Identification and Functional Characterization of a K+ Channel α-Subunit with Regulatory Properties Specific to Brain (original) (raw)
Abstract
The physiological diversity of K+ channels mainly depends on the expression of several genes encoding different α-subunits. We have cloned a new K+ channel α-subunit (Kv2.3r) that is unable to form functional channels on its own but that has a major regulatory function. Kv2.3r can coassemble selectively with other α-subunits to form functional heteromultimeric K+channels with kinetic properties that differ from those of the parent channels. Kv2.3r is expressed exclusively in the brain, being concentrated particularly in neocortical neurons. The functional expression of this regulatory α-subunit represents a novel mechanism without precedents in voltage-gated channels, which might contribute to further increase the functional diversity of K+ channels necessary to specify the intrinsic electrical properties of individual neurons.
Keywords: K+ channels, α-subunit, cloning, regulatory mechanism, channel diversity, neurons
Electrical signaling in neurons and other excitable cells is determined mainly by the functional characteristics of their K+ channels. The best known K+channels are the voltage-gated, which comprise a diverse family of membrane proteins participating in action potential repolarization and the regulation of repetitive firing (see Connor and Stevens, 1971;Rogawski, 1985; Rudy, 1988; Hille, 1992). These K+ channels are composed of four homologous α-subunits, forming a transmembrane aqueous-conducting pore selective for K+ ions. All α-subunits share a common general design: a central core with six putative transmembrane segments, flanked by hydrophilic N- and C-terminal domains of variable length, facing the cytosol (Tempel et al., 1987; MacKinnon, 1991; Jan and Jan, 1994). There are five major families of genes encoding voltage-gated K+ channels, designated as Kv1 (Shaker), Kv2 (Shab), Kv3 (Shaw), Kv4 (Shal), and Kv5. At least two additional α-subunits, unable to produce functional K+currents, have been cloned (Drewe et al., 1992). The sequence homology among α-subunits of the same family is >70%, but this value decreases to <50% among members of different families (Kamb et al., 1987; Papazian et al., 1987; Pongs et al., 1988; Stühmer et al., 1989; Drewe et al., 1992; Salkoff et al., 1992; Chandy and Gutman, 1993; Zhao et al., 1994). The marked functional variability of the K+ currents, a characteristic particularly apparent in mammalian neurons (Llinás, 1988; Rudy, 1988), arises from several mechanisms. These include the differential tissue and cellular expression of the various genetic families (Drewe et al., 1992; Hwang et al., 1992; Deal et al., 1994; Weiser et al., 1994) and the ability of α-subunits within a family to aggregate into oligomers. The formation of heteromeric K+ channels has been demonstrated in heterologous expression systems (Christie et al., 1990; Isacoff et al., 1990; Ruppersberg et al., 1990; Covarrubias et al., 1991; Li et al., 1992) as well as in brain cells (Sheng et al., 1993; Wang et al., 1993). In addition, there are regulatory hydrophilic β-subunits that can coassemble selectively with α-subunits (see Rettig et al., 1994;Yu et al., 1996).
Here, we report the identification of a protein (which we call Kv2.3r) with a structural design similar to K+ channel α-subunits, which seems to have a major regulatory function. Kv2.3r does not appear to produce functional channels by itself; however, it can form functional heteromers with other K+ channel α-subunits, such as Kv2.1 (Drk1; Frech et al., 1989). The coexpression of Kv2.3r and Kv2.1 results in channels exhibiting striking modifications in kinetics, if compared with homomultimeric Kv2.1 channels. Kv2.3r has no effect when it is coexpressed with Rbk1 (Kv1.1) or Shaker B channels (members of the Kv1 family;Tempel et al., 1987; Christie et al., 1989). Kv2.3r appears to be expressed almost exclusively in the brain, where it may contribute to the physiological diversity of K+ channels.
A preliminary account of these data has appeared in abstract form (Castellano et al., 1996).
MATERIALS AND METHODS
Isolation of Kv2.3r cDNA. A fragment of the Kv2.3r cDNA was identified initially from poly(A+) RNA obtained from rat cerebral cortex by reverse transcription and amplification with degenerate oligonucleotides, using PCR. The PCR primers had the following sequences: forward primer, CCTCTAGAA(TC)GAGTA(TC)TT(TC)TT(TC)GA(TC)(AC)G; reverse primer, GGGGATCC(GA)TA(ATG)CC(CAT)AC(AC)GT(GT)GTCAT. These primers were designed to hybridize two sequences highly conserved in K+channels: part of box B (amino acids NEYFFDR) and the pore (MTTVGYG), respectively (see Fig. 1 below). The full-length Kv2.3r cDNA was cloned by screening rat forebrain and hippocampus cDNA libraries with probes derived from the PCR-amplified fragment and using conditions described previously (Castellano et al., 1993).
