Lck availability during thymic selection determines the recognition specificity of the T cell repertoire (original) (raw)
. Author manuscript; available in PMC: 2014 Sep 12.
Summary
Thymic selection requires signaling by the protein tyrosine kinase Lck to generate T cells expressing αβ T cell antigen receptors (TCR). For reasons not understood, the thymus selects only αβTCR that are restricted by major histocompatibility complex (MHC) encoded determinants. Here, we report that Lck proteins that were coreceptor-associated promoted thymic selection of conventionally MHC-restricted TCR, but Lck proteins that were coreceptor-free promoted thymic selection of MHC-independent TCR. Transgenic TCR with MHC-independent specificity for CD155 utilized coreceptor-free Lck to signal thymic selection in the absence of MHC, unlike any transgenic TCR previously described. Thus, the thymus can select either MHC-restricted or MHC-independent αβTCR depending on whether Lck is coreceptor-associated or coreceptor-free. We conclude that the intracellular state of Lck determines the specificity of thymic selection, and that Lck association with coreceptor proteins during thymic selection is the mechanism by which MHC restriction is imposed on a randomly generated αβTCR repertoire.
Introduction
Antigen receptors on cells of the adaptive immune system must be capable of recognizing existing pathogens as well as new pathogens that will arise in the future. To do so, T and B lymphocytes use gene recombination to randomly generate antigen receptors with hugely diverse recognition specificities (Davis and Bjorkman, 1988). Although generated by the same recombination machinery, antigen receptors on mature T and B lymphocytes recognize fundamentally different types of antigenic ligands. Antigen receptors on B lymphocytes recognize conformational epitopes on native antigenic proteins and glycolipids, as do antigen receptors on the minor subset of T cells bearing γδTCR (Chien and Konigshofer, 2007). However, antigen receptors on the major subset of T cells bearing αβTCR do not recognize conformational antigenic epitopes but instead recognize linear peptide fragments of antigenic proteins bound to MHC encoded determinants, the feature of antigen recognition referred to as MHC restriction (Davis and Bjorkman, 1988). MHC restriction is unique to αβTCR and allows identification of cells containing intracellular pathogens, foreign proteins, or genetic mutations. As a result, MHC restriction is critical for T lymphocyte recognition and function, but how it is imposed on a randomly generated αβTCR repertoire remains a major unsolved problem.
Two different explanations have been proposed for the exclusive expression of MHC-restricted αβTCR on mature T cells. The germline model of MHC restriction proposes that MHC restriction is intrinsic to germline encoded αβTCR structural elements (Feng et al., 2007; Garcia et al., 2009; Marrack et al., 2008; Scott-Browne et al., 2009). According to the germline model, specific amino acids in the complementary determining regions (CDR) 1 and 2 of TCRα and TCRβ have been conserved during evolution because they contact MHC chains and impose MHC specificity on αβTCR recognition. Consequently, αβTCR are limited by germline imposed structural constraints to be MHC-specific and to bind only to MHC-dependent ligands, with the exception of a few exceedingly rare αβTCR that cross-reactively bind an MHC-independent ligand with very low affinity (Barnd et al., 1989; Hanada et al., 2011; Rao et al., 1984). In contrast to the germline model, the selection model of MHC restriction proposes that MHC restriction is the result of TCR-signaled thymic selection and is not an intrinsic feature of αβTCR structure (Collins and Riddle, 2008; Tikhonova et al., 2012; Van Laethem et al., 2007; Van Laethem et al., 2012). According to the selection model, αβTCR are randomly generated in the thymus so that pre-selection CD4+CD8+ (double positive, DP) thymocytes express αβTCR with a huge diversity of recognition specificities. However, only MHC-restricted αβTCR signal DP thymocytes to undergo thymic selection because CD4 and CD8 coreceptors on DP thymocytes sequester the signaling protein tyrosine kinase (PTK) p56lck (Lck) (Haughn et al., 1992) so that only αβTCR with the same MHC specificity as either CD4 or CD8 coreceptor proteins can access Lck (Doyle and Strominger, 1987; Norment et al., 1988).
Lck is a Src family PTK that is expressed in all T lineage cells and inserts in the inner leaf of their plasma membrane as a result of being myristilated or palmitoylated at its amino terminus (Paige et al., 1993). Membrane Lck initiates TCR signal transduction by first phosphorylating signaling motifs in the TCR complex and then phosphorylating ZAP-70 PTK molecules that are recruited into the TCR complex by the phosphorylated signaling motifs (Abraham et al., 1991; Chan et al., 1992; Gascoigne and Palmer, 2011; Nika et al., 2010). Although p59fyn (Fyn) can also initiate TCR signaling, Lck is the PTK that initiates TCR signaling in the vast majority of developing thymocytes (Palacios and Weiss, 2004). Notably, membrane Lck has two cytosolic cysteines that mediate non-covalent interactions with cysteines in the cytosolic tails of CD4 and CD8 (Rudd et al., 1988; Shaw et al., 1989; Turner et al., 1990; Veillette et al., 1988). As a result, membrane Lck can be either coreceptor-associated or coreceptor-free if it is not bound to CD4 or CD8. In pre-selection DP thymocytes, membrane Lck is overwhelmingly coreceptor-associated because of the large quantities of both CD4 and CD8 coreceptors expressed, with the result that αβTCR signaling in DP thymocytes is only initiated by co-engagement of TCR with coreceptor proteins (Wiest et al., 1996).
The selection model of MHC restriction predicts that MHC-independent αβTCR would signal thymic selection and generate an MHC-independent peripheral αβTCR repertoire if pre-selection thymocytes contained coreceptor-free Lck instead of coreceptor-associated Lck. This prediction was tested in Quad-KO mice that were simultaneously both MHC-deficient (b2m−/−H-2Ab−/−) and coreceptor-deficient(Cd4−/−Cd8−/−) because Quad-KO thymocytes contained Lck that was necessarily coreceptor-free (Van Laethem et al., 2007). Thymocytes in Quad-KO mice were found to be signaled in vivo and many thymocytes were signaled intensely enough to undergo clonal deletion. Bcl-2 transgene (Bcl-2Tg) expression prevented clonal deletion in Quad-KO mice and resulted in large numbers of peripheral T cells expressing αβTCR with MHC-independent recognition specificities (Tikhonova et al., 2012; Van Laethem et al., 2007). Two MHC-independent αβTCR were cloned from Quad-KO.Bcl-2Tg mice and displayed antibody-like recognition properties that fundamentally differed from conventionally MHC-restricted TCR. The two MHC-independent αβTCR did not recognize peptide/MHC (pMHC) complexes but instead bound to conformational epitopes on the membrane protein CD155, and they did so independently of MHC with an affinity that resembled that of primary antibodies (Tikhonova et al., 2012).
The generation in Quad-KO mice of peripheral T cells bearing MHC-independent αβTCR fulfilled predictions of the thymic selection model and contradicted requirements of the germline model. Nevertheless, central issues related to MHC-independent thymic selection were not resolved, including the ligand-dependence of thymic selection by coreceptor-free Lck.
We undertook the present study to evaluate the impact of Lck availability on thymic selection and to assess the differentiation of MHC-independent αβTCR in the thymus. We now report that generation of an MHC-independent αβTCR repertoire only required mutation of the two cysteines in Lck's N-terminal region that promote association with CD4 and CD8 coreceptors in the thymus. We also demonstrate that, unlike coreceptor-associated Lck which promoted thymic selection of MHC-restricted αβTCR, coreceptor-free Lck preferentially promoted thymic selection of MHC-independent αβTCR. Moreover we characterize thymic selection of two MHC-independent transgenic αβTCR specific for different epitopes on the self-protein CD155. Thymic selection of both MHC-independent αβTCR utilized coreceptor-free Lck, did not require MHC expression, but strictly required ligand engagement. Thus this study demonstrates that thymic selection of either MHC-independent or MHC-restricted αβTCR depends on the presence or absence of coreceptor-free Lck, and it is Lck's association with CD4/CD8 coreceptor proteins during thymic selection that is responsible for thymic selection of an exclusively MHC-restricted αβTCR repertoire.
Results
Signaling and selection in the thymus by coreceptor-free Lck
MHC restriction is the cardinal feature of αβTCR selected in the thymus but its basis remains uncertain. The Lck sequestration model of thymic selection (Van Laethem et al., 2007) proposes that MHC restriction is not intrinsic to αβTCR structure but is the result of thymic selection (Fig. S1). According to this hypothesis, αβTCR specific for MHC-independent ligands fail to transduce positive selection signals because Lck is bound to the cytosolic tails of CD4/CD8 coreceptor proteins which sequester Lck away from all TCR except those that engage peptide-MHC (pMHC) ligands together with CD4/CD8 coreceptor proteins (Fig. S1). A prediction of the Lck sequestration model is that coreceptor-free Lck would promote signaling by MHC-independent TCR in the thymus and selection of MHC-independent αβT cells in the periphery.
