Murine thymic selection quantified using a unique method to capture deleted T cells (original) (raw)
Abstract
Thymic positive and negative selection events generate a T-cell repertoire that is MHC restricted and self-tolerant. The number of T cells undergoing positive and negative selection in normal mice has never been firmly established. We generated mice that lack the proapoptotic molecule Bim (bcl2l11) together with a Nur77GFP transgene, which allowed the identification and enumeration of T cells that would normally undergo clonal deletion. Using this method, we report the striking observation that six times more cells undergo negative selection than complete positive selection. Seventy-five percent of the negatively selected cells are deleted at the double positive stage in the thymic cortex, compared with 25% at the single positive stage in the medulla. The fact that more thymocytes are highly reactive to MHC than are weakly reactive is inconsistent with a random model of recognition and suggests that T-cell recognition is MHC biased. Furthermore, Bim−/− mice had an increased number of GFPhi cells in the peripheral lymphoid tissue and a corresponding increase in antigen experienced or anergic cell phenotype. Our data also show that the CD4+ T cells that are clonally deleted experienced only slightly stronger T-cell receptor signaling than those that developed into regulatory T cells.
Keywords: lymphocyte development, thymus
Positive and negative selection events in the thymus create a T-cell repertoire that is both major histocompatibility complex (MHC) restricted and self-tolerant. It is well established that the affinity of the T-cell receptor (TCR) for self-MHC ligands is critical in determining these fates. Low-affinity interactions promote survival and maturation, whereas high-affinity interactions promote deletion, with a surprisingly narrow threshold distinguishing these two (1). Nonetheless, it is unclear how many T-cell clones achieve this threshold and become deleted relative to the number that are positively selected. Because the T-cell receptor is formed by somatic recombination with nontemplated nucleotide addition, the amino acids in the antigen binding region are highly diverse and essentially random. Thus, it has been assumed that the number of clones that can interact with any given peptide MHC complex with high affinity (negative selection) would be smaller than the number of clones that could interact more weakly (positive selection). However, attempts to understand what fraction of the repertoire undergoes positive and negative selection have led to disparate findings.
The murine thymus exports ∼1–4 × 106 cells per day, and these are predominantly naïve CD4 and CD8 T cells (2). Thus, based on the previous estimates of negative selection, one might predict that the number of self-reactive clones generated and deleted per day would be much smaller than this number. However, because apoptotic cells are so efficiently engulfed by thymic macrophages, it has been challenging to quantify clonal deletion. Consequently, the frequency with which clonal deletion occurs relative to positive selection has been controversial. Using a TUNEL assay to measure apoptosis in situ, Sprent and colleagues (3) observed little difference between WT and MHC-deficient mice, suggesting that the majority of thymocytes die from neglect, as opposed to clonal deletion. Laufer et al. (4) created mice where MHC class II was only expressed on cortical epithelial cells and concluded that 5% of the selected repertoire is normally negatively selected. In contrast, van Meerwijk and colleagues (5) estimated that 50% of the selected repertoire is negatively selected via analysis of bone marrow chimeras where MHC class II-deficient bone marrow was transplanted into wild-type mice. Finally, Merkenschlager’s (6) group estimated that 2 times as many cells undergo negative selection compared with positive selection through determination of the percent of preselection thymocytes that respond to MHC expressing antigen presenting cell. Thus, the estimates of relative thymic positive and negative selection incidence vary widely.
In this study, we determined the relative rates of positive and negative selection using an approach involving Bim-deficient mice. Bim is a proapoptotic molecule that is required for clonal deletion of self-reactive T cells (7). Those self-reactive T-cell clones rescued from deletion can, in principle, be enumerated in Bim-deficient mice. However, it is unclear whether Bim deficiency solely prevents deletion of strongly self-reactive clones, or if it also enhances the survival of weakly self-reactive clones—those that are normally positively selected. Thus, we combined Bim-deficient mice with Nur77GFP reporter mice. Nur77 is a TCR immediate early gene, and we previously reported that in T cells from Nur77GFP mice, GFP expression reflects the signal strength perceived by the antigen receptor (8). We reasoned that when combined with Bim deficiency, the GFP level of rescued cells would report the TCR signaling experience of that cell and thereby allow the identification of normally deleted clones. Here we report the surprising finding that ∼6 times more cells undergo negative selection in the thymus than complete positive selection. The implications of this for our understanding of T-cell recognition are discussed.
Results
Highly Self-Reactive Thymocytes Accumulate in Bim−/− Mice.
