Structure of an RNA Silencing Complex of the CRISPR-Cas immune system (original) (raw)
. Author manuscript; available in PMC: 2014 Oct 10.
Published in final edited form as: Mol Cell. 2013 Oct 10;52(1):10.1016/j.molcel.2013.09.008. doi: 10.1016/j.molcel.2013.09.008
Summary
Bacterial and archaeal CRISPR (Clustered Regularly Interspaced Short Palindromic Repeat) loci capture virus and plasmid sequences and use them to recognize and eliminate these invaders. CRISPR (cr)RNAs containing the acquired sequences are incorporated into effector complexes that destroy matching invader nucleic acids. The multi-component Cmr effector complex cleaves RNA targets complementary to the crRNAs. Here we report cryo-electron microscopy reconstruction of a functional Cmr complex bound with a target RNA at ∼12Å. Pairs of the Cmr4 and Cmr5 proteins form a helical core that is asymmetrically capped on each end by distinct pairs of the four remaining subunits – Cmr2 and Cmr3 at the conserved 5′ crRNA tag sequence and Cmr1 and Cmr6 near the 3′ end of the crRNA. The shape and organization of the RNA-targeting Cmr complex is strikingly similar to the DNA-targeting Cascade complex. Our results reveal a remarkably conserved architecture among very distantly related CRISPR-Cas complexes.
Introduction
Many bacteria and archaea employ Clustered Regularly Interspaced Short Palindromic Repeats (CRISPRs) and CRISPR-associated proteins (Cas proteins) to defend themselves against invading nucleic acid elements (Makarova et al., 2011b; Sorek et al., 2013; Terns and Terns, 2011; Wiedenheft et al., 2012). A typical CRISPR-Cas module includes a set of Cas protein-encoding genes (cas genes) and a CRISPR array of identical repeats interspaced with distinct spacer or guide sequences acquired from invaders. The CRISPR locus is transcribed and processed into small CRISPR RNAs. Cas proteins act in all three functional phases of CRISPR-mediated immunity: incorporation of new spacer sequences, biogenesis of CRISPR RNAs (crRNAs), and degradation of invader nucleic acids. The CRISPR repeat sequence functions as an important recognition element in the processes of crRNA biogenesis, invader interference (Brouns et al., 2008; Carte et al., 2008; Hale et al., 2008; Haurwitz et al., 2010) and likely also spacer incorporation (Barrangou et al., 2007; Datsenko et al., 2012; Erdmann and Garrett, 2012; Garneau et al., 2010; Horvath et al., 2008; Swarts et al., 2012; Yosef et al., 2012). The CRISPR spacers, ∼35 nt sequences derived from past invaders (Bolotin et al., 2005; Mojica et al., 2005; Pourcel et al., 2005), give rise to the crRNA guide sequences used to target invading nucleic acids. Together, the transcribed CRISPR repeat-spacer array and Cas proteins provide an RNA-mediated defense mechanism to a wide range of microorganisms (Haft et al., 2005; Makarova et al., 2011b).
The effector complexes that mediate target destruction by the various CRISPR-Cas systems are comprised of distinct sets of Cas proteins and crRNA species. The crRNAs identify and bind target nucleic acids through base pairing interactions, and Cas proteins cleave the bound target. Both DNA and RNA have been found to be interference targets. There are three major types of CRISPR-Cas systems that each harbor different effector complexes. The effector complex of Escherichia coli(E. coli), a Type I CRISPR-Cas system, is formed from the Cascade complex composed of five different Cse proteins and a crRNA with CRISPR repeat sequence flanking the guide sequence at both ends (Jore et al., 2011; Lintner et al., 2011; Westra et al., 2012). The Cascade complex uses its bound crRNA to target double-stranded DNA (Sashital et al., 2012). The Cascade-bound DNA is partially unwound by formation of an R-loop structure with the crRNA, which recruits the nuclease Cas3 to further unwind and cleave the target DNA (Sinkunas et al., 2013; Westra et al., 2012). Type II CRISPR-Cas effector complexes from Streptococcus thermophilus and Streptococcus pyogenes use the dual active site nuclease Cas9 to cleave DNA targets, also via formation of the R-loop structure (Jinek et al., 2012). These single protein effector complexes utilize crRNAs with a shortened guide region and a 3′ CRISPR repeat sequence tag, and require a trans-activating crRNA (tracrRNA) in addition to the crRNA for activity (Deltcheva et al., 2011; Gasiunas et al., 2012; Jinek et al., 2012). Both Type I and Type II effector complexes have an additional requirement for a protospacer adjacent motif (or PAM) sequence adjacent to the target sequence recognized by the crRNA to ensure that the DNA target is foreign (Marraffini and Sontheimer, 2010; Mojica et al., 2009; Shah et al., 2013). Unlike the DNA-targeting Type I and Type II effector complexes (Brouns et al., 2008; Jinek et al., 2012; Jore et al., 2011; Marraffini and Sontheimer, 2008), the Type III effector complexes isolated from Pyrococcus furiosus(P. furiosus) (Hale et al., 2012; Hale et al., 2009) and Sulfolobus solfataricus(S. solfataricus) (Zhang et al., 2012) cleave target RNA (Cong et al., 2013; Hale et al., 2012; Hale et al., 2009; Zhang et al., 2012). These RNA-targeting complexes are comprised of 6-7 Cmr proteins and crRNAs with repeat sequence tags at the 5′ end. The apparent differences among the three types of effector complexes highlight the need for detailed mechanistic and structural studies of these complexes.
