The Effects of Estrogen in Ischemic Stroke (original) (raw)
. Author manuscript; available in PMC: 2014 Dec 24.
Published in final edited form as: Transl Stroke Res. 2012 Dec 7;4(4):390–401. doi: 10.1007/s12975-012-0230-5
Abstract
Stroke is a leading cause of death and the most common cause of long-term disability in the USA. Women have a lower incidence of stroke compared with men throughout most of the lifespan which has been ascribed to protective effects of gonadal steroids, most notably estrogen. Due to the lower stroke incidence observed in pre-menopausal women and robust preclinical evidence of neuroprotective and anti-inflammatory properties of estrogen, researchers have focused on the potential benefits of hormones to reduce ischemic brain injury. However, as women age, they are disproportionately affected by stroke, coincident with the loss of estrogen with menopause. The risk of stroke in elderly women exceeds that of men and it is clear that in some settings estrogen can have pro-inflammatory effects. This review will focus on estrogen and inflammation and its interaction with aging.
Keywords: Stroke, Aging, Sex, Estrogen
Stroke
Stroke is the fourth leading cause of death in the USA and ranks as one of the leading causes of long-term disability [1]. Given the current population trend of increasing life expectancy in the USA with its concurrent expansion of the elderly population, the annual number of stroke patients is expected to increase with every given year. The most important nonmodifiable risk factor for stroke is aging. The majority of stroke patients are over 65 [2, 3], and since 75– 89 % of strokes occur in individuals over the age of 65 [4], the aging population bears the major brunt of stroke-related mortality and disability. At these current trends, the increased prevalence of stroke victims suffering from disability will place serious burdens on both the families caring for stroke victims and the capacity and costs of the healthcare system.
Ischemic stroke accounts for 87 % of all stroke cases and offers the greatest potential for therapeutic prevention and treatment [1]. Currently, there are only two therapies available to patients who present with an acute ischemic stroke: thrombolytic agents and endovascular interventions for acute clot retrieval. If the patient is examined and diagnosed within three hours of stroke onset and there are no contra-indications to lytic use, then intravenous tissue plasminogen activator (tPA) can be given [5, 6]. Treatment with tPA carries risks, including possible intracerebral hemorrhage as demonstrated in Fig. 1. In any case, less than 5 % of the nearly 800,000 patients who present with stroke annually are treated with tPA, mostly due to its narrow time window [1, 7, 8]. For patients that fall outside of this 3-h window, alternative treatments include mechanical clot removal or local application of lytic agents through an intravascular catheter. Even with these advanced treatments (that are often not available outside of large comprehensive stroke centers), many patients still fall outside the therapeutic treatment window [1, 7]. Thus, given the current population trends and the limited availability of treatments, it is imperative that focus turns to stroke prevention, new therapeutic options that can be utilized in clinically realistic time windows, and strategies that improve recovery once a stroke has occurred.
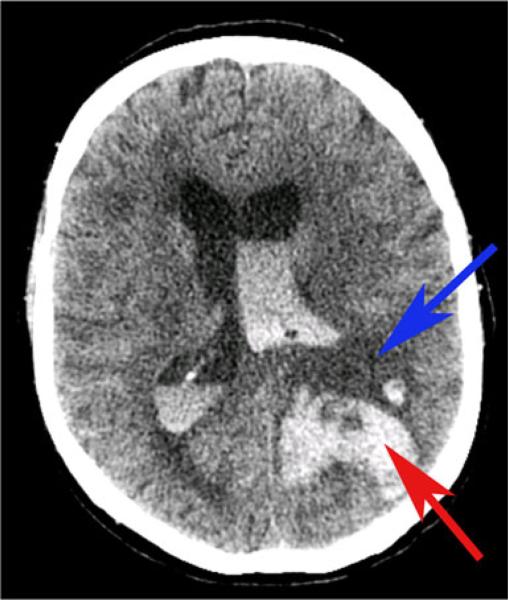
Fig. 1.
Case of intraventricular and intracerebral hemorrhage following treatment with tPA 70-year-old man with left middle cerebral artery stroke who was administered tPA and subsequently worsened. Repeat CT scan showed intraventricular and intracerebral hemorrhage as well as midline shift. Blue arrow indicates ischemic infarct and red arrow indicates hemorrhage
Aging and Stroke
Aging is a complex process that leads to an altered innate immune environment in the brain [9]. Chronic low-grade inflammation is seen in older individuals, which contributes to the development of age-related diseases including stroke [10–13]. Aging leads to both higher baseline and stimulus-induced activation of central pro-inflammatory cytokines [1, 14–17] compared with the young brain. Systemic inflammation (such as can occur with infections) increases brain inflammation in both animals and in humans [18], but the detrimental effects are primarily seen in the aged. Peripheral or central LPS administration (the major component of the outer membrane of Gram-negative bacteria) dramatically increases brain cytokine synthesis (i.e., interleukin (IL)-1β, IL-18, and IFN-γ), and this is much more robust in the aged brain [15, 16, 19]. To make matters worse, the aging brain also loses endogenous anti-inflammatory and protective substances, such as IL-10, IL-4, and brain-derived neurotrophic factor, making it even more difficult to cope with ischemic stress [20, 21]. Aging primates and humans have been described as having higher microglial numbers (although differentiation from other myeloid cells with currently available markers can be difficult) with a more “activated” phenotype [22, 23]. As microglia are a major source of pro-inflammatory cytokines, this results in a heightened basal level of cytokines even in the absence of ischemic injury in aged brain [24]. The biochemical, structural, and metabolic changes that occur in the aging brain have only recently been recognized (see review by [25]. These are extremely important factors in the response to stroke, and recent epidemiology and preclinical evidence suggest that aging is a major contributor to the sex differences seen in stroke epidemiology and outcomes.
Sex Differences in Stroke
Clinical Epidemiology
Clinical evidence that suggests intrinsic sex differences play a role in the response to stroke comes from the extreme ends of the lifespan—in the very young and in the elderly. Clinically, if one collapses across all age groups, women are disproportionately more likely to be affected by stroke than men each year [1]. However, almost all of this excess risk is due to the dramatic increase in stroke incidence seen in elderly women, as throughout most of adulthood, the overall incidence of stroke in men is estimated to be 33 % higher than that of women [26]. This “male-sensitive” phenotype is also observed in the perinatal, neonatal, and childhood population; males are at higher risk of both hemorrhagic and ischemic stroke [27–32]. A sex disparity in outcomes after ischemic injury also exists, with female infants faring better than males [33–37]. Considering that sex hormone levels are low in both females and males at this age, these differences in outcomes may be influenced by sex-specific hormone-independent factors (i.e., XX vs. XY) or by early “organizational” (perinatal) effects of gonadal steroid exposure (see below).
Importantly, the epidemiology of stroke changes as women age. After the age of 75, stroke rates begin to increase and vastly exceed that of age-matched men by the age of 80 [17, 38]. Elderly women have more severe strokes, poorer recovery, and greater long-term disability [26, 39–41] compared with age-matched men. There are numerous potential confounding factors that contribute to this change in clinical stroke epidemiology, including the older age at which women have strokes. The age of a first stroke in women is on average four years higher than men [42] and advanced age is a well-established risk factor for poor outcomes and increased stroke-related mortality [43–45]. Aged patients also have additional co-morbid illnesses, such as diabetes and hypertension, which can influence outcome [42]. In addition, differences in stroke etiology also may contribute to the poorer outcomes seen in elderly women, most notably in cardioembolic strokes secondary to atrial fibrillation (AF). The incidence of atrial fibrillation is greater among men at all ages but given that there are almost twice as many women aged >75 years with AF compared with men, the absolute number of women with AF is growing [46–48]. Women are more likely to experience a cardioembolic stroke secondary to AF than men and this is true regardless of anticoagulant use [49, 50]. Female sex is an independent risk factor for stroke in AF with a hazard ratio of 1.5, and this is especially notable in women over 75. Risk stratification schemes now take female sex into account when considering risk of ischemic stroke from AF [49]. In a recent analysis of the AFFIRM study, women with AF spent less time in the therapeutic range (an INR of 2–3) when treated with warfarin, which not surprisingly led to increased stroke rates [51]. Sex-specific differences in the pharmacodynamics and therapeutic efficacy of warfarin anticoagulation may exist and remain to be explored. This is a critical point as embolic strokes lead to large territorial infarcts (i.e., MCA) with high NIHSS scores and greater resulting disability [52] compared with small vessel and atheromatous etiologies. There are also several social factors that likely contribute to the poor outcomes in elderly women. Many have outlived their spouses and are less likely to be able to return home after a stroke occurs leading to significantly higher institutionalization rates for women after stroke [51]. However, even after controlling for these confounders, evidence for an age-related loss of intrinsic female protection remains. It remains to be determined if this is loss of a beneficial factor in women (i.e., estrogen), or loss of a detrimental factor with age (i.e., androgens) in males, which is the subject of a review elsewhere in this issue.