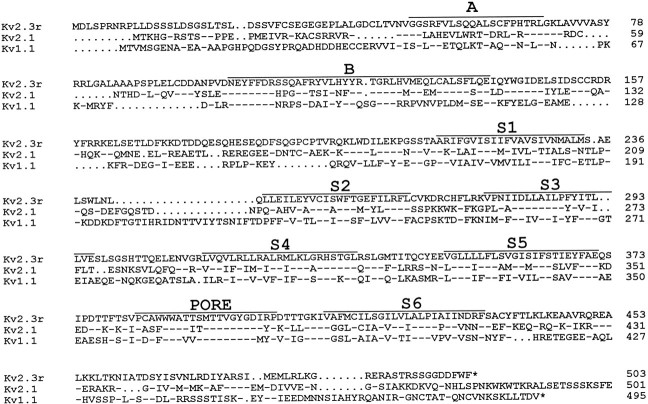
Fig. 1.
Amino acid sequence of Kv2.3r and alignment with the sequence of two other K+ channel α-subunits: Kv2.1 (Drk1) and Kv1.1 (Rbk1). The single letter code is used for amino acid identification. Hyphens indicate identity to the sequence at the top (Kv2.3r), and_dots_ represent gaps introduced to maintain alignment. The numbers indicate the positions in the respective sequence. Note that the C termini of Kv2.1 and Kv1.1 are shown incomplete. Bars delineate the extent of the conserved regions, including the putative transmembrane domains (S1–S6), the pore (PORE), and the_A_ and B boxes of the N terminal. The nucleotide sequence of Kv2.3r cDNA has been deposited in GenBank (accession number X98564).
Northern blot analysis and in situ _hybridization.For Northern blot analysis poly(A+) RNA was isolated from different rat tissues or CHO cells transfected with cDNA. Approximately 3 μg of poly(A+) RNA was applied in each lane, and Northern blot was performed as described (Lucas et al., 1993). We used as hybridization probe the_Eco_RI–_Cla_I fragment (nucleotides 1–442) of Kv2.3r cDNA, which has low sequence similarity with other cloned K+ channels. Washing after hybridization was done under high stringency conditions, and autoradiography developed after a few hours. Longer exposure (several days) confirmed the absence of hybridization signal in peripheral tissues. Hybridization of the blot with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or cyclophilin probes was done to evaluate the amount of RNA loaded in each lane. For_in situ hybridization cRNA antisense and sense probes for Kv2.3r were synthesized in vitro from linearized plasmid pBluescript–Kv2.3r, using 35S-UTP as described previously (Mellström et al., 1993), or with digoxigenin (DIG)-UTP, as suggested by the manufacturer (Boehringer Mannheim, Mannheim, Germany).In situ hybridization on fresh frozen rat brain sections (10 μm) was performed as described (Mellström et al., 1993). Detection of DIG was done by using anti-DIG Fab fragment conjugated to alkaline phosphatase (dilution 1:500) and nitro blue tetrazolium chloride (NBT) and 5-bromo-4-chloro-3-indolyl phosphate (BCIP) as substrates. For control of nonspecific hybridization, consecutive sections were hybridized with the corresponding sense probe.
_Functional expression and electrophysiological techniques._Kv2.3r, Kv2.1, Kv1.1, and Shaker B Δ6–46 cDNAs were subcloned in plasmid p513. The tandem dimer Kv2.1/Kv2.3r was generated by ligating a DNA fragment containing the entire Kv2.3r coding sequence into a p513–Kv2.1 construct with a deletion of its last 48 amino acids. This construction included amino acids 1–805 of Kv2.1, followed by a serine and the 503 amino acids of Kv2.3r. In some experiments cDNA of green fluorescent protein (GFP) included in plasmid pRK5 was cotransfected with K+ channel α-subunits to detect by fluorescence those cells expressing K+ currents (Marshall et al., 1995). In these experiments 100% of the cells that appeared as intensely fluorescent also expressed measurable K+currents. CHO cells were transiently transfected with 2–4 μg of the cDNAs by electroporation. When two α-subunits were coexpressed, we used for transfection equal amounts of each cDNA. Potassium currents were recorded 20–48 hr after transfection by the whole-cell configuration of the patch-clamp technique (Hamill et al., 1981). We used low-resistance electrodes (1–3 MΩ), capacity compensation, and subtraction of linear leakage and capacity currents. Full compensation of series resistance was not attempted systematically. Compensation was in all experiments <50%. Solution composition was (in mm): for the bath solution, 140 NaCl, 2.7 KCl, 2.5 CaCl2, 4 MgCl2, and 10 HEPES, pH 7.4; for the solution in the pipette and inside the cell, 80 KCl, 30 K-glutamate, 20 K-fluoride, 4 ATP·Mg, 10 HEPES, and 10 EGTA, pH 7.2. In the high K+ external solutions, 70 mm NaCl was replaced for 70 mm KCl. In some experiments ZnCl2 (up to 1 mm) was added to the external solution.