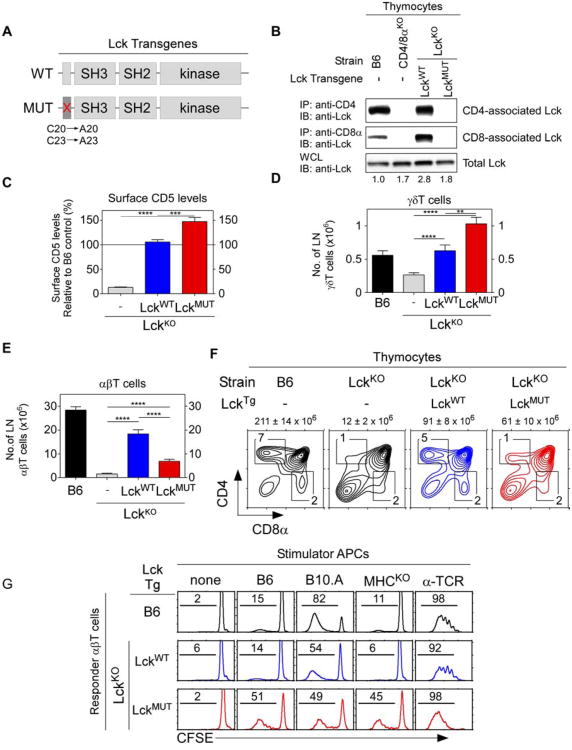
To determine if coreceptor-free Lck promoted thymic selection of MHC-independent αβTCR, we constructed hCD2-driven transgenes encoding either wildtype Lck proteins (Lckwt) or mutant Lck proteins (Lckmut) whose cysteines at positions 20 and 23 were converted to alanines to impair binding to coreceptor proteins (Fig. 1A) (Turner et al., 1990). Both Lck transgenes were introduced into LckKO mice to generate mice referred to as LckwtTg and LckmutTg mice, respectively. Immunoblotting of thymocyte lysates revealed that LckmutTg thymocytes contained Lck in amounts that were intermediate between B6 and LckwtTg mice (Fig. 1B). Immunoprecipitation of thymocyte lysates with coreceptor-specific antibodies further revealed that Lckmut proteins bound neither CD4 nor CD8, unlike Lckwt proteins which bound to both CD4 and CD8 (Fig. 1B). Nevertheless, CD5 surface expression was restored in LckKO mice by both Lckmut and Lckwt transgenic proteins, with LckmutTg thymocytes expressing higher CD5 levels as an indication of stronger in vivo signaling by Lckmut Tg transgenic proteins (Fig. 1C).
Figure 1. Effect of coreceptor-free and coreceptor-associated Lck on thymic selection.
(A) hCD2-driven transgenic constructs encoding wildtype (top) and C20A/C23A mutant (bottom) Lck proteins.
(B) Abrogation of Lck binding to coreceptor proteins. Thymocyte lysates were precipitated with anti-CD4 or anti-CD8α and blotted for Lck. Numbers indicate total Lck band intensities relative to B6 (set equal to 1.0). Data represent 4 experiments.
(C) CD5 levels on thymocytes relative to control B6 mice (mean ± SE, n=7 mice/group).
(D and E) Numbers of γδ (D) and αβ (E) LN T cells (mean ± SE, n=9 mice/group).
(F) Numbers above profiles indicate thymus cellularity (mean ± SE, n=10mice/group).
(G) Ligand specificity of αβT cells in mixed lymphocyte reactions as assessed byproliferation-induced CFSE dye dilution on day 4. Numbers represent percentage of cellsthat underwent at least one division. Data represent 3 experiments.
**** P<0.0001; *** P<0.001; ** P<0.01.
See also Figures S1 and S2.
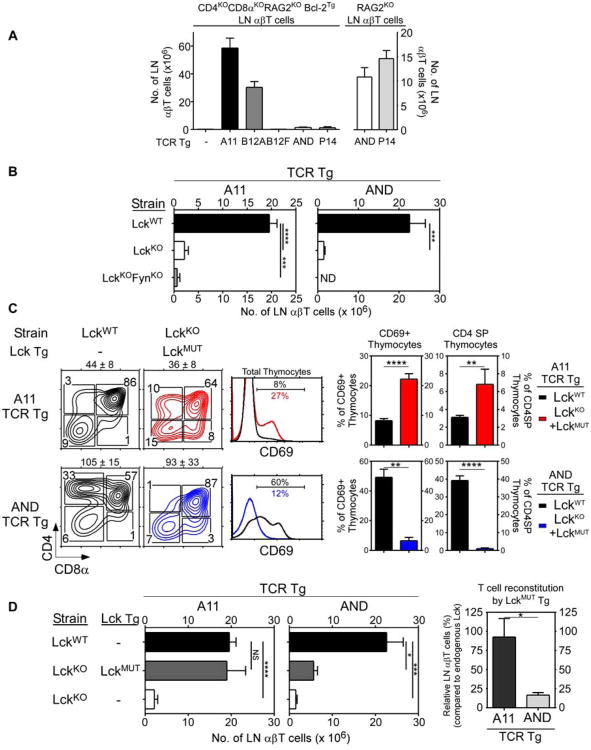
To assess the function of Lck transgenic proteins in thymic selection, we examined γδ and αβ T cell generation (Fig. 1D,E). γδTCR arise at the DN stage of thymocyte development when CD4/CD8 coreceptors are not expressed, so Lckwt and Lckmut proteins would both be available to γδTCR. Indeed γδT cell generation which was reduced in LckKOmice was restored by both Lckmut and Lckwt transgenic proteins, although significantly more γδT cells were present in Lckmut transgenic mice, another indication of stronger in vivo signaling by Lckmut transgenic proteins (Fig. 1D).
Unlike γδTCR, αβTCR are expressed at the DP stage of thymocyte differentiation when CD4/CD8 coreceptor proteins are present that bind Lck. Interestingly, αβT cell generation which was abrogated in LckKO mice was only poorly restored by Lckmut proteins compared to Lckwt proteins, with significantly fewer peripheral αβT cells and SP thymocytes in LckmutTg mice (Fig. 1E,F). Impaired αβT cell generation by Lckmut proteins contrasted markedly with robust γδT cell generation in the same mice and raised the possibility that αβT cells might have been signaled so strongly by coreceptor-free Lck that they underwent clonal deletion. However, introduction of the pro-survival Bcl-2Tg to impair in vivo clonal deletion (Pobezinsky et al., 2012) revealed that LckmutTg.Bcl-2Tg mice still contained fewer αβT cells and SP thymocytes than LckwtTg.Bcl-2Tg mice (Fig. S2 A,B). Consequently, coreceptor-free Lckmut proteins signaled the generation of fewer αβ T cells than coreceptor-associated Lck proteins despite stronger in vivo signaling.
We then assessed the specificity of peripheral αβT cells generated in LckmutTg mice in in vitro mixed lymphocyte reactions. Unlike B6 and LckwtTg αβT cells which only reacted against MHC-expressing stimulator cells, Lckmut αβT cells reacted vigorously against both MHC-deficient and MHC-expressing stimulator cells (Fig. 1G). That is, αβT cells generated by coreceptor-free Lck in LckmutTg mice expressed αβTCR that recognized ligands independently of MHC on MHC-deficient stimulator cells that were also present on MHC-expressing stimulator cells. These results demonstrate that coreceptor-free Lck during thymic selection is all that is required to generate an MHC-independent αβTCR repertoire.