Experimental Studies
Preclinical studies confirm that the effects of stroke vary based on both the sex and age of the animal examined. Although decreased ischemic damage occurs in adult female vs. male rodents in many models of induced global [53] and focal [48] cerebral ischemia (for reviews, see [54, 55]), we have found that female mice are more susceptible to stroke damage than males [24] after the age of 15 months. This has also been seen by others [56, 57] and the loss of circulating E2 with gonadal senescence has been one accepted etiology of this sex difference [58]. However, as hormone levels are similar in males and females prior to puberty yet females show reduced ischemic damage, there must be an unknown interaction with age and hormone loss or an intrinsic sex difference mediated by chromosome compliment and/or organizational effects of steroids (see recent reviews [25, 59]).
Estrogen Mediated Neuroprotection
Estrogen, specifically 17β-estradiol, is a neuroprotective hormone in the vast majority of animal studies (see the review by [60]. Sex steroid hormones can act via organizational effects which irreversibly commit tissues to a male or female phenotype, or activational effects which are reversible and dependent on the continued presence of the hormone [61–63]. Sex differences in stroke incidence and outcome are present in neonatal populations, long before the onset of the activational effects of gonadal hormones at puberty. Thus, recent attention has been given to the possible organizational effects of steroids which occur during prenatal and early postnatal life [64]. Males convert testosterone to estradiol within the brain through the action of aromatase, while the organization of the female brain occurs due to the lack of this locally produced estradiol. In females, estradiol produced by the ovaries circulates bound to α-fetoprotein, which prevents it from crossing the blood–brain barrier (BBB) [65]. These sex-specific organizational effects contribute to the respective sex-specific behaviors of males and females [66], as they appear to be permanent and are incapable of reversal via gonadectomy. Experimental evidence suggests that estradiol can protect the brain from stroke via activational mechanisms, as ovariectomy extinguishes this neuroprotective effect [24, 66]. These activational effects of acute estrogen exposure are also independent of chromosomal sex as evidenced by findings that estradiol administered at reperfusion also decreases infarct size in males [67]. However, very recent data suggest that ischemic sensitivity can also be patterned very early in development secondary to organizational effects of gonadal steroids [68]. Neonatal male rats were administered either exogenous testosterone proprionate (TP), the non-aromatizable androgen, dihydrotestosterone (DHT), or vehicle for 5 days after birth. At 3 months of age, a focal stroke was induced. Testosterone treated rats (but not DHT-treated animals) had significantly smaller infarct volumes as well as increased estradiol levels compared with oil-treated animals. TP-injected males also had increased testicular aromatase (P450arom) levels compared with oil-treated males. This suggests that neonatal exposure to exogenous testosterone (an aromatizable androgen) chronically up-regulates aromatase expression in the testes of the male rats, leading to higher serum estradiol and neuroprotection against ischemic injury in adulthood [68]. Interestingly peri-natal exposure to testosterone also increased the adult expression of X-linked inhibitor of apoptosis (XIAP), an anti-apoptotic protein that may be especially important in reducing ischemic injury in the female brain [69]. This study suggests that early exposure to gonadal hormones can have dramatic effects on the response to adult cerebrovascular injury.
Sex Differences in Inflammatory Signaling
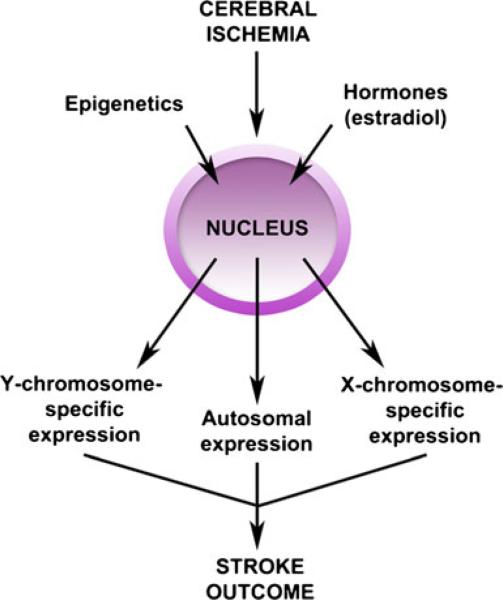
Genetic differences leading to alterations in the inflammatory response have been noted in other models [70, 71], but very little is known regarding ischemic stroke. Differential expression by sex of autosomal [72] and X-chromosome genes [73] have been identified in blood samples of ischemic stroke patients, and differential expression of Y-chromosome genes have been identified in male stroke patients compared with male controls [74]. Animal models have recently been developed that may help dissect out the effects of chromosomal sex and hormone exposure including the “Four Core Genotype” and the “Y consomic rat” model, but each has limitations (see reviews [75, 76]). Each of these models involves manipulation of the Y chromo-some or Y chromosome genes and are currently being evaluated in stroke models. Recent experimental studies assessed chromosome dosage effects in two separate strains of female mice with either an XX or XO chromosome compliment, which had no differences in infarct following induced stroke, regardless of acute hormone status. This suggests that the effects of X-chromosomal dose on acute ischemic sensitivity are minimal, at least in a reperfusion model. However, these animals are difficult to breed, animal numbers were small, and further studies using these models are warranted [77]. There are likely to be complex interactions between genetic sex and hormonal contributions to ischemic sensitivity that lead to subtle differences in pheno-type. More translationally relevant (yet costly) models, such as examining stroke sensitivity in aged XO mice, or examining spontaneously occurring strokes, may lead to very different results. Epigenetic mechanisms in stroke are also beginning to be explored and are reviewed extensively elsewhere (see [78–80]). Experimental challenges arise in identifying purely genetic, epigenetic, and hormonal effects as these all contribute to the complex inflammatory response to ischemic stroke (see Fig. 2).
Fig. 2.
Influence of epigenetics and hormones on genetic expression and stroke outcome
Sexual Dimorphism in Stroke-Induced Cell Death
Males Preferentially Utilize Caspase-Independent Cell Death Mechanisms
Several divergent cell death pathways are activated by ischemic injury, and these represent signaling cascades that could differ based on sex or hormonal patterning. Laboratory evidence demonstrates that males and females utilize different cell death pathways when confronted chemic with an is-insult, illustrated in Fig. 3. Males tend to exhibit caspase-independent cell death [81, 82] via the induction of nNOS [83], the formation of peroynitrite (ONOO–) with subsequent DNA damage through oxidation and nitration [84]. This DNA damage induces poly(ADP-ribose) polymerase-1 activation, translocation of apoptosis inducing factor from the mitochondrion to the nucleus [85, 86], and subsequent cell death. This has been supported by numerous experimental studies (see Table 1) and has been a subject of several recent reviews [18, 59, 87].
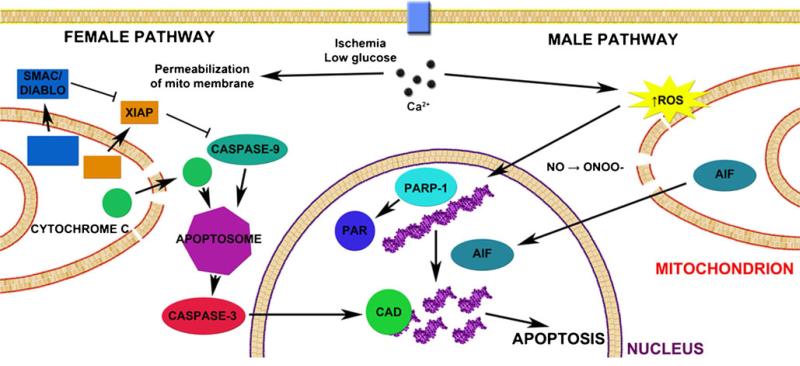
Fig. 3.
Sexual dimorphism in cell death following cerebral ischemia Caspase-dependent death predominates in females, where the influx of Ca2+ induces formation of the MAC which permeabilizes the mitochondrial membrane. Cytochrome c, Smac/DIABLO, and X-linked inhibitor of apoptosis protein (XIAP) are released into the cytosol. Cytochrome c and caspase-9 facilitate formation of the apoptosome which in turn activates caspase-3. Caspase-3 cleaves the inhibitor of caspase-activated DNase to give caspase-activated DNase (CAD) which subsequently cleaves DNA and results in apoptosis. Caspase-independent cell death predominates in males, in which ischemia results in an increase in reactive oxygen species (ROS) which combine with nitric oxide (NO) to form peroxynitrite (_ONOO_–). ONOO– damages DNA via oxygenation and nitration which activates polymerase (ADP-ribose), polymerase-1 (_PARP_-1), and formation of poly(ADP-ribose) (PAR) polymers. Permeabilization of the mitochondrial membrane enables apoptosis-inducing factor (AIF) to translocate from the intermembrane space of the mitochondrion to the nucleus where it causes specific DNA fragmentation patterns and chromatin condensation, ultimately resulting in apoptosis
Table 1.
