The lymphatic vascular system in liver diseases: its role in ascites formation (original) (raw)
Abstract
The lymphatic system is part of the circulatory system and plays a key role in normal vascular function. Its failure plays a crucial role in the development and maintenance of various diseases including liver diseases. Lymphangiogenesis (the growth of lymphatic vessels) and changes in the properties of lymphatic vessels are associated with pathogenesis of tumor metastases, ascites formation, liver fibrosis/cirrhosis and portal hypertension. Despite its significant role in liver diseases and its importance as a potential therapeutic target for those diseases, the lymphatic vascular system of the liver is poorly understood. Therefore, how the lymphatic vascular system in general and lymphangiogenesis in particular are mechanistically related to the pathogenesis and maintenance of liver diseases are largely unknown. This article summarizes: 1) the lymphatic vascular system; 2) its role in liver tumors, liver fibrosis/cirrhosis and portal hypertension; and 3) its role in ascites formation.
Keywords: Lymphangiogenesis, Ascites, Portal hypertension, Nitric oxide
INTRODUCTION
A common and devastating complication of liver cirrhosis is the development of ascites. Most generally, ascites can be defined as the pathological accumulation of fluid in the peritoneal cavity. The lymphatic vascular system plays a critical role in ascites formation.1 The lymphatic vascular system removes interstitial fluid from tissues in the body and returns it to the bloodstream. This interstitial fluid is called lymph when it enters lymphatic capillaries. Lymphatic fluid resembles blood plasma in its composition and contains white blood cells. Failure of normal lymphatic function results in a build-up of interstitial fluid and can lead to the clinical manifestations such as lymphedema and ascites.1-4 While structural analyses of the lymphatic vascular system have been conducted extensively in the liver, its regulatory mechanisms are largely unknown. This article summarizes the lymphatic vascular system and its role in liver diseases with special attention to ascites formation.
THE LYMPHATIC VASCULAR SYSTEM
The lymphatic vascular system is essential for tissue fluid homeostasis, immune surveillance and lipid uptake in the gastrointestinal organs.2-4 Disruption of normal lymphatic vascular structure and function plays a crucial role in the pathogenesis of various disease conditions. In many forms of cancer, for example, metastatic spreading of tumor cells often occurs through the lymphatic vascular network. Furthermore, an impairment of its function causes lymphedema and ascites. Thus, understanding the regulatory mechanisms of the lymphatic vascular system is important for the development of therapeutic strategies in these pathological conditions. In this section, general knowledge of the lymphatic vascular system is presented.
The structure of the lymphatic vascular system
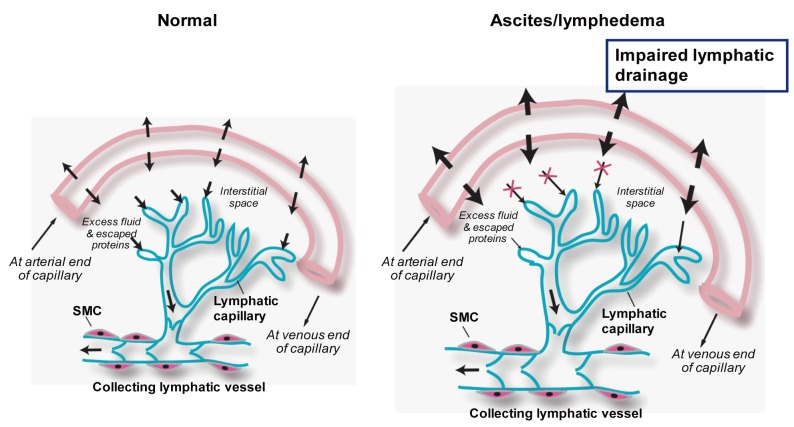
The lymphatic vascular system consists of closed-ended capillaries and larger collecting lymphatic vessels (also called collecting ducts) (Fig. 1). The lymphatic capillaries are composed of a single layer of lymphatic endothelial cells and absence of a continuous basement membrane. Smooth muscle cells and pericytes (i.e., the smooth muscle-like contractile cells that wrap around the outer surface of blood vessels) are absent in the terminal lymphatic capillaries but are present in the collecting lymphatic vessels.5 Patency of the lymphatic capillaries is maintained by wispy connective tissue fibers that attach to surrounding connective tissue. Whether cirrhosis results in disruption of these connective tissue fibers, thereby disrupting patency of lymphatic vessels is unknown.