Signaling of MHC-independent αβT cells in the thymus
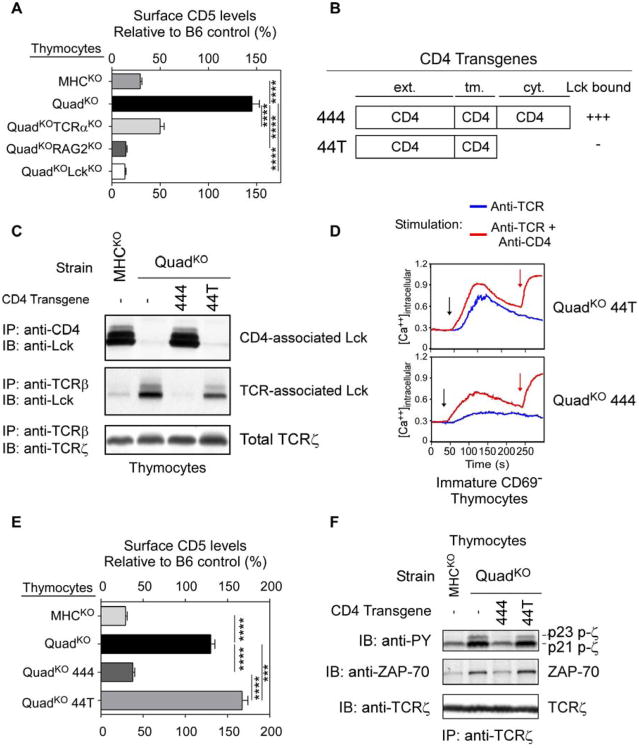
To further characterize MHC-independent αβTCR selection and signaling in the thymus, we examined thymocytes from so-called QuadKO mice that were MHC-deficient as well as deficient in both CD4 and CD8 coreceptors (Cd4−/−Cd8−/−b2m−/−Ab−/−)(Van Laethem et al., 2007). Endogenous Lck proteins in QuadKO thymocytes were necessarily coreceptor-free because coreceptor proteins were not expressed. As indicated by CD5 surface expression levels, coreceptor-deficient QuadKO thymocytes were strongly signaled in vivo while coreceptor-sufficient MHCKO thymocytes were not (Fig. 2A). Importantly, MHC-independent in vivo signaling of QuadKO thymocytes required αβTCR and Lck proteins, as deficiency in TCRα, RAG2, or Lck reduced or abolished CD5 up-regulation (Fig. 2A).
Figure 2. Lck sequestration and TCR signaling in the thymus.
(A) CD5 on thymocytes relative to B6 (set at 100%). Mean ± SE, n=3 mice/group.
(B) hCD2-driven transgenic constructs encoding wildtype (444) and tailless (44T) CD4 proteins named for their extra-cellular, transmembrane, and cytosolic domains.
(C) Lck is sequestered away from TCR on thymocytes by the cytosolic tail of coreceptor proteins. Thymocyte lysates were precipitated by anti-CD4 or anti-TCRβ and blotted for Lck or TCRζ. Data represent 3 experiments.
(D) In vitro TCR induced calcium mobilization. Thymocytes were loaded at 31oC with Indo-1 and coated with biotinylated anti-TCR (5μg/ml) either alone or together with biotinylated anti-CD4 (1μg/ml). Crosslinking by avidin (black arrow) and addition of ionomycin (red arrow) are indicated. Data represent 2 experiments.
(E) In vivo signaling of thymocytes. CD5 on thymocytes is shown relative tocontrol B6 mice (mean ± SE, n=10 mice/group).
(F) In vivo signaling of thymocytes assessed biochemically. Lysates wereprecipitated with anti-TCRζ and blotted for phospho-zeta, ZAP70, or TCRζ. Datarepresent 2 experiments.
**** P<0.0001; *** P<0.001.
Lck sequestration by coreceptor proteins on developing thymocytes
Because MHC-independent TCR signaling of QuadKO thymocytes was transduced by coreceptor-free Lck, we wished to determine biochemically if CD4/CD8 coreceptors, by binding Lck, would sequester Lck away from αβTCR. To do so, we introduced hCD2-driven CD4 transgenes encoding either full length (444) or tailless (44T) CD4 proteins (Van Laethem et al., 2007) into QuadKO mice to generate 444.QuadKO and 44T.QuadKO mice whose endogenous Lck proteins bound to 444 but not 44T proteins as revealed by anti-CD4 immunoprecipitation of thymocyte lysates (Fig. 2B,C). Most importantly, endogenous Lck could also be immunoprecipitated by anti-TCRβ but only in thymocytes in which Lck was not bound to CD4 coreceptor proteins (Fig. 2C), biochemically documenting that Lck binding to CD4 prevented Lck association with TCR.
Lck sequestration impairs coreceptor-independent TCR signaling
To determine the impact of Lck sequestration on thymocyte signaling, we compared TCR signaling in 444.QuadKO and 44T.QuadKO thymocytes. By in vitro assessment, 44T.QuadKO thymocytes with coreceptor-free Lck could be triggered to mobilize intracellular calcium by anti-TCR crosslinking alone (Fig. 2D top), whereas 444.QuadKO thymocytes lacking coreceptor-free Lck required anti-TCR/CD4 co-crosslinking (Fig. 2D bottom). These in vitro results demonstrated that Lck sequestration markedly impaired coreceptor-independent TCR signaling. By in vivo assessment, TCR signaling in MHC-deficient thymi was greater in 44T.QuadKO than 444.QuadKO thymocytes as revealed by dramatically higher CD5 expression, greater TCRζ phosphorylation, and greater amounts of TCR-associated ZAP-70 proteins (Fig. 2E,F).These in vivo results reveal that Lck sequestration markedly impairs TCR signaling in MHC-deficient thymi.
Thymic selection of MHC-independent TCR
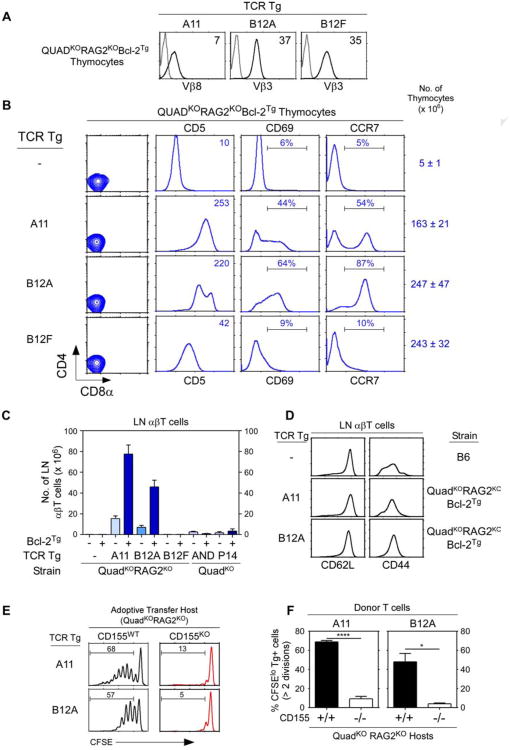
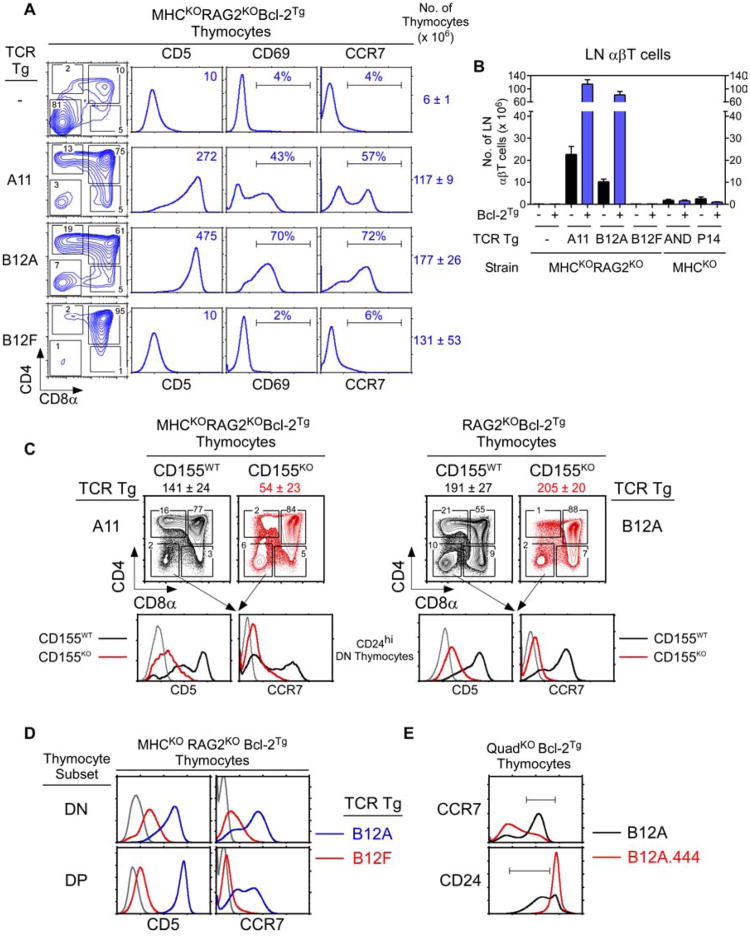
To better understand αβTCR signaling of thymic selection in the absence of MHC, we generated TCR transgenes encoding αβTCR from two T cell clones that were derived from QuadKOBcl-2Tg mice with specificity for an MHC-independent ligand (Fig. S3A) (Tikhonova et al., 2012). The T-hybridoma cells were derived from QuadKO mice expressing the Bcl-2Tg that protected strongly signaled MHC-independent αβTCR from clonal deletion (Van Laethem et al., 2007). Three hCD2-driven TCR transgenes were constructed from the A11 and B12 T-hybridoma cell lines described previously (Tikhonova et al., 2012) and are referred to as A11, B12A, and B12F. The A11 TCR was TCR-Vα8Vβ8, whereas B12A and B12F TCR were identically TCR-Vα4Vβ3 but with different VαJα-encoded CDR3 sequences (Fig. S3B). A11 and B12A TCR recognized different conformational epitopes on the self-ligand CD155, the murine analog of the human polio virus receptor, and bound to CD155 proteins independently of MHC with affinities that were unusually high for αβTCR (Tikhonova et al., 2012). However, the B12F TCR displayed no known ligand specificity (Fig. S3C). In vivo all three TCR transgenes (A11, B12A, and B12F) induced TCR expression on developing thymocytes (Fig. 3A).