RESULTS
Identification and cloning of Kv2.3r
A fragment of K+ channel-like cDNA initially was identified by PCR amplification of poly(A+) RNA obtained from rat cerebral cortex. This fragment was used to isolate the full-length cDNA clone from a rat brain cDNA library. We have designated this cDNA sequence Kv2.3r, referring to its sequence similarity and selective regulatory effect on Kv2.1 (see below). The clone contains 2318 nucleotides with an open reading frame of 1509 nucleotides. The 3′ untranslated region has ∼800 nucleotides, and the 5′ untranslated region is short, with only nine nucleotides and no upstream stop codon preceding the assigned initiation codon (the first ATG found). However, this codon is flanked by an A at position −3 and a G at position 4, which conforms with the main requirements of the consensus sequence for initiation of translation (Kozak, 1987). A short 5′ untranslated region (13 nucleotides) also was reported in the Kv2.1 clone (Frech et al., 1989). The Kv2.3r cDNA encodes a protein of 503 amino acids with general characteristics similar to previously cloned K+ channel α-subunits (Fig. 1). The hydropathy plot (data not shown) suggests that Kv2.3r also is composed of six putative transmembrane segments (S1–S6) and of cytoplasmic N- and C-terminal domains. This protein has several potential intracellular phosphorylation sites: two for cAMP-dependent protein kinase, eight for caseine kinase II, and six for protein kinase C.
Kv2.3r has a relatively low degree of sequence identity with other known K+ channel subunits. This is illustrated in Figure 1where, to facilitate the comparison, we have included the amino acid sequences of two representative K+ channel α-subunits, Kv2.1 and Kv1.1. The highest similarity of Kv2.3r (44%) was found with members of the Kv2 family (Kv2.1 and Kv2.2; Frech et al., 1989; Drewe et al., 1992; Hwang et al., 1992), although important sequence divergence is observed in the loops among the transmembrane segments and in the amino and carboxyl ends. The degree of identity of Kv2.3r with channels of the Kv1 family is ∼35%. When the core regions (S1–S6) are compared, similarity of Kv2.3r with Kv2.1 increases to slightly <50%. Kv2.3r shares several important structural features with the channels of the Kv2 family, including the five positively charged amino acids in the S4 segment and the conservation of amino acids 132–136, which are part of the distinct Kv2 region described byWei et al. (1990). These observations suggest that Kv2.3r may have evolved from the Kv2 channel family.
Tissue distribution
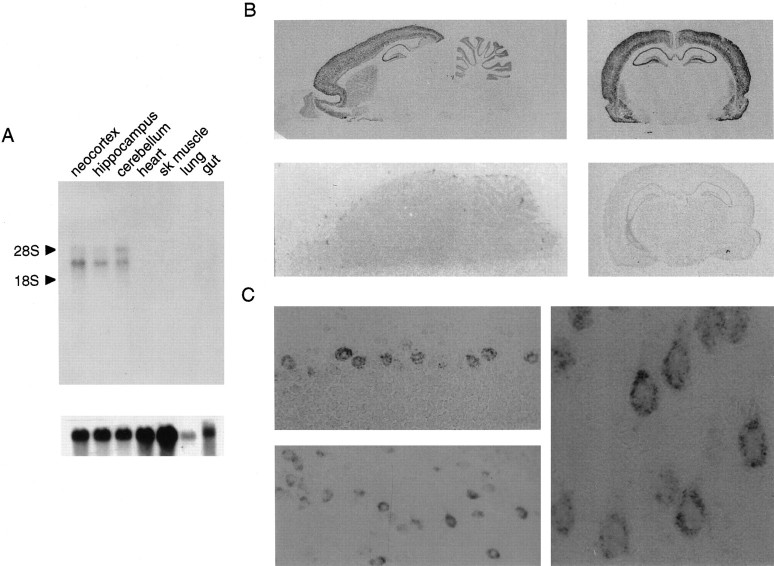
Although Kv2.3r does not seem to have intrinsic channel activity (see below), a regulatory role for this α-subunit was suggested by its distinctive tissue distribution. Whereas Kv2.1 and Kv2.2 channels are highly expressed in both brain and non-neural tissues (e.g., heart and skeletal muscle) (Drewe et al., 1992; Hwang et al., 1992), Kv2.3r seems to be expressed almost exclusively in the brain (Fig.2A). Northern blot analysis indicates that there are two Kv2.3r mRNA species of ∼5 and 3.3 kb that also seem to be distributed differentially within the brain. The 5 kb transcript is more abundant in cerebellum, whereas the 3.3 kb species is more prominent in the neocortex (Fig. 2A).In situ hybridization studies indicate that the greatest density of Kv2.3r mRNA is found in the neocortical layers II, III, and VI (particularly in the frontal cortex), olfactory tubercle, hippocampus (C1–C4 as well as dentate gyrus), piriform cortex, amygdala, and cerebellum (Purkinje and granular cells). Moderate densities of labeling occur in the olfactory bulb, striatum, septum, supraoptic nucleus, and lateral reticular nucleus (Fig.2B). Notable is the near absence of labeling in large areas like the diencephalon and the brainstem. The expression of Kv2.3r mRNA within representative neurons, including the large neocortical pyramidal cells, is illustrated in Figure 2C. Thus, there are several brain regions with high density of Kv2.3r mRNA overlapping the distribution of other K+ channels (Beckh and Pongs, 1990; Séquier et al., 1990; Drewe et al., 1992; Hwang et al., 1992; Weiser et al., 1994). This fact leads us to hypothesize that Kv2.3r might be regulating the function of these K+channels at specific locations.
Fig. 2.