We carried out biochemical and structural characterization of a Type III effector complex, the Cmr complex of P. furiosus (Pf). The Pf Cmr complex belongs to Type III subtype B (III-B) CRISPR-Cas systems (Hale et al., 2012; Hale et al., 2009; Zhang et al., 2012) and includes six proteins (Cmr1-Cmr6) (Fig. 1A). Two forms of the crRNA co-purify with the complex and both can mediate RNA-guided RNA cleavage (Hale et al., 2012; Hale et al., 2009). The 39- and 45-nt Cmr crRNAs consist of an 8-nt CRISPR repeat sequence (5′ tag) and a 31- or 37-nt guide sequence (Hale et al., 2012; Hale et al., 2009) (Fig. 1B). The reconstituted complex specifically cleaves single-stranded RNAs complementary to the crRNA guide and can be directed with customized crRNAs to cleave novel RNA targets (Hale et al., 2012; Hale et al., 2009). In the studies described here, we have determined the functional organization of the Pf Cmr complex in the presence of a target RNA.
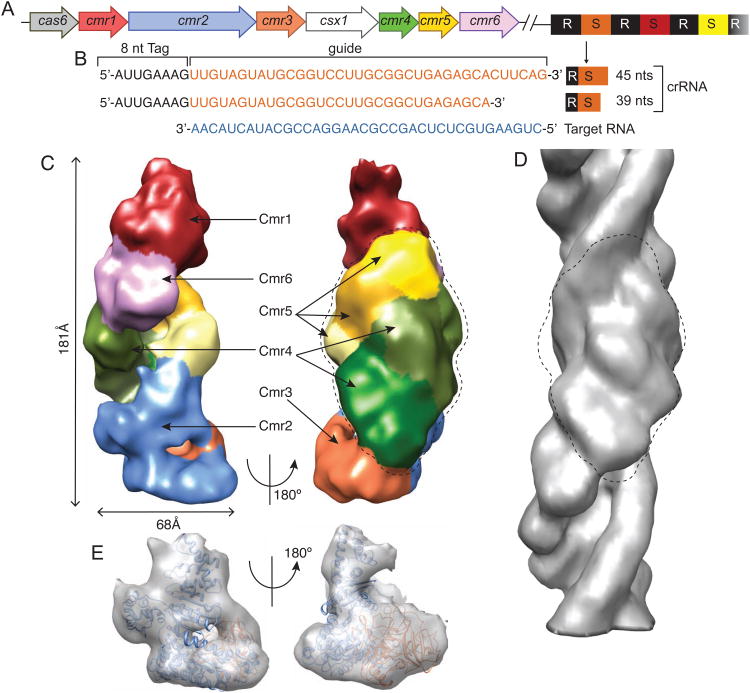
Fig. 1.
Overview of the P. furiosus CRISPR/Cas locus and the Cmr complex structure determined by cryo electron microscopy. (A) The genes encoding the Cmr complex protein subunits are color-coded to match those used for structure images in subsequent figures. The CRISPR repeats are shown in black and spacers in various colors. (B) The mature 39-nt and 45-nt crRNAs contain a 5′ repeat-derived 8-nt sequence (5′-tag) and a 31-nt or 37-nt spacer-derived sequence (guide), respectively. The sequence of the target RNA used in RNA cleavage assays and assembly with the Cmr complex is shown in blue. (C) Color-coded EM density of the Cmr complex bound with the 45-nt crRNA and the target RNA is shown in two orientations. Cmr1 (red), Cmr2 (light blue), Cmr3 (orange), Cmr4 (three different shades of green), Cmr5 (three different shades of yellow), and Cmr6 (magenta). (D) Fitting of the crystal structure of Cmr2-Cmr3 complex to the ‘foot’ of the Cmr complex in two orthogonal orientations. (E) Helical reconstruction of the Cmr4-Cmr5 filament structure. Circled regions indicate matched densities in both the Cmr complex and the Cmr4-Cmr5 filament. See also Figures S1, S2 and S3.
Results and Discussions
Structure of the Cmr complex
We carried out 3D electron microscopy (EM) studies of a holo Cmr complex reconstituted from recombinantly expressed Pf Cmr proteins, a synthetically made 45-nt crRNA and a target RNA under the noncleaving condition without added magnesium ions (Fig. S1). The EM structural models of the Cmr complex were first obtained by random conical tilt (RCT) and single particle reconstruction from negatively stained specimens (Fig. S2A-S2D, supplementary materials), and subsequently by high throughput cryoEM reconstruction to ∼12Å resolution (by the FSC0.5 criterion) (supplementary materials) (Fig. 1C). The structure was reconstructed from particles that measured 68Å×181Å. A small fraction of the particles exhibited varying lengths and these were excluded from the reconstruction (Fig. S2E, supplementary materials). Assignment of protein subunits in EM densities was accomplished from 3D reconstructions of negatively stained Cmr complexes lacking specific subunits and from fitting crystal structures of the Cmr2-Cmr3 complex (Shao et al., 2013) (PDB ID 4H4K) and Cmr5 (Park et al., 2013) (PDB ID 4GKF) (supplementary materials). The final 3D EM structure of the Cmr complex is shown in Figure 1 (Fig. 1, and supplemental movie 1).
In the orientation shown in Figure 1, the lower ‘foot’ region of the particle was identified first. The contours of the Cmr2-Cmr3 crystal structure closely match those of the EM density in this region of the Cmr complex (Fig. 1D). It was evident that slight conformational changes in Cmr2 and Cmr3 occurred when the intact complex assembled, and these were modeled by flexibly fitting the crystal structure into the EM density (Fig. S2F, supplementary materials).