Figure 3. Thymic selection of MHC-independent αβTCR in the absence of both MHC and coreceptors.
(A) TCR expression on thymocytes from transgenic host mice (QuadKORAG2KOBcl-2Tg). Black lines indicate TCR expression, grey lines indicate control staining. Numbers in panels indicate mean fluorescence intensity (MFI). Data represent 5 experiments.
(B) Thymocyte profiles from host transgenic mice expressing transgenic TCR. Numbers in CD5 histograms indicate CD5 MFI. Numbers in CD69 and CCR7 histogramsindicate frequency of positive cells. Thymus cellularity is shown as mean ± SE (n=5 mice/group).
(C) Numbers of αβ LN T cells in TCR transgenic mice (mean ± SE, n=5 mice/group).
(D) Expression of CD62L and CD44 on αβT cells from TCR transgenic mice. Data represent 3 experiments.
(E and F) Lymphopenia-induced proliferation triggered by A11 and B12A TCR is dependent on CD155 recognition. A11 and B12A αβT cells were CFSE labeled and injected (5×106 cells/mouse) into CD155+/+ or CD155−/− lymphopenic hosts for 1 week. Numbers in histograms (E) represent the percentage of cells that underwent at least two divisions and summarized in (F). Mean ± SE of 3 mice/group.
**** p<0.0001; * p<0.05.
See also Figure S3.
To determine if these TCR could signal positive selection in the absence of MHC, we expressed them in the same host mice from which they were originally derived, i.e. QuadKOBcl-2Tg mice, except that transgenic host mice were additionally RAG2-deficient to prevent endogenous TCR rearrangements (Fig. 3B). We refer to QuadKORagKOBcl-2Tg mice simply as ‘transgenic host mice’ and we observed that all three TCR reconstituted thymus cellularity, an indicator of pre-TCR signaling (Fig. 3B). Despite absent MHC and coreceptor expression, A11 and B12A TCR markedly up-regulated thymocyte expression of CD5, CD69, and CCR7 which indicated positive selection signaling, whereas B12F TCR only minimally up-regulated CD5 expression consistent with pre-TCR signaling (Fig. 3B). The inability of B12F TCR to markedly up-regulate CD5, CD69, and CCR7 expression in QuadKO thymocytes indicated that coreceptor-free Lck only transduced positive selection signals by TCR that engaged an intra-thymic ligand. Indeed, coreceptor-free Lck in QuadKO thymocytes did not transduce positive selection signals by either MHC-II restricted AND TCR or MHC-I restricted P14 TCR whose MHC ligands were not expressed in the thymus (Fig. S3D).
Because A11 and B12A TCR signaled positive selection in the absence of MHC, A11 and B12A TCR transgenic mice should contain mature αβT cells in the MHC-deficient periphery (Fig. 3C). In fact A11 and B12A TCR generated peripheral LNT cells in huge numbers (50-80 million) in transgenic host mice expressing Bcl-2Tg and in substantial numbers (10-20 million) without Bcl-2Tg (Fig. 3C). The LNT cells generated by A11 and B12A TCR displayed a CD44loCD62Lhi naïve phenotype, indicating that their huge numbers were due to extensive thymic selection and not to extensive peripheral expansion (Fig. 3D). In contrast, MHC-restricted AND and P14 TCR did not generate mature T cells in MHC- and coreceptor-deficient mice (Fig. 3C).
To document that A11 and B12A transgenic TCR displayed the same ligand specificity as the T-hybridomas from which they were derived (Tikhonova et al., 2012), we examined the ability of A11 and B12A TCR to trigger lymphopenia-induced T cell proliferation upon CD155 recognition. A11 and B12A LNT cells from transgenic host mice that were not lymphopenic were labeled with the intracellular dye CFSE and then transferred into lymphopenic host mice (QuadKORagKO) that either expressed or lacked CD155 (Fig. 3E,F). Assessed one week after transfer, both A11 and B12A T cells proliferated vigorously in CD155+/+ but not CD155−/− lymphopenic hosts (Fig. 3E,F), documenting that both transgenic TCR recognized CD155.
We conclude that A11 and B12A TCR transgenes encode MHC-independent and CD155-specific TCR that are uniquely capable of signaling positive selection in thymi that lack MHC expression, unlike any αβTCR previously identified.
Identification of the thymic selecting ligand
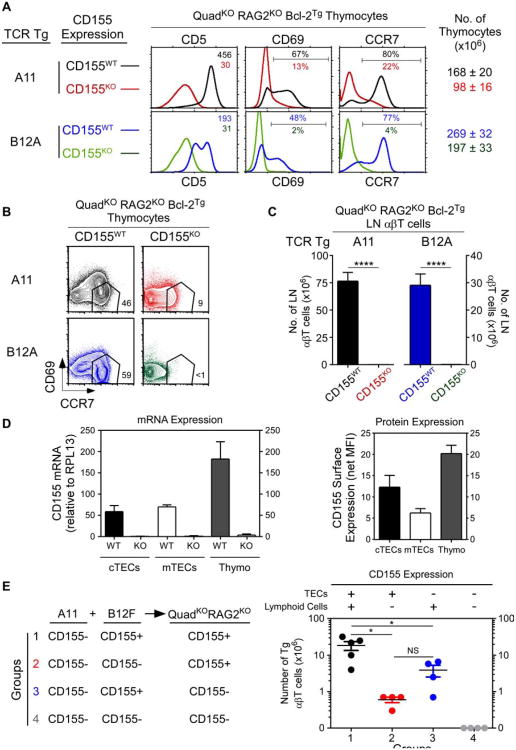
Having discovered transgenic αβTCR that signaled positive selection in MHC-deficient thymi, we wished to identify the selecting ligand responsible. Because CD155 was recognized by both A11 and B12A TCR and was a self-protein in host transgenic mice, we expressed A11 and B12A TCR in transgenic host mice in which thymocytes would either encounter CD155 or not. Interestingly, A11 and B12A TCR signaled positive selection only in CD155+/+ but not CD155−/− transgenic host mice as indicated by CD5, CD69, and CCR7 up-regulation (Fig. 4A) and by thymocyte differentiation into mature CD69−CCR7+ cells (Fig. 4B). Moreover, A11 and B12A TCR generated peripheral T cells only in CD155+/+ transgenic host mice, while CD155−/− transgenic host mice were devoid of peripheral T cells (Fig. 4C). Thus CD155 is the thymic selecting ligand for both A11 and B12A TCR.
Figure 4. Identification of CD155 as the thymic selecting ligand.
(A) In vivo positive selection signaling by A11 and B12A TCR requires CD155. Numbers in CD5 histograms indicate MFI. Numbers in CD69 and CCR7 histograms indicate frequency of positive cells. Thymus cellularity is shown as mean ± SE (n=5 mice/group).
(B) CD69 versus CCR7 profiles of A11 and B12A thymocytes. Numbers indicate frequency of mature CD69−CCR7hi thymocytes (n=5 mice/group).
(C) Numbers of αβ LN T cells (mean ± SE of 5 mice/group).
(D) CD155 expression by electronically sorted thymic elements by quantitativePCR (left panel) and surface protein expression (right panel). Mean ± SE (n=3).
(E) Thymic elements that induce CD155-specific thymic selection. Four groups ofmixed donor bone marrow chimeras were constructed with 1:1 mixtures of A11 andB12F donor cells and injected into irradiated host (QuadKORAG2KO) mice that expressedor lacked CD155. Numbers of A11 αβT cells in LN were determined after 8-10wks.Each circle represents one mouse (n=4).
* p <0.05; **** p<0.0001; NS, not significant.