Summary of experimental evidence suggestive of sexual dimorphism in cellular death following cerebral ischemia
| Experimental setting | Authors | Outcome on stroke severity | ||
|---|---|---|---|---|
| Males | Females | |||
| Caspase independent | PARP-1–/– | [11, 145] | ↓ | ↑ |
| PARP-1 inhibitor (minocycline) | [146] | ↓ | ↑1 | |
| [147] | ↓ | ↑1 | ||
| nNOS–/– | [148] | ↓ | ↑ | |
| nNOS inhibitor | [67, 148] | ↓ | ↑ | |
| Harlequin (low AIF) | [87, 149] | ↓ | ↔ | |
| Caspase dependent | Broad spectrum caspase inhibitor | [18] | ↔ | ↓ |
| XIAP small molecule inhibitor | [69] | ↔ | ↑ |
Females Favor Caspase-Dependent Cell Death
In females, caspase-induced cell death predominates, illustrated in Fig. 3. The influx of Ca2+ induces formation of the mitochondrial apoptosis-induced channel (MAC) which permeabilizes the mitochondrial membrane, prompting the release of cytochrome c into the cytosol activating the caspase cascade and subsequent cell death [88]. Importantly, this differential sensitivity to specific cell death mechanisms contributes to differential responsiveness to neuroprotective agents. Although this preclinical work is in its infancy, the future translational relevance could be high as many potential neuroprotective drugs interfere with these cell death pathways.
Clinical Evidence
Clinical studies are revealing clear sex differences in therapies utilized in the treatment of stroke patients, including thrombolytics and antiplatelet agents [15, 89]. For example, in the Women's Health Study, aspirin lowered the risk of stroke by 24 % in women in direct contrast to findings in the Physicians Health Study, a similar, male-only cohort, in which there was no effect of aspirin on stroke [90]. Similar sex-specific effects are also seen in infancy, implying that sex dimorphisms are established very early in development, either via organizational effects or chromosomal effects. For example, indomethacin significantly lowered the incidence and severity of intraventricular hemorrhage in preterm infants but did not improve later childhood cognitive performance. Subsequent reports in animals showed sex-specific effects of this drug, leading to a reanalysis of the clinical data. In boys, indomethacin reduced the incidence of IVH by half, and led to higher verbal IQ scores, but the drug had no effect in girls [37, 91, 92]. In pediatric stroke, when levels of androgens in males and estrogens in females are low, epidemiological findings indicate that boys have a higher incidence rate of stroke and worse outcomes than their corresponding female cohorts [2, 19, 30]. This indicates that hormones are not the only component of sexual dimorphism in ischemic stroke and that there may be a genetic or epigenetic component. Future studies of sex as an independent response variable are needed to optimize the development of future stroke therapies.
The observation that reproductive-aged, estrogen-producing women are at a much lower risk for stroke than their age- matched male and postmenopausal female counterparts suggests that estrogen replacement therapy (ERT) could have potential for preventative treatment against ischemic stroke. However, attempts to use estrogen to reduce stroke risk have been unsuccessful. In both the Women Estrogen Stroke Trial (WEST; conjugated equine estrogen, 0.625 mg/day) and the Women's Health Initiative (WHI) trial (estradiol, 1 mg/day) there was an increase in stroke incidence in women treated with estrogen [93, 94]. Current recommendations state that hormone replacement therapy (HRT) is not indicated for primary or secondary prevention of cardiovascular disease or dementia, nor for preventing deterioration of cognitive function in postmenopausal women [95]. However, several issues have been extensively discussed in the literature regarding the design of these trials, specifically the timing of initiation of ERT and the chosen dose. High doses of estrogen correlated with an elevated serum level of C-reactive protein (CRP) which is a marker of vascular risk [96–98]. Interestingly, in reproductiveage women, estrogen fluctuations that occur during the menstrual cycle inversely correlate with CRP protein levels [96, 99]. This suggests that endogenous levels of estrogen produce an anti-inflammatory effect, however higher doses of estrogen predispose women to increased vascular risk.
Recent data emerging from the Kronos Early Estrogen Prevention Study also suggest that increasing age is, as suspected, a major contributing factor in the response to ERT. Low-dose estrogen and cyclic monthly progesterone in healthy women initiated within 3 years after menopause did not increase the risk of myocardial infarction (MI), transient ischemic attacks, stroke, or venous thromboembolic disease (http://www.keepstudy.org/news/pr_100312_a.cfm). Consistent with these results, a European trial of women who were followed for over 10 years after randomization into hormone replacement therapy cohorts (triphasic estradiol and norethisterone acetate, or 2 mg estradiol a day in women with previous hysterectomy) initiated early after menopause found that treated women had a significantly reduced risk of mortality, heart failure, or MI, without any apparent increase in risk of cancer, venous thromboembolism, or stroke [100]. This is in contrast with results from the WEST and WHI trials, where ERT increased stroke incidence and severity when ERT was initiated after a prolonged period of hypestrogenicity. Importantly, these new findings correlate well with animal studies that demonstrated that estrogen can enhance the inflammatory response and worsen stroke damage if ERT was initiated after prolonged ovariectomy [101] or given months after cessation of ovarian cycling to aged female mice [102]. These studies highlight the importance of using appropriate preclinical animal paradigms to model human disease.
Stroke Risk and Hormonal Variations
An early age of menarche (<12 years of age) has been associated with increased risk of cardiovascular disease events including stroke; however, this was associated with increased adiposity [103]. Adiposity is associated with increased levels of estrogen, as white adipose tissue is capable of producing endogenous estrogen [104, 105]. High levels of estrogen may be associated with stroke risk, especially when they are in the nonphysiological range, as evidenced by the increase in stroke risk seen with older “high-estrogen” birth control [106, 107]. Recent studies have suggested that higher serum estrogen levels are associated with stroke [59], independent of BMI. However, as serum levels were measured post-stroke, this could be a response to the injury or even an attempt to control or reduce damage.
Another well-known period of high risk for women is during pregnancy and the peripartum. During pregnancy estrogen levels steadily climb and increases production of clotting factors, which may account for the increased risk of stroke in women in the peripartum period [108, 109]. More research is necessary to understand the underlying mechanisms of how estradiol, or perhaps fluctuations or changes in estradiol, could predispose women to a higher risk of stroke.
Effects of Estrogen on the Inflammatory Response in Stroke
Estrogen is a neuroprotective agent in most experimental studies, and this may be due in large part to a suppressive action of estrogen on inflammation [110–112]. Tumor necrosis factor (TNF) is involved in many neurodegenerative diseases including multiple sclerosis, Alzheimer's disease, and Parkinson's disease [113]. It has been known for some time that postmenopausal women have higher levels of circulating TNF [114]. Here, we will briefly discuss the role of estrogen on the TNF pathway and its downstream effects on nuclear factor-κB (NFκB) signaling in ischemic stroke.
Estrogen and TNF
TNF is a pro-inflammatory cytokine that acts on receptors found on neuronal [115, 116], glial [117], and endothelial cell populations [118]. Growing evidence suggests that while TNF is intended to aid in injury repair, excessive TNF can be neurotoxic [119–121]. Levels of TNF can increase to 100- to 1,000-fold that of basal levels following stroke in rats [122, 123] and is significantly elevated in stroke patients [124]. Female rats with low estrogen levels tend to produce more TNF than female rats with higher estrogen levels [125]. However, chronic administration of estradiol in mice stimulates the secretion of a variety of pro-inflammatory cytokines, including TNF itself [126]. Physiologic levels of estrogen appear to attenuate TNF expression [125] while estrogen deficiency (as in menopause) represents a loss of this attenuation and excessive TNF expression, as illustrated in Fig. 4.
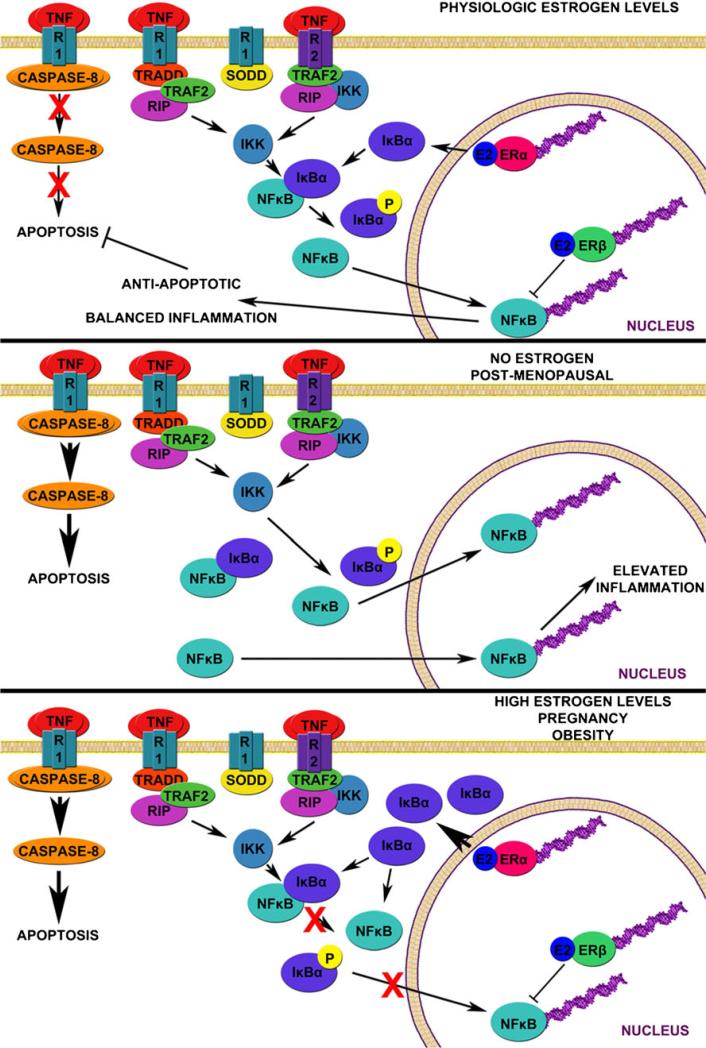
Fig. 4.