The central ‘backbone’ of the particle was identified through its similarity to a helical filament formed by Cmr4 and Cmr5 alone (Fig. 1E). The EM density for the repeating units of this Cmr4-Cmr5 filament and the Cmr holo particle are well accounted for by a modeled Cmr4-Cmr5 heterodimer structure (Fig. 1E and Fig. S2D), suggesting that the helical structure is comprised of repeating Cmr4-Cmr5 heterodimers. Three Cmr4-Cmr5 repeats closely match the size and shape of the central region of the intact Cmr complex (Fig. 1E). The 29.3 Å rise and 48° twist of the Cmr4-Cmr5 filament are almost identical to the rise and twist estimated from the repeating density in the central domain of the Cmr complex (Fig. 1E). The groove between the Cmr4 and the Cmr5 ridges is fuller in the Cmr complex than in the isolated Cmr4-Cmr5 filament (Fig. 1C & 1E), which may be attributable to the bound crRNA-target RNA duplex. These structural results indicate that the central region of the complex is comprised of a helical ladder of three Cmr4-Cmr5 units.
The ‘top’ of the particle was assigned through difference density methods. Cmr1 was assigned to this region by comparing the EM densities of the particles with and without Cmr1 (Fig. S3A, supplementary materials). The only substantial remaining unaccounted density is located immediately below Cmr1 and this was assigned to Cmr6. In the absence of Cmr6, Cmr1 does not associate with the complex, consistent with a possible interaction between Cmr1 and Cmr6 (Fig. S3A, supplementary materials).
Together, the cryo-EM map of the Cmr complex, the helical reconstruction of the Cmr4-Cmr5 filament, the available crystal structures of Cmr2-Cmr3 (Shao et al., 2013) and Cmr5 (Park et al., 2013) and the homology-built structures of other subunits (Fig. S4, supplementary materials) allowed us to construct a pseudo-atomic model of the Cmr complex (Fig. 2A). The intact Cmr complex bound with the target RNA has a helical architecture with two asymmetric ends (Fig. 1). The foot of the particle contains Cmr2 and Cmr3. Emanating from the Cmr2-Cmr3 dimer and stacking upwards in a right-handed twist are three Cmr4-Cmr5 repeats arranged in a head-to-tail fashion (Fig. 1). At the top of the Cmr4-Cmr5 dimers is Cmr6, capped by Cmr1.
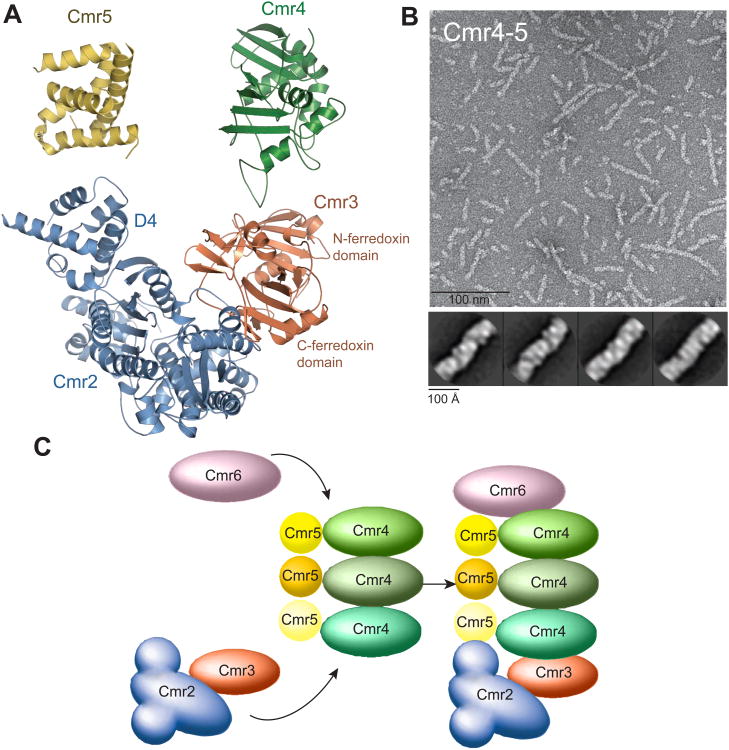
Fig. 2.
Assembly process of the Cmr complex. (A) Left, a ribbon model of the assembled protein subunits of an intact target-bound Cmr complex. Right, capping at the foot of the Cmr complex by interaction of the homologous domain of Cmr2 with Cmr5 and of Cmr3 with Cmr4. Structural similarity between Cmr5 ((Park et al., 2013), PDB ID 4GKF) and the D4 domain of Cmr2 is shown (r.m.s.d = 3.3 Å for 62 aligned Cα atoms). A homology model of Cmr4 constructed from Cas6 (sequence similarity 30%, (Carte et al., 2008), PDB ID 3PKM) is also homologous to that of Cmr3 (r.m.s.d = 3.4 Å for 136 aligned Cα atoms). (B) A micrograph of the Cmr4-Cmr5 filament and the class averages of Cmr4-Cmr5 filaments. (C) Model for assembly method of the Cmr complex. By acting as a non-extendable Cmr4-Cmr5 dimer, Cmr6 and Cmr2-Cmr3 complex cap the growth of the Cmr4-Cmr5 filament at the top and foot of the Cmr complex, respectively. See also Figures S2E and S4.