See also Figure S4.
It was then important to determine which cells in the thymus expressed CD155. CD155 was expressed by all elements in the thymus (i.e. cortical thymic epithelial cells (cTECs), medullary thymic epithelial cells (mTECs), and thymocytes) but thymocytes expressed the most (Fig. 4D). To determine which thymic cellular element(s) induced positive selection signaling, we constructed four different mixed bone marrow (bm) chimeras in which CD155-deficient A11 thymocytes only encountered CD155 on other cells in the thymus. All four groups of chimeras were constructed by injecting equal mixtures of donor bm stem cells from A11 and B12F transgenic mice into irradiated QuadKORagKO host mice. LNT cells in chimeric host mice were examined 8-10 weeks later (Fig. 4E). It should be appreciated that B12F thymocytes could not contribute to peripheral T cell numbers which resulted entirely from positive selection by A11 TCR. We found the greatest numbers of peripheral T cells resulted from CD155 expressed on both bm derived elements and TEC (Fig. 4E group 1), followed by CD155 expressed only on bm derived cells (Fig. 4E group 3), followed by CD155 expressed only on TEC (Fig. 4E group 2). Thus the number of peripheral A11 T cells corresponded to the overall amount of CD155 expressed in the thymus, and did not correspond to CD155 expression on any particular thymic element.
Because ligands on lymphoid elements in the thymus have been shown to positively select innate-like αβT cells that are characterized by expression of the transcription factor PLZF (Constantinides and Bendelac, 2013; Lee et al., 2011), we assessed CD155-selected αβT cells for expression of Zbtb16, the gene that encodes PLZF, and for Sox13, a gene expressed by many γδ-lineage T cells (Melichar et al., 2007). In fact CD155-selected A11 and B12A T cells neither expressed Zbtb16 nor Sox13 (Fig. S4).
We conclude that CD155 is the thymic selecting ligand for A11 and B12A TCR, and that A11 and B12A peripheral T cells are neither innate-like nor γδ-like T cells.
Effect of coreceptor expression on MHC-independent selection
Our results demonstrated that αβTCR can engage MHC-independent ligands in the thymus and can utilize coreceptor-free Lck to signal positive selection. We then asked if thymocyte expression of CD4 and CD8 coreceptor proteins would affect selection signaling by MHC-independent transgenic TCR.
We introduced A11, B12A, and B12F TCR transgenes into coreceptor-sufficient (Cd4+/+Cd8+/+) mice that were MHCKORAGKOBcl-2Tg (Fig. 5A). Contrary to our expectation, A11 and B12A transgenic TCR did signal positive selection in coreceptor-sufficient mice as indicated by marked up-regulation of CD5, CD69, and CCR7 (Fig. 5A). Indeed, A11 and B12A TCR signaled coreceptor-sufficient thymocytes to differentiate into SP thymocytes that were mostly CD4SP (Fig. 5A). MHC-independent positive selection signaling by A11 and B12A TCR also generated peripheral LN T cells in huge numbers (70-100 million) with Bcl-2Tg and in substantial numbers (10-20 million) without Bcl-2Tg (Fig. 5B). In contrast, AND and P14 MHC-restricted TCR did not generate T cells in MHC-deficient mice despite CD4/CD8 coreceptor expression (Fig. 5B).
Figure 5. Positive selection signaling by MHC-independent TCR in coreceptor-sufficient mice lacking MHC.
(A) Thymocyte profiles. Numbers in CD4 vs CD8α plots indicate cell frequencies. Numbers in CD5 histograms indicate MFI. Numbers in CD69 and CCR7 histograms indicate frequency of positive cells. Thymus cellularity is shown as mean ± SE (n=6 mice/group).
(B) Numbers of αβ LN T cells (mean ± SE, n=6 mice/group).
(C) Positive selection signaling occurs in DN thymocytes and is ligand specific. CD4 vs CD8α profiles (top) and CD5 and CCR7 expression on CD24hiDN thymocytes (bottom). Data represent 4 experiments.
(D) Early positive selection signaling is TCR specific. CD5 and CCR7 expression on gated DN and DP thymocytes. Data represent 4 experiments.
(E) Early positive selection signaling is inhibited by early CD4 expression. CCR7 and CD24 expression on thymocytes from B12A and B12A.444 transgenic mice. Data represent 4 individual mice/group.
See also Figure S5.
To understand MHC-independent A11 and B12A TCR signaling of thymocytes in coreceptor-sufficient mice, we considered that transgenic αβTCR differed from endogenous αβTCR in first being expressed on thymocytes at the DN stage of differentiation when Lck was necessarily coreceptor-free. Consequently, we wondered if A11 and B12A transgenic TCR initiated positive selection signaling at the DN stage of thymocyte differentiation. To identify positive selection signaling in TCR transgenic DN thymocytes, we decided on three criteria that would distinguish positive selection from pre-TCR signaling: (a) marked up-regulation of both CD5 and CCR7, because pre-TCR signaling minimally upregulates only CD5; (b) ligand-dependence, because pre-TCR signaling is ligand-independent; and (c) TCR specificity, because pre-TCR signaling is induced by transgenic αβTCR regardless of recognition specificity.
We observed that A11 and B12A TCR signaling in immature HSAhiDN thymocytes markedly up-regulated expression of both CD5 and CCR7 (Fig. 5C); such up-regulation occurred in CD155+/+ but not CD155−/− mice, and so was ligand-dependent (Fig. 5C); and such up-regulation did not occur with B12F TCR and so was TCR-specific (Fig. 5D). Thus, A11 and B12A TCR had indeed initiated positive selection signaling in DN thymocytes. Interestingly, thymocytes signaled by A11 and B12A TCR underwent progressive changes in CD4/CD8 and CD69/CCR7 expression expected of thymocytes signaled to undergo positive selection by conventional αβTCR (Fig. S5A). Moreover, like peripheral CD4 and CD8 T cells selected by conventional αβTCR, A11 and B12A CD4 T cells expressed the helper-lineage genes Zbtb7b and Cd40l, while A11 and B12A CD8 T cells expressed the cytotoxic-lineage genes Runx3d and Tbx21(Fig. S5B). Thus, thymocyte differentiation and lineage specification signaled by MHC-independent αβTCR resembled that of conventional αβTCR.
We think that positive selection signaling by A11 and B12A TCR is initiated in DN thymocytes that had already been pre-TCR signaled to differentiate into DP cells, and that positive selection signaling continues until coreceptor proteins are expressed in sufficient amounts to sequester Lck away from the TCR. As confirmation that positive selection signaling by transgenic TCR was initiated in DN thymocytes, DP thymocytes in B12A transgenic mice were not pre-selection CD5loCCR7− cells as in unsignaled B12F transgenic mice, but instead were CD5hiCCR7+ cells that had received positive selection signals at their prior (i.e. DN) stage of thymocyte development (Fig. 5D). And as confirmation that Lck sequestration by coreceptor proteins could inhibit early positive selection signaling by transgenic TCR, prematurely-expressed CD4 transgenes inhibited generation of mature (CCR7hiCD24lo) thymocytes in B12A transgenic mice (Fig. 5E). Interestingly, early Lck sequestration by 444 CD4 transgenic proteins also inhibited γδ T cell generation in non-TCR transgenic mice (Fig. S5C).
We conclude that positive selection signaling by MHC-independent TCR is mediated by coreceptor-free Lck which, in coreceptor-sufficient mice, is present in DN and early (i.e. coreceptor-low) DP thymocytes.
Lck availability and the specificity of positive selection
Because coreceptor-free Lck transduced positive selection signals by MHC-independent TCR, we wished to determine if coreceptor-free Lck also transduced positive selection signals by MHC-restricted TCR. To do so, we first examined MHC-sufficient mice whose thymocytes were coreceptor-deficient so that endogenous Lck would be coreceptor-free (Fig. 6A). Coreceptor-deficiency did not interfere with peripheral T cell generation by A11 and B12A MHC-independent TCR, but it abrogated peripheral T cell generation by MHC-restricted AND and P14 TCR despite Bcl-2Tg expression (Fig. 6A left). Note that AND and P14 TCR did generate peripheral T cells in coreceptor-sufficient mice (Fig. 6A right). Thus, unlike positive selection signaling by MHC-independent TCR, positive selection signaling by MHC-restricted TCR required coreceptor proteins.
Figure 6. Effect on thymic selection of coreceptor-associated and coreceptor-free Lck.
(A) Numbers of αβ LN T cells in coreceptor-deficient (left side) or coreceptor-sufficient (right side) mice (mean ± SE, n=3 mice/group).
(B) Numbers of αβ LN T cells in kinase-deficient mice (mean ± SE, n=3 mice/group). ND, not done.