Figure 1.
Lymphatic vascular systems in normal and pathological conditions. A filtrate (essentially plasma) escapes from blood vessels into the interstitial space of the surrounding tissues. The lymphatic vascular system removes this excess interstitial fluid and returns it to the bloodstream. The lymphatic vascular system consists of closed-ended capillaries and larger collecting lymphatic vessels. The lymphatic capillaries are composed of a single layer of lymphatic endothelial cells and absence of a continuous basement membrane, making it highly permeable to fluid and macromolecules. Smooth muscle cells (SMCs) are absent in the lymphatic capillaries but are present in the collecting lymphatic vessels. The collecting lymphatic vessels have intraluminal valves that prevent lymphatic backflow. Insufficient lymphatic drainage results in lymphedema and ascites.
Regulation of lymph flows
The lymphatic capillaries first gather into precollecting lymphatic vessels, which merge into larger collecting lymphatic vessels. The collecting lymphatic vessels are covered by smooth muscle cells, which provide contractile activity to assist the flow of lymph, and possess a continuous basement membrane. The lymph collected in the collecting lymphatic vessels is drained into the thoracic duct and is then returned to the blood circulation through the lymphatic-blood vessel connections at the junction of the jugular and subclavian veins.
When the surrounding interstitial pressure changes, these lymphatic vessels either expand and fill with lymph or contract and push lymph.6 The contractility derived by surrounding smooth muscle cells of the collecting lymphatic vessels has been proposed as one of the major driving forces of the lymphatic circulation.7 Nitric oxide (NO) produced in lymphatic endothelial cells is also thought to contribute to the regulation of lymphatic flows by modulating the contractility of smooth muscle cells.8 Ribera et al9 reported increased endothelial NO synthase (eNOS) levels in endothelial cells isolated from mesenteric lymphatic vessels of cirrhotic rats generated by carbon tetrachloride (CCl4) inhalation. They also showed decreased smooth muscle cell coverage in mesenteric lymphatic vessels in those rats. This decrease in smooth muscle cell coverage was reversed by a treatment with a NOS inhibitor, suggesting that NO produced by lymphatic endothelial cells inhibits smooth muscle cell coverage of mesenteric lymphatic vessels in cirrhotic rats. Thus, similar to the case of blood vessels, NO decreases contractility as well as recruitment of smooth muscle cells in lymphatic vessels.
THE LYMPHATIC VASCULAR SYSTEM IN LIVER DISEASES
The liver produces a large amount of lymph, which is estimated to be 25 to 50% of the lymph flowing through the thoracic duct.10 Similar to those in other organs, lymphatic vessels in the liver function to retain fluid and regulate the immune system. The hepatic lymph derives primarily from the hepatic sinusoids, and to a lesser extent from the peribiliary plexus. Fluid filtered out of the sinusoids into the space of Disse flows through the channels traversing the limiting plate either independently of blood vessels or along blood vessels and enters the interstitial space of either portal tract or sublobular veins. Fluid in the space of Disse also flows through similar channels traversing the hepatocytes intervening between the space of Disse and the hepatic capsule and drains into the interstitial space of the capsule. Fluid and migrating cells in the interstitial space pass through prelymphatic vessels to finally enter lymphatic vessels. The hepatic lymphatic system is an integral part of the liver microcirculation. Studies have shown that significant structural changes of lymphatic vessels occur in liver diseases. This section discusses a potential role of the lymphatic vascular system in the formation of liver tumors, fibrosis/cirrhosis and portal hypertension, starting with identification of lymphatic vessels.
