitis (3).
To date, the detection of Aire-mediated thymic negative selection has relied on the use of T-cell receptor (TCR) transgenic mice (7, 8). In these TCR transgenic models, the TCRs are specific for model foreign antigens, and “neo”-self antigen expression is provided by a second transgene where the model antigen is expressed under the control of a tissue-specific promoter that results in mTEC expression. Although such transgenic models have been instructive in demonstrating the potential role of Aire in negative selection, the physiological meaning of these studies is limited by a number of factors, including the elevated precursor frequencies of the transgenic T cells, the timing of transgenic TCR expression, and how well the “neo”-self transgene mimics expression of TSAs in mTECs (9). Thus, it is not clear how complete or efficient Aire-dependent deletion of autoreactive T cells with TSA-reactivity is in the polyclonal setting.
Previous studies have established a method for detecting rare antigen-specific populations in the setting of a polyclonal T-cell repertoire (10–12). Here we used that approach to study Aire-mediated thymic deletion of IRBP-specific T cells, and found that autoreactive T cells specific for a peptide epitope of IRBP is deleted via an Aire-dependent mechanism, whereas T cells specific for another epitope of IRBP escape Aire-mediated thymic deletion.
Results
Design and Validation of IRBP-Specific Tetramer Reagents.
Given that posterior uveitis in Aire-deficient mice is dependent on a CD4+ T-cell response to IRBP (3), we sought to develop tetramer reagents to detect such cells in the polyclonal repertoire. To identify an immunoreactive epitope of IRBP for tetramer loading, we generated CD4+ T-cell hybridoma clones from Aire−/− animals on the C57BL/6 (B6) background that were immunized with full-length IRBP protein emulsified in complete Freund’s adjuvant (CFA). By screening with a combination of truncated constructs of IRBP and peptide scanning, we identified a CD4+ T-cell hybridoma clone, LB4, that was specific for a peptide epitope of amino acids 271–290 of the IRBP protein (Fig. S1). Screening of the 271–290 region narrowed the reactive epitope down to 277–290, which is what we used to generate a tetramer reagent (Fig. S1).
Immunoreactivity to different peptide epitopes of IRBP has been reported in B6 mice that are Aire-sufficient (13). To identify additional peptides for tetramer design, we analyzed the epitopes defined by Cortes et al. (14) using the Immune Epitope Database computer-modeling program. IRBP(771–790) was predicted to have high-affinity binding for the I-Ab MHC molecule and was selected to generate a second tetramer reagent. We determined the affinity of the two peptides for I-Ab binding through a peptide competition assay (15). Although both peptides bind to I-Ab, IRBP(771–790) has a higher affinity for I-Ab compared with IRBP(271–290) (Fig. S2).
We generated tetramer reagents for both peptide epitope specificities. To help validate the specificity of both tetramers, we immunized B6 IRBP−/− mice, which in theory should not be tolerant of IRBP. Eight days after immunization, cells from the spleen, cervical lymph nodes, submandibular lymph nodes, axillary lymph nodes, inguinal lymph nodes, and mesenteric lymph nodes were isolated and pooled for each individual mouse. Cells were stained with both tetramer reagents that were labeled with different fluorophores. IRBP−/− mice immunized with IRBP(271-290) in CFA had a higher frequency of IRBP(277–290) tetramer-reactive CD4+ T cells, herein designated P2-specific cells, compared with mice immunized with IRBP(771–790) in CFA or CFA alone (Fig. S3). Similarly, IRBP−/− mice immunized with IRBP(771–790) in CFA had a higher frequency of IRBP(771–790) tetramer-reactive CD4+ T cells, or P7-specific cells, compared with mice immunized with IRBP(271–290) in CFA or CFA alone (Fig. S3).
Detection of IRBP-Specific T Cells in the Polyclonal Repertoire of Aire−/− and Aire+/+ Mice.
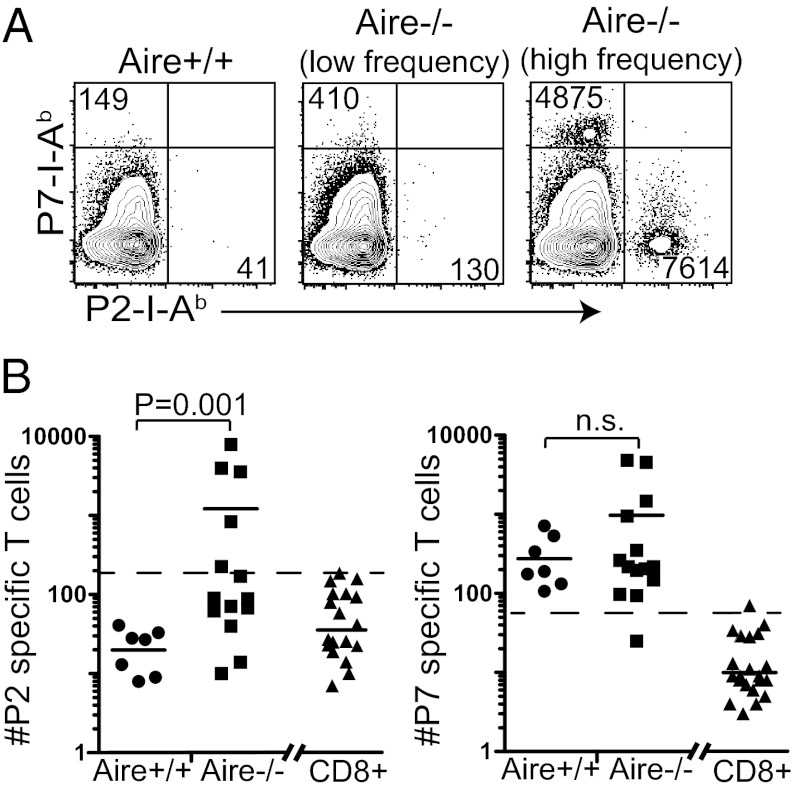
We determined the frequency of tetramer-binding cells from the periphery of cohorts of Aire+/+ and Aire−/− mice at 10–20 wk of age. There was a clear increase in the frequency of the P2-I-Ab–specific T-cell population in Aire−/− mice, whereas no Aire+/+ mouse had P2-specific cells above the limit of detection (LOD), defined as the average number of tetramer-positive CD8+ T cells plus 3 SDs of this average (16) (Fig. 1). In contrast, both Aire+/+ and Aire−/− mice exhibited P7-I-Ab tetramer binding CD4+ T cells above the LOD, and there was no significant difference in the frequency of P7-specific CD4+ T cells between Aire+/+ and Aire−/− mice.