Hypothesized interaction between estradiol, TNF, and NFκB signaling At physiological levels of estradiol (E2), tumor necrosis factor (TNF) preferentially binds to TNF receptor 2 (TNFR2), denoted R2, leading to activation of nuclear factor-κ B (NFκB) which translocates to the nucleus. Balance between estrogen receptor-α (ERα) transcription of inhibitor of κ B-α (IκBα) and estrogen receptor-β (ERβ) inhibition of NFκB binding is present, resulting in balanced inflammation and net anti-apoptotic signaling. With physiological levels of E2, TNF levels are lower and there is reduced stimulation of TNF receptor 1 (TNFR1), denoted R1, and subsequent caspase signaling. In postmenopausal women, E2 levels decline and there is a lack of ERα-induced IκBα expression and ERβ-mediated inhibition of NFκB. Higher circulating TNF levels stimulate both TNFR2 and TNFR1, leading to relatively uninhibited inflammation and induction of caspase-dependent apoptosis. With high levels of E2 as seen in pregnancy and obesity, there is increased expression of IκBα which inhibits NFκB. The NFκB that translocates to the nucleus is inhibited by ERβ binding, inhibiting expression of anti-apoptotic genes by NFκB. Lack of inhibition of TNFR1 signaling results in caspase-dependent apoptosis. See reviews [120, 144]
Several experiments suggest that targeting TNF may be a translationally relevant target for ischemic stroke therapy. Infliximab (Remicade) and adalimumab (Humira) are anti-TNF therapies that have been approved for use in other clinical conditions. As we are already in the era of “biological” therapies, it is possible that some of these existing therapies have the potential to be developed for emergency care. Recently, a fusion protein of the extracellular domain of human TNFR2 was fused to the C-terminus of the heavy chain mouse transferrin receptor (TfR), named cTfRMAb-TNFR [127]. Administration of this antibody 45 min after arterial occlusion led to a significant reduction in both infarct and neurobehavioral deficits, suggesting that these agents can cross the BBB and have significant therapeutic effects for estrogen-deficient postmenopausal women. However, the effect of this agent in aged mice and in females has not yet been examined. If the detrimental effects of TNF signaling are normally suppressed by estrogen, this could be a viable therapy for the majority of women with stroke (who are postmenopausal).
Estrogen and NFκB
NFκB is an inducible transcription factor that functions to mediate inflammatory signaling in a variety of cell types, including neurons [128, 129], and is illustrated in Fig. 4. Hypoxic conditions [130] and oxidative stress [131] lead to the immediate activation of NFκB [132]. Contradictory views on the role of NFκB in stroke stem from its dual function: to induce pro-inflammatory cytokine expression [133–135] and to induce survival signaling [136–139]. The difference may reside within the extent and severity of the ischemia and is reviewed elsewhere [140, 141]; however, here we focus on estrogen effects on NFκB signaling.
In vitro experiments in rat aortic smooth muscle cells have shown that estradiol binds to intracellular estrogen receptor β (ERβ) and promotes synthesis of inhibitor of κB-α (IκBα) [142]. This increase in IκBα levels is responsible for increased inhibition of NFκB signaling in the presence of estradiol [142]. A deficiency of IκBα in the brain predisposes the brain to a higher basal level of neuro-inflammation [143]. Pre-treatment with estradiol enhances binding of estradiol-ERβ complexes at promoters of vascular inflammatory genes MCP-1 and CINC-2β and reduces binding of NFκB, thereby reducing their expression [142]. While neurons express NFκB, some argue that the primary cell type subject to NFκB regulation in the CNS is the astrocyte [143]. Thus, while the interplay of estrogen in the NFκB pathway is being elucidated, more research needs to be done to understand the role of astrocytes in communicating responses to neurons following ischemic insult.
Final Remarks
The mechanisms underlying ischemic sexual dimorphism are only beginning to be understood and investigated. Differences in the cell death pathways activated by an ischemic injury in males and females require therapeutic exploitation. We require a better understanding of the immune response to injury and how both sex and gonadal hormones can influence the post-stroke immune response. Biological agents targeting stroke-induced inflammatory cytokines will be developed and these agents will need to be tested in clinically relevant animal models prior to administration to stroke patients [127]. The role of genes, epigenetic regulation, and how hormones interact with these will require considerable research.
Acknowledgments
This work was supported by the NINDS RO1 NS055215-06 to Dr. McCullough.
Footnotes
Conflict of Interest None.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–220. doi: 10.1161/CIR.0b013e31823ac046. doi:10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration. Stroke. 1997;28(3):491–9. doi: 10.1161/01.str.28.3.491. [DOI] [PubMed] [Google Scholar]
- 3.Plesnila N, Zhu C, Culmsee C, Groger M, Moskowitz MA, Blomgren K. Nuclear translocation of apoptosis-inducing factor after focal cerebral ischemia. J Cereb Blood Flow Metab. 2004;24(4):458–66. doi: 10.1097/00004647-200404000-00011. doi:10.1097/00004647-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Yu SW, Wang H, Dawson TM, Dawson VL. Poly(ADP-ribose) polymerase-1 and apoptosis inducing factor in neurotoxicity. Neurobiol Dis. 2003;14(3):303–17. doi: 10.1016/j.nbd.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333(24):1581–7. doi: 10.1056/NEJM199512143332401. doi:10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 6.Adams HP, Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38(5):1655–711. doi: 10.1161/STROKEAHA.107.181486. doi:10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 7.Penumbra Pivotal Stroke Trial Investigators The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40(8):2761–8. doi: 10.1161/STROKEAHA.108.544957. doi:10.1161/STROKEAHA.108.544957. [DOI] [PubMed] [Google Scholar]
- 8.California Acute Stroke Pilot Registry (CASPR) Investigators Prioritizing interventions to improve rates of thrombolysis for ischemic stroke. Neurology. 2005;64(4):654–9. doi: 10.1212/01.WNL.0000151850.39648.51. doi:10.1212/01.WNL.0000151850.39648.51. [DOI] [PubMed] [Google Scholar]
- 9.Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, et al. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297(5579):259–63. doi: 10.1126/science.1072221. doi:10.1126/science.1072221297/5579/259. [DOI] [PubMed] [Google Scholar]
- 10.Eliasson MJ, Huang Z, Ferrante RJ, Sasamata M, Molliver ME, Snyder SH, et al. Neuronal nitric oxide synthase activation and peroxynitrite formation in ischemic stroke linked to neural damage. J Neurosci. 1999;19(14):5910–8. doi: 10.1523/JNEUROSCI.19-14-05910.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, et al. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997;3(10):1089–95. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- 12.Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265(5180):1883–5. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 13.Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991;88(14):6368–71. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du L, Bayir H, Lai Y, Zhang X, Kochanek PM, Watkins SC, et al. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem. 2004;279(37):38563–70. doi: 10.1074/jbc.M405461200. doi:10.1074/jbc.M405461200M405461200. [DOI] [PubMed] [Google Scholar]
- 15.Turtzo LC, McCullough LD. Sex-specific responses to stroke. Future Neurol. 2010;5(1):47–59. doi: 10.2217/fnl.09.66. doi:10.2217/fnl.09.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H, Pin S, Zeng Z, Wang MM, Andreasson KA, McCullough LD. Sex differences in cell death. Ann Neurol. 2005;58(2):317–21. doi: 10.1002/ana.20538. doi:10.1002/ana.20538. [DOI] [PubMed] [Google Scholar]