Identification of lymphatic vessels in the liver
Markers that distinguish lymphatic vessels from blood vessels are critical to understand lymphatic vessel formation and function in the liver.11 These markers include vascular endothelial growth factor receptor-3 (VEGFR3)/FLT4, lymphatic vessel endothelial hyaluronan receptor (LYVE)-1, Prox-1 (a transcription factor), podoplanin, macrophage mannose receptor 1, Chemokine (C-C motif) ligand 2 (CCL21), Desmoplakin, Plakoglobin and integrin α9 (see review by Adams and Alitalo).12 However, these proteins are not exclusively found in lymphatic vessels and their expression patterns may differ depending on the disease type (e.g., fibrosis vs. tumor). For example, VEGFR3/FLT4, which is also a receptor for VEGF-C and D, was originally described as restricted to adult lymphatic endothelial cells, but later found in angiogenic blood vessels of retinas, wounds and tumors, and fenestrated capillaries of normal tissues.13 LYVE-1, a CD44 homologue, is frequently used as a marker of lymphatic vessels. However, Mouta et al11 demonstrated that LYVE-1 is not exclusive to lymphatic vessels. Indeed, LYVE-1 is also present in normal liver sinusoidal endothelial cells and Kupffer cells in mice and humans.11 Surprisingly, LYVE-1 is absent from angiogenic blood vessels of human liver tumors and only weakly present in the microcirculation of regenerative hepatic nodules in cirrhosis, although both vessels are largely derived from liver sinusoids. A combination of lymphatic markers, such as Prox-1 or podoplanin, with LYVE-1, may be necessary for accurate identification of lymphatic vessels.
Liver tumors and lymphangiogenesis
By double immunostaining LYVE-1 and Prox-1, Mouta et al showed in human HCC liver specimens that lymphatic vessels are more developed in fibrous areas that circumscribe tumors but absent within the core of tumors.11 It was also reported that LYVE-1 and Prox1-positive lymphatic vessels are abundant in the immediate vicinity of HCC and liver metastasis.11 HCCs expressing VEGF-C are more liable to metastasis.14 Since VEGF-C is known to enhance new lymphatic vessel formation, i.e., lymphangiogenesis, these observations may indicate that metastasis is associated with increased VEGF-C expression and resulting increased lymphangiogenesis.15
Fibrosis and lymphangiogenesis
The number of lymphatic vessels increases in fibrotic/cirrhotic livers.11,16,17 Previous studies of experimental fibrosis have shown that VEGF levels are increased in regions of fibrosis but whether VEGF-C expression specifically is elevated remains unknown.18
Both the number and area of lymphatic vessels are positively correlated with the severity of fibrosis around the portal tracts of human liver specimens.16 Similarly, in cirrhotic rats generated by CCl4 inhalation, increased lymphatic vessels (podoplanin positive vessels) were observed in portal areas.17 Interestingly, a significant increase in VEGF-D expression, another inducer of lymphangiogenesis, was observed in cirrhotic livers. In addition, VEGF-D expression was positively correlated with the progression of liver fibrosis. Collectively, these observations in both rats and humans suggest that lymphatic vessel enlargement and density coincides with the severity of liver fibrosis/cirrhosis. The molecular mechanisms underlying the increased lymphatic vessel formation in fibrosis/cirrhosis are largely unknown.
Portal hypertension and lymphangiogenesis
An increase in portal lymph flow occurs in liver fibrosis/cirrhosis.19,20 Barrowman and Granger21 showed a 30-fold increase in lymph flow from cirrhotic livers of rats and a positive correlation of hepatic lymph flow with increasing portal pressures. Most remarkably, they showed that in cirrhotic livers the functional capacity of lymphatic vessels to absorb interstitial fluid was compromised. Oikawa et al22 reported that the area of portal lymphatic vessels is increased in idiopathic portal hypertension. Collectively, it is speculated that the new lymphatic vessels are formed in cirrhosis, which could accommodate increased lymphatic flows. This compensatory lymphangiogenic response may help to reduce the high portal pressure observed in idiopathic portal hypertension as well as portal hypertension that develops with liver cirrhosis.
ASCITES FORMATION AND THE LYMPHATIC VASCULAR SYSTEM
Failure of the lymphatic vascular system is commonly manifested in the development of ascites. An impairment of lymphatic drainage and a build-up of interstitial fluid are direct causes of ascites formation. Although ascites is often related to chronic liver disease, the lymphatic vascular system in the liver can be overwhelmed in diseases where injury to the liver occurs secondarily to processes that originate elsewhere or primarily affect non-parenchymal cells.1-4 Therefore, this section is particularly dedicated to the formation of cirrhotic ascites and non-cirrhotic ascites in relation to the lymphatic vascular system.