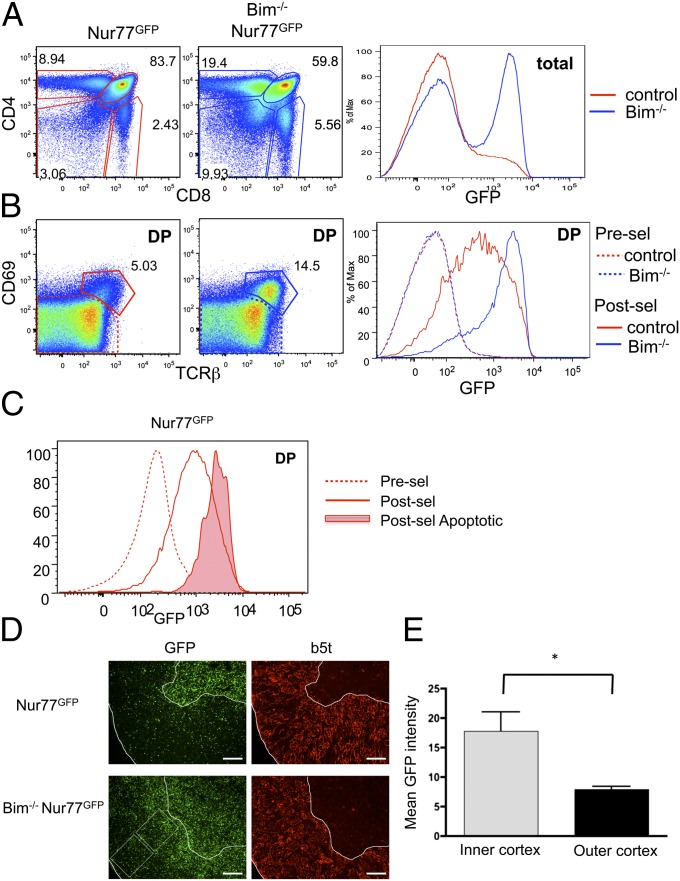
Bim−/− mice have a significant increase in CD4 single positive (SP) and CD8SP cells (Fig. 1_A_) (9). This finding is consistent with previous studies showing that Bim is required for the deletion of self-reactive T cells in both class II and class I restricted selection models (7). However, its absence might also improve the lifespan of nonselected or positively selected cells as well. Thus, to determine if Bim deficiency is preferentially rescuing highly self-reactive T cells from deletion or if it is also enhancing survival of low-affinity or nonreactive clones, we crossed the Bim−/− mouse to the Nur77GFP reporter mouse (8). GFP expression in the Nur77GFP mouse reports the overall TCR signal strength perceived. As previously reported, the majority of thymocytes from control Nur77GFP mice were GFP low (Fig. 1_A_). Remarkably, thymocytes from Bim−/− Nur77GFP mice showed a large GFP high subpopulation indicating a robust overall rescue of clones that had received a strong signal through the TCR (Fig. 1_A_). We then looked specifically at the double positive (DP) or cortical thymocyte population. DP cells that are TCRβlo and CD69lo are considered “preselection” (before positive or negative selection), and these cells express low levels of GFP in both control and Bim−/− mice (Fig. 1_B_). Postselection (TCRβ+CD69hi) DP in control mice showed an increase in GFP (8). However, postselection DP from Bim−/− mice had an even greater increase in GFP expression with the majority of cells being GFPhi (Fig. 1_B_). Furthermore, using active caspase 3 as a way to identify cells undergoing apoptosis in normal Bim+/+ mice, we found that active caspase 3 positive (dying) cells in the postselection pool had Nur77GFP levels similar to the rescued cells in the Bim−/− mice (Fig. 1_C_). Immunofluorescence of thymi from Bim−/− Nur77GFP mice showed a large accumulation of GFP positive cells in both the cortex and medulla (Fig. 1_D_). Furthermore, the overall level of GFP in the cortex was higher toward the medulla (inner) than toward the capsule (outer) (Fig. 1_E_), consistent with a previous report suggesting that clonal deletion preferentially occurs in this area (10). Together, these data show that Bim deficiency is primarily rescuing self-reactive T cells (high affinity) from deletion and that such cells accumulate in the Bim−/− thymus.
Fig. 1.
Highly self-reactive thymocytes accumulate in Bim−/− mice. (A and B) Thymocytes from Nur77GFP mice that were either Bim deficient (Bim−/−) or control (Bim+/+ or Bim+/−), were analyzed by flow cytometry. (C) GFP level was assessed on DP thymocytes from Bim+/+ Nur77GFP mice stained and gated on CD69loCD5lo (presel), CD69+CD5+ (postsel), or CD69+CD5+Active caspase 3+ (postsel apoptotic). (D) Bim−/− or control thymi were analyzed using immunofluoresence. β5t was stained to determine the cortex region. (E) The mean fluorescence intensity of the inner and outer cortex of Bim−/− mice. In B, cells were gated as indicated on the left: preselection CD69−,TCRβlo; postselection, CD69+,TCRβhi. Data are representative of more than eight (A and B), 5 (C), or 4 (D and E) animals from two to six independent analyses. Asterisks indicated P < 0.05 using a Student t test.
Enumeration of Progenitors with Intermediate or High GFP Expression.
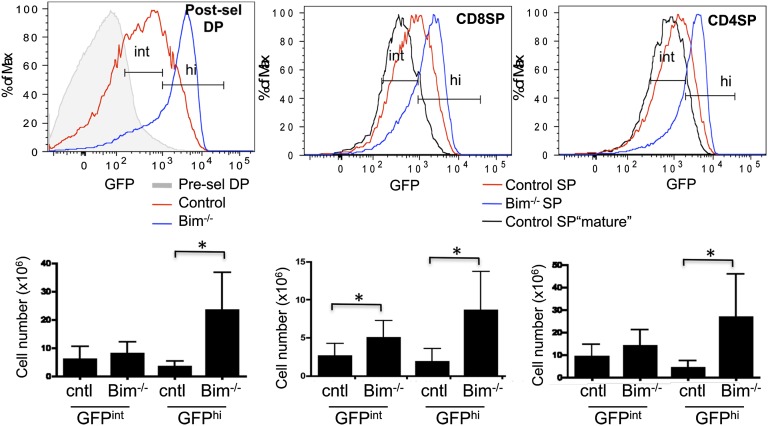
Because GFP allows us to differentiate between cells that have received a weak TCR signal and those that have received a strong TCR signal, we used Nur77GFP Bim−/− mice to enumerate the cells undergoing positive and negative selection. We defined a “GFP intermediate” (positive selection) gate based on the level of GFP expressed by the most mature (HSAlow) naïve SP thymocytes from control Nur77GFP mice (Fig. 2). This intermediate level was higher than preselection thymocytes and maintained in naïve peripheral T cells via tonic interactions with MHC (8). Bim-deficient thymi have about a 1.5-fold increase in total cell number, which was used to calculate absolute number of cells falling into the GFPhi and GFPint gate (Fig. S1). The absolute number of DP cells with an intermediate level of GFP was similar between control and Bim−/− mice (Fig. 2). However, the number of DP cells with a high level of GFP was increased sevenfold in Bim−/− mice. This result shows that Bim deficiency rescues predominantly high-affinity clones and is not influencing positive selection to a significant degree (Fig. 2). Bim deficiency also did not alter the number of preselection GFP negative DP thymocytes (Fig. S2), suggesting that it does not influence the lifespan of this population. These findings are consistent with the observation that Bim is much more highly expressed in cells undergoing negative selection than positive selection or no selection (11, 12).