Tissue distribution of Kv2.3r.A, Northern blot analysis indicating the selective expression of Kv2.3r in the brain. Two transcripts of ∼3.3 and 5 kb were identified in neocortex, hippocampus, and cerebellum, but no signal was detected in the other tissues studied (heart, skeletal muscle, lung, and gut). B, Parasagittal and coronal sections of the entire rat brain showing the cellular distribution of Kv2.3r mRNA. In the top panels the incubation with a Kv2.3r cRNA antisense probe shows its preferential expression in neocortex, cerebellum, hippocampus, and amygdala. The bottom panels demonstrate the lack of signal when the Kv2.3r cRNA sense probe was used. C, Microphotographs of in situ hybridization demonstrating the expression of Kv2.3r in Purkinje cells of the cerebellum (left,top), hippocampal CA4 neurons (left,bottom), and neocortical pyramidal cells (right). Magnifications are 900× (left) and 2700× (right).
Expression of Kv2.3r
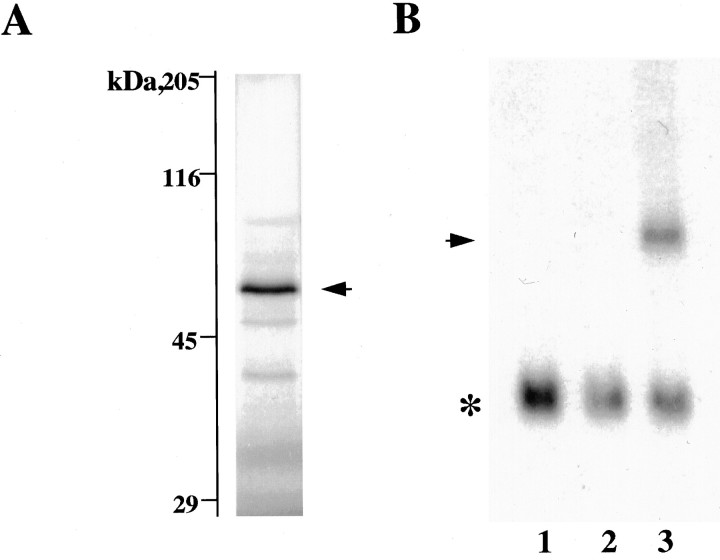
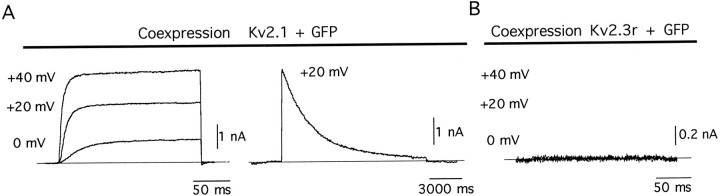
In vitro translation of Kv2.3r cRNA in reticulocyte lysates produced a protein of molecular weight similar to that predicted from the cDNA-derived amino acid sequence (∼56 kDa; Fig. 3A). In addition, Northern blot analysis in cells transfected with Kv2.3r cDNA indicated that it can be transcribed into the corresponding mRNA (Fig. 3B). However, under standard experimental conditions we were unable to detect measurable voltage-dependent K+ currents after injection of Kv2.3r cRNA into Xenopus oocytes or after transfection of CHO or HEK cells with cDNA. We also have studied cells cotransfected with K+ channels and GFP cDNAs to discard the possibility that Kv2.3r is expressed with very low efficiency and, therefore, only present in a few cells difficult to identify in patch-clamp experiments. All CHO cells cotransfected with GFP and Kv2.1 appearing as intensely fluorescent under the microscope exhibited large macroscopic K+ currents with normal activation and inactivation kinetics (Fig. 4A). In contrast, we never saw detectable K+ currents in intensely fluorescent cells cotransfected with GFP and Kv2.3r cDNA. In Figure4B we show recordings at high gain of a representative example of this last type of experiment. The expression of Kv2.3r also was studied in high external K+, which is known to facilitate the opening of some K+ channels (Pardo et al., 1992; López-Barneo et al., 1993). Because the effect of external K+ is influenced by the amino acid in the position equivalent to R402 of Kv2.3r (Pardo et al., 1992; López-Barneo et al., 1993), we tested whether the replacement of arginine by tyrosine (amino acid present at this position in Kv2.1 and Kv1.1) resulted in functional channels. Neither high external K+ nor the R402Y mutation of Kv2.3r yielded any measurable current. Thus, it seems reasonable to conclude that Kv2.3r is unable to form homomultimeric functional channels in heterologous expression systems. Among other factors, the lack of functional expression of Kv2.3r could be related to the presence of several amino acids (e.g., S234, K268, V382, W386, or A412) in Kv2.3r, which differ from residues highly conserved in voltage-gated K+ channels (see Drewe et al., 1992).
Fig. 3.
Expression of Kv2.3r. A,In vitro translation of Kv2.3r cDNA in reticulocyte lysates (Promega, TNT). Protein analysis was done on a 9% SDS-polyacrylamide gel. The arrow indicates the band corresponding to the Kv2.3r protein with a molecular weight of ∼59 kDa. B, Northern blot analysis of RNA obtained from CHO cells, using the same Kv2.3r probe as in Figure 2. Lane 1, Untransfected cells; lane 2, cells transfected with Kv2.1 cDNA; lane 3, cells cotransfected with Kv2.1 plus Kv2.3r. The arrow indicates a band of ∼1.6 kb corresponding to Kv2.3r cRNA. The _asterisk_indicates the hybridization with cyclophilin mRNA used as a control for the loading on each lane.