Fig. 1.
Adult Aire−/− mice exhibit a high frequency of P2- and P7-specific T cells. (A) Peripheral lymphoid organs were pooled from adult Aire+/+ or Aire−/− mice and stained with the P2 and P7 tetramers. Representative FACS plots of tetramer-enriched, peripheral CD4+ T cells from an Aire+/+ mouse, an Aire−/− mouse with a low frequency of tetramer-positive T cells, and an Aire−/− mouse with a high frequency of tetramer-positive cells are shown. The absolute number of tetramer-positive cells from the individual mice is noted in each FACS plot. (B) The total number of P2- and P7-specific binding cells from individual mice were quantified for Aire+/+ and Aire−/−. Each data point represents one mouse, and the horizontal bar is the average of each group. The dotted line in each graph is the limit of detection (LOD). The LOD is the average number of tetramer-positive CD3+CD8+ cells (shown on the right side of the graph) plus 3 SDs of this average (16).
Approximately one-third of 10- to 20-wk-old B6 Aire−/− mice develop spontaneous autoimmune uveitis, as identified by lymphocyte infiltration and damage to the retina (1, 3). To determine whether P2-specific and P7-specific tetramer frequencies could be correlated with the development of autoimmune uveitis, we segregated tetramer analyses on the cohort of 10- to 20-wk-old Aire−/− mice based on the presence or absence of histological uveitis. Aire−/− mice with uveitis had higher frequencies of both P2-specific and P7-specific cells compared with Aire−/− mice with no eye disease (Fig. 2 A and B). Furthermore, in Aire−/− mice with uveitis, P2-specific and P7-specific cells showed evidence of expansion based on up-regulation of CD44 on tetramer-binding cells (Fig. 2_C_). We also detected higher frequencies of such cells in the eye-draining cervical lymph nodes, submandibular lymph nodes (17), and spleen compared with other lymphoid organs (Fig. 2_D_). Taken together, these data demonstrate a dramatic increase of tetramer-positive cells in Aire−/− mice with uveitis, and suggest that proliferation in the eye-draining lymph nodes and in the spleen drives expansion of these cells during active disease.
Fig. 2.
High frequency of activated P2 and P7 specific T cells correlates with the presence of uveitis in Aire−/− mice. (A) H&E-stained retina sections (200× magnification) taken from 10- to 20-wk-old Aire−/− mice with low (Left) and high (Right) frequencies of P2- and P7-specific T cells which correlates with no uveitis (Left) and severe uveitis (Right). (B) The total number of P2 and P7 specific binding cells from 10- to 20-wk-old Aire−/− mice, as shown in Fig. 1_B_, were quantified. Each data point represents one mouse, and the horizontal bar is the average of each group. The dotted line in each graph is the LOD, as described previously. (C and D) Individual lymph nodes from 10- to 20-wk-old 5 Aire+/+ or 5 Aire−/− mice were pooled and analyzed by flow cytometry after tetramer-binding T-cell enrichment. (C) The absolute number of tetramer-positive cells is noted in each FACS plot. (D) The total number of tetramer-positive cells in the designated peripheral lymphoid organs pooled from five mice was calculated. Data in C and D are representative of three independent experiments.
P2-Specific Precursors Can Be Expanded in Aire−/− Mice, but Not in Aire+/+ Mice, Whereas P7-Specific T Cells Can Be Expanded in both Aire−/− and Aire+/+ Mice.