- 17.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7(10):915–26. doi: 10.1016/S1474-4422(08)70193-5. doi:10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu F, Li Z, Li J, Siegel C, Yuan R, McCullough LD. Sex differences in caspase activation after stroke. Stroke. 2009;40(5):1842–8. doi: 10.1161/STROKEAHA.108.538686. doi:10.1161/STROKEAHA.108.538686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang JT, McCullough LD. Pathways to ischemic neuronal cell death: are sex differences relevant? J Transl Med. 2008;6:33. doi: 10.1186/1479-5876-6-33. doi:10.1186/1479-5876-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Renolleau S, Fau S, Charriaut-Marlangue C. Gender-related differences in apoptotic pathways after neonatal cerebral ischemia. Neuroscientist. 2008;14(1):46–52. doi: 10.1177/1073858407308889. doi:10.1177/1073858407308889. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Nair A, Krady K, Corpe C, Bonneau RH, Simpson IA, et al. Estrogen stimulates microglia and brain recovery from hypoxia-ischemia in normoglycemic but not diabetic female mice. J Clin Invest. 2004;113(1):85–95. doi: 10.1172/JCI200418336. doi:10.1172/JCI18336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harry GJ, Kraft AD. Microglia in the developing brain: a potential target with lifetime effects. Neurotoxicology. 2012;33(2):191–206. doi: 10.1016/j.neuro.2012.01.012. doi:10.1016/j.neuro.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheffield LG, Berman NE. Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol Aging. 1998;19(1):47–55. doi: 10.1016/s0197-4580(97)00168-1. [DOI] [PubMed] [Google Scholar]
- 24.Liu F, Yuan R, Benashski SE, McCullough LD. Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab. 2009;29(4):792–802. doi: 10.1038/jcbfm.2009.5. doi:10.1038/jcbfm.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu F, McCullough LD. Interactions between age, sex, and hormones in experimental ischemic stroke. Neurochem Int. 2012 doi: 10.1016/j.neuint.2012.10.003. doi:10.1016/j.neuint.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40(4):1082–90. doi: 10.1161/STROKEAHA.108.540781. doi:10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong-Wells J, Johnston SC, Wu YW, Sidney S, Fullerton HJ. Prevalence and predictors of perinatal hemorrhagic stroke: results from the kaiser pediatric stroke study. Pediatrics. 2009;123(3):823–8. doi: 10.1542/peds.2008-0874. doi:10.1542/peds.2008-0874. [DOI] [PubMed] [Google Scholar]
- 28.Raju TN, Nelson KB, Ferriero D, Lynch JK. Ischemic perinatal stroke: summary of a workshop sponsored by the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke. Pediatrics. 2007;120(3):609–16. doi: 10.1542/peds.2007-0336. doi:10.1542/peds.2007-0336. [DOI] [PubMed] [Google Scholar]
- 29.Golomb MR, Dick PT, MacGregor DL, Curtis R, Sofronas M, de Veber GA. Neonatal arterial ischemic stroke and cerebral sinovenous thrombosis are more commonly diagnosed in boys. J Child Neurol. 2004;19(7):493–7. doi: 10.1177/08830738040190070301. [DOI] [PubMed] [Google Scholar]
- 30.Golomb MR, Fullerton HJ, Nowak-Gottl U, Deveber G. Male predominance in childhood ischemic stroke: findings from the international pediatric stroke study. Stroke. 2009;40(1):52–7. doi: 10.1161/STROKEAHA.108.521203. doi:10.1161/STROKEAHA.108.521203. [DOI] [PubMed] [Google Scholar]
- 31.Salih MA, Abdel-Gader AG, Al-Jarallah AA, Kentab AY, Alorainy IA, Hassan HH, et al. Stroke in Saudi children. Epidemiology, clinical features and risk factors. Saudi Med J. 2006;27(Suppl 1):S12–20. [PubMed] [Google Scholar]
- 32.Bonduel M, Sciuccati G, Hepner M, Pieroni G, Torres AF, Frontroth JP, et al. Arterial ischemic stroke and cerebral venous thrombosis in children: a 12-year Argentinean registry. Acta Haematol. 2006;115(3–4):180–5. doi: 10.1159/000090932. doi:10.1159/000090932. [DOI] [PubMed] [Google Scholar]
- 33.Ingemarsson I. Gender aspects of preterm birth. BJOG. 2003;110(Suppl 20):34–8. doi: 10.1016/s1470-0328(03)00022-3. [DOI] [PubMed] [Google Scholar]
- 34.Hintz SR, Kendrick DE, Vohr BR, Kenneth Poole W, Higgins RD. Gender differences in neurodevelopmental outcomes among extremely preterm, extremely-low-birthweight infants. Acta Paediatr. 2006;95(10):1239–48. doi: 10.1080/08035250600599727. doi:10.1080/08035250600599727. [DOI] [PubMed] [Google Scholar]
- 35.Sheiner E, Levy A, Katz M, Hershkovitz R, Leron E, Mazor M. Gender does matter in perinatal medicine. Fetal Diagn Ther. 2004;19(4):366–9. doi: 10.1159/000077967. doi:10.1159/00007796777967. [DOI] [PubMed] [Google Scholar]
- 36.Di Renzo GC, Rosati A, Sarti RD, Cruciani L, Cutuli AM. Does fetal sex affect pregnancy outcome? Gend Med. 2007;4(1):19–30. doi: 10.1016/s1550-8579(07)80004-0. [DOI] [PubMed] [Google Scholar]
- 37.Johnston MV, Hagberg H. Sex and the pathogenesis of cerebral palsy. Dev Med Child Neurol. 2007;49(1):74–8. doi: 10.1017/s0012162207000199.x. doi:10.1111/j.1469-8749.2007.0199a.x. [DOI] [PubMed] [Google Scholar]
- 38.Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA. Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke. 2009;40(4):1032–7. doi: 10.1161/STROKEAHA.108.542894. doi:10.1161/STROKEAHA.108.542894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niewada M, Kobayashi A, Sandercock PA, Kaminski B, Czlonkowska A. Influence of gender on baseline features and clinical outcomes among 17,370 patients with confirmed ischaemic stroke in the international stroke trial. Neuroepidemiology. 2005;24(3):123–8. doi: 10.1159/000082999. doi:10.1159/000082999. [DOI] [PubMed] [Google Scholar]
- 40.Roquer J, Campello AR, Gomis M. Sex differences in first-ever acute stroke. Stroke. 2003;34(7):1581–5. doi: 10.1161/01.STR.0000078562.82918.F6. doi:10.1161/01.STR.0000078562.82918. [DOI] [PubMed] [Google Scholar]
- 41.Fukuda M, Kanda T, Kamide N, Akutsu T, Sakai F. Gender differences in long-term functional outcome after first-ever ischemic stroke. Intern Med. 2009;48(12):967–73. doi: 10.2169/internalmedicine.48.1757. [DOI] [PubMed] [Google Scholar]
- 42.Arnold M, Halpern M, Meier N, Fischer U, Haefeli T, Kappeler L, et al. Age-dependent differences in demographics, risk factors, co-morbidity, etiology, management, and clinical outcome of acute ischemic stroke. J Neurol. 2008;255(10):1503–7. doi: 10.1007/s00415-008-0949-9. doi:10.1007/s00415-008-0949-9. [DOI] [PubMed] [Google Scholar]
- 43.Bhatnagar P, Sinha D, Parker RA, Guyler P, O'Brien A. Intravenous thrombolysis in acute ischaemic stroke: a systematic review and meta-analysis to aid decision making in patients over 80 years of age. J Neurol Neurosurg Psychiatry. 2011;82(7):712–7. doi: 10.1136/jnnp.2010.223149. doi:10.1136/jnnp.2010.223149. [DOI] [PubMed] [Google Scholar]
- 44.Engelter ST, Reichhart M, Sekoranja L, Georgiadis D, Baumann A, Weder B, et al. Thrombolysis in stroke patients aged 80 years and older: Swiss survey of IV thrombolysis. Neurology. 2005;65(11):1795–8. doi: 10.1212/01.wnl.0000183702.04080.27. doi:10.1212/01.wnl.0000183702.04080.27. [DOI] [PubMed] [Google Scholar]
- 45.Zeevi N, Kuchel GA, Lee NS, Staff I, McCullough LD. Interventional stroke therapies in the elderly: are we helping? AJNR Am J Neuroradiol. 2012;33(4):638–42. doi: 10.3174/ajnr.A2845. doi:10.3174/ajnr.A2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155(5):469–73. [PubMed] [Google Scholar]
- 47.Lane DA, Lip GY. Female gender is a risk factor for stroke and thromboembolism in atrial fibrillation patients. Thromb Haemost. 2009;101(5):802–5. [PubMed] [Google Scholar]
- 48.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29(1):159–65. doi: 10.1161/01.str.29.1.159. discussion 66. [DOI] [PubMed] [Google Scholar]
- 49.Hart RG, Eikelboom JW, Pearce LA. Sex, Stroke, and Atrial Fibrillation. Arch Neurol. 2012:1–3. doi: 10.1001/archneurol.2012.2691. doi:10.1001/archneurol.2012.26911362172. [DOI] [PubMed] [Google Scholar]
- 50.Avgil Tsadok M, Jackevicius CA, Rahme E, Humphries KH, Behlouli H, Pilote L. Sex differences in stroke risk among older patients with recently diagnosed atrial fibrillation. JAMA. 2012;307(18):1952–8. doi: 10.1001/jama.2012.3490. doi:10.1001/jama.2012.3490. [DOI] [PubMed] [Google Scholar]
- 51.Sullivan RM, Zhang J, Zamba G, Lip GY, Olshansky B. Relation of Gender-Specific Risk of Ischemic Stroke in Patients With Atrial Fibrillation to Differences in Warfarin Anticoagulation Control (from AFFIRM). Am J Cardiol. 2012 doi: 10.1016/j.amjcard.2012.08.014. doi:10.1016/j.amjcard.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 52.Forster A, Gass A, Kern R, Wolf ME, Ottomeyer C, Zohsel K, et al. Gender differences in acute ischemic stroke: etiology, stroke patterns and response to thrombolysis. Stroke. 2009;40(7):2428–32. doi: 10.1161/STROKEAHA.109.548750. doi:10.1161/STROKEAHA.109.548750. [DOI] [PubMed] [Google Scholar]