(C) Profiles of thymocytes with endogenous Lck (black lines) or Lckmut (colored lines) proteins. Numbers in CD4 vs CD8α plots indicate cell frequencies. Numbers in CD69 histograms indicate frequency of positive cells and is summarized in right panels (mean ± SE, n=3 mice/group).
(D) Numbers of αβ LN T cells in TCR transgenic mice with endogenous Lck (black bar) or Lckmut (gray bar) proteins. Reconstitution by Lckmut proteins relative to endogenous Lck proteins (set at 100%) is displayed in the right panel (mean ± SE, n=3 mice/group).
* p<0.05; **, p<.01; ***, p<0.001; ****, p<0.0001; NS, not significant.
To determine if the requirement by MHC-restricted TCR for coreceptor proteins reflected, at least partly, a requirement for coreceptor-associated Lck, we introduced A11 and AND transgenic TCR into Lck−/− mice that expressed coreceptor proteins as well as MHC. Relative to Lck+/+ mice, peripheral T cell generation by A11 and AND TCR were both significantly reduced in Lck−/− mice (Fig. 6B). The few peripheral T cells present in Lck−/− mice were generated by Fyn, as A11 Lck−/−Fyn−/− mice were essentially devoid of T cells (Fig. 6B). We then reconstituted A11.Lck−/− and AND.Lck−/− mice with coreceptor-free Lckmut transgenic proteins (see Fig. 1A,B) and compared positive selection signaling by Lckmut and endogenous Lck proteins as indicated by CD69 up-regulation and CD4SP thymocyte generation (Fig. 6C). Coreceptor-free Lckmut proteins were far more effective than endogenous Lck+/+ proteins in transducing positive selection signals by MHC-independent A11 TCR, whereas Lckmut proteins were far less effective for MHC-restricted AND TCR (Fig. 6C). In fact, coreceptor-free Lckmut proteins fully restored peripheral T cell generation in Lck−/− mice by A11 TCR (>95%), but only minimally restored peripheral T cell generation by AND TCR (<20%) (Fig. 6D). Thus coreceptor-free Lck was optimal for positive selection signaling by MHC-independent A11 TCR but was only minimally effective for positive selection signaling by MHC-restricted AND TCR.
We conclude that coreceptor-free Lck preferentially promotes positive selection signaling by MHC-independent TCR, whereas coreceptor-associated Lck is required for positive selection signaling by MHC-restricted TCR.
Structural TCR constraints on MHC-independent thymic selection
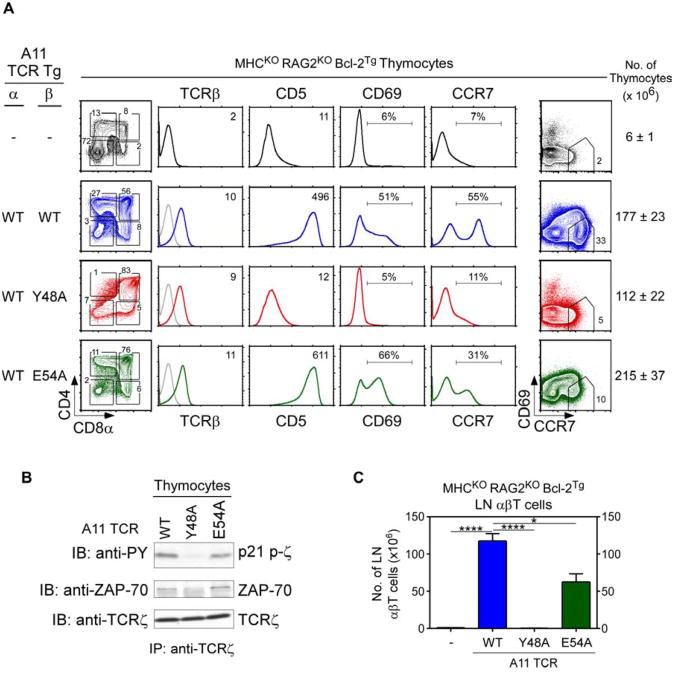
The germline concept of MHC restriction proposed that germline-encoded amino acid (aa) residues in CDR1 and CDR2 of TCRα and TCRβ have been evolutionarily conserved to engage MHC molecules and to impose MHC specificity on thymic selection (Garcia et al., 2009; Marrack et al., 2008; Scott-Browne et al., 2009). A prediction of the germline concept is that evolutionarily conserved CDR2 aa would not promote TCR selection by MHC-independent ligands. To test this prediction, we constructed hCD2-driven transgenes in which the CDR2 region of the A11 TCR-Vβ8 chain contained a point mutation in an evolutionarily conserved aa, either Y48 or E54. A11 TCR variants (referred to as A11WT, A11Y48A, and A11E54A) were introduced into MHC-deficient (MHCKORAGKOBcl-2Tg) host mice that were CD155+/+ and all three TCR variants were expressed at comparable surface levels (Fig. 7A). All three TCR variants reconstituted the cellularity of host thymi and induced the generation of DP thymocytes, indicative of pre-TCR signaling (Fig. 7A). However, positive selection signaling which was robust in A11WT thymocytes was absent in A11Y48A and reduced in A11E54A thymocytes (Fig. 7A). A11Y48A thymocytes did not up-regulate CD5, CD69, or CCR7 (Fig. 7A middle panels); did not differentiate into CD4SP cells (Fig. 7A left panel); and did not differentiate into mature CD69−CCR7+ cells (Fig. 7A right panel). Biochemical analysis revealed that in vivo TCR signaling was absent in A11Y48A thymocytes as TCRζ was neither phosphorylated nor associated with ZAP-70 (Fig. 7B). In the lymphoid periphery, peripheral T cells were absent in A11Y48A and reduced in A11E54A transgenic mice (Fig. 7C). These results reveal that the evolutionarily conserved Y48 aa residue, and to a lesser degree the E54 aa residue, contributed to CD155-specific positive selection signaling by A11 TCR, even though MHC recognition was not involved.
Figure 7. Role of evolutionarily conserved CDR2β residues in thymic selection by an MHC-independent TCR.
(A) Thymocyte profiles from MHC-deficient mice expressing A11 TCR whose TCRβ chain was wildtype or contained a point mutation in CDR2, either Y48A or E54A. Numbers in TCRβ and CD5 histograms indicate MFI. Numbers in CD69 and CCR7 histograms indicate frequency of positive cells. Numbers in CD69 vs CCR7 plots indicate frequency of mature cells. Thymus cellularity is shown as mean ± SE (n=4 mice/group).
(B) Assessment of in vivo signaling of thymocytes. Unstimulated thymocyte lysates were precipitated with anti-TCRζ and blotted for phospho-zeta, ZAP70, or TCRζ. Data represent 2 experiments.
(C) LN αβT cell numbers (mean ± SE, n=4 mice/group).
* p<0.05; ****, p<0.0001.
Discussion
The present study demonstrates that thymic selection imposes MHC-restriction on a randomly generated αβTCR repertoire that is not intrinsically MHC-specific. It was specifically Lck's availability during thymic selection that determined if the thymus selected a mature αβTCR repertoire that was MHC-restricted or selected one that was MHC-independent. Coreceptor-associated Lck promoted thymic selection of conventionally MHC-restricted αβTCR, but coreceptor-free Lck preferentially promoted thymic selection of MHC-independent αβTCR. This study also characterized two unique transgenic TCR, A11 and B12A, that utilized coreceptor-free Lck to signal positive selection and to generate mature αβT cells in the absence of MHC, unlike any αβTCR previously described. The selecting ligand in the thymus for both TCR was the self-ligand CD155 for which these MHC-independent TCR had high affinity (Tikhonova et al., 2012). Finally, the evolutionarily conserved and germline-encoded TCR-Vβ8 residues Y48 and E54 in CDR2β were shown to be necessary for MHC-independent thymic selection of A11, suggesting that these TCR residues might have been conserved during evolution for reasons other than MHC recognition. Thus, the thymus can select either MHC-restricted or MHC-independent TCR, and it is Lck's association with CD4/CD8 coreceptor proteins on pre-selection thymocytes that limits the thymus to selecting an exclusively MHC-restricted αβTCR repertoire.