An assembly mechanism underlies Cmr complex flexibility
The pseudo-atomic model of the Cmr complex suggests a mechanism of assembly based on interactions between homologous domains found on the proteins (Fig. 2). At the foot region of the particle, the D4 domain of Cmr2 contacts Cmr5, and the N-terminal ferredoxin domain of Cmr3 contacts Cmr4. Interestingly, the D4 domain of Cmr2 has the same overall fold as Cmr5 while the ferredoxin domain of Cmr3 resembles that of the homology model of Cmr4 (Zhu and Ye, 2012) (Fig. 2A), thus they can cap the foot end of the Cmr4-Cmr5 repeats by mimicking the helical binding sites for those proteins. At the top of the complex, Cmr6 contacts Cmr4. Like Cmr4, Cmr6 is a RAMP (Repeat-Associated Mysterious Proteins) protein and thus may share a structural homology with Cmr4. This suggests that the top end of the Cmr4-Cmr5 repeats is also capped by mimicking the helical binding sites. The noted structural homologies and the filamentous nature of the isolated Cmr4-Cmr5 subcomplex (Fig. 2B) indicate that Cmr complex assembly makes use of homologous proteins and domains in terminating polymerization on both ends of the filament (Fig. 2C).
The proposed assembly mechanism suggests that formation of Cmr complexes with variable numbers of Cmr4-Cmr5 repeats is possible, and indeed, particles of other lengths were observed in raw EM images of negatively stained, reconstituted Cmr complexes (Fig. S2E, supplementary materials). Flexibility in the length of the Cmr4-Cmr5 helix may offer a structure solution for the Cmr complex to utilize crRNAs of two different sizes.
Directional tracking of central protein core by the crRNA
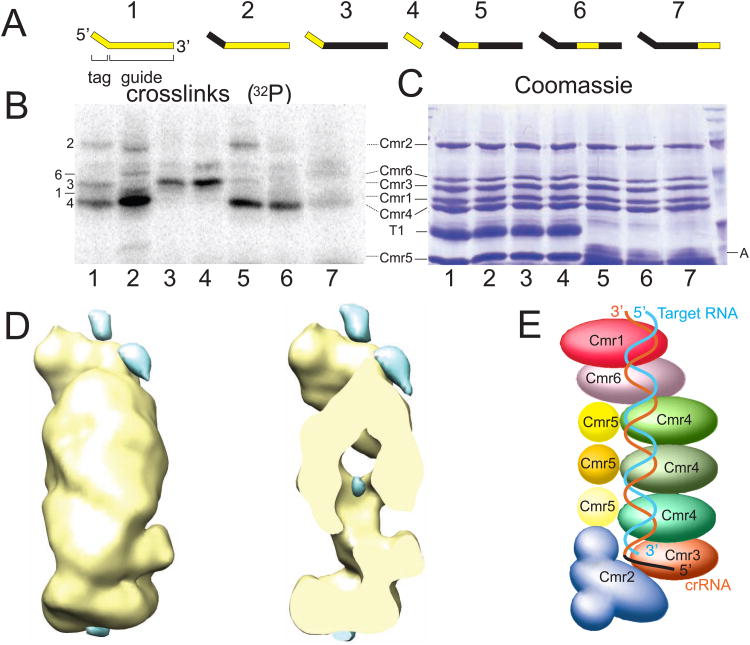
In order to determine the orientation of the crRNA relative to the Cmr protein subunits in the complex, we carried out protein-RNA crosslinking studies using radiolabeled crRNAs containing photoreactive 4-thiouridine residues. By changing the location of the radioactive nucleotides, various regions of the crRNA were probed for nearby Cmr protein components (Fig. 3A). Five of the Cmr proteins – Cmr1-4 and Cmr6 – were detected by crosslinking to crRNAs labeled throughout (Fig. 3B & 3C, Lane 1). Cmr3 was specifically crosslinked to the 5′ tag of the crRNA and was also detected when label was included in the guide region of the crRNA most proximal to the 5′ tag (Fig. 3B & 3C, Lanes 3-5). Cmr1, Cmr2, Cmr4 and Cmr6 were all crosslinked to crRNAs labeled in the guide region (Fig. 3B & 3C, Lane 2). Three sections of the guide region were independently radiolabeled (Fig. 3B & 3C, Lanes 5-7). Cmr2 was specifically crosslinked to the section of the guide region proximal to the 5′ tag of the crRNA. Cmr4 protein was crosslinked to the 5′ and middle sections, and to a lesser extent the 3′ end of the guide sequence. Cmr1 and 6 were specifically detected at the 3′ end of the crRNA (Fig. 3B & 3C, Lane 7).
Fig. 3.
Positions of the crRNA and target RNA within the complex. (A) RNA Substrates used for crosslinking assays. The 45-nt crRNAs (1-3, 5-8) or tag RNAs (4) were generated by in vitro transcription and subsequent Cas6 digestion. Regions marked in yellow contain radiolabeled nucleotides. Labeled (i.e. crosslinked) (B) and Coomassie-stained (i.e. total) (C) proteins are shown. The RNA from (A) used in each reaction is indicated beneath the lanes, and correlates with (A). T1 and A denote RNases used in the experiment and visible in the Coomassie-stained gel (C). (D) Difference density to identify bound target RNA. The difference density was obtained by subtracting the negatively stained density of the Cmr complex in the absence of target RNA from that in the presence of target RNA. Left, the common density +/- target RNA is shown in yellow and the difference density in blue (10σ). Right, the same view as that on the left but clipped to expose the difference density in the center of the complex. (E) Schematic structure of the holo Cmr complex showing the deduced path of the crRNA and target RNA. See also Table S1.