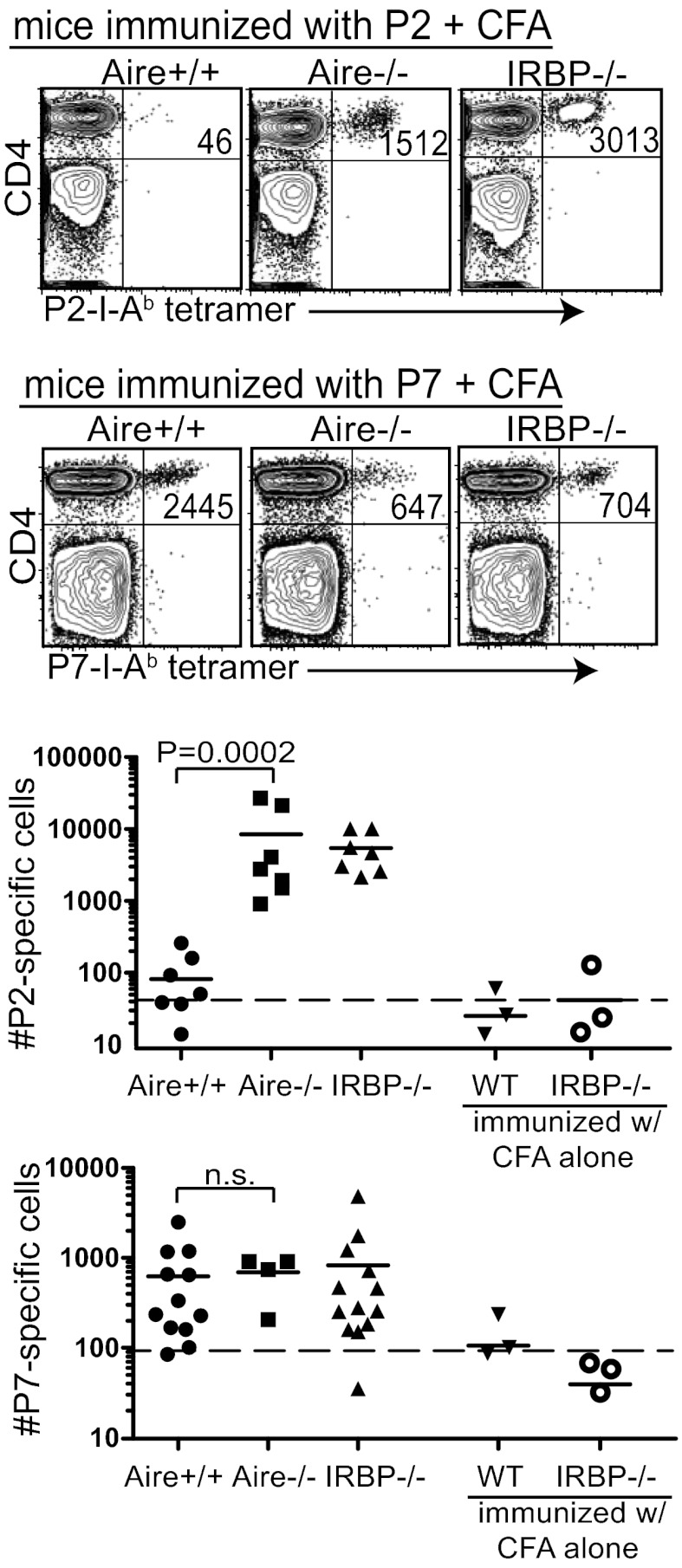
Aire+/+ mice have P7-specific T cells above the LOD but few, if any, P2-specific T cells above the LOD. To enhance the detection of P2-specific and P7-specific cells in Aire+/+ and Aire−/− mice, we immunized mice with P2 or P7 in CFA before tetramer analysis. We included IRBP−/− mice as a control in these experiments to determine the full expansion of T cells in mice not tolerized to the IRBP antigen. Immunization of Aire−/− and IRBP−/− mice with P2 peptide in CFA resulted in a substantial increase in P2-specific T cells, whereas very few P2-specific T cells expanded in the P2-immunized Aire+/+ mice (Fig. 3). In contrast, immunization with P7 peptide in CFA resulted in a similar increase in P7-specific T cells in Aire+/+, Aire−/−, and IRBP−/− mice. These data suggest that although the T-cell repertoires in the 4- to 6-wk-old Aire+/+and Aire−/− mice contain similar frequencies of P7-specific cells, the Aire−/− repertoire contains significantly more P2-specific cells than the Aire+/+ repertoire.
Fig. 3.
P2-specific CD4+ T cells can be expanded in Aire−/− mice, but not in Aire+/+ mice, whereas P7-specific cells can be expanded in both Aire−/− and Aire+/+ mice. Here, 4- to 7-wk-old Aire+/+, Aire−/−, and IRBP−/− mice were immunized with either P2 peptide or P7 peptide in CFA. At 8 d after immunization, tetramer-positive T cells in the secondary lymphoid organs were quantified. Representative FACS plots (Upper) and a summary of all data collected (Lower) are shown. Each graphed data point represents one mouse. The dotted line in each graph is the LOD.
To address whether Aire-mediated thymic selection affects the avidity of IRBP-specific TCRs for antigen in the periphery, we compared the mean fluorescence intensity (MFI) of P2 and P7 tetramer stains on T cells from immunized Aire+/+, Aire−/−, and IRBP−/− mice. These analyses made use of the linear relationship between tetramer MFI and TCR avidity for peptide MHC (18). Aire+/+, Aire−/−, and IRBP−/− T cells demonstrated similar avidity for P7 tetramer (Fig. S4). In contrast, P2-specific cells had a lower avidity for P2 tetramer in Aire+/+ mice compared with Aire−/− and IRBP−/− mice.
Immunization with P2 Peptide Can Provoke Uveitis in Young Aire−/− Mice.
We analyzed a cohort of young (4 wk) Aire+/+ and Aire−/− mice for the development of uveitis at 3 wk after immunization with P2 or P7 in CFA (Fig. S5). Uveitis developed in five of six Aire−/− mice after immunization with P2 in CFA, compared with two of six Aire−/− mice after immunization with P7 in CFA. In contrast, no WT mice developed uveitis after immunization with either P2 or P7 in CFA, and no Aire−/− mice developed uveitis in the absence of immunization at 7 wk of age (Fig. S5) (1). These data support previous work demonstrating that P2-specific cells are more pathogenic than P7-specific T cells (13). Taken together, these data suggest that Aire mediates the deletion of pathogenic, high-avidity, P2-specific T cells to prevent autoimmune uveitis.