Fig. 4.
Coexpression of green fluorescent protein (GFP) and K+ channel α-subunits.A, Recordings from a cell expressing the_GFP_ and Kv2.1 currents. B, Recordings from a cell expressing the GFP that also was transfected with Kv2.3r cDNA. Note the absence of detectable K+ current. Current records obtained during depolarizing pulses to 0, +20, and_+40 mV_ are superimposed. Patch-clamp experiments were done 24–30 hr after cotransfection of the cells with 2 μg of_GFP_ and 2 μg of either Kv2.1 or_Kv2.3r_ cDNAs.
Functional characteristics and regulatory properties
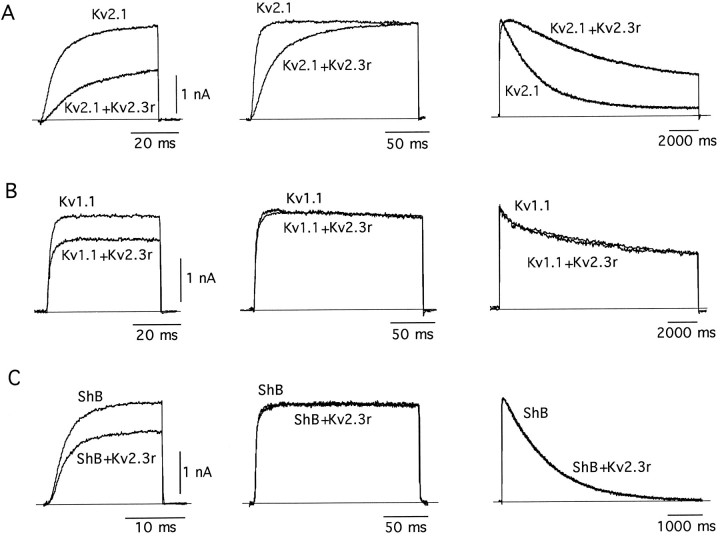
The fact that Kv2.3r mRNA is highly concentrated in specific areas of the brain (see above) suggested that it could have a regulatory role by forming heteromultimeric channels. This idea was tested by coexpressing Kv2.3r with other K+ channel α-subunits in CHO cells. Cotransfection of CHO cells with Kv2.3r plus Kv2.1, its most closely related structural K+ channel homolog, resulted in macroscopic K+ currents with somewhat smaller amplitude and profoundly different kinetics when compared with the currents produced by Kv2.1 alone. The Kv2.3r plus Kv.2.1 currents exhibited a striking deceleration of kinetics, with four- to fivefold slowing of activation and inactivation time courses observed at some membrane potentials (see Figs. 5, 6, Table1). In contrast, the coexpression of Kv2.3r with either Kv1.1 (Fig. 5B) or Shaker BΔ6–46 (Fig.5C) led to the expression of currents that, although of smaller amplitude, were kinetically indistinguishable from the currents generated by Kv1.1 or Shaker BΔ6–46 alone (see Table1).
Fig. 5.
Effect of coexpression of Kv2.3r with Kv2.1 (A), Kv1.1 (B), and Shaker_BΔ6–46 (ShB; C). In each case we superimposed K+ current traces obtained in cells transfected with a K+ channel α-subunit alone (Kv2.1, Kv1.1, or ShB) with the recordings obtained from cells transfected with a mixture of each type of α-subunit plus an equal amount of Kv2.3r (Kv2.1+Kv2.3r,Kv1.1+Kv2.3r, and ShB+Kv2.3r).Traces in the middle and right columns have been scaled to facilitate the comparison of activation and inactivation time courses, respectively. Note the marked and selective effect of Kv2.3r on Kv2.1 currents. In the_left and middle columns the depolarizing pulses used to open the channels were applied to 0 mV. In the_right column_ the pulses were applied to +20 mV. In all cases the holding potential was −80 mV.
Fig. 6.