Cirrhotic ascites
In cirrhotic patients, interstitial fluid is increased. The lymphatic vascular system reabsorbs excess fluid in the liver and splanchnic region and helps to prevent ascites formation. Consequently, lymph flows in the liver are increased, which stimulates hepatic lymphangiogenesis.20 Ascites is developed due to increased intravascular hydrostatic pressure, enlarged splanchnic arterial vasculature and decreased plasma oncotic pressure.23 These changes in pressure and structure of the vasculature facilitate excessive fluid filtration, which is exacerbated by the concomitant transformation of the hepatic microvasculature into a capillarized and defenestrated endothelium,24,25 which is often observed in liver cirrhosis. As liver cirrhosis progresses, the antidiuretic and antinatriuretic properties of compensatory neuroendocrine systems, which are activated secondary to arterial vasodilation, worsen the edematous condition and contributes to ascites formation.1 However, the role of the lymphatic vascular system in the pathogenesis of ascites remains to be fully elucidated.
NON-MALIGNANT HEPATIC ASCITES
A common cause of ascites originating from a non-hepatic disease is cardiac ascites. Typically classified as a 'post-sinusoidal' cause of ascites, a myriad of cardiac diseases comprising right-sided heart failure can result in hepatic congestion. These include constrictive pericarditis, valvular heart disease (especially tricuspid regurgitation), cor pulmonale, cardiomyopathy, and others. Tricuspid valve dysfunction and regurgitation, in particular, results in severe hepatic congestion because right ventricular pressures are directly transmitted to the draining vessels of the liver.26 Elevated pressures directly affect the hepatic veins and small venules that drain the hepatic acini. This forces protein-rich fluid into the space of Disse due, in part, to the inability of hepatic lymphatics to compensate for the increase in interstitial fluid. This protein-rich fluid is in contrast to the protein-poor content fluid that develops in liver cirrhosis.
A clinical picture similar to that seen in the development of cardiac ascites is the hepatic venous outflow obstruction known eponymously as Budd-Chiari Syndrome (BCS).27 Causes of BCS include myeloproliferative disorders such as polycythemia vera and hypercoagulable states that stem from diseases such as systemic lupus erythematosis (SLE) and paroxysmal nocturnal hemoglobinuria. Hepatic venous outflow can also occur from disorders that predispose to thrombosis formation such as anti-thrombin III, Protein S and C deficiencies, Factor V Leiden mutations, drugs such as oral contraceptives, and others. In addition, the inciting injury in the sinusoidal obstruction syndrome (previously known as veno-occlusive disease) originates in the sinusoidal endothelial cells but eventually involves the hepatic veins resulting in a vascular outflow obstruction pattern.28 In these conditions, it is likely that elevated pressures within the space of Disse overwhelm the capacity of hepatic lymphatics to carry interstitial fluid back into the systemic circulation. Whether compensatory lymphangiogenesis occurs in these conditions and can be manipulated to increase the functional capacity to reasborb fluid, however, remains unknown.
Malignancy-related hepatic ascites
The presence of metastatic lesions to the liver can lead to ascites without the development of cirrhosis.29,30 Massive liver infiltration from metastases can lead to portal hypertension and ascites formation. Lymphomas can disrupt the normal lymphatics with resulting ascites that is often chylous in nature. In these conditions, the normal lymphatic flow is disrupted by either tumor infiltration directly in the lymph vessels or in the outflow vascular tracts thereby resulting in increased fluid within the space of Disse and the accompanying inability of existing lymphatics to absorb this fluid.
CONCLUSION
Lymphangiogenesis is associated with tumor metastasis and increases in liver fibrosis. It is, however, not known whether or how increased lymphangiogenesis contributes to the development of liver fibrosis. Since an increase in hepatic lymphatic vessels occurs in cirrhotic rats with portal hypertension, it is speculated that increased lymphatic vessels may be a compensatory response to alleviate elevated portal pressures by handling the increased lymphatic flow that derived from increased perfusion. These observations linking the lymphatic vascular system to liver diseases indicate its potential as a therapeutic target. How the lymphatic vascular system in general and lymphangiogenesis in particular are mechanistically related to the pathogenesis and maintenance of those liver diseases is largely unknown and warrants future investigation.