Detection of Thymic Deletion of P2-Specific, but Not P7-Specific, CD4+CD8− Thymocytes.
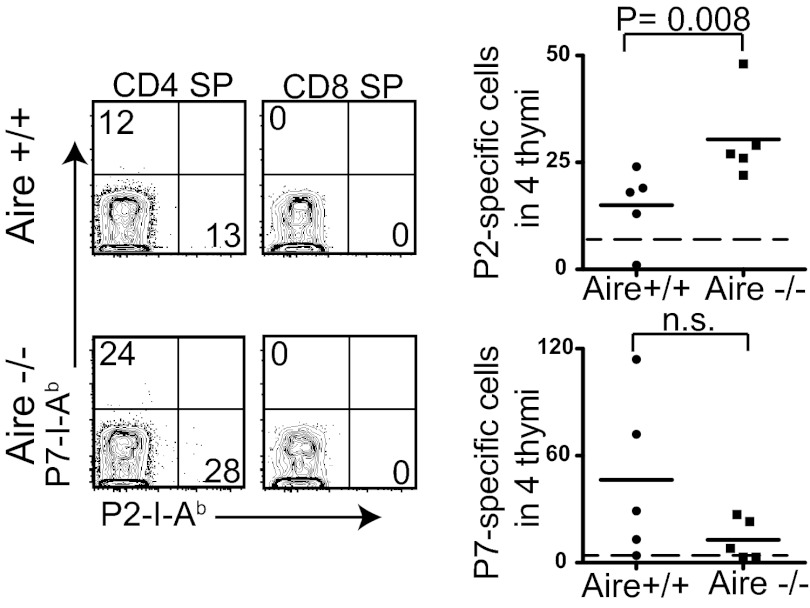
We next sought to determine whether the difference in the precursor frequency of P2-specific cells in Aire+/+ and Aire−/− mice could be explained by thymic deletion of P2-specific T cells. Because the frequency of tetramer-binding thymocytes is extremely low in the polyclonal repertoire (19), four thymi from Aire+/+ and Aire−/− mice were pooled for each analysis using the tetramer enrichment technique. Representative flow cytometery plots of CD8 single-positive (SP) thymocytes showed low nonspecific tetramer staining of thymocytes compared with that of peripheral T cells, resulting in a lower LOD (Figs. 1_B_ and 4). More P2-specific CD4+CD8− thymocytes were detected in Aire−/− thymi compared with Aire+/+ thymi; however, the difference in the number of P7-specific CD4+CD8− thymocytes in Aire+/+ and Aire−/− thymi was not consistent (Fig. 4).
Fig. 4.
Thymic deletion of P2-specific cells, but not of P7-specific cells, is dependent on Aire expression. Four thymi from Aire+/+ and Aire−/− mice were pooled and the tetramer-binding T cells quantified. Representative FACS plots of CD3+CD4+ SP thymocytes and CD3+CD8+ SP thymocytes from pooled Aire+/+ and Aire−/− thymi are shown. Each data point in the summary graph represents the number of tetramer-positive cells in the four thymi. The dotted line in each graph is the LOD.
Thymic Antigen-Presenting Cells Preferentially Present P2 Peptide Epitope over P7 Peptide Epitope.
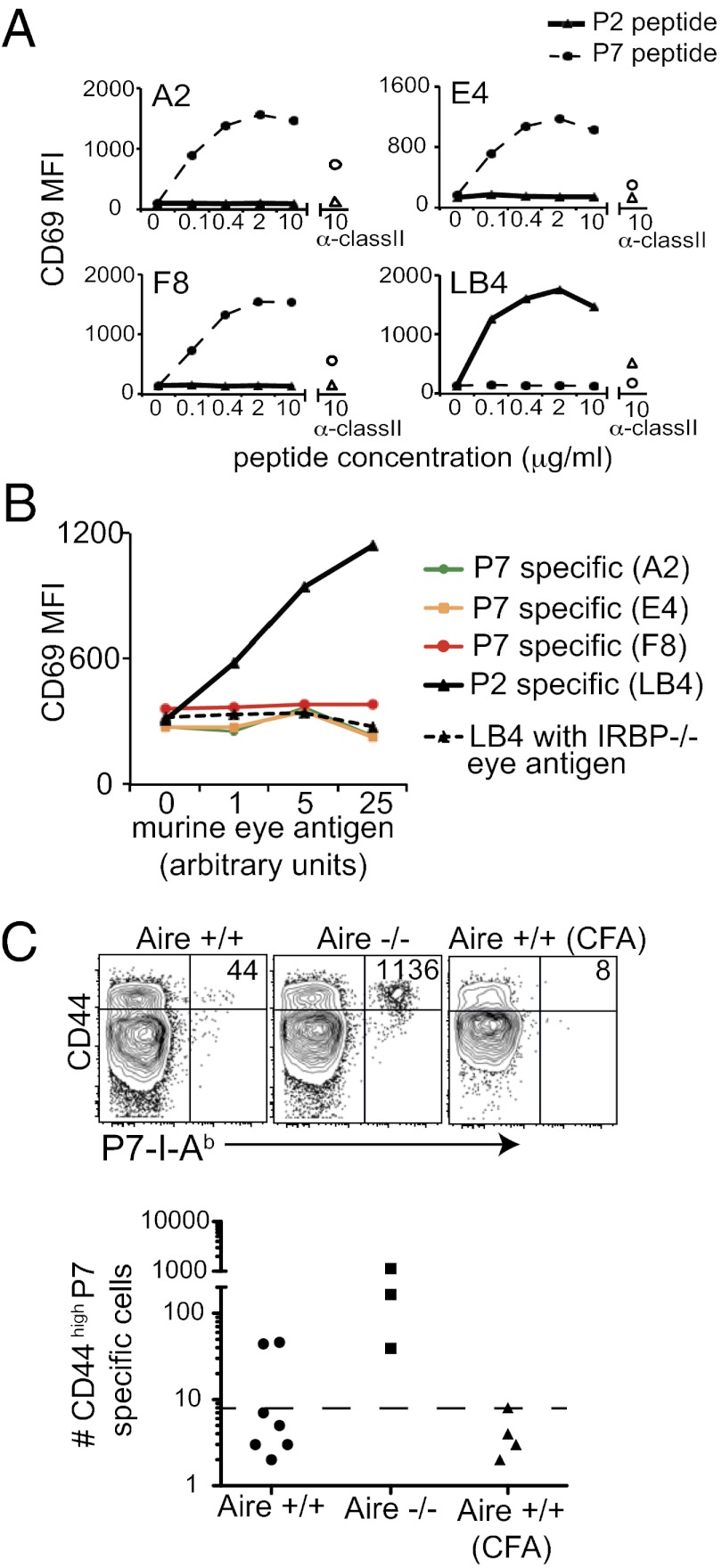
To explain the difference in thymic selection between the two IRBP epitopes, we hypothesized that the P7 epitope might not be effectively presented in the thymus despite its high affinity for I-Ab (Fig. S2). In other model systems, alternative splicing of protein in the thymus compared with peripheral tissues can result in a differential display of peptide epitopes (20). However, because both the P2 and P7 epitopes are contained within the same exon of IRBP, we reasoned that differential display of IRBP in the thymus and periphery could not have been due to alternative splicing. We next considered whether differences in antigen processing and presentation of the two epitopes within the thymus could explain the differential display. To test this hypothesis, we used T-cell hybridomas specific for either the P2 or P7 peptide of IRBP to detect both peptide and whole antigen presentation efficiency by antigen-presenting cells (APCs). T-cell hybridoma lines were stimulated with irradiated splenocytes pulsed with either P2 or P7 peptide. The P7-specific hybridomas (A2, E4, and F8) were specifically stimulated by the P7 peptide, and the P2-specific hybridoma (LB4) was specifically stimulated by the