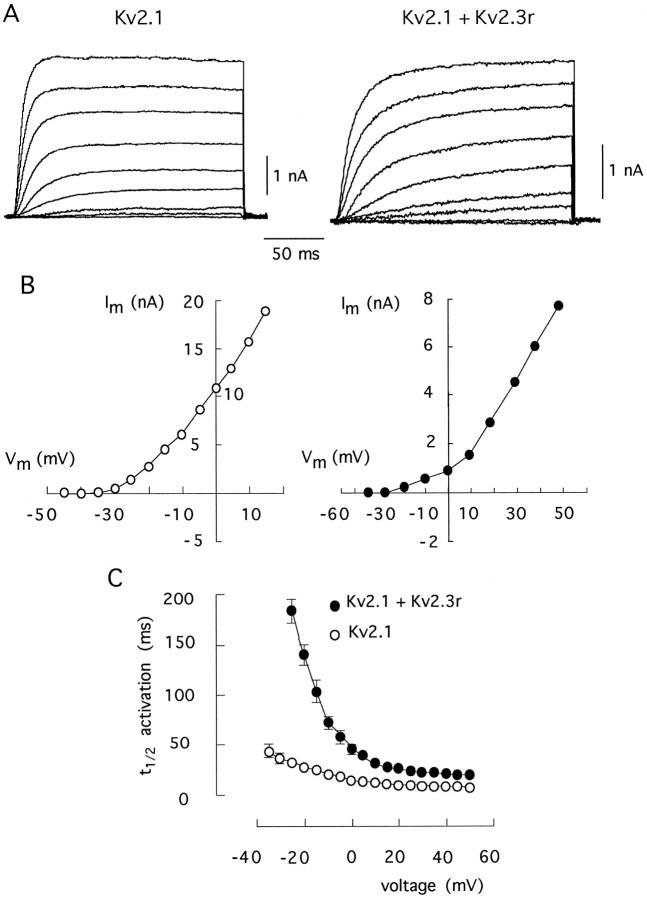
Comparison of the kinetic properties of homomultimeric Kv2.1 channels and of channels formed by the coexpression of Kv2.1 plus Kv2.3r. A, Families of macroscopic K+ currents obtained during depolarizing pulses to various membrane potentials (from −40 to +40 in steps of 10 mV) from a holding potential of −80 mV. B, Current–voltage curves obtained by plotting current amplitude measured at the end of each pulse (ordinate) as a function of membrane potential during the pulse (abscissa). C, Time to reach half-maximal (t1/2) activation of Kv2.1 and Kv2.1+Kv2.3r currents at various membrane potentials. Each point represents the mean ± SD of measurements done in seven experiments.
Table 1.
Comparison of the kinetic parameters of Kv2.1, Kv1.1, and_Shaker_ BΔ6–46 K+ channels expressed alone or coexpressed with Kv2.3r
| _t_1/2 activation (msec) | τ inactivation (msec) | τ closing (msec) | ||||
|---|---|---|---|---|---|---|
| −20 mV | 0 mV | +20 mV | +20 mV | −80 mV | −60 mV | |
| Kv2.1 | 38 ± 6 (7) | 13 ± 4 (8) | 11 ± 3 (8) | 3300 ± 420 (10) | 3.5 ± 0.2 (5) | 4.3 ± 0.4 (5) |
| Kv2.1 plus Kv2.3r | 161 ± 33 (5) | 52 ± 6 (8) | 21 ± 6 (7) | 19,400 ± 2,400 (8) | 3.6 ± 0.2 (2) | 7.4 ± 0.3 (2) |
| Kv1.1 | 3.4 ± 0.9 (6) | – | 2.6 ± 0.9 (7) | 5200 ± 1,100 (5) | – | – |
| Kv1.1 plus Kv2.3r | 3.8 ± 1.3 (4) | – | 2.8 ± 0.9 (4) | 6800 ± 2,200 (4) | – | – |
| Sh BΔ6–46 | 4.9 ± 1.3 (6) | – | 1.8 ± 0.6 (8) | 1300 ± 200 (8) | – | – |
| Sh BΔ6–46 plus Kv2.3r | 4.7 ± 1.4 (6) | – | 1.9 ± 0.3 (6) | 1200 ± 300 (7) | – | – |
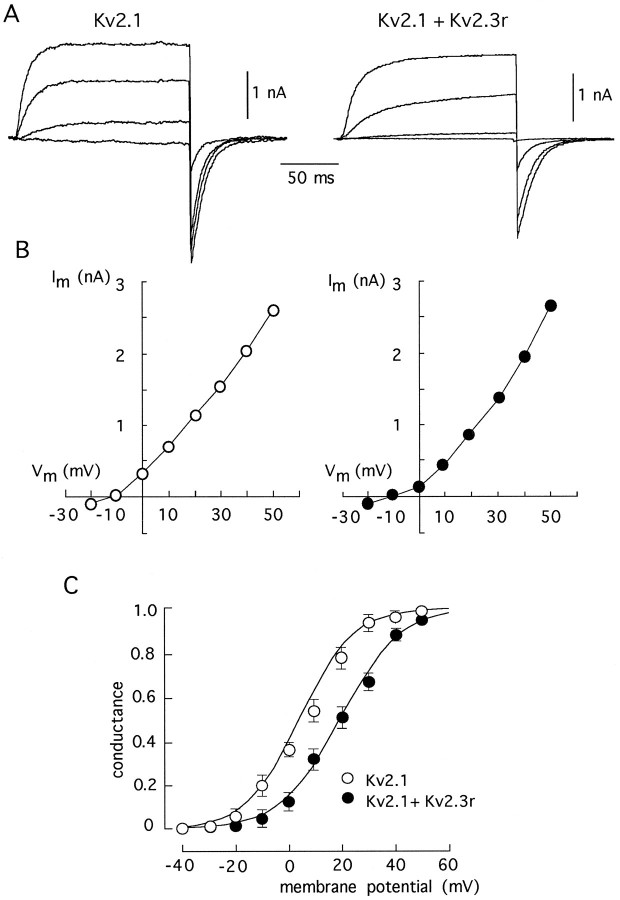
The effects of Kv2.3r on the Kv2.1 currents are illustrated further in Figures 6 and 7. Figure 6_A_shows representative current traces recorded at various membrane potentials in cells transfected with either Kv2.1 alone or with Kv2.1 plus Kv2.3r. The corresponding current–voltage curves, plotted in Figure 6B, indicate that the presence of Kv2.3r produced a displacement toward positive membrane potentials of the voltage dependence of activation. The marked voltage dependence of the slowing of activation time course induced by Kv2.3r is illustrated in Figure 6C. Notably, the effects of Kv2.3r on channel closing were also voltage-dependent (see Table 1). Figure 7 shows current traces obtained from cells transfected with either Kv2.1 alone or with Kv2.1 plus Kv2.3r, using high K+ (70 mm) in the external solution (Fig. 7A). Tail currents recorded at the end of the pulses represent the deactivation time course of the channels. The current–voltage curves in Figure 7B show that, despite all of the kinetics modifications caused by Kv2.3r on Kv2.1 channels, the reversal potential (at approximately −10 mV) was unchanged, indicating that the selectivity for K+ was not greatly altered. The conductance–voltage relationships shown in Figure7C illustrate that the presence of Kv2.3r leads to a shift in the voltage dependence of activation. At half-maximal activation voltage, the shift induced by Kv2.3r was between 10 and 15 mV.