Acknowledgement
This work was supported by grants R01DK082600 from the National Institutes of Health (Iwakiri) and VA merit award (Chung).
Abbreviations
BCS
Budd-Chiari Syndrome
CCl4
carbon tetrachloride
eNOS
endothelial nitric oxide synthase
LYVE-1
lymphatic vessel endothelial hyaluronan receptor
NO
nitric oxide
SMC
smooth muscle cell
VEGFR3
vascular endothelial growth factor receptor-3
Footnotes
The authors have no conflicts to disclose.
References
- 1.Cardenas A, Bataller R, Arroyo V. Mechanisms of ascites formation. Clin Liver Dis. 2000;4:447–465. doi: 10.1016/s1089-3261(05)70118-5. [DOI] [PubMed] [Google Scholar]
- 2.Harvey NL, Oliver G. Choose your fate: artery, vein or lymphatic vessel? Curr Opin Genet Dev. 2004;14:499–505. doi: 10.1016/j.gde.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Oliver G, Alitalo K. The lymphatic vasculature: recent progress and paradigms. Annu Rev Cell Dev Biol. 2005;21:457–483. doi: 10.1146/annurev.cellbio.21.012704.132338. [DOI] [PubMed] [Google Scholar]
- 4.Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 5.Schmid-Schonbein GW. Microlymphatics and lymph flow. Physiol Rev. 1990;70:987–1028. doi: 10.1152/physrev.1990.70.4.987. [DOI] [PubMed] [Google Scholar]
- 6.Leak LV, Burke JF. Fine structure of the lymphatic capillary and the adjoining connective tissue area. Am J Anat. 1966;118:785–809. doi: 10.1002/aja.1001180308. [DOI] [PubMed] [Google Scholar]
- 7.Aukland K, Reed RK. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993;73:1–78. doi: 10.1152/physrev.1993.73.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Hagendoorn J, Padera TP, Kashiwagi S, Isaka N, Noda F, Lin MI, et al. Endothelial nitric oxide synthase regulates microlymphatic flow via collecting lymphatics. Circ Res. 2004;95:204–209. doi: 10.1161/01.RES.0000135549.72828.24. [DOI] [PubMed] [Google Scholar]
- 9.Ribera J, Pauta M, Melgar-Lesmes P, Tugues S, Fernández-Varo G, Held KF, et al. Increased nitric oxide production in lymphatic endothelial cells causes impairment of lymphatic drainage in cirrhotic rats. Gut. 2013;62:138–145. doi: 10.1136/gutjnl-2011-300703. [DOI] [PubMed] [Google Scholar]
- 10.Barrowman JA. Hepatic lymph and lymphatics. In: McIntyre N, Benhamou JP, Bircher J, Rizzetto M, editors. Oxford textbook of clinical hepatology. New York: Oxford University Press; 1991. pp. 37–40. [Google Scholar]
- 11.Mouta Carreira C, Nasser SM, di Tomaso E, Padera TP, Boucher Y, Tomarev SI, et al. LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res. 2001;61:8079–8084. [PubMed] [Google Scholar]
- 12.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 13.Partanen TA, Arola J, Saaristo A, Jussila L, Ora A, Miettinen M, et al. VEGF-C and VEGF-D expression in neuroendocrine cells and their receptor, VEGFR-3, in fenestrated blood vessels in human tissues. FASEB J. 2000;14:2087–2096. doi: 10.1096/fj.99-1049com. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi R, Yano H, Nakashima O, Akiba J, Nishida N, Kurogi M, et al. Expression of vascular endothelial growth factor-C in human hepatocellular carcinoma. J Gastroenterol Hepatol. 2006;21:152–160. doi: 10.1111/j.1440-1746.2005.04217.x. [DOI] [PubMed] [Google Scholar]
- 15.Ohtani O, Ohtani Y. Lymph circulation in the liver. Anat Rec (Hoboken) 2008;291:643–652. doi: 10.1002/ar.20681. [DOI] [PubMed] [Google Scholar]