P2 peptide, with similar dose–response curves in all four clones (Fig. 5_A_). We next stimulated the P2-specific and P7-specific hybridomas with sorted thymic CD11c+CD45+ APCs loaded with whole IRBP protein from a mouse eye extract (Fig. 5_B_). APCs loaded with eye extract stimulated the P2-specific hybridoma (LB4), but did not stimulate any of the P7-specific hybridomas (A2, E4, and F8). The LB4 hybridoma could not be stimulated with APC loaded with eye extract prepared from IRBP−/− eyes, confirming that LB4 is specific for an IRBP peptide epitope (Fig. 5_B_). Thus, thymic APCs loaded with IRBP seem to preferentially present the P2 peptide over the P7 peptide, despite the fact that P2 has a lower affinity for I-Ab compared with P7 (Fig. S2). These data support the hypothesis that P2-specific thymocytes are deleted more efficiently than P7-specific thymocytes because the P2 epitope is presented more efficiently by thymic APCs.
Fig. 5.
Thymic APCs preferentially present the P2 peptide epitope over the P7 peptide epitope. P7-specific (A2, E4, and F8) and P2-specific (LB4) hybridoma stimulation was measured by CD69 up-regulation. (A) T-cell hybridomas were incubated with irradiated WT B6 splenocytes and P2 or P7 peptide for 12 h. The empty triangle and circle data points indicate hybridoma stimulated in the presence of anti-class II blocking mAb plus 10 μg/mL of P2 peptide and P7 peptide, respectively. Data are representative of three independent experiments. (B) P7-specific (A2, E4, and F8) and P2-specific (LB4) hybridoma clones were incubated with CD45+CD11c+ thymic APC and soluble eye antigen from WT or IRBP−/− mice. Data are representative of two independent experiments. (C) Four- to 5-wk-old WT and Aire−/− mice were immunized with eye lysate from WT in CFA or CFA alone. At 8 d after immunization, CD44high tetramer-positive T cells in the secondary lymphoid organs were quantified. Each graphed data point represents one mouse. The dotted line in each graph is the LOD.
Although preferential presentation of the P2 epitope by APCs in thymus might account for the low frequency of P2-specific thymocytes, we detected a high frequency of antigen-experienced (CD44high) P2- and P7-specific T cells in Aire−/− mice with uveitis (Fig. 2). We hypothesized that APCs in the periphery could present the P7 epitope on inflammation in vivo. To test this hypothesis, we investigated the expansion of P7-specific T cells after immunization with murine eye extract as a source of IRBP protein. We found that immunization with whole IRBP resulted in the expansion of P7-specific cells in all Aire−/− mice and at least some Aire+/+ mice (Fig. 5_C_). Thus, it appears that the P7 epitope can be presented by APCs loaded with whole IRBP in the context of immunization-driven inflammation in vivo.