Fig. 2.
Enumeration of progenitors with intermediate or high GFP expression. Thymocytes from Nur77GFP mice that were either Bim deficient (Bim−/−) or control (Bim+/+ or Bim+/−), were analyzed by flow cytometry. (Upper) Gates used to define GFP intermediate (int) and GFP high (hi) populations. The intermediate gate, which defines the level of GFP expressed in positively selected naïve T cells, was established by gating on the GFP level of mature (HSAlo) CD4SP or CD8SP, CD25- thymocytes from the same experiment (black lines). The high gate was extended from the upper end of the intermediate gate. (Lower) The total numbers of thymocytes in the indicated gates. Data are representative or calculated from eight mice from six independent analyses. Error bars indicated SD. Asterisks indicated P < 0.05 using a Student t test.
We also observed a significant increase in the numbers of both CD4SP and CD8SP thymocytes that are GFPhi in Bim−/− mice relative to controls (Fig. 2). Accumulation of SP GFPhi cells in the Bim−/− mice was not due to increased proliferation of the normally deleted GFPhi cells (Fig. S3). Such GFPhi SP cells are likely cells rescued from deletion in response to self-antigens presented by medullary antigen presenting cells (13). In theory, GFPhi SP cells could be the descendents of GFPhi DP cells that maintained a high level of GFP as they differentiated. The latter possibility is unlikely, however, because several studies have shown that, although Bim deficiency can rescue DP thymocytes from acute apoptosis, it does not allow their differentiation to the SP stage (10, 14, 15). Thus, we consider it most likely that GFPhi SP thymocytes in Bim−/− mice represent cells that were positively selected by the classical low-affinity interactions and are responding to new high-affinity interactions in the medulla.
Lifespan Estimates for Major Thymocyte Populations.
We noted that the number of GFPhi cells rescued by Bim deficiency is substantially higher than the number of cells that are GFPint (Fig. 2). However, this does not necessarily mean that more cells are undergoing negative selection than positive selection, because GFPhi Bim−/− DP cells may persist longer in the thymus than GFPint cells persist before differentiating to SP cells. Thus, to understand the relative frequency of positive and negative selection events, we first needed to determine the lifespan of specific subsets of Bim−/− thymocytes relative to control subsets. By lifespan, we refer to the average time a cell persists in a particular differentiation state, thus either death or differentiation can define this value. Previous studies have estimated the lifespan of normal preselection DPs to be 60 h and that postselection DP and SP thymocytes persist for 16 h and 96 h respectively, before differentiating, dying, or emigrating (Table 1, and references therein). We presumed the lifespan of preselection DP would be fairly similar between control and Bim−/− mice, because preselection DP express very low levels of Bim (12). However, it was important to establish the relative lifespan of postselection DP and SP thymocytes from Bim−/− mice. To do this, we sorted preselection DP, postselection DP, and SP thymocytes from congenic control and Bim−/− animals and injected them together into the thymus of a recipient mouse, measuring the relative recovery at various time points afterward. As expected, the same number of Bim−/− and control preselection DPs were recovered (Table 1). However, postselection DP thymocytes from Bim−/− mice had a lifespan 2.1 times longer than control, which translates to an estimated lifespan of 34 h (Table 1). Bim−/− CD4 and CD8SP cells also had increased lifespans that were 1.4 and 1.7 times longer than control CD4 and CD8SPs, respectively.
Table 1.
Lifespan estimates for major thymocyte populations
| Source | Preselection DP | Postselection DP | SP |
|---|---|---|---|
| Historical data (WT) | 60 h | 16 h | 96 h |
| Ref(s). | 33 | 34 | 35 |
| Bim−/−* | 60 h | 34 h | 130 h, 162 h |
Murine Thymic Selection Rates.
With numbers and lifespan estimates from both control and Bim−/− mice, we were able to determine the rate of positive and negative selection, in cells per hour (Table 2). The rate of positive selection shown in Table 2 was calculated using the number of GFPint cells from control mice, although similar results were obtained using the number of GFPint cells from Bim−/− mice (Table S1). Negative selection rates were calculated using the difference in number of GFPhi cells in Bim−/− mice and control mice (i.e., the number of “rescued” cells) (see Methods for details). To our surprise, the rate of cells undergoing negative selection at the DP stage was not smaller than the number of cells undergoing positive selection, but nearly 1.4 times larger. Furthermore, nearly half of the cells that are positively selected at the DP stage in the cortex were subsequently deleted at the SP or medullary stage (Table 2). As an internal control, we calculated the rate of cells positively selected at the DP stage and compared it to the sum of both positive and negative selection at the SP stage and found they were very similar, further supporting our assumption that GFPhi SP cells do not develop directly from GFPhi DP, but represent medullary deletion events (Table 2). Furthermore, multiplying our estimated hourly rate of positive selection by 24 yields a number (2.9 × 106) that is very close to what was previously suggested to be the daily thymocyte export rate for young mice (1–4 × 106) (2). Considering cortical and medullary events together, we found that the number of cells that undergo negative selection per hour is about 5.7 times higher than the number of cells that complete positive selection.
Table 2.