Location of the target RNA was assessed by calculating a difference map between the Cmr complexes with and without the target RNA (Fig. 3D). The basic shape of the particle remains similar upon binding the target RNA (Fig. 1C & Fig. 3D). Difference peaks were observed at the top, in a central cavity and along the helical back of the complex (Fig. 3D). We attribute the difference peak in the central cavity to a conformational change because its adjacent region is also associated with a strong negative difference peak (data not shown). We attribute the difference peaks along the helical back and at the top of the particle to the bound target RNA. Interestingly, the difference peak at the top of the particle is near but not overlapping the density identified for Cmr1 (Fig. 3D and Fig. S3A), suggesting a co-localization of Cmr1 and part of the target RNA.
Based on the structural and crosslinking results, the crRNA-target duplex is modeled to bind along the elongated Cmr assembly (Fig. 3E). Our results indicate that the 5′ end of the crRNA is anchored in the Cmr2-Cmr3 foot portion of the Cmr complex. In particular, Cmr3 is found in close proximity to the signature 5′ tag sequence, suggesting a role for Cmr3 in recognition of CRISPR guide RNAs within the cellular environment. The guide sequence is associated with Cmr4, consistent with location of the crRNA along the helical groove formed by the repeating Cmr4-Cmr5 subunits, however no crosslinking was observed to Cmr5. The 3′ end of the crRNA is specifically associated with Cmr1 and Cmr6 at the top of the particle. Cleavage of target RNAs by Cmr complexes occurs 14 nucleotides from the 3′ end of the CRISPR guide RNA (Hale et al., 2009). Our findings suggest that cleavage occurs in the vicinity of the junction of Cmr1/6 and Cmr4/5.
The Cmr structure reveals the striking organizational conservation of multi-protein CRISPR-Cas effector complexes
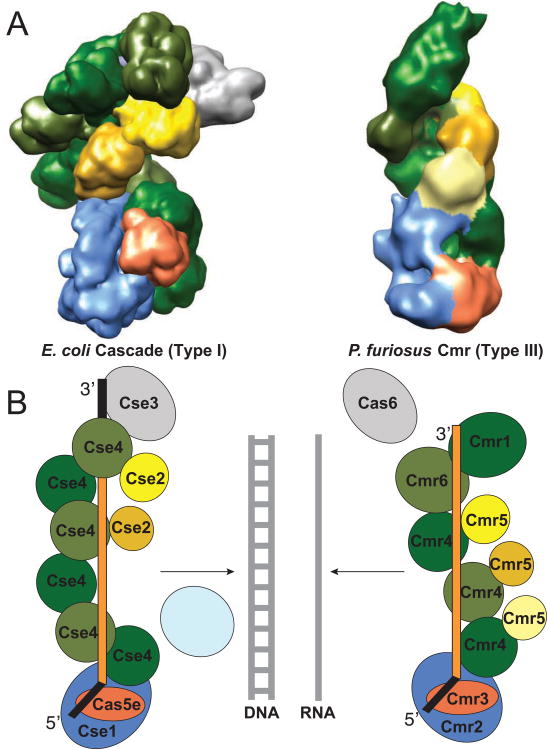
A truly surprising similarity between the organization of the Type III-B Cmr complex and the previously characterized Type I-E Cse effector complex, the E. Coli Cascade complex, emerges from analysis of our findings. These two CRISPR-Cas effector complexes are comprised of distinct protein subunits (Makarova et al., 2011a) and exhibit different activities. Cascade does not target RNA, nor does it cleave DNA on its own; Cascade prepares DNA for cleavage by Cas3. However, Cascade also exhibits a helical arrangement of proteins along the length of the crRNA (Wiedenheft et al., 2011). Moreover, these two effector complexes share a truly striking similarity in the organization of broad classes of distantly related proteins within the structures (Fig. 4 and supplemental movie 2). The Cmr3 and Cas5e proteins found at the 5′ ends of the crRNAs in the structures are both members of the Cas5 superfamily of RAMP-type proteins (Makarova et al., 2011a). Cmr2 and Cse1 are both members of the “large subunit” category of Cas proteins. Extending from the foot of both complexes along the guide region of the crRNAs is a helical region populated on one side by Cas7 superfamily RAMP proteins – Cmr4, Cmr6 and Cmr1 in the RNA-targeting complex, and Cse4 in the DNA-targeting complex (Fig. 4). “Small subunit” category Cas proteins – Cmr5 and Cse2 – are found on the other side of the helical region in both complexes. Cse3 (or Cas6e), the enzyme that processes crRNA transcripts, remains associated with the repeat sequence retained at the 3′ end of Cascade crRNAs (Fig. 4). The equivalent enzyme, Cas6, is not associated with the Cmr complex, whose crRNAs lack repeat sequence at the 3′ end (Carte et al., 2008) (Hale et al., 2008) and Fig. 4).
Fig. 4.
Conserved organization of an RNA- and DNA-targeting CRISPR-Cas effector complex. The Cse and Cmr CRISPR-Cas systems include distantly related members of several broad categories of Cas proteins: large subunit (blue), small subunit (shades of yellow), Cas5 superfamily (orange), Cas7 superfamily (shades of green) and Cas6 superfamily proteins (gray). Our findings reveal a conserved functional organization of these classes of Cas proteins within the effector complexes. (A). Comparison of cryoEM structures of E. coli Cascade (left) and P. furiosus Cmr complex (right). Segmented densities assigned to the subunits are color-coded according to their broad classification. (B). Cartoon representations of the functional organization of the complexes. The Cas5 superfamily RAMP proteins – Cmr3 and Cas5e – are found near the 5′ tag of the crRNAs in both complexes. Large subunit proteins – Cmr2 (member of the Cas10 superfamily) and Cse1 (member of the Cas8 superfamily) – are also found near the 5′ end of the crRNAs. Cas7 superfamily RAMP proteins – Cmr4, Cmr6 and Cmr1 in the Cmr complex and Cse4 in the Cse complex – and the small subunit of proteins – Cmr5 and Cse2 – form the backbones of the helical structure that extends along the guide region of the crRNAs. In Cascade, the Cas6 superfamily RAMP protein – Cse3 – remains associated with the CRISPR repeat sequence retained at the 3′ end of the crRNA. The Cse complex binds DNA targets that are then cleaved by Cas3. The Cmr complex cleaves complementary RNAs.