- 53.Hall ED, Pazara KE, Linseman KL. Sex differences in postischemic neuronal necrosis in gerbils. J Cereb Blood Flow Metab. 1991;11(2):292–8. doi: 10.1038/jcbfm.1991.61. doi:10.1038/jcbfm.1991.61. [DOI] [PubMed] [Google Scholar]
- 54.Herson PS, Koerner IP, Hurn PD. Sex, sex steroids, and brain injury. Semin Reprod Med. 2009;27(3):229–39. doi: 10.1055/s-0029-1216276. doi:10.1055/s-0029-1216276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hurn PD, Vannucci SJ, Hagberg H. Adult or perinatal brain injury: does sex matter? Stroke. 2005;36(2):193–5. doi: 10.1161/01.STR.0000153064.41332.f6. doi:10.1161/01.STR.0000153064.41332.f6. [DOI] [PubMed] [Google Scholar]
- 56.Lewis DK, Thomas KT, Selvamani A, Sohrabji F. Age-related severity of focal ischemia in female rats is associated with impaired astrocyte function. Neurobiol Aging. 2012;33(6):1123, e1–16. doi: 10.1016/j.neurobiolaging.2011.11.007. doi:10.1016/j.neurobiolaging.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dinapoli VA, Benkovic SA, Li X, Kelly KA, Miller DB, Rosen CL, et al. Age exaggerates proinflammatory cytokine signaling and truncates signal transducers and activators of transcription 3 signaling following ischemic stroke in the rat. Neuroscience. 2010;170(2):633–44. doi: 10.1016/j.neuroscience.2010.07.011. doi:10.1016/j.neuroscience.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turtzo LC, McCullough LD. Sex differences in stroke. Cerebrovasc Dis. 2008;26(5):462–74. doi: 10.1159/000155983. doi:10.1159/000155983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manwani B, McCullough LD. Estrogen in ischaemic stroke: the debate continues. Eur J Neurol. 2012;19(10):1276–7. doi: 10.1111/j.1468-1331.2012.03746.x. doi:10.1111/j.1468-1331.2012.03746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hurn PD, Macrae IM. Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab. 2000;20(4):631–52. doi: 10.1097/00004647-200004000-00001. doi:10.1097/00004647-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 61.Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146(4):1650–73. doi: 10.1210/en.2004-1142. doi:10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 62.Arnold AP. Mouse models for evaluating sex chromosome effects that cause sex differences in non-gonadal tissues. J Neuroendocrinol. 2009;21(4):377–86. doi: 10.1111/j.1365-2826.2009.01831.x. doi:10.1111/j.1365-2826.2009.01831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30(1):1–9. doi: 10.1016/j.yfrne.2008.11.001. doi:10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cohen-Bendahan CC, van de Beek C, Berenbaum SA. Prenatal sex hormone effects on child and adult sex-typed behavior: methods and findings. Neurosci Biobehav Rev. 2005;29(2):353–84. doi: 10.1016/j.neubiorev.2004.11.004. doi:10.1016/j.neubiorev.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 65.Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, et al. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci. 2006;9(2):220–6. doi: 10.1038/nn1624. doi:10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- 66.Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav. 2009;55(5):570–8. doi: 10.1016/j.yhbeh.2009.03.011. doi:10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab. 2003;14(5):228–35. doi: 10.1016/s1043-2760(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 68.Persky RW, Liu F, Xu Y, Weston G, Levy S, Roselli CE, et al. Neonatal testosterone exposure protects adult male rats from stroke. Neuroendocrinology. 2012 doi: 10.1159/000343804. doi:10.1159/000343804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Siegel C, Li J, Liu F, Benashski SE, McCullough LD. miR-23a regulation of X-linked inhibitor of apoptosis (XIAP) contributes to sex differences in the response to cerebral ischemia. Proc Natl Acad Sci U S A. 2011;108(28):11662–7. doi: 10.1073/pnas.1102635108. doi:10.1073/pnas.1102635108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10(5):338–49. doi: 10.1016/S1473-3099(10)70049-9. doi:10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chrousos GP. Stress and sex versus immunity and inflammation. Sci Signal. 2010;3(143) doi: 10.1126/scisignal.3143pe36. doi:10.1126/scisignal.3143pe36.pe36. [DOI] [PubMed] [Google Scholar]
- 72.Tian Y, Stamova B, Jickling GC, Liu D, Ander BP, Bushnell C, et al. Effects of gender on gene expression in the blood of ischemic stroke patients. J Cereb Blood Flow Metab. 2012;32(5):780–91. doi: 10.1038/jcbfm.2011.179. doi:10.1038/jcbfm.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stamova B, Tian Y, Jickling G, Bushnell C, Zhan X, Liu D, et al. The X-chromosome has a different pattern of gene expression in women compared with men with ischemic stroke. Stroke. 2012;43(2):326–34. doi: 10.1161/STROKEAHA.111.629337. doi:10.1161/STROKEAHA.111.629337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tian Y, Stamova B, Jickling GC, Xu H, Liu D, Ander BP, et al. Y chromosome gene expression in the blood of male patients with ischemic stroke compared with male controls. Gend Med. 2012;9(2):68–75. e3. doi: 10.1016/j.genm.2012.01.005. doi:10.1016/j.genm.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sampson AK, Jennings GL, Chin-Dusting JP. Y are males so difficult to understand?: a case where “X” does not mark the spot. Hypertension. 2012;59(3):525–31. doi: 10.1161/HYPERTENSIONAHA.111.187880. doi:10.1161/HYPERTENSIONAHA.111.187880. [DOI] [PubMed] [Google Scholar]
- 76.Manwani B, McCullough LD. Sexual dimorphism in ischemic stroke: lessons from the laboratory. Womens Health (Lond Engl) 2011;7(3):319–39. doi: 10.2217/whe.11.22. doi:10.2217/whe.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turtzo LC, Siegel C, McCullough LD. X chromosome dosage and the response to cerebral ischemia. J Neurosci. 2011;31(37):13255–9. doi: 10.1523/JNEUROSCI.0621-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qureshi IA, Mehler MF. The emerging role of epigenetics in stroke: III. Neural stem cell biology and regenerative medicine. Arch Neurol. 2011;68(3):294–302. doi: 10.1001/archneurol.2011.6. doi:10.1001/archneurol.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qureshi IA, Mehler MF. The emerging role of epigenetics in stroke: II. RNA regulatory circuitry. Arch Neurol. 2010;67(12):1435–41. doi: 10.1001/archneurol.2010.300. doi:10.1001/archneurol.2010.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qureshi IA, Mehler MF. Emerging role of epigenetics in stroke: part 1: DNA methylation and chromatin modifications. Arch Neurol. 2010;67(11):1316–22. doi: 10.1001/archneurol.2010.275. doi:10.1001/archneurol.2010.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiong ZG, Pignataro G, Li M, Chang SY, Simon RP. Acid-sensing ion channels (ASICs) as pharmacological targets for neurodegenerative diseases. Curr Opin Pharmacol. 2008;8(1):25–32. doi: 10.1016/j.coph.2007.09.001. doi:10.1016/j.coph.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.MacDonald JF, Xiong ZG, Jackson MF. Paradox of Ca2+ signaling, cell death and stroke. Trends Neurosci. 2006;29(2):75–81. doi: 10.1016/j.tins.2005.12.001. doi:10.1016/j.tins.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 83.Beckman JS, Crow JP. Pathological implications of nitric oxide, superoxide and peroxynitrite formation. Biochem Soc Trans. 1993;21(2):330–4. doi: 10.1042/bst0210330. [DOI] [PubMed] [Google Scholar]
- 84.Crow JP, Beckman JS. Reactions between nitric oxide, superoxide, and peroxynitrite: footprints of peroxynitrite in vivo. Adv Pharmacol. 1995;34:17–43. doi: 10.1016/s1054-3589(08)61079-0. [DOI] [PubMed] [Google Scholar]