Murine thymic selection rates
| Selection event (stage) | Rate, per hour | |
|---|---|---|
| Positive selection (control) | Negative selection | |
| DP | 3.7 × 105 | 5.0 × 105 |
| SP | 1.23 × 105 | 1.99 × 105 |
| SP-CD8 | 0.27 × 105 | 0.33 × 105 |
| SP-CD4 | 0.96 × 105 | 1.66 × 105 |
| Total | 1.23 × 105 | 6.99 × 105 |
Analysis of Active Caspase 3 Yields Similar DP to SP Deletion Ratios.
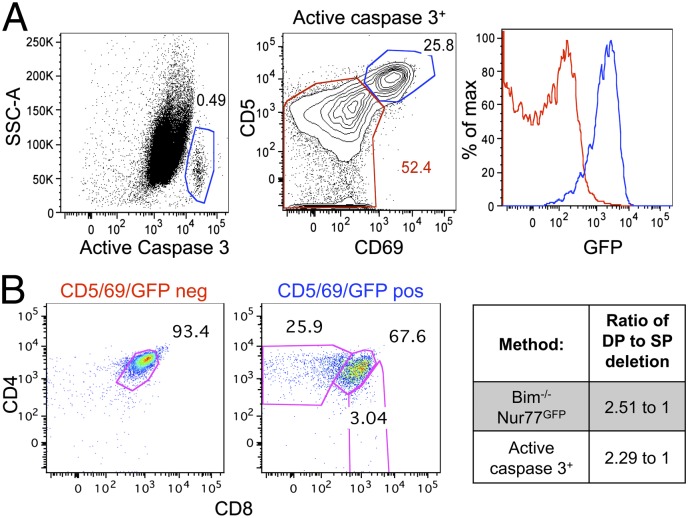
Because our rate estimates are highly dependent on accurate calculations about the lifespan of Bim−/− thymocytes, we sought to verify our findings using another method. T cells undergoing apoptosis activate a cascade of death inducers, including caspase 3. Therefore, in the thymus, caspase 3 is activated in cells undergoing “death by neglect” as well as by negative selection. To distinguish between these two fates, we used CD69 and CD5, which are up-regulated upon antigen receptor signaling during both positive and negative selection (16, 17). As expected, GFP expression was low in the CD69−CD5low cells and high in the CD69+CD5hi cells (Fig. 3_A_). The CD69/CD5/GFP low cells that were active caspase 3 positive are presumably cells undergoing death by neglect, whereas the CD69/CD5/GFP high cells that were active caspase 3 positive are cells undergoing clonal deletion. Consistent with this idea, the former cells were exclusively DN and DP thymocytes, whereas the latter cells were comprised of both DP and SP thymocyte populations (Fig. 3_B_), as expected for negative selection. The ratio of DP to SP cells undergoing deletion should be similar when determined via this method or via the rates method we applied above. Indeed, the ratio of DP to SP that were active caspase 3 positive and CD69/CD5/GFP high was 2.29–1, and the calculated ratio of DP to SP deletion using the Bim−/− Nur77 GFP method was very similar, 2.51–1 (Fig. 3_B_). These data support the accuracy of our method by validation with an independent approach, and show that 2.5 times more cells undergo clonal deletion at the DP (cortical) stage, compared with the SP (medullary) stage.
Fig. 3.
Analysis of active caspase 3 yields similar DP to SP deletion ratios. Thymi from Bim control Nur77GFP mice were analyzed by flow cytometry. (A) (Left) The gating scheme for active caspase 3. (Center) CD69 and CD5 expression on active-caspase 3+ cells. The red gate defines death by neglect; the blue gate defines clonal deletion. (Right) GFP levels in cells undergoing death by neglect versus clonal deletion is shown in the histogram overlay. (B) (Left and Center) The CD4/8 profiles of cells undergoing death by neglect or clonal deletion. (Right) Comparison of the ratio of DP to SP deletion using the methods used in Figs. 2 and 3 (Bim−/− Nur77GFP and active caspase 3, respectively). Data are representative from five to eight mice from at least three independent analyses.
Cells Rescued from Clonal Deletion Express a Similar Level of GFP Compared with Treg Cells.
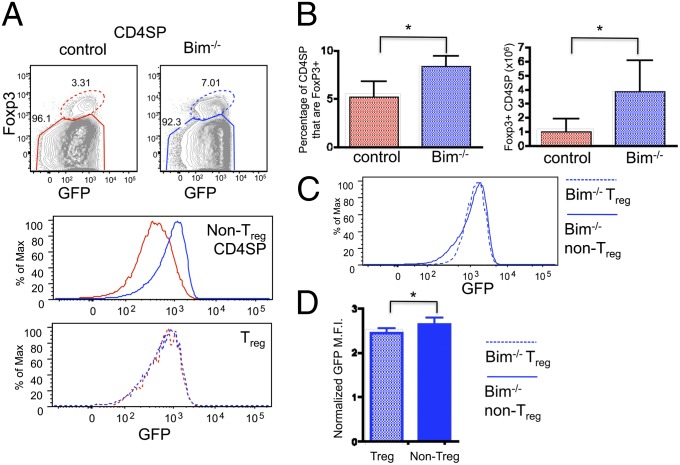
Development of natural T regulatory cells (Treg) is dependent on high-affinity TCR interactions during selection in the thymus (18). In fact, studies suggest that high-affinity TCR ligands are rare, and that competition for these ligands can limit Treg cell development (8, 19, 20). It is unclear whether TCR affinity for self is the only limiting factor or if other factors, such as cytokine availability and/or costimulation might also limit Treg development. Bim−/− mice accumulate viable CD4 T cells that have been strongly signaled through the TCR, so we wondered whether this would result in increased Treg cell development. Indeed, we found about a twofold increase in the percentage, and fourfold increase in the number, of Foxp3+ (forkhead box P3) Treg cells (Fig. 4 A and B). However, the majority of highly self-reactive (GFPhi) CD4 T cells rescued by Bim deficiency did not become Treg cells (Fig. 4 A Top and B). Furthermore, Treg cells from Bim-deficient mice express similar levels of GFP compared with control Treg cells, unlike the significant increase in GFP in Bim−/− CD4SP non-Treg cells compared with controls (Fig. 4_A_). We then compared the mean fluorescence intensity of GFP on “rescued” cells from Bim−/− mice to the level of GFP of Treg cells. Interestingly, the level of GFP expressed by rescued self-reactive T cells was only slightly higher than that of Treg cells, albeit statistically significant (Fig. 4 C and D). These data confirm that not all self-reactive T cells become Treg cells and moreover suggest that many of the cells that normally undergo clonal deletion have similar levels of self-reactivity as Treg cells.