It should be noted that the structure of the Pf Cmr complex differs significantly from the EM structure reported for the Sulfolobus solfataricus (Ss) Cmr particle (Zhang et al., 2012). The Ss Cmr particle has multiple protuberances within an overall globular shape rather than an elongated character. The basis for the difference in particle shape is not clear, however the Ss complex also includes a seventh Cmr protein subunit that is restricted to the Sulfolobales and displays a distinct sequence-specific cleavage pattern (Zhang et al., 2012).
The analogous positions of the members of the very distantly related members of superfamilies of Cas proteins within the Cmr and Cse complexes provide structural validation for the relationships initially identified between the proteins on the basis of CRISPR-Cas module operon organization and weak sequence similarities (Makarova et al., 2011a). Our findings reveal a common paradigm for the organization of distantly related CRISPR-Cas effector complexes that suggests divergent evolution from a common ancestral complex and predicts the organization of other effector complexes as well as common functional roles for analogous components.
Experimental Procedures
Sample Preparation and Electron Microscopy Reconstruction of the Cmr complex
Detailed preparation and EM reconstruction methods for the Cmr complex and subcomplexes are included in the online Supplementary Materials. Briefly, individual Cmr proteins were purified according to previously published methods (Cocozaki et al., 2012) and incubated with the 45-nt crRNA with or without the 37-nt target RNA. The Cmr complexes were isolated by a size exclusion procedure. Samples of the Cmr complex and subcomplexes were studied with 2 % (w/v) uranyl formate negative-stain and by cryo-EM. Grids were prepared by placing 3 μl aliquots of sample onto grids that had been plasma cleaned for 5 seconds using a Gatan SOLARIS model 950 plasma cleaner. The cryo-EM samples were prepared using C-flat 2/2 400 mesh holey carbon grids and were flash frozen by plunging into liquid ethane using an FEI Vitrobot. Data were collected on a Phillips CM120 equipped with a Tietz 2k × 2k slow scan CCD camera and an FEI Titan Krios that was equipped with a Gatan Ultrascan 4k × 4k pixel CCD camera and the Leginon software for automatic data acquisition (Suloway et al., 2005). Data were collected with a range of defocus from -1.5 to -3 um, and an electron dose of 20 e-/Å2. Images were processed using Appion (Lander et al., 2009). Particles were picked using difference of Gaussian (DoG) (Voss et al., 2009) for the RCT data and template-based matching for the negative stain and cryo data. Incorrect picks were removed using junk sort in XMIPP (Scheres et al., 2008). This resulted in more than 10,000 for negatively stained and 100,000 for the cryo particles. CTFs were estimated with the Automated CTF Estimation (ACE) (Mallick et al., 2005), rejecting images with an estimation confidence value of less than 0.7 and flipping phases for individual particles according to their defocus. Particles were reconstructed using a combination of EMAN refinement (Ludtke et al., 1999) and multivariate data analysis with SPIDER (Frank et al., 1996) to limit particle heterogeneity. The Random Conical Tile (RCT) reconstruction was used as the initial model (Fig. S2). To identify individual subunits within the density, a combination of crystal structure fitting, helical reconstruction of Cmr4-Cmr5 filament, and pair-wise interaction data were used (Supplementary Materials). The helical reconstruction of Cmr4-Cmr5 was accomplished using the IHRSR software package (Egelman, 2007) combined with EMAN and in-house software. EM density maps were visualized using UCSF Chimera (Pettersen et al., 2004).
RNA-protein crosslinking
Detailed sample preparation, UV crosslinking reactions are described in the online supplementary materials. Briefly, internally labeled crRNA at different regions were incubated with the Cmr proteins and irradiated by 312 nm UV light. Irradiated reaction mixtures were treated by RNases and analyzed on SDS-PAGE gels for the location of the crosslink.
Supplementary Material
01
02
03
Highlights.
- The helical Cmr complex structure runs along the length of the guide and target RNA
- Cmr2 and Cmr3 proteins anchor the Cmr complex at the conserved 5′ tag of the crRNA
- At the 3′ end of the crRNA, Cmr1 and Cmr6 cap a filament of Cmr4-5 heterodimers
- Distantly related CRISPR-Cas effector complexes share a common functional design
Acknowledgments
This work was supported by National Institutes of Health grant R01 GM99604 to H.L., American Heart Association predoctoral fellowship 11PRE7090000 to A.I.C., and National Institutes of Health Grant R01 GM54682 to M.T. and R.T.