Coreceptor-associated and coreceptor-free Lck are alternative states of membrane Lck that were mutually exclusive in pre-selection DP thymocytes. Association with CD4 and CD8 coreceptors physically sequestered membrane Lck away from αβTCR, documenting that membrane Lck molecules in pre-selection DP thymocytes were present in insufficient amounts to bind to coreceptor proteins as well as TCR complexes. Because membrane Lck bound to the cytosolic tails of CD4 and CD8 coreceptors whose external domains interacted with MHC-II and MHC-I determinants, respectively, DP thymocytes could only be signaled by αβTCR that engaged intra-thymic pMHC ligands together with one or the other coreceptor, whereas coreceptor-free Lck was available to all αβTCR. In fact, when pre-selection thymocytes contained coreceptor-free Lck, either because of mutation of Lck's N-terminal domain cysteines that eliminated coreceptor binding or because of absent coreceptor protein expression, MHC-independent αβTCR were able to signal thymic selection and generate an MHC-independent αβTCR repertoire. Thus, this study establishes that MHC-independent αβTCR do exist in the thymus but are normally prevented from signaling thymic selection by Lck sequestration.
This analysis leads to the novel insight that TCR gene rearrangements may be specifically timed during normal development to determine the recognition specificities of the TCR expressed by mature γδ and αβ T cells. TCR-γ,-δ, and -β gene loci rearrange at the DN stage of thymocyte differentiation but TCR-α gene loci do not rearrange until the DP stage. As a result, γδTCR and preTCR complexes (consisting of TCRβ and invariant preTα chains) are formed prior to CD4/CD8 coreceptor expression on DN thymocytes which contain coreceptor-free Lck, while αβTCR complexes are formed after CD4/CD8 coreceptor expression on DP thymocytes that contain coreceptor-associated Lck. In fact we suggest that the timing of endogenous γδ and αβ TCR expression has specifically evolved to allow different TCR complexes selective access to either coreceptor-free or coreceptor-associated Lck, so that ligand recognition by γδTCR would be mainly MHC-independent and ligand recognition by αβTCR would be MHC-restricted.
This study also characterized two different transgenic αβTCR that signaled thymic selection in the absence of MHC, demonstrating the existence of αβTCR that do not require MHC for thymic selection. Even though these TCR were MHC-independent, thymic selection signaling by A11 and B12A TCR strictly required engagement of a unique selecting ligand in the thymus that we identified as the self-protein CD155. Identification of CD155 as the unique thymic selecting ligand for A11 and B12A TCR contrasts with the inability to identify unique thymic selecting ligands for all but one conventionally MHC restricted TCR (Lo et al., 2009). We suspect this is because A11 and B12A bind to CD155 with relatively high affinity - and therefore with a high degree of specificity - as a result of their binding to ligand independently of coreceptors. In contrast, we think that MHC-restricted TCR bind to their thymic pMHC selecting ligands with extremely low affinity - and therefore with a low degree of specificity - because these TCR must co-engage those ligands together with coreceptor proteins in order to access coreceptor-associated Lck molecules for transduction of thymic selection signals.
Affinity considerations also explain why coreceptor-free Lck proteins preferentially promoted thymic selection of MHC-independent rather than MHC-restricted TCR. While coreceptor-free Lck was available to all αβTCR, coreceptor-free Lck promoted thymic selection signaling by MHC-independent A11 TCR much more efficiently than MHC-restricted AND TCR. In our view, coreceptor-free Lck is inefficient in promoting TCR signal transduction because coreceptor-free Lck must be passively captured within micro-clusters of ligand-engaged αβTCR, whereas CD4/CD8 coreceptors bring coreceptor-associated Lck directly into micro-clusters of pMHC-engaged αβTCR (Holdorf et al., 2002). As a result, signaling by coreceptor-free Lck requires αβTCR with significantly higher ligand affinities than signaling by coreceptor-associated Lck. In fact the two MHC-independent αβTCR characterized in this study bind their MHC-independent selecting ligand CD155 with high affinity that is in the 150-200 nanomolar range. Consequently, we think that coreceptor-free Lck preferentially promotes thymic selection of MHC-independent αβTCR because MHC-independent αβTCR bind their selecting ligand in the thymus with relatively high affinity while MHC-restricted αβTCR bind their selecting ligands in the thymus with very low affinity.
Affinity considerations can also explain why coreceptor-free Lck proteins generated fewer polyclonal αβT cells than coreceptor-associated Lck proteins. This experimental observation may appear discordant with the a priori expectation that coreceptor-free Lck would promote signaling by αβTCR with a broader range of ligand specificities than coreceptor-associated Lck. However, αβTCR that bind thymic selecting ligands with sufficiently high affinity to utilize coreceptor-free Lck would be less frequent than αβTCR that bind thymic selecting ligands with the much lower affinity needed to utilize coreceptor-associated Lck. Consequently, we do not think that the fewer polyclonal αβT cells generated by coreceptor-free than coreceptor-associated Lck is indicative of the relative numbers of MHC-independent and MHC-restricted αβTCR expressed in the pre-selection repertoire.
Positive selection signaling by MHC-independent αβTCR in the thymus resembled that of MHC-restricted αβTCR in their requirements for the evolutionarily conserved Y48 and E54 residues in the CDR2 region of Vβ8 TCR (Feng et al., 2007; Garcia et al., 2009; Marrack et al., 2008). Positive selection signaling by a transgenic MHC-restricted Vβ8 TCR was previously shown to require Y48 and E54, arguably because of their roles as MHC contact residues that promoted TCR engagement of pMHC selecting ligands in the thymus and fulfilling a prediction of the germline model of MHC restriction (Scott-Browne et al., 2009). However, the current study showed that the transgenic MHC-independent A11 Vβ8 TCR also required Y48 and E54 for optimal positive selection signaling, even though MHC was not involved. Reciprocally, it was recently shown that an MHC-restricted TCR repertoire could still be generated without evolutionarily conserved germline-encoded CDR1 and CDR2 sequences (Holland et al., 2012). We suggest that germline-encoded CDR1 and CDR2 amino acid residues may have been conserved in evolution for reasons unrelated to their role as MHC contact residues but possibly related to their role in maintaining the integrity of the TCR combining site.
Because thymic differentiation of MHC-independent αβTCR has not previously been characterized, it is worth noting that, because transgenic MHC-independent αβTCR are expressed in DN thymocytes and signal in the absence of coreceptors, transgenic MHC-independent αβTCR initiated positive selection signaling in thymocytes at the DN stage of differentiation. However, we think that DN thymocytes that were signaled to undergo positive selection had already been signaled by their pre-TCR to differentiate into DP thymocytes, in which case positive selection signaling continued until coreceptor protein expression was high enough to sequester Lck and extinguish further MHC-independent TCR signaling. Nevertheless, thymocytes signaled by transgenic MHC-independent αβTCR underwent similar changes in CD4/CD8 and CD69/CCR7 expression in the thymus as thymocytes signaled by conventional MHC-restricted αβTCR, and gave rise to similar CD4/helper- and CD8/cytotoxic-lineage T cells in the periphery. Thus, thymocyte development and lineage-specification appear to progress identically regardless of the phenotypic stage in thymocyte differentiation that αβTCR-mediated positive selection signaling occurs.
In conclusion, this study has documented the existence of MHC-independent αβTCR in the thymus and identified the basis for thymic selection of an MHC-restricted αβTCR repertoire. This study has also demonstrated that generation of an unconventional MHC-independent αβTCR repertoire expressed by peripheral T cells requires the presence of coreceptor-free Lck during thymic selection. As a result, TCR gene rearrangements appear to be carefully timed during development in the thymus so that γδTCR specifically encounter coreceptor-free Lck and αβTCR specifically encounter coreceptor-associated Lck so that T cells expressing these different TCR complexes will primarily recognize MHC-independent and MHC-restricted ligands, respectively.
Experimental Procedures
Animals
New hCD2-driven transgenes constructed for this study encoded: wildtype or mutant Lck (Lckwt and Lckmut); αβTCR (A11, B12A, and B12F); and αβTCR with CDR2β point mutations (A11Y48 and A11E54). For a description of all strains see Extended Experimental Procedures. Animal care was in accordance with National Institutes of Health (NIH) guidelines.
Antibodies and reagents
For a detailed list see Extended Experimental Procedures.
Flow cytometry and cell sorting
See Extended Experimental Procedures for details.
Calcium mobilization and protein immunoblotting
Assays were performed as described (Van Laethem et al., 2007). For details see Extended Experimental Procedures.
Construction of bone marrow chimeras
RAGKO host mice were irradiated with 6 Gy and reconstituted with 107 T-depleted donor bm cells.
Statistical analyses
Student's t-test with two-tailed distributions was used for statistical analyses. P values of 0.05 or less were considered significant.
Supplementary Material
1
2
Highlights.