Fig. 4.
Cells rescued from clonal deletion express a similar level of GFP compared with Treg cells. Cells from thymi of control and Bim−/− Nur77GFP mice were analyzed by flow cytometry. (A) (Top) Cells were gated on Foxp3+ (dotted oval gate) or Foxp3− (solid polygonal gate). (Middle and Bottom) Overlays of GFP on the different cell populations. (B) The percent and number of Treg cells in control and Bim−/− thymi. (C) GFP expression on Treg cells and non-Treg cells from Bim−/− thymi. (D) Normalized mean fluorescence intensity of GFP expression on Bim−/− cell populations. Data are representative from seven animals from at least four independent analyses. Asterisks indicated P < 0.05 using a Student t test.
Bim−/− Peripheral T Cells Are Enriched for “Anergic Phenotype” Cells.
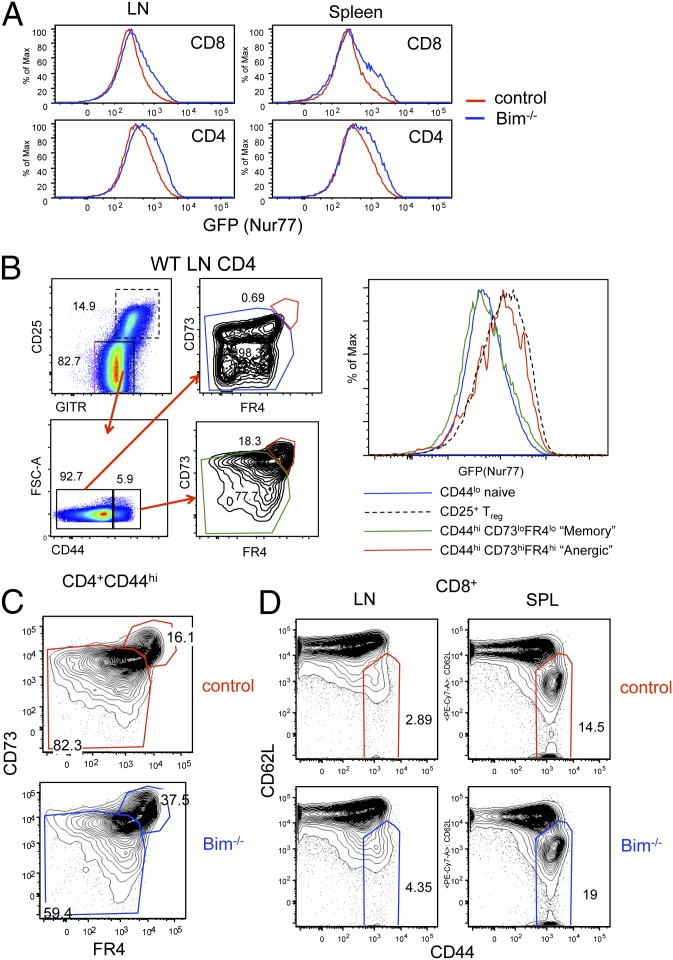
Because self-reactive T cells are rescued in the thymus of Bim−/− mice, we wanted to determine whether they were also present in peripheral lymphoid tissues. Bim−/− CD4 and CD8 T cells from both the spleen and lymph nodes showed an increase in Nur77GFP compared with control CD4 and CD8 T cells (Fig. 5_A_). Even though Bim-deficient mice have this marked increase in self-reactive T cells in the peripheral lymphoid tissue, they rarely develop autoimmune diseases until later in life (9). Peripheral tolerance mechanisms that might control self-reactive T cells include deletion, T regulatory cell induced suppression and anergy. As mentioned above, Bim−/− mice have increased percentages and numbers of Treg cells in the thymus, and this is the case in the peripheral lymphoid tissue as well (Fig. S4). Increased Treg cells could potentially provide a mechanism by which self-reactive clones outside of the thymus are controlled. Recently, cell surface proteins CD73 and FR4 were reported to be up-regulated on “anergic” CD4 T cells (21). In the lymph nodes and spleens of normal polyclonal mice there is a population of CD4+CD44hi non-Treg cells expressing CD73 and FR4. Interestingly, such cells expressed a high level of GFP in Nur77GFP mice, supporting the idea that anergic cells are more self-reactive than the overall naïve CD4 T-cell population (Fig. 5_B_). To determine whether Bim−/− CD4 T cells that have escaped deletion have an anergic phenotype we stained lymph nodes from Bim−/− and control mice. Bim−/− CD4 T cells have a notable increase in both the percentage and number of anergic phenotype cells compared with control animals (Fig. 5_C_). These data suggest that another potential way Bim−/− mice keep self-reactive CD4 T cells in the periphery in check is through the induction of clonal anergy. Like CD4 T cells, Bim−/− CD8 T cells also showed an increased GFP expression overall (Fig. 5_A_). CD8 T cells from Bim−/− mice showed a greater proportion of CD62Llo CD44hi effector/memory phenotype cells compared with control mice (Fig. 5_D_). The increase of these cells in Bim−/− mice suggests that self-reactive CD8 T cells exist in the peripheral lymphoid tissues, but again, the overall health of young Bim−/− mice shows that they do not cause an autoimmune reaction.