Deletion of P2-Specific T Cells Involves Intercellular Transfer of IRBP.
We previously mapped thymic IRBP expression to the mTEC compartment (3); however, whether mTECs directly present IRBP to the developing thymocyte repertoire, or whether IRBP-antigen is transferred to other thymic APC populations to promote negative selection, is unclear. To examine this question, we generated bone marrow (BM) chimeric mice by transfer of WT or CIITA−/− BM into WT irradiated hosts. Animals reconstituted with CIITA−/− BM will express MHC class II only on thymic epithelial cells, and not on hematopoetically derived thymic APCs. We analyzed thymi from reconstituted mice for the frequency of tetramer-binding thymocytes. Mice reconstituted with CIITA−/− BM had a higher frequency of P2-specific thymocytes compared with mice with WT BM (Fig. 6), indicating that the deletion of these T cells is heavily dependent on class II expression on BM-derived APCs. In contrast, no significant difference was seen in the frequency of P7-specific thymocytes in mice reconstituted with WT or CIITA−/− BM (Fig. 6), reinforcing the finding that P7-specific cells do not undergo negative selection because of a lack of P7 presentation in the thymus. Taken together, these data demonstrate that intercellular transfer of IRBP between Aire-expressing mTECs and BM-derived APCs plays an important role in the negative selection of P2-specific T cells.
Fig. 6.
Thymic deletion of P2-specific cells, but not of P7-specific cells, is dependent on antigen presentation by BM-derived APCs. Irradiated WT mice were reconstituted with either WT or CIITA−/− BM. Four thymi were pooled and the tetramer-binding T cells quantified. Representative FACS plots of CD3+CD4+ SP and CD3+CD8+ SP thymocytes are shown. Each data point in the summary graph represents the number of tetramer-positive cells in the four thymi. The dotted line in each graph is the LOD.
Discussion
In this study, we developed two tetramer reagents to demonstrate that Aire+/+ and Aire−/− mice have distinctly different frequencies of IRBP-specific T cells in their polyclonal T-cell repertoires. We found that thymic deletion of P2-specific T cells is highly dependent on Aire, as demonstrated by the detection of little or no P2-specific T cells in Aire+/+ mice even after immunization. In contrast, T cells specific for the P7 epitope of IRBP are detectable and expand after immunization in Aire+/+ mice. This difference appears to be related to differences in antigen processing by thymic APCs that pick up the IRBP antigen from mTECs, because both peptide epitopes bind to the I-Ab complex. These results provide several important insights into Aire-mediated selection in the more physiological setting of a polyclonal repertoire.
IRBP expression within the thymus maps to mTECs, where it is expressed at relatively low levels and requires robust PCR-based methods for reliable detection (3). In addition, TSA expression within individual Aire-expressing mTECs appears to be stochastic, with only limited subsets of mTECs expressing a given TSA (21, 22). Despite these low expression levels and the scattered distribution of TSA expression within the mTEC compartment, our results clearly demonstrate that thymic deletion mediated by such endogenous TSAs is remarkably efficient in the polyclonal setting. The relatively limited spatiotemporal expression of TSAs suggests that additional processes, such as chemokine-mediated migration of APCs to TSA-expressing mTECs and extensive APC scanning by thymocytes, help ensure that the developing T-cell repertoire is exposed to the full array of TSAs present in the medullary compartment (23–26).
We also found that deletion of P2-specific T cells relies on intercellular transfer of IRBP between the radioresistant mTEC compartment and BM-derived thymic APCs. Thus, such a handoff also might improve the efficiency of thymic deletion by allowing a larger number of cells to display the P2 epitope beyond the mTEC cells that are the expression source of the IRBP antigen. Although we have demonstrated the importance of hematopoetically derived APCs in the negative selection of P2-specific cells, in this study we did not address the extent of negative selection that occurs via direct presentation of IRBP by mTECs. Aire-expressing mTECs can present self-antigens via autophagy, and this mechanism has been shown to play an important role in central tolerance (27). Further study is needed to determine the extent of thymic deletion of IRBP-specific T cells via presentation of antigen by Aire-expressing mTECs.
In contrast to P2-specific cells, T cells specific for the P7 epitope of IRBP are not efficiently deleted in the thymus, despite the P7 epitope’s higher binding affinity for I-Ab. Differential deletion of T cells specific for different epitopes of a single autoantigen has been described previously (28, 29). However, unlike in previous studies, here we found that the lack of autoreactive T-cell deletion can arise from inefficient presentation of particular peptide epitopes, like P7, by thymic APCs. Despite the fact that P7-specific T cells escape thymic deletion in Aire+/+ mice, these mice do not spontaneously succumb to uveitis, even on immunization with P7 in CFA (Fig. S5). We speculate that this is due to the fact that the P7 epitope is also inefficiently presented by APCs loaded with endogenous IRBP antigen in the eye-draining lymph nodes. Thus, tolerance to the eye is maintained despite the persistence of a detectable pool of IRBP-specific T cells in the polyclonal repertoire.