Footnotes
Accession numbers: The frozen hydrated Cmr complex EM map was deposited in the EM databank under the accession number EMD-5740. The Cmr 4-5 filament map was deposited with the accession number EMD-5737.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709–1712. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- Bolotin A, Quinquis B, Sorokin A, Ehrlich SD. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology. 2005;151:2551–2561. doi: 10.1099/mic.0.28048-0. [DOI] [PubMed] [Google Scholar]
- Brouns SJ, Jore MM, Lundgren M, Westra ER, Slijkhuis RJ, Snijders AP, Dickman MJ, Makarova KS, Koonin EV, van der Oost J. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science. 2008;321:960–964. doi: 10.1126/science.1159689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carte J, Wang R, Li H, Terns RM, Terns MP. Cas6 is an endoribonuclease that generates guide RNAs for invader defense in prokaryotes. Genes Dev. 2008;22:3489–3496. doi: 10.1101/gad.1742908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocozaki AI, Ramia NF, Shao Y, Hale CR, Terns RM, Terns MP, Li H. Structure of the Cmr2 subunit of the CRISPR-Cas RNA silencing complex. Structure. 2012;20:545–553. doi: 10.1016/j.str.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA, Pougach K, Tikhonov A, Wanner BL, Severinov K, Semenova E. Molecular memory of prior infections activates the CRISPR/Cas adaptive bacterial immunity system. Nat Commun. 2012;3:945. doi: 10.1038/ncomms1937. [DOI] [PubMed] [Google Scholar]
- Deltcheva E, Chylinski K, Sharma CM, Gonzales K, Chao Y, Pirzada ZA, Eckert MR, Vogel J, Charpentier E. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature. 2011;471:602–607. doi: 10.1038/nature09886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelman EH. The iterative helical real space reconstruction method: surmounting the problems posed by real polymers. J Struct Biol. 2007;157:83–94. doi: 10.1016/j.jsb.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Erdmann S, Garrett RA. Selective and hyperactive uptake of foreign DNA by adaptive immune systems of an archaeon via two distinct mechanisms. Mol Microbiol. 2012;85:1044–1056. doi: 10.1111/j.1365-2958.2012.08171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eswar N, Eramian D, Webb B, Shen MY, Sali A. Protein structure modeling with MODELLER. Methods Mol Biol. 2008;426:145–159. doi: 10.1007/978-1-60327-058-8_8. [DOI] [PubMed] [Google Scholar]
- Frank J, Radermacher M, Penczek P, Zhu J, Li Y, Ladjadj M, Leith A. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J Struct Biol. 1996;116:190–199. doi: 10.1006/jsbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- Garneau JE, Dupuis M, Villion M, Romero DA, Barrangou R, Boyaval P, Fremaux C, Horvath P, Magadán AH, Moineau S. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature. 2010;468:67–71. doi: 10.1038/nature09523. [DOI] [PubMed] [Google Scholar]
- Gasiunas G, Barrangou R, Horvath P, Siksnys V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci U S A. 2012;109:E2579–2586. doi: 10.1073/pnas.1208507109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haft DH, Selengut J, Mongodin EF, Nelson KE. A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR/Cas subtypes exist in prokaryotic genomes. PLoS Comput Biol. 2005;1:e60. doi: 10.1371/journal.pcbi.0010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale C, Kleppe K, Terns RM, Terns MP. Prokaryotic silencing (psi)RNAs in Pyrococcus furiosus. Rna. 2008;14:2572–2579. doi: 10.1261/rna.1246808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale CR, Majumdar S, Elmore J, Pfister N, Compton M, Olson S, Resch AM, Glover CV, 3rd, Graveley BR, Terns RM, et al. Essential features and rational design of CRISPR RNAs that function with the Cas RAMP module complex to cleave RNAs. Mol Cell. 2012;45:292–302. doi: 10.1016/j.molcel.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale CR, Zhao P, Olson S, Duff MO, Graveley BR, Wells L, Terns RM, Terns MP. RNA-guided RNA cleavage by a CRISPR RNA-Cas protein complex. Cell. 2009;139:945–956. doi: 10.1016/j.cell.2009.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haurwitz RE, Jinek M, Wiedenheft B, Zhou K, Doudna JA. Sequence- and structure-specific RNA processing by a CRISPR endonuclease. Science. 2010;329:1355–1358. doi: 10.1126/science.1192272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath P, Romero DA, Coute-Monvoisin AC, Richards M, Deveau H, Moineau S, Boyaval P, Fremaux C, Barrangou R. Diversity, activity, and evolution of CRISPR loci in Streptococcus thermophilus. J Bacteriol. 2008;190:1401–1412. doi: 10.1128/JB.01415-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jore MM, Lundgren M, van Duijn E, Bultema JB, Westra ER, Waghmare SP, Wiedenheft B, Pul U, Wurm R, Wagner R, et al. Structural basis for CRISPR RNA-guided DNA recognition by Cascade. Nat Struct Mol Biol. 2011;18:529–536. doi: 10.1038/nsmb.2019. [DOI] [PubMed] [Google Scholar]
- Lander GC, Stagg SM, Voss NR, Cheng A, Fellmann D, Pulokas J, Yoshioka C, Irving C, Mulder A, Lau PW, et al. Appion: an integrated, database-driven pipeline to facilitate EM image processing. J Struct Biol. 2009;166:95–102. doi: 10.1016/j.jsb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintner NG, Kerou M, Brumfield SK, Graham S, Liu H, Naismith JH, Sdano M, Peng N, She Q, Copie V, et al. Structural and functional characterization of an archaeal clustered regularly interspaced short palindromic repeat (CRISPR)-associated complex for antiviral defense (CASCADE) J Biol Chem. 2011;286:21643–21656. doi: 10.1074/jbc.M111.238485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludtke SJ, Baldwin PR, Chiu W. EMAN: semiautomated software for high-resolution single-particle reconstructions. J Struct Biol. 1999;128:82–97. doi: 10.1006/jsbi.1999.4174. [DOI] [PubMed] [Google Scholar]