Fig. 5.
Bim−/− peripheral T cells are enriched for anergic phenotype cells. Peripheral CD4+ (non-Treg) T cells and CD8 T cells from Bim−/− and control mice were analyzed by flow cytometry. (A) The level of GFP in CD4 and CD8 T cells from spleen and lymph nodes. (B) (Left) The gating strategy used to define “anergic phenotype” cells in the peripheral lymphoid tissue. (Right) Nur77GFP expression of the indicated cell populations. (C) Dot plots show the percentage of CD4+ CD44hi cells from Bim−/− or control spleens that expressed an anergic phenotype. (D) CD8+ T cells from the spleen or lymph nodes of Bim−/− and Bim+/− were stained for CD62L and CD44. Data shown are representative of at least four animals from at least three independent analyses.
Discussion
The rate and number of cells undergoing negative selection in the thymus has been difficult to estimate. In previous reports, the number of cells undergoing negative selection ranged from a small percentage to more cells than undergo positive selection (3–6). In this report we used mice deficient in the proapoptotic molecule Bim crossed to Nur77GFP reporter mice. This system allowed us to calculate the number of cells that have received a weak TCR signal and underwent positive selection compared with those that had received a strong TCR signal and would normally have undergone deletion. Using knowledge of the relative lifespan of different thymocyte populations, we were able to deduce positive and negative selection rates. The number of cells undergoing negative selection per hour was far greater than the number of cells being positively selected. Moreover, the number of cortical DP thymocytes that were negatively selected was 2–3 times more than the number of medullary cells deleted at the SP stage, a finding that we confirmed using active caspase 3 analysis. Overall, we estimate that nearly 5.5–6 times as many T cells undergo negative selection compared with positive selection, a ratio much higher than previously appreciated.
This estimate is much higher than appreciated by two previous studies (4, 5), although both of those approached only examined the effect when MHC class II was absent from select APC. Thus, the disparate results may reflect our incomplete understanding of the efficiency of different APC in inducing negative selection. Another previous study (3) suggested that the vast majority of thymocytes die from neglect, rather than negative selection. Our study did not directly compare the rate of death by neglect to that of negative selection. However, the active caspase 3 data in Fig. 3 suggests that about one quarter of the ongoing apoptosis in the thymus is due to negative selection. It is possible that the active caspase 3 and TUNEL assays differ in their sensitivity to detect distinct forms of apoptosis, or that immunofluorescence does not have the same quantitative power as flow cytometry. Our results are most consistent with those of Merkenschlager et al., who examined the fraction of preselection thymocytes that respond to MHC expressing cells (6). It should be noted that our method presumes that Bim deficiency rescues the majority of self-reactive T cells from clonal deletion. A recent study that examined mice that were deficient in both Bim and a related BH3-only family member, Puma, suggested that double deficiency rescued an even higher number of T cells from clonal deletion (22). It could be argued that the effect they observed largely reflects Puma mediated rescue from peripheral deletion rather than thymic, because the rescued cells in double deficient mice resembled recirculating T cells. Nonetheless, if true, the negative selection rate could be even higher than we estimate.
A high negative selection rate is consistent with the high level of MHC reactivity observed in hybridomas cloned from preselection cells (23) and with the large proportion of preselection cells that up-regulate CD69 when presented with MHC-bearing antigen presenting cells (6). These findings insinuate that T cells are inherently self-reactive. T-cell receptors do not have an obvious conservation of amino acids that contact MHC residues, yet close analysis of multiple structures suggests a potential germ-line bias toward MHC recognition (24–27). Germ-line encoded amino acids in the TCR were also demonstrated to influence thymic selection (25). Nonetheless, the idea of a germ-line bias for MHC reactivity continues to be debated (27, 28). However, almost all of the structures published so far involve TCRs that have survived normal negative selection (24). Thus, in the future it would be interesting to study TCRs cloned from cells destined for clonal deletion (GFPhi DP cells from Bim−/− mice) as these would represent the maximum reactivity of the TCR for MHC, and might have a greater potential to reveal germ-line bias in the TCR.
Previous models of T-cell selection suggested that TCR affinity for self can define which cells will become conventional CD4 T cells, Treg cells, or be deleted from the repertoire (29). Bim deficiency in our studies allowed for the survival of a huge population of self-reactive T cells. However, the number of Treg cells in Bim−/− mice was only modestly increased. These data support a previously published two-step model (30, 31) of Treg differentiation. In this model, to become a Treg cell, a clone would not only need to be highly self-reactive but also need a second signal such as IL-2 or IL-15. Therefore, the number of Treg cells developing in Bim−/− mice is potentially limited by the availability of the second signal. Interestingly, the level of GFP in cells rescued from negative selection was only very slightly higher than the level observed in Treg cells, suggesting that affinity thresholds are unlikely to be a major fate determinant between Treg development and negative selection. This finding is also consistent with a recent study that examined ovalbumin reactive TCRs in RIP-mOVA mice (32). They found that the propensity for negative selection versus Treg cell differentiation increased with TCR reactivity, although there was substantial overlap within the same clone.
Our data show that Bim deficiency led to a build up of self-reactive T cells in the thymus. Some of these self-reactive T cells were exported to the peripheral lymphoid tissues where they remained GFPhi. Nevertheless, Bim−/− mice are relatively healthy and only some develop autoimmunity later in life (9). Our data would suggest that peripheral tolerance mechanisms are highly effective in keeping self-reactive T cells in check. Using markers that have been previously described to be expressed on “anergic” CD4 T cells, we showed that Bim-deficient mice had a two- to threefold increase in the number of anergic CD4 T cells compared with control mice (21). Anergy is one potential mechanism that allows self-reactive T cells to exist in the periphery without causing autoimmune diseases.