Fig. 7.
Comparison of the kinetic properties of homomultimeric Kv2.1 channels and of channels formed by the coexpression of Kv2.1 plus Kv2.3r in high external K+ (70 mm). A, Families of macroscopic K+ currents obtained during depolarizing pulses to various membrane potentials (from −20 to +40 in steps of 20 mV) from a holding potential of −80 mV. Note the tail currents recorded on repolarization, representing the deactivation time course of the channels. B, Current–voltage curves obtained by plotting current amplitude measured at the end of each pulse (ordinate) as a function of membrane potential during the pulse (abscissa). The intersection of the curves with the x_-axis indicates the reversal potential.C, Average conductance–voltage relationships. Conductance was estimated from the amplitude of tail currents recorded on repolarization to −80 mV of voltage pulses delivered to variable membrane potentials. The values in the ordinate were normalized to the amplitude of the tails at +50 mV. Each_point represents the mean ± SD of measurements done in five to eight experiments. Curves drawn on the data points are least-squares fits to a Boltzmann function of the form:
G=1−(Gmax/[1+exp(V−V1/2)/k])
in which _G_max is maximal conductance, V the membrane potential during the pulse,V1/2 the potential at which 50% of Gmax is obtained (+9 mV for Kv2.1 and +19 mV for Kv2.1+Kv2.3r currents), and k a slope factor that indicates the steepness of the curve (7 mV for Kv2.1 and 7.9 mV for Kv2.1+Kv2.3r currents).
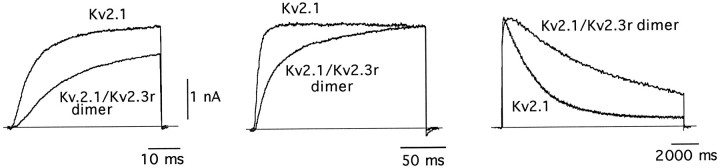
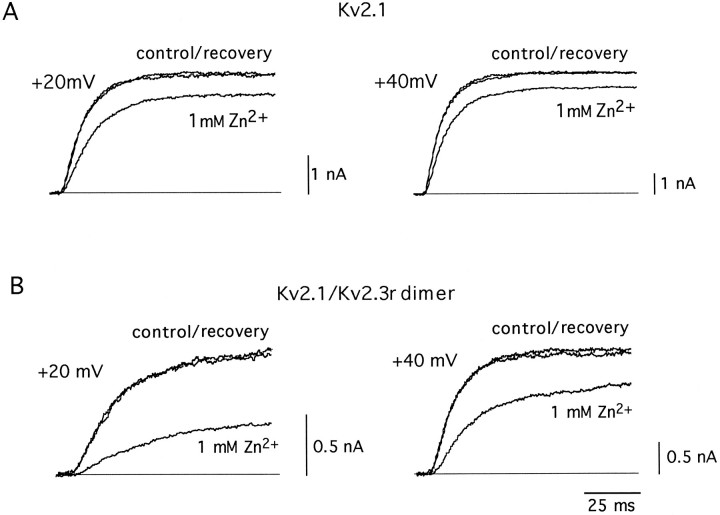
Further demonstration that Kv2.3r can, indeed, interact with Kv2.1 to form heteromultimeric K+ channels was obtained by the construction of a tandem dimer with the two α-subunits. This dimer was created by ligation of the 3′ end of the translated region of Kv2.1 and the 5′ end of Kv2.3r. The expression of this cDNA construct in CHO cells resulted in robust macroscopic K+ currents that presumably represent the activity of a homogeneous population of heteromultimeric Kv2.1/Kv2.3r channels (Fig.8). The currents recorded by the expression of the Kv2.1/Kv2.3r dimer exhibited modifications in activation and inactivation kinetics qualitatively similar to those produced by the coexpression of Kv2.1 and Kv2.3r (see Figure 8 legend). The formation of heteromultimeric channels by the Kv2.1/Kv2.3r dimer also was evaluated by studying its sensitivity to external Zn2+. The activity of many K+ channels is reduced by externally applied Zn2+ (see Gilly and Armstrong, 1982). However, it is known that the Kv2.1 channels are rather insensitive to the cation, with a Kd value of 14 mm (De Biasi et al., 1993). Because Kv2.1 and Kv2.3r have a high degree of sequence divergence in the extracellular loops between the transmembrane segments (see Fig. 1), we hypothesized that the heteromultimeric Kv2.1/Kv2.3r channels also could have modified their sensitivity to external Zn2+. In accord with this idea, Figure 9 shows that Zn2+ had a clearly larger effect on the dimer Kv2.1/Kv2.3r than on the homomultimeric Kv2.1 channels. Whereas at +20 mV 1 mm Zn2+ reduced Kv2.1 currents to only 84 ± 10% of the control value (Fig. 9A; also see De Biasi et al., 1993), it diminished Kv2.1/Kv2.3r currents to almost one-half of the control amplitude (53 ± 12%; n = 4; Fig.9B). Together, these data suggest that Kv2.3r can coassemble with Kv2.1, forming heteromultimeric K+ channels with characteristic functional properties.