In the absence of Aire, both P2-specific and P7-specific T cells are detectable in the T-cell repertoire. With the onset of active uveitis, the frequency of both P2-specific and P7-specific T cells increases in Aire−/− mice, and both populations exhibit up-regulation of the activation marker CD44. Our findings suggest that the expansion of the P7 population with uveitis might be related to the phenomenon of epitope spreading (30). We propose a model in which the P2-specific response drives the initial stages of uveitis and the resultant damage to the posterior eye allows subsequent presentation of the P7-specific epitope by APCs. In support of our idea that the P2 epitope is pathogenic-specific and a driver of uveitis, previous work has demonstrated that P2-specific cells can induce uveitis in an adoptive transfer model (13). In addition, we found that immunizing young Aire−/− mice with P2 peptide in CFA led to the development of uveitis at a time point before spontaneous disease would normally arise in Aire−/− mice (Fig. S5) (1). Taken together, these data support the hypothesis that P2-specific cells may be able to initiate disease, whereas P7-specific cells may accelerate disease pathogenesis in autoimmune-susceptible hosts such as Aire−/− mice. Further studies are needed to more closely analyze the relative pathogenicity of P2, P7, and other epitopes of IRBP in this model system.
Our development of a P2-specific tetramer provides a valuable tool to directly assess defects in negative selection to an endogenous self-antigen within a polyclonal repertoire. Recent work has suggested that genetic defects in genes other than Aire (e.g., CCR7, RANK-L, TRAF6, Bim) also may affect central tolerance to thymic TSAs (31–34). Application of our P2-specific tetramer reagent in such mutant strains and in other models that invoke defects in central tolerance will allow for a more refined assessment of their potential effect in the maintenance of thymic tolerance.
We have demonstrated that thymic deletion to a naturally occurring TSA in the thymus, IRBP, can be detected in the polyclonal repertoire using a tetramer enrichment technique. We found that deletion of P2-specific cells relies not only on Aire-dependent thymic expression of IRBP, but also on proper antigen processing and presentation of IRBP by thymic APCs. In contrast, P7-specific T cells escape negative selection but remain quiescent in the periphery until the introduction of IRBP in the context of inflammation. Therefore, it will be of interest to investigate how hidden self-epitopes like P7 are revealed to autoreactive T cells and may serve as targets to obstruct the autoimmune process.
Materials and Methods
Mice.
Aire−/− mice were generated as described previously (1). IRBP−/− mice were provided by R. Caspi (National Institutes of Health, National Eye Institute, Bethesda, MD) (35). CIITA−/− mice were purchased from Jackson Laboratory (36). All mice were on the C57BL/6 background (>10 generations) and were housed in a pathogen-free barrier facility at the University of California at San Francisco in compliance with Animal Welfare Act and National Institutes of Health guidelines.
Tetramer Analysis.
Tetramers were generated by J.J.M. as described previously (10). Tetramer staining is described in detail in SI Materials and Methods.
Histology.
Uveitis was identified based on the presence or absence of histological infiltrates and tissue disruption/damage in the retina (37). Further details of slide preparation are provided in SI Materials and Methods.
Immunization.
Mice were immunized with 100 μg of P2 or P7 peptide emulsified in 100 μL of CFA. Further details of mouse immunization are provided in SI Materials and Methods.
Hybridoma Generation and Stimulation.
Hybridomas were generated according to standard protocols (38). Details of hybridoma generation and stimulation are provided in SI Materials and Methods.
BM Chimeras.
BM was depleted of T cells using antibodies specific for CD4 (GK1.5) and CD8 (YTS-169) and rabbit complement (Pel-Freez Biologicals). Depleted BM was resuspended at a final concentration of 10E6 cells/mL, and 100 μL was injected into WT or CIITA−/−C57BL/6 recipients that had received a lethal dose of irradiation (500 × 2 rads). After 5–6 wk, thymocytes from chimeras were analyzed for tetramer reactivity.
Statistical Analysis.
Statistical analyses were performed by the unpaired, one-tailed, Mann–Whitney test using Prism (GraphPad Software). The limits of detection were calculated as described by Armbruster et al. (16).