Overall, our data show that a surprisingly high number of developing thymocytes are self-reactive and undergo deletion in the thymus. Furthermore, the rate of negative selection is much higher than the rate of positive selection. Together, our data support a model where a majority of developing thymocytes are self-reactive and deleted by an efficient process in the thymus thus providing protection against the development of autoimmunity.
Methods
Mice.
Nur77GFP reporter mice were described (8). Nur77GFP mice were crossed to _bcl2l11_−/− (Bim-deficient) mice provided by Andreas Strasser (Walter and Eliza Hall Institute, Melbourne, Australia). Controls used were Nur77GFP Bim+/+ or Nur77GFP Bim+/−. All mice were on a C57BL/6NCr (B6) background from the National Cancer Institute Animal Production Program.
Flow Cytometry.
Surface staining of cells was performed using the indicated antibodies from eBioscience, BD, or BioLegend. Cells were incubated with antibodies for 20 min on ice. For intracellular Foxp3 staining, cells were stained using the eBioscience Foxp3 staining kit and manufacturer’s protocol. Data were collected using an LSR II instrument (BD Biosciences) and analyzed using FlowJo (Tree Star).
Immunofluorescence.
Thymi were harvested and placed immediately in 4% (vol/vol) PFA overnight. Tissues were then washed three times in PBS and placed in 15% (wt/vol) sucrose overnight. Following overnight incubation, tissue was put in O.C.T compound and frozen in 2-Methylbutane with dry ice. Tissue was stored in −80 °C until sections were cut. Frozen sections (10 μm) were cut using a CM1800 cryostat (Leica). Slides were dried for 30 min and then were immerged in 0.1% Triton-X100/PBS (PBST) for 5 min at room temperature. The sections were incubated in PBS with 3% BSA (PBSB) for 1 h at room temperature before incubation overnight with the primary antibody (anti-beta5t rabbit polyclonal antibody, MBL International) in PBSB at 4 °C. This was followed by washing in PBST and incubation with the secondary antibody (Alexa Fluor 555 Goat anti-rabbit IgG antibody, Invitrogen) in PBSB (1 h at room temperature). VECTASHIELD Mounting Media (Vector) was used for preventing photobleaching. Images were obtained using a microscope (DM5500B; Leica) with a camera (DFC 340FX; Leica) operating with the Leica Application Suite Advanced Fluorescence (LAS AF; Leica) software and analyzed using Photoshop (Adobe) software.
Active Caspase 3 Flow Cytometry.
Detection of active caspase 3 was done by flow cytometry. Cells were first surface stained as described above. Following surface staining, cells were fixed and permeablized using Cytofix/Cytoperm solution (BD Biosciences) on ice for 10 min followed by two washes with permeabilization buffer. Cells were then incubated with cleaved Caspase-3 (Asp175) antibody (Cell Signaling) at a concentration of 1:5 for 30 min at room temperature. After two additional washes, cells were stained with secondary antibody conjugated to Alexa Fluor 555 goat anti-rabbit IgG (Invitrogen) at a concentration of 1:10 for 30 min at room temperature. Samples were then processed using a LSR II (BD Biosciences) and data were analyzed with FlowJo software (Tree Star).
Relative Thymocyte Lifespan.
To determine the relative lifespan of WT and Bim−/− thymocytes, we sorted DP or SP thymocytes from WT and Bim−/− mice, and injected them intrathymically into B6.SJL (CD45.1; NCI) recipient mice, along with a sentinel or control population. See SI Text for more information.
Selection Rate Determination.
To determine positive and negative selection rates for DP thymocytes, we used the following equations:
- Positive selection: # GFPint A/t A (t = lifespan of the relevant population, see Table 1).
- Negative selection: (# GFPhi A′/t _A_′) − (# GFPhi A/t A). A represents the number of postselection DP cells, and was obtained from either control (A) or Bim−/− (A′) mice.
Separate calculations were made for three relevant populations: A = cortical postselection DP (CD69+TCRβ+CD4+CD8+), B = medullary CD4SP (CD4+CD8−CD25−TCRβ+), or C = medullary CD8SP (CD4−CD8+TCRβ+).
Statistical Analysis.
Prism (GraphPad software) was used to calculate statistical significance.