- 85.Andrabi SA, Kim NS, Yu SW, Wang H, Koh DW, Sasaki M, et al. Poly(ADP-ribose) (PAR) polymer is a death signal. Proc Natl Acad Sci U S A. 2006;103(48):18308–13. doi: 10.1073/pnas.0606526103. doi:10.1073/pnas.0606526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu SW, Andrabi SA, Wang H, Kim NS, Poirier GG, Dawson TM, et al. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc Natl Acad Sci U S A. 2006;103(48):18314–9. doi: 10.1073/pnas.0606528103. doi:10.1073/pnas.0606528103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yuan M, Siegel C, Zeng Z, Li J, Liu F, McCullough LD. Sex differences in the response to activation of the poly(ADP-ribose) polymerase pathway after experimental stroke. Exp Neurol. 2009;217(1):210–8. doi: 10.1016/j.expneurol.2009.02.012. doi:10.1016/j.expneurol.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reubold TF, Eschenburg S. A molecular view on signal transduction by the apoptosome. Cell Signal. 2012;24(7):1420–5. doi: 10.1016/j.cellsig.2012.03.007. doi:10.1016/j.cellsig.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 89.Bushnell CD. Stroke and the female brain. Nat Clin Pract Neurol. 2008;4(1):22–33. doi: 10.1038/ncpneuro0686. doi:10.1038/ncpneuro0686. [DOI] [PubMed] [Google Scholar]
- 90.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352(13):1293–304. doi: 10.1056/NEJMoa050613. doi:10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 91.Ment LR, Vohr BR, Makuch RW, Westerveld M, Katz KH, Schneider KC, et al. Prevention of intraventricular hemorrhage by indomethacin in male preterm infants. J Pediatr. 2004;145(6):832–4. doi: 10.1016/j.jpeds.2004.07.035. doi:10.1016/j.jpeds.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 92.Ohlsson A, Roberts RS, Schmidt B, Davis P, Moddeman D, Saigal S, et al. Male/female differences in indomethacin effects in preterm infants. J Pediatr. 2005;147(6):860–2. doi: 10.1016/j.jpeds.2005.07.032. doi:10.1016/j.jpeds.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 93.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345(17):1243–9. doi: 10.1056/NEJMoa010534. doi:10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- 94.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women's Health Iniative: a randomized trial. JAMA. 2003;289(20):2673–84. doi: 10.1001/jama.289.20.2673. doi:10.1001/jama.289.20.2673289/20/2673. [DOI] [PubMed] [Google Scholar]
- 95.Marjoribanks J, Farquhar C, Roberts H, Lethaby A. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2012:7. doi: 10.1002/14651858.CD004143.pub4. doi:10.1002/14651858.CD004143.pub4. [DOI] [PubMed] [Google Scholar]
- 96.Gaskins AJ, Wilchesky M, Mumford SL, Whitcomb BW, Browne RW, Wactawski-Wende J. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle Study. Am J Epidemiol. 2012;175(5):423–31. doi: 10.1093/aje/kwr343. doi:10.1093/aje/kwr343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carcaillon L, Garcia-Garcia FJ, Tresguerres JA, Gutierrez Avila G, Kireev R, Rodriguez-Manas L. Higher levels of endogenous estradiol are associated with frailty in postmenopausal women from the toledo study for healthy aging. J Clin Endocrinol Metab. 2012;97(8):2898–906. doi: 10.1210/jc.2012-1271. doi:10.1210/jc.2012-1271jc.2012-1271. [DOI] [PubMed] [Google Scholar]
- 98.Lakoski SG, Herrington DM. Effects of hormone therapy on C-reactive protein and IL-6 in postmenopausal women: a review article. Climacteric. 2005;8(4):317–26. doi: 10.1080/13697130500345109. doi:10.1080/13697130500345109. [DOI] [PubMed] [Google Scholar]
- 99.Wander K, Brindle E, O'Connor KA. C-reactive protein across the menstrual cycle. Am J Phys Anthropol. 2008;136(2):138–46. doi: 10.1002/ajpa.20785. doi:10.1002/ajpa.20785. [DOI] [PubMed] [Google Scholar]
- 100.Schierbeck LL, Rejnmark L, Tofteng CL, Stilgren L, Eiken P, Mosekilde L, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ. 2012;345:e6409. doi: 10.1136/bmj.e6409. doi:10.1136/bmj.e6409bmj.e6409. [DOI] [PubMed] [Google Scholar]
- 101.Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc Natl Acad Sci U S A. 2007;104(14):6013–8. doi: 10.1073/pnas.0610394104. doi:10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu F, Benashski SE, Xu Y, Siegel M, McCullough LD. Effects of chronic and acute oestrogen replacement therapy in aged animals after experimental stroke. J Neuroendocrinol. 2012;24(2):319–30. doi: 10.1111/j.1365-2826.2011.02248.x. doi:10.1111/j.1365-2826.2011.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw KT, et al. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab. 2009;94(12):4953–60. doi: 10.1210/jc.2009-1789. doi:10.1210/jc.2009-1789. [DOI] [PubMed] [Google Scholar]
- 104.Meseguer A, Puche C, Cabero A. Sex steroid biosynthesis in white adipose tissue. Horm Metab Res. 2002;34(11–12):731–6. doi: 10.1055/s-2002-38249. doi:10.1055/s-2002-38249. [DOI] [PubMed] [Google Scholar]
- 105.Bulun SE, Simpson ER. Competitive reverse transcription-polymerase chain reaction analysis indicates that levels of aromatase cytochrome P450 transcripts in adipose tissue of buttocks, thighs, and abdomen of women increase with advancing age. J Clin Endocrinol Metab. 1994;78(2):428–32. doi: 10.1210/jcem.78.2.8106632. [DOI] [PubMed] [Google Scholar]
- 106.Lidegaard O, Lokkegaard E, Jensen A, Skovlund CW, Keiding N. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366(24):2257–66. doi: 10.1056/NEJMoa1111840. doi:10.1056/NEJMoa1111840. [DOI] [PubMed] [Google Scholar]
- 107.Zakharova MY, Meyer RM, Brandy KR, Datta YH, Joseph MS, Schreiner PJ, et al. Risk factors for heart attack, stroke, and venous thrombosis associated with hormonal contraceptive use. Clin Appl Thromb Hemost. 2011;17(4):323–31. doi: 10.1177/1076029610368670. doi:10.1177/1076029610368670. [DOI] [PubMed] [Google Scholar]
- 108.Kittner SJ, Stern BJ, Feeser BR, Hebel R, Nagey DA, Buchholz DW, et al. Pregnancy and the risk of stroke. N Engl J Med. 1996;335(11):768–74. doi: 10.1056/NEJM199609123351102. doi:10.1056/NEJM199609123351102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tettenborn B. Stroke and pregnancy. Neurol Clin. 2012;30(3):913–24. doi: 10.1016/j.ncl.2012.06.002. doi:10.1016/j.ncl.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 110.Das A, Smith JA, Gibson C, Varma AK, Ray SK, Banik NL. Estrogen receptor agonists and estrogen attenuate TNF-alpha-induced apoptosis in VSC4.1 motoneurons. J Endocrinol. 2011;208(2):171–82. doi: 10.1677/JOE-10-0338. doi:10.1677/JOE-10-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tenenbaum M, Azab AN, Kaplanski J. Effects of estrogen against LPS-induced inflammation and toxicity in primary rat glial and neuronal cultures. J Endotoxin Res. 2007;13(3):158–66. doi: 10.1177/0968051907080428. doi:10.1177/0968051907080428. [DOI] [PubMed] [Google Scholar]
- 112.Suzuki S, Brown CM, Wise PM. Neuroprotective effects of estrogens following ischemic stroke. Front Neuroendocrinol. 2009;30(2):201–11. doi: 10.1016/j.yfrne.2009.04.007. doi:10.1016/j.yfrne.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Montgomery SL, Bowers WJ. Tumor necrosis factor-alpha and the roles it plays in homeostatic and degenerative processes within the central nervous system. J Neuroimmune Pharmacol. 2012;7(1):42–59. doi: 10.1007/s11481-011-9287-2. doi:10.1007/s11481-011-9287-2. [DOI] [PubMed] [Google Scholar]
- 114.Kamada M, Irahara M, Maegawa M, Ohmoto Y, Takeji T, Yasui T, et al. Postmenopausal changes in serum cytokine levels and hormone replacement therapy. Am J Obstet Gynecol. 2001;184(3):309–14. doi: 10.1067/mob.2001.109940. doi:10.1067/mob.2001.109940. [DOI] [PubMed] [Google Scholar]
- 115.Dziewulska D, Mossakowski MJ. Cellular expression of tumor necrosis factor a and its receptors in human ischemic stroke. Clin Neuropathol. 2003;22(1):35–40. [PubMed] [Google Scholar]
- 116.Figiel I, Dzwonek K. TNFalpha and TNF receptor 1 expression in the mixed neuronal-glial cultures of hippocampal dentate gyrus exposed to glutamate or trimethyltin. Brain Res. 2007;1131(1):17–28. doi: 10.1016/j.brainres.2006.10.095. doi:10.1016/j.brainres.2006.10.095. [DOI] [PubMed] [Google Scholar]
- 117.Dopp JM, Mackenzie-Graham A, Otero GC, Merrill JE. Differential expression, cytokine modulation, and specific functions of type-1 and type-2 tumor necrosis factor receptors in rat glia. J Neuroimmunol. 1997;75(1–2):104–12. doi: 10.1016/s0165-5728(97)00009-x. [DOI] [PubMed] [Google Scholar]
- 118.Bebo BF, Jr, Linthicum DS. Expression of mRNA for 55-kDa and 75-kDa tumor necrosis factor (TNF) receptors in mouse cerebrovascular endothelium: effects of interleukin-1 beta, interferon-gamma and TNF-alpha on cultured cells. J Neuroimmunol. 1995;62(2):161–7. doi: 10.1016/0165-5728(95)00113-5. [DOI] [PubMed] [Google Scholar]