- Lck sequestration during thymic selection is the basis for T cell MHC restriction
- Coreceptor-independent Lck generates an MHC-independent TCR repertoire
- Identification of a thymic selecting ligand for MHC-independent TCR
- Differentiation of transgenic αβTCR in the absence of thymic MHC
Acknowledgments
We thank Richard Hodes and Hyun Park for critically reading the manuscript; Joy Williams for thymic epithelial cell preparations; and Peter Sun and Jinghua Lu for helpful discussion. This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham N, Miceli MC, Parnes JR, Veillette A. Enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine protein kinase p56lck. Nature. 1991;350:62–66. doi: 10.1038/350062a0. [DOI] [PubMed] [Google Scholar]
- Acuto O, Cantrell D. T cell activation and the cytoskeleton. Annu Rev Immunol. 2000;18:165–184. doi: 10.1146/annurev.immunol.18.1.165. [DOI] [PubMed] [Google Scholar]
- Barnd DL, Lan MS, Metzgar RS, Finn OJ. Specific, major histocompatibility complex-unrestricted recognition of tumor-associated mucins by human cytotoxic T cells. Proc Natl Acad Sci U S A. 1989;86:7159–7163. doi: 10.1073/pnas.86.18.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AC, Iwashima M, Turck CW, Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992;71:649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- Chien YH, Konigshofer Y. Antigen recognition by gammadelta T cells. Immunological reviews. 2007;215:46–58. doi: 10.1111/j.1600-065X.2006.00470.x. [DOI] [PubMed] [Google Scholar]
- Collins EJ, Riddle DS. TCR-MHC docking orientation: natural selection, or thymic selection? Immunologic research. 2008;41:267–294. doi: 10.1007/s12026-008-8040-2. [DOI] [PubMed] [Google Scholar]
- Constantinides MG, Bendelac A. Transcriptional regulation of the NKT cell lineage. Curr Opin Immunol. 2013;25:161–167. doi: 10.1016/j.coi.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Doyle C, Strominger JL. Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature. 1987;330:256–259. doi: 10.1038/330256a0. [DOI] [PubMed] [Google Scholar]
- Feng D, Bond CJ, Ely LK, Maynard J, Garcia KC. Structural evidence for a germline-encoded T cell receptor-major histocompatibility complex interaction ‘codon’. Nat Immunol. 2007;8:975–983. doi: 10.1038/ni1502. [DOI] [PubMed] [Google Scholar]
- Garcia KC, Adams JJ, Feng D, Ely LK. The molecular basis of TCR germline bias for MHC is surprisingly simple. Nat Immunol. 2009;10:143–147. doi: 10.1038/ni.f.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascoigne NR, Palmer E. Signaling in thymic selection. Curr Opin Immunol. 2011;23:207–212. doi: 10.1016/j.coi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K, Wang QJ, Inozume T, Yang JC. Molecular identification of an MHC-independent ligand recognized by a human {alpha}/{beta} T-cell receptor. Blood. 2011;117:4816–4825. doi: 10.1182/blood-2010-11-317743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughn L, Gratton S, Caron L, Sekaly RP, Veillette A, Julius M. Association of tyrosine kinase p56lck with CD4 inhibits the induction of growth through the alpha beta T-cell receptor. Nature. 1992;358:328–331. doi: 10.1038/358328a0. [DOI] [PubMed] [Google Scholar]
- Holdorf AD, Lee KH, Burack WR, Allen PM, Shaw AS. Regulation of Lck activity by CD4 and CD28 in the immunological synapse. Nat Immunol. 2002;3:259–264. doi: 10.1038/ni761. [DOI] [PubMed] [Google Scholar]
- Holland SJ, Bartok I, Attaf M, Genolet R, Luescher IF, Kotsiou E, Richard A, Wang E, White M, Coe DJ, et al. The T-cell receptor is not hardwired to engage MHC ligands. Proc Natl Acad Sci U S A. 2012;109:E3111–3118. doi: 10.1073/pnas.1210882109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Jameson SC, Hogquist KA. Alternative memory in the CD8 T cell lineage. Trends in immunology. 2011;32:50–56. doi: 10.1016/j.it.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo WL, Felix NJ, Walters JJ, Rohrs H, Gross ML, Allen PM. An endogenous peptide positively selects and augments the activation and survival of peripheral CD4+ T cells. Nat Immunol. 2009;10:1155–1161. doi: 10.1038/ni.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P, Scott-Browne JP, Dai S, Gapin L, Kappler JW. Evolutionarily conserved amino acids that control TCR-MHC interaction. Annu Rev Immunol. 2008;26:171–203. doi: 10.1146/annurev.immunol.26.021607.090421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melichar HJ, Narayan K, Der SD, Hiraoka Y, Gardiol N, Jeannet G, Held W, Chambers CA, Kang J. Regulation of gammadelta versus alphabeta T lymphocyte differentiation by the transcription factor SOX13. Science. 2007;315:230–233. doi: 10.1126/science.1135344. [DOI] [PubMed] [Google Scholar]
- Nika K, Soldani C, Salek M, Paster W, Gray A, Etzensperger R, Fugger L, Polzella P, Cerundolo V, Dushek O, et al. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity. 2010;32:766–777. doi: 10.1016/j.immuni.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norment AM, Salter RD, Parham P, Engelhard VH, Littman DR. Cell-cell adhesion mediated by CD8 and MHC class I molecules. Nature. 1988;336:79–81. doi: 10.1038/336079a0. [DOI] [PubMed] [Google Scholar]
- Paige LA, Nadler MJ, Harrison ML, Cassady JM, Geahlen RL. Reversible palmitoylation of the protein-tyrosine kinase p56lck. The Journal of biological chemistry. 1993;268:8669–8674. [PubMed] [Google Scholar]
- Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23:7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- Pobezinsky LA, Angelov GS, Tai X, Jeurling S, Van Laethem F, Feigenbaum L, Park JH, Singer A. Clonal deletion and the fate of autoreactive thymocytes that survive negative selection. Nat Immunol. 2012;13:569–578. doi: 10.1038/ni.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Ko WW, Faas SJ, Cantor H. Binding of antigen in the absence of histocompatibility proteins by arsonate-reactive T-cell clones. Cell. 1984;36:879–888. doi: 10.1016/0092-8674(84)90037-0. [DOI] [PubMed] [Google Scholar]
- Rudd CE, Trevillyan JM, Dasgupta JD, Wong LL, Schlossman SF. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc Natl Acad Sci U S A. 1988;85:5190–5194. doi: 10.1073/pnas.85.14.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Browne JP, White J, Kappler JW, Gapin L, Marrack P. Germline-encoded amino acids in the alphabeta T-cell receptor control thymic selection. Nature. 2009;458:1043–1046. doi: 10.1038/nature07812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AS, Amrein KE, Hammond C, Stern DF, Sefton BM, Rose JK. The lck tyrosine protein kinase interacts with the cytoplasmic tail of the CD4 glycoprotein through its unique amino-terminal domain. Cell. 1989;59:627–636. doi: 10.1016/0092-8674(89)90008-1. [DOI] [PubMed] [Google Scholar]
- Tikhonova AN, Van Laethem F, Hanada K, Lu J, Pobezinsky LA, Hong C, Guinter TI, Jeurling SK, Bernhardt G, Park JH, et al. alphabeta T cell receptors that do not undergo major histocompatibility complex-specific thymic selection possess antibody-like recognition specificities. Immunity. 2012;36:79–91. doi: 10.1016/j.immuni.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JM, Brodsky MH, Irving BA, Levin SD, Perlmutter RM, Littman DR. Interaction of the unique N-terminal region of tyrosine kinase p56lck with cytoplasmic domains of CD4 and CD8 is mediated by cysteine motifs. Cell. 1990;60:755–765. doi: 10.1016/0092-8674(90)90090-2. [DOI] [PubMed] [Google Scholar]
- Van Laethem F, Sarafova SD, Park JH, Tai X, Pobezinsky L, Guinter TI, Adoro S, Adams A, Sharrow SO, Feigenbaum L, et al. Deletion of CD4 and CD8 coreceptors permits generation of alphabetaT cells that recognize antigens independently of the MHC. Immunity. 2007;27:735–750. doi: 10.1016/j.immuni.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Van Laethem F, Tikhonova AN, Singer A. MHC restriction is imposed on a diverse T cell receptor repertoire by CD4 and CD8 co-receptors during thymic selection. Trends in immunology. 2012;33:437–441. doi: 10.1016/j.it.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillette A, Bookman MA, Horak EM, Bolen JB. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988;55:301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Wiest DL, Ashe JM, Abe R, Bolen JB, Singer A. TCR activation of ZAP70 is impaired in CD4+CD8+ thymocytes as a consequence of intrathymic interactions that diminish available p56lck. Immunity. 1996;4:495–504. doi: 10.1016/s1074-7613(00)80415-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
2