- 16.Yamauchi Y, Michitaka K, Onji M. Morphometric analysis of lymphatic and blood vessels in human chronic viral liver diseases. Am J Pathol. 1998;153:1131–1137. doi: 10.1016/S0002-9440(10)65657-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tugues S, Morales-Ruiz M, Fernandez-Varo G, Ros J, Arteta D, Muñoz-Luque J, et al. Microarray analysis of endothelial differentially expressed genes in liver of cirrhotic rats. Gastroenterology. 2005;129:1686–1695. doi: 10.1053/j.gastro.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Corpechot C, Barbu V, Wendum D, Kinnman N, Rey C, Poupon R, et al. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology. 2002;35:1010–1021. doi: 10.1053/jhep.2002.32524. [DOI] [PubMed] [Google Scholar]
- 19.Ludwig J, Linhart P, Baggenstoss AH. Hepatic lymph drainage in cirrhosis and congestive heart failure. A postmortem lymphangiographic study. Arch Pathol. 1968;86:551–562. [PubMed] [Google Scholar]
- 20.Witte MH, Dumont AE, Cole WR, Witte CL, Kintner K. Lymph circulation in hepatic cirrhosis: effect of portacaval shunt. Ann Intern Med. 1969;70:303–310. doi: 10.7326/0003-4819-70-2-303. [DOI] [PubMed] [Google Scholar]
- 21.Barrowman JA, Granger DN. Effects of experimental cirrhosis on splanchnic microvascular fluid and solute exchange in the rat. Gastroenterology. 1984;87:165–172. [PubMed] [Google Scholar]
- 22.Oikawa H, Masuda T, Sato S, Yashima A, Suzuki K, Sato S, et al. Changes in lymph vessels and portal veins in the portal tract of patients with idiopathic portal hypertension: a morphometric study. Hepatology. 1998;27:1607–1610. doi: 10.1002/hep.510270621. [DOI] [PubMed] [Google Scholar]
- 23.Henriksen JH, Møller S. Alterations of hepatic and splanchnic microvascular exchange in cirrhosis: local factors in the formation of ascites. In: Gines P, Arroyo V, Rodes J, Schrier R, editors. Dysfunction in Liver Disease: Pathogenesis, Diagnosis, and Treatment. Second Edition. MA: Blackwell Publishing; 2005. pp. 174–185. [Google Scholar]
- 24.Bhunchet E, Fujieda K. Capillarization and venularization of hepatic sinusoids in porcine serum-induced rat liver fibrosis: a mechanism to maintain liver blood flow. Hepatology. 1993;18:1450–1458. [PubMed] [Google Scholar]
- 25.Mori T, Okanoue T, Sawa Y, Hori N, Ohta M, Kagawa K. Defenestration of the sinusoidal endothelial cell in a rat model of cirrhosis. Hepatology. 1993;17:891–897. [PubMed] [Google Scholar]
- 26.Hou W, Sanyal AJ. Ascites: diagnosis and management. Med Clin North Am. 2009;93:801–817. doi: 10.1016/j.mcna.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Plessier A, Rautou PE, Valla DC. Management of hepatic vascular diseases. J Hepatol. 2012;56(Suppl 1):S25–S38. doi: 10.1016/S0168-8278(12)60004-X. [DOI] [PubMed] [Google Scholar]
- 28.DeLeve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease) Semin Liver Dis. 2002;22:27–42. doi: 10.1055/s-2002-23204. [DOI] [PubMed] [Google Scholar]
- 29.Almakdisi T, Massoud S, Makdisi G. Lymphomas and chylous ascites: review of the literature. Oncologist. 2005;10:632–635. doi: 10.1634/theoncologist.10-8-632. [DOI] [PubMed] [Google Scholar]
- 30.Sultan S, Pauwels A, Poupon R, Levy VG. Chylous ascites in cirrhosis. Retrospective study of 20 cases. Gastroenterol Clin Biol. 1990;14:842–847. [PubMed] [Google Scholar]