- 119.Tobinick E, Kim NM, Reyzin G, Rodriguez-Romanacce H, Depuy V. Selective TNF inhibition for chronic stroke and traumatic brain injury: an observational study involving 629 consecutive patients treated with perispinal etanercept. CNS Drugs. 2012 doi: 10.1007/s40263-012-0013-2. doi:10.1007/s40263-012-0013-2. [DOI] [PubMed] [Google Scholar]
- 120.Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab. 2012;32(9):1677–98. doi: 10.1038/jcbfm.2012.88. doi:10.1038/jcbfm.2012.88jcbfm201288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Smith JA, Das A, Ray SK, Banik NL. Role of pro-inflammatory cytokines released from microglia in neurode-generative diseases. Brain Res Bull. 2012;87(1):10–20. doi: 10.1016/j.brainresbull.2011.10.004. doi:10.1016/j.brainresbull.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, et al. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke. 1994;25(7):1481–8. doi: 10.1161/01.str.25.7.1481. [DOI] [PubMed] [Google Scholar]
- 123.Maddahi A, Kruse LS, Chen QW, Edvinsson L. The role of tumor necrosis factor-alpha and TNF-alpha receptors in cerebral arteries following cerebral ischemia in rat. J Neuroinflammation. 2011;8:107. doi: 10.1186/1742-2094-8-107. doi:10.1186/1742-2094-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cui G, Wang H, Li R, Zhang L, Li Z, Wang Y, et al. Polymorphism of tumor necrosis factor alpha (TNF-alpha) gene promoter, circulating TNF-alpha level, and cardiovascular risk factor for ischemic stroke. J Neuroinflammation. 2012;9(1):235. doi: 10.1186/1742-2094-9-235. doi:10.1186/1742-2094-9-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liao SL, Chen WY, Chen CJ. Estrogen attenuates tumor necrosis factor-alpha expression to provide ischemic neuroprotection in female rats. Neurosci Lett. 2002;330(2):159–62. doi: 10.1016/s0304-3940(02)00754-1. [DOI] [PubMed] [Google Scholar]
- 126.Calippe B, Douin-Echinard V, Laffargue M, Laurell H, Rana-Poussine V, Pipy B, et al. Chronic estradiol administration in vivo promotes the proinflammatory response of macrophages to TLR4 activation: involvement of the phosphatidylinositol 3-kinase pathway. J Immunol. 2008;180(12):7980–8. doi: 10.4049/jimmunol.180.12.7980. [DOI] [PubMed] [Google Scholar]
- 127.Sumbria RK, Boado RJ, Pardridge WM. Brain protection from stroke with intravenous TNFalpha decoy receptor-Trojan horse fusion protein. J Cereb Blood Flow Metab. 2012 doi: 10.1038/jcbfm.2012.97. doi:10.1038/jcbfm.2012.97jcbfm201297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Matthews JR, Hay RT. Regulation of the DNA binding activity of NF-kappa B. Int J Biochem Cell Biol. 1995;27(9):865–79. doi: 10.1016/1357-2725(95)00071-v. [DOI] [PubMed] [Google Scholar]
- 129.Emmanouil M, Taoufik E, Tseveleki V, Vamvakas SS, Probert L. A role for neuronal NF-kappaB in suppressing neuroinflammation and promoting neuroprotection in the CNS. Adv Exp Med Biol. 2011;691:575–81. doi: 10.1007/978-1-4419-6612-4_60. doi:10.1007/978-1-4419-6612-4_60. [DOI] [PubMed] [Google Scholar]
- 130.Rupec RA, Baeuerle PA. The genomic response of tumor cells to hypoxia and reoxygenation. Differential activation of transcription factors AP-1 and NF-kappa B. Eur J Biochem. 1995;234(2):632–40. doi: 10.1111/j.1432-1033.1995.632_b.x. [DOI] [PubMed] [Google Scholar]
- 131.Dudek EJ, Shang F, Taylor A. H(2)O(2)-mediated oxidative stress activates NF-kappa B in lens epithelial cells. Free Radic Biol Med. 2001;31(5):651–8. doi: 10.1016/s0891-5849(01)00634-7. [DOI] [PubMed] [Google Scholar]
- 132.Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science. 2002;298(5596):1241–5. doi: 10.1126/science.1071914. doi:10.1126/science.1071914298/5596/1241. [DOI] [PubMed] [Google Scholar]
- 133.Carroll JE, Howard EF, Hess DC, Wakade CG, Chen Q, Cheng C. Nuclear factor-kappa B activation during cerebral reperfusion: effect of attenuation with N-acetylcysteine treatment. Brain Res Mol Brain Res. 1998;56(1–2):186–91. doi: 10.1016/s0169-328x(98)00045-x. [DOI] [PubMed] [Google Scholar]
- 134.Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med. 1999;5(5):554–9. doi: 10.1038/8432. doi:10.1038/8432. [DOI] [PubMed] [Google Scholar]
- 135.Nurmi A, Lindsberg PJ, Koistinaho M, Zhang W, Juettler E, Karjalainen-Lindsberg ML, et al. Nuclear factor-kappaB contributes to infarction after permanent focal ischemia. Stroke. 2004;35(4):987–91. doi: 10.1161/01.STR.0000120732.45951.26. doi:10.1161/01.STR.0000120732.45951.26. [DOI] [PubMed] [Google Scholar]
- 136.Culmsee C, Siewe J, Junker V, Retiounskaia M, Schwarz S, Camandola S, et al. Reciprocal inhibition of p53 and nuclear factor-kappaB transcriptional activities determines cell survival or death in neurons. J Neurosci. 2003;23(24):8586–95. doi: 10.1523/JNEUROSCI.23-24-08586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hill WD, Hess DC, Carroll JE, Wakade CG, Howard EF, Chen Q, et al. The NF-kappaB inhibitor diethyldithiocarba-mate (DDTC) increases brain cell death in a transient middle cerebral artery occlusion model of ischemia. Brain Res Bull. 2001;55(3):375–86. doi: 10.1016/s0361-9230(01)00503-2. [DOI] [PubMed] [Google Scholar]
- 138.Foehr ED, Lin X, O'Mahony A, Geleziunas R, Bradshaw RA, Greene WC. NF-kappa B signaling promotes both cell survival and neurite process formation in nerve growth factor-stimulated PC12 cells. J Neurosci. 2000;20(20):7556–63. doi: 10.1523/JNEUROSCI.20-20-07556.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Duckworth EA, Butler T, Collier L, Collier S, Pennypacker KR. NF-kappaB protects neurons from ischemic injury after middle cerebral artery occlusion in mice. Brain Res. 2006;1088(1):167–75. doi: 10.1016/j.brainres.2006.02.103. doi:10.1016/j.brainres.2006.02.103. [DOI] [PubMed] [Google Scholar]
- 140.Sarnico I, Lanzillotta A, Benarese M, Alghisi M, Baiguera C, Battistin L, et al. NF-kappaB dimers in the regulation of neuronal survival. Int Rev Neurobiol. 2009;85:351–62. doi: 10.1016/S0074-7742(09)85024-1. doi:10.1016/S0074-7742(09)85024-1. [DOI] [PubMed] [Google Scholar]
- 141.Ridder DA, Schwaninger M. NF-kappaB signaling in cerebral ischemia. Neuroscience. 2009;158(3):995–1006. doi: 10.1016/j.neuroscience.2008.07.007. doi:10.1016/j.neuroscience.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 142.Xing D, Oparil S, Yu H, Gong K, Feng W, Black J, et al. Estrogen modulates NFkappaB signaling by enhancing IkappaBalpha levels and blocking p65 binding at the promoters of inflammatory genes via estrogen receptor-beta. PLoS One. 2012;7(6):e36890. doi: 10.1371/journal.pone.0036890. doi:10.1371/journal.pone.0036890PONE-D-11-18299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lian H, Shim DJ, Gaddam SS, Rodriguez-Rivera J, Bitner BR, Pautler RG, et al. IkappaBalpha deficiency in brain leads to elevated basal neuroinflammation and attenuated response following traumatic brain injury: implications for functional recovery. Mol Neurodegener. 2012;7(1):47. doi: 10.1186/1750-1326-7-47. doi:10.1186/1750-1326-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev. 2006;210:171–86. doi: 10.1111/j.0105-2896.2006.00375.x. doi:10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 145.Liu F, Lang J, Li J, Benashski SE, Siegel M, Xu Y, et al. Sex differences in the response to poly(ADP-ribose) polymerase-1 deletion and caspase inhibition after stroke. Stroke. 2011;42(4):1090–6. doi: 10.1161/STROKEAHA.110.594861. doi:10.1161/STROKEAHA.110.594861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hoda MN, Li W, Ahmad A, Ogbi S, Zemskova MA, Johnson MH, et al. Sex-independent neuroprotection with minocycline after experimental thromboembolic stroke. Exp Transl Stroke Med. 2011;3(1):16. doi: 10.1186/2040-7378-3-16. doi:10.1186/2040-7378-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li J, McCullough LD. Sex differences in minocycline-induced neuroprotection after experimental stroke. J Cereb Blood Flow Metab. 2009;29(4):670–4. doi: 10.1038/jcbfm.2009.3. doi:10.1038/jcbfm.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25(4):502–12. doi: 10.1038/sj.jcbfm.9600059. doi:10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- 149.Klein JA, Longo-Guess CM, Rossmann MP, Seburn KL, Hurd RE, Frankel WN, et al. The harlequin mouse mutation down-regulates apoptosis-inducing factor. Nature. 2002;419(6905):367–74. doi: 10.1038/nature01034. doi:10.1038/nature01034nature01034. [DOI] [PubMed] [Google Scholar]