Supplementary Material
Supporting Information
Acknowledgments
We thank Marc Jenkins, Sara Hamilton, Cara Skon, Keli Holzapfel, Janelle Olson, and Amy Moran for helpful discussions and for reading the manuscript. This work was supported by National Institutes of Health Grants P01 AI35296 (to K.A.H. and D.L.M.), R37 AI39560 (to K.A.H.), and T32 CA009138 (to G.L.S.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Palmer E. Negative selection—clearing out the bad apples from the T-cell repertoire. Nat Rev Immunol. 2003;3(5):383–391. doi: 10.1038/nri1085. [DOI] [PubMed] [Google Scholar]
- 2.Scollay RG, Butcher EC, Weissman IL. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980;10(3):210–218. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- 3.Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372(6501):100–103. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 4.Laufer TM, DeKoning J, Markowitz JS, Lo D, Glimcher LH. Unopposed positive selection and autoreactivity in mice expressing class II MHC only on thymic cortex. Nature. 1996;383(6595):81–85. doi: 10.1038/383081a0. [DOI] [PubMed] [Google Scholar]
- 5.van Meerwijk JP, et al. Quantitative impact of thymic clonal deletion on the T cell repertoire. J Exp Med. 1997;185(3):377–383. doi: 10.1084/jem.185.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merkenschlager M, et al. How many thymocytes audition for selection? J Exp Med. 1997;186(7):1149–1158. doi: 10.1084/jem.186.7.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouillet P, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415(6874):922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 8.Moran AE, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208(6):1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouillet P, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286(5445):1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 10.McCaughtry TM, Baldwin TA, Wilken MS, Hogquist KA. Clonal deletion of thymocytes can occur in the cortex with no involvement of the medulla. J Exp Med. 2008;205(11):2575–2584. doi: 10.1084/jem.20080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liston A, et al. Impairment of organ-specific T cell negative selection by diabetes susceptibility genes: genomic analysis by mRNA profiling. Genome Biol. 2007;8(1):R12. doi: 10.1186/gb-2007-8-1-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baldwin TA, Hogquist KA. Transcriptional analysis of clonal deletion in vivo. J Immunol. 2007;179(2):837–844. doi: 10.4049/jimmunol.179.2.837. [DOI] [PubMed] [Google Scholar]
- 13.Derbinski J, Kyewski B. How thymic antigen presenting cells sample the body’s self-antigens. Curr Opin Immunol. 2010;22(5):592–600. doi: 10.1016/j.coi.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Hu Q, Sader A, Parkman JC, Baldwin TA. Bim-mediated apoptosis is not necessary for thymic negative selection to ubiquitous self-antigens. J Immunol. 2009;183(12):7761–7767. doi: 10.4049/jimmunol.0902181. [DOI] [PubMed] [Google Scholar]
- 15.Kovalovsky D, Pezzano M, Ortiz BD, Sant’Angelo DB. A novel TCR transgenic model reveals that negative selection involves an immediate, Bim-dependent pathway and a delayed, Bim-independent pathway. PLoS ONE. 2010;5(1):e8675. doi: 10.1371/journal.pone.0008675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fowlkes BJ, Edison L, Mathieson BJ, Chused TM. Early T lymphocytes. Differentiation in vivo of adult intrathymic precursor cells. J Exp Med. 1985;162(3):802–822. doi: 10.1084/jem.162.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bendelac A, Matzinger P, Seder RA, Paul WE, Schwartz RH. Activation events during thymic selection. J Exp Med. 1992;175(3):731–742. doi: 10.1084/jem.175.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsieh CS, Lee HM, Lio CW. Selection of regulatory T cells in the thymus. Nat Rev Immunol. 2012;12(3):157–167. doi: 10.1038/nri3155. [DOI] [PubMed] [Google Scholar]
- 19.Leung MW, Shen S, Lafaille JJ. TCR-dependent differentiation of thymic Foxp3+ cells is limited to small clonal sizes. J Exp Med. 2009;206(10):2121–2130. doi: 10.1084/jem.20091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bautista JL, et al. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat Immunol. 2009;10(6):610–617. doi: 10.1038/ni.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez RJ, et al. Arthritogenic self-reactive CD4+ T cells acquire an FR4hiCD73hi anergic state in the presence of Foxp3+ regulatory T cells. J Immunol. 2012;188(1):170–181. doi: 10.4049/jimmunol.1101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray DH, et al. The BH3-only proteins Bim and Puma cooperate to impose deletional tolerance of organ-specific antigens. Immunity. 2012;37(3):451–462. doi: 10.1016/j.immuni.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zerrahn J, Held W, Raulet DH. The MHC reactivity of the T cell repertoire prior to positive and negative selection. Cell. 1997;88(5):627–636. doi: 10.1016/s0092-8674(00)81905-4. [DOI] [PubMed] [Google Scholar]
- 24.Marrack P, Scott-Browne JP, Dai S, Gapin L, Kappler JW. Evolutionarily conserved amino acids that control TCR-MHC interaction. Annu Rev Immunol. 2008;26:171–203. doi: 10.1146/annurev.immunol.26.021607.090421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott-Browne JP, White J, Kappler JW, Gapin L, Marrack P. Germline-encoded amino acids in the alphabeta T-cell receptor control thymic selection. Nature. 2009;458(7241):1043–1046. doi: 10.1038/nature07812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stadinski BD, et al. A role for differential variable gene pairing in creating T cell receptors specific for unique major histocompatibility ligands. Immunity. 2011;35(5):694–704. doi: 10.1016/j.immuni.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Laethem F, et al. Deletion of CD4 and CD8 coreceptors permits generation of alphabetaT cells that recognize antigens independently of the MHC. Immunity. 2007;27(5):735–750. doi: 10.1016/j.immuni.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Huseby ES, et al. How the T cell repertoire becomes peptide and MHC specific. Cell. 2005;122(2):247–260. doi: 10.1016/j.cell.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Stritesky GL, Jameson SC, Hogquist KA. Selection of self-reactive T cells in the thymus. Annu Rev Immunol. 2012;30:95–114. doi: 10.1146/annurev-immunol-020711-075035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burchill MA, et al. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28(1):112–121. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28(1):100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee HM, Bautista JL, Scott-Browne J, Mohan JF, Hsieh CS. A broad range of self-reactivity drives thymic regulatory T cell selection to limit responses to self. Immunity. 2012;37(3):475–486. doi: 10.1016/j.immuni.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Egerton M, Scollay R, Shortman K. Kinetics of mature T-cell development in the thymus. Proc Natl Acad Sci USA. 1990;87(7):2579–2582. doi: 10.1073/pnas.87.7.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saini M, et al. Regulation of Zap70 expression during thymocyte development enables temporal separation of CD4 and CD8 repertoire selection at different signaling thresholds. Sci Signal. 2010;3(114):ra23. doi: 10.1126/scisignal.2000702. [DOI] [PubMed] [Google Scholar]
- 35.McCaughtry TM, Wilken MS, Hogquist KA. Thymic emigration revisited. J Exp Med. 2007;204(11):2513–2520. doi: 10.1084/jem.20070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information