- Makarova KS, Aravind L, Wolf YI, Koonin EV. Unification of Cas protein families and a simple scenario for the origin and evolution of CRISPR-Cas systems. Biol Direct. 2011a;6:38. doi: 10.1186/1745-6150-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova KS, Haft DH, Barrangou R, Brouns SJ, Charpentier E, Horvath P, Moineau S, Mojica FJ, Wolf YI, Yakunin AF, et al. Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol. 2011b;9:467–477. doi: 10.1038/nrmicro2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick SP, Carragher B, Potter CS, Kriegman DJ. ACE: automated CTF estimation. Ultramicroscopy. 2005;104:8–29. doi: 10.1016/j.ultramic.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Marraffini LA, Sontheimer EJ. CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science. 2008;322:1843–1845. doi: 10.1126/science.1165771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marraffini LA, Sontheimer EJ. Self versus non-self discrimination during CRISPR RNA-directed immunity. Nature. 2010;463:568–571. doi: 10.1038/nature08703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojica FJ, Diez-Villasenor C, Garcia-Martinez J, Almendros C. Short motif sequences determine the targets of the prokaryotic CRISPR defence system. Microbiology. 2009;155:733–740. doi: 10.1099/mic.0.023960-0. [DOI] [PubMed] [Google Scholar]
- Mojica FJ, Diez-Villasenor C, Garcia-Martinez J, Soria E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J Mol Evol. 2005;60:174–182. doi: 10.1007/s00239-004-0046-3. [DOI] [PubMed] [Google Scholar]
- Park JH, Sun J, Park SY, Hwang HJ, Park MY, Shin M, Kim JS. Crystal structure of Cmr5 from Pyrococcus furiosus and its functional implications. FEBS Lett. 2013 doi: 10.1016/j.febslet.2013.01.029. [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Pourcel C, Salvignol G, Vergnaud G. CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology. 2005;151:653–663. doi: 10.1099/mic.0.27437-0. [DOI] [PubMed] [Google Scholar]
- Sashital DG, Wiedenheft B, Doudna JA. Mechanism of foreign DNA selection in a bacterial adaptive immune system. Mol Cell. 2012;46:606–615. doi: 10.1016/j.molcel.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres SH, Nunez-Ramirez R, Sorzano CO, Carazo JM, Marabini R. Image processing for electron microscopy single-particle analysis using XMIPP. Nat Protoc. 2008;3:977–990. doi: 10.1038/nprot.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SA, Erdmann S, Mojica FJ, Garrett RA. Protospacer recognition motifs Mixed identities and functional diversity. RNA Biol. 2013;10 doi: 10.4161/rna.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Cocozaki AI, Ramia NF, Terns RM, Terns MP, Li H. Structure of the Cmr2-Cmr3 Subcomplex of the Cmr RNA Silencing Complex. Structure. 2013 doi: 10.1016/j.str.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkunas T, Gasiunas G, Waghmare SP, Dickman MJ, Barrangou R, Horvath P, Siksnys V. In vitro reconstitution of Cascade-mediated CRISPR immunity in Streptococcus thermophilus. Embo J. 2013;32:385–394. doi: 10.1038/emboj.2012.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorek R, Lawrence CM, Wiedenheft B. CRISPR-mediated Adaptive Immune Systems in Bacteria and Archaea. Annu Rev Biochem. 2013 doi: 10.1146/annurev-biochem-072911-172315. [DOI] [PubMed] [Google Scholar]
- Suloway C, Pulokas J, Fellmann D, Cheng A, Guerra F, Quispe J, Stagg S, Potter CS, Carragher B. Automated molecular microscopy: the new Leginon system. J Struct Biol. 2005;151:41–60. doi: 10.1016/j.jsb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Swarts DC, Mosterd C, van Passel MW, Brouns SJ. CRISPR interference directs strand specific spacer acquisition. PLoS One. 2012;7:e35888. doi: 10.1371/journal.pone.0035888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terns MP, Terns RM. CRISPR-based adaptive immune systems. Curr Opin Microbiol. 2011;14:321–327. doi: 10.1016/j.mib.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss NR, Yoshioka CK, Radermacher M, Potter CS, Carragher B. DoG Picker and TiltPicker: software tools to facilitate particle selection in single particle electron microscopy. J Struct Biol. 2009;166:205–213. doi: 10.1016/j.jsb.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westra ER, van Erp PB, Kunne T, Wong SP, Staals RH, Seegers CL, Bollen S, Jore MM, Semenova E, Severinov K, et al. CRISPR immunity relies on the consecutive binding and degradation of negatively supercoiled invader DNA by Cascade and Cas3. Mol Cell. 2012;46:595–605. doi: 10.1016/j.molcel.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenheft B, Lander GC, Zhou K, Jore MM, Brouns SJ, van der Oost J, Doudna JA, Nogales E. Structures of the RNA-guided surveillance complex from a bacterial immune system. Nature. 2011;477:486–489. doi: 10.1038/nature10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenheft B, Sternberg SH, Doudna JA. RNA-guided genetic silencing systems in bacteria and archaea. Nature. 2012;482:331–338. doi: 10.1038/nature10886. [DOI] [PubMed] [Google Scholar]
- Yosef I, Goren MG, Qimron U. Proteins and DNA elements essential for the CRISPR adaptation process in Escherichia coli. Nucleic Acids Res. 2012;40:5569–5576. doi: 10.1093/nar/gks216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Rouillon C, Kerou M, Reeks J, Brugger K, Graham S, Reimann J, Cannone G, Liu H, Albers SV, et al. Structure and mechanism of the CMR complex for CRISPR-mediated antiviral immunity. Mol Cell. 2012;45:303–313. doi: 10.1016/j.molcel.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Ye K. Crystal structure of Cmr2 suggests a nucleotide cyclase-related enzyme in type III CRISPR-Cas systems. FEBS Lett. 2012;586:939–945. doi: 10.1016/j.febslet.2012.02.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01
02
03