Supplementary Material
Supporting Information
Acknowledgments
We thank Navdeep Grewal for technical assistance and Rachel Caspi for helpful discussions and her generous gift of bovine IRBP for our experiments. We also thank Adam Savage, Todd Metzger, Mickie Cheng, and Mike Waterfield for helpful comments on the manuscript. This work was supported by National Institutes of Health Grants EY16408 and DK063720.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Anderson MS, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 2.Anderson MS, Su MA. Aire and T cell development. Curr Opin Immunol. 2011;23:198–206. doi: 10.1016/j.coi.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeVoss J, et al. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J Exp Med. 2006;203:2727–2735. doi: 10.1084/jem.20061864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shum AK, et al. Identification of an autoantigen demonstrates a link between interstitial lung disease and a defect in central tolerance. Sci Transl Med. 2009;1:9ra20. doi: 10.1126/scitranslmed.3000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeVoss JJ, et al. An autoimmune response to odorant binding protein 1a is associated with dry eye in the Aire-deficient mouse. J Immunol. 2010;184:4236–4246. doi: 10.4049/jimmunol.0902434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gavanescu I, Kessler B, Ploegh H, Benoist C, Mathis D. Loss of Aire-dependent thymic expression of a peripheral tissue antigen renders it a target of autoimmunity. Proc Natl Acad Sci USA. 2007;104:4583–4587. doi: 10.1073/pnas.0700259104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson MS, et al. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 9.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: Learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 10.Moon JJ, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obar JJ, Khanna KM, Lefrançois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moon JJ, et al. Tracking epitope-specific T cells. Nat Protoc. 2009;4:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortes LM, et al. Repertoire analysis and new pathogenic epitopes of IRBP in C57BL/6 (H-2b) and B10.RIII (H-2r) mice. Invest Ophthalmol Vis Sci. 2008;49:1946–1956. doi: 10.1167/iovs.07-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vita R, et al. The immune epitope database 2.0. Nucleic Acids Res. 2010;38(Database issue):D854–D862. doi: 10.1093/nar/gkp1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sette A, Buus S, Colon S, Miles C, Grey HM. Structural analysis of peptides capable of binding to more than one Ia antigen. J Immunol. 1989;142:35–40. [PubMed] [Google Scholar]
- 16.Armbruster DA, Pry T. Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev. 2008;29(Suppl 1):S49–S52. [PMC free article] [PubMed] [Google Scholar]
- 17.Egan RM, et al. Peptide-specific T cell clonal expansion in vivo following immunization in the eye, an immune-privileged site. J Immunol. 1996;157:2262–2271. [PubMed] [Google Scholar]
- 18.Crawford F, Kozono H, White J, Marrack P, Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- 19.Chu HH, Moon JJ, Kruse AC, Pepper M, Jenkins MK. Negative selection and peptide chemistry determine the size of naive foreign peptide-MHC class II-specific CD4+ T cell populations. J Immunol. 2010;185:4705–4713. doi: 10.4049/jimmunol.1002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein L, Klugmann M, Nave KA, Tuohy VK, Kyewski B. Shaping of the autoreactive T-cell repertoire by a splice variant of self protein expressed in thymic epithelial cells. Nat Med. 2000;6:56–61. doi: 10.1038/71540. [DOI] [PubMed] [Google Scholar]
- 21.Derbinski J, Pinto S, Rösch S, Hexel K, Kyewski B. Promiscuous gene expression patterns in single medullary thymic epithelial cells argue for a stochastic mechanism. Proc Natl Acad Sci USA. 2008;105:657–662. doi: 10.1073/pnas.0707486105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villaseñor J, Besse W, Benoist C, Mathis D. Ectopic expression of peripheral-tissue antigens in the thymic epithelium: Probabilistic, monoallelic, misinitiated. Proc Natl Acad Sci USA. 2008;105:15854–15859. doi: 10.1073/pnas.0808069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lei Y, et al. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J Exp Med. 2011;208:383–394. doi: 10.1084/jem.20102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hubert FX, et al. Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood. 2011;118:2462–2472. doi: 10.1182/blood-2010-06-286393. [DOI] [PubMed] [Google Scholar]
- 25.Le Borgne M, et al. The impact of negative selection on thymocyte migration in the medulla. Nat Immunol. 2009;10:823–830. doi: 10.1038/ni.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Derbinski J, Kyewski B. How thymic antigen presenting cells sample the body’s self-antigens. Curr Opin Immunol. 2010;22:592–600. doi: 10.1016/j.coi.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Nedjic J, Aichinger M, Emmerich J, Mizushima N, Klein L. Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature. 2008;455:396–400. doi: 10.1038/nature07208. [DOI] [PubMed] [Google Scholar]
- 28.Klein L, Klein T, Rüther U, Kyewski B. CD4 T cell tolerance to human C-reactive protein, an inducible serum protein, is mediated by medullary thymic epithelium. J Exp Med. 1998;188:5–16. doi: 10.1084/jem.188.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrington CJ, et al. Differential tolerance is induced in T cells recognizing distinct epitopes of myelin basic protein. Immunity. 1998;8:571–580. doi: 10.1016/s1074-7613(00)80562-2. [DOI] [PubMed] [Google Scholar]
- 30.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: Implications for immunotherapy. Nat Rev Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- 31.Kurobe H, et al. CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity. 2006;24:165–177. doi: 10.1016/j.immuni.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Hikosaka Y, et al. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity. 2008;29:438–450. doi: 10.1016/j.immuni.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 33.Ohshima D, et al. RANK signaling induces interferon-stimulated genes in the fetal thymic stroma. Biochem Biophys Res Commun. 2011;408:530–536. doi: 10.1016/j.bbrc.2011.04.049. [DOI] [PubMed] [Google Scholar]
- 34.Kovalovsky D, Pezzano M, Ortiz BD, Sant’Angelo DB. A novel TCR transgenic model reveals that negative selection involves an immediate, Bim-dependent pathway and a delayed, Bim-independent pathway. PLoS ONE. 2010;5:e8675. doi: 10.1371/journal.pone.0008675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liou GI, et al. Early onset photoreceptor abnormalities induced by targeted disruption of the interphotoreceptor retinoid-binding protein gene. J Neurosci. 1998;18:4511–4520. doi: 10.1523/JNEUROSCI.18-12-04511.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang CH, Guerder S, Hong SC, van Ewijk W, Flavell RA. Mice lacking the MHC class II transactivator (CIITA) show tissue-specific impairment of MHC class II expression. Immunity. 1996;4:167–178. doi: 10.1016/s1074-7613(00)80681-0. [DOI] [PubMed] [Google Scholar]
- 37.Pennesi G, et al. A humanized model of experimental autoimmune uveitis in HLA class II transgenic mice. J Clin Invest. 2003;111:1171–1180. doi: 10.1172/JCI15155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kruisbeek AM. Production of mouse T cell hybridomas. Curr Protoc Immunol. 2001;24:3.14.1–3.14.11. doi: 10.1002/0471142735.im0314s24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information