Fig. 8.
Macroscopic K+ currents recorded from cells expressing either Kv2.1 channels or the Kv2.1/Kv2.3r tandem dimer. Traces are superimposed (and scaled in the_middle_ and right panels) to facilitate the comparison of the activation and inactivation time courses in the two types of currents. Because we wanted to stress the similarity between the Kv2.1+Kv2.3r and the Kv2.1/Kv2.3r tandem dimer currents, the Kv2.1 current traces of this figure are the same as in Figure5A. Depolarizing pulses are applied to 0 mV (left and middle panels) or +20 mV (right panels). Holding potential is −80 mV. For Kv2.1/Kv2.3r currents, t1/2 of activation at 0 mV is 25 ± 4 msec (mean ± SD, n = 6), and inactivation time constant at +20 mV is 9500 ± 2600 msec (n = 7).
Fig. 9.
Differential effects of external Zn2+(1 mm) on Kv2.1 and Kv2.1/Kv2.3r dimer currents.A, Reversible inhibition of Kv2.1 currents recorded at two different membrane potentials. B, Reversible inhibition of Kv2.1/Kv2.3r dimer K+ currents. Currents were generated by depolarization to the indicated membrane potentials. Holding potential is −80 mV.
DISCUSSION
The major finding in this paper is the cloning and functional characterization of a new K+ channel α-subunit that seems to have a regulatory function. Kv2.3r appears to be unable to form functional channels on its own but it seems to coassemble with other K+ channel α-subunits to form functional heteromultimeric channels with special biophysical features. Kv2.3r is expressed exclusively in the brain, partially overlapping the expression of other K+-channel α-subunits (Drewe et al., 1992; Hwang et al., 1992; Weiser et al., 1994). These facts suggest that Kv2.3r may contribute to specify the intrinsic electrophysiological properties of neurons. The detailed study of the interaction of Kv2.3r with the various families of K+ channel α-subunits currently is being undertaken; however, the available data indicate that it selectively may regulate channels of the Kv2 family. These channels are highly concentrated in neocortex, cerebellum, and hippocampus (Drewe et al., 1992; Hwang et al., 1992), which are the areas with the highest expression of Kv2.3r.
Although the molecular mechanism underlying the interaction of Kv2.3r with other K+ channel α-subunits is unknown for the moment, it may depend, at least in part, on the degree of structural similarity among their N termini. It is known that this domain is important for subunit recognition and assembly (Li et al., 1992; Lee et al., 1994). In addition, recently the existence of highly conserved regions within the N-terminal of α-subunits belonging to the same family (A and B boxes in Fig. 1) has been reported, which seem to determine subfamily-specific associations (Xu et al., 1995; Yu et al., 1996). The A box of Kv2.3r has low sequence identity with the same region of the other α-subunits studied here (40, 40, and 45% with Kv2.1, Kv1.1, and Shaker BΔ6–46, respectively); however, the B box of Kv2.3r has clearly higher similarity with the B box of Kv2.1 (68%) than with the same region of Kv1.1 (43%) or_Shaker_ BΔ6–46 (44%). Thus, the higher similarity of their respective B regions possibly permits Kv2.3r to select Kv2.1 against Kv1.1 or Shaker B. Part of the effects of the expression of Kv2.3r might result from altered subunit assembly, because the heteromeric Kv2.1/Kv2.3r channels share many kinetic properties with those exhibited by Kv2.1 channels with large N-terminal deletions (VanDongen et al., 1990).
During the final stages of preparation of this paper, an article appeared (Hugnot et al., 1996) reporting the cloning from hamster tissues of a K+ channel α-subunit (called by these authors Kv8.1) with essentially the same structure and tissue distribution as the Kv2.3r described here. Although there are only minor sequence differences between the two clones (three amino acid substitutions in the N-terminal, one substitution in the S2–S3 linker, and the insertion of a glycine in position 20), the reported functional properties are quite different. Like Kv2.3r, Kv8.1 does not have intrinsic channel activity; however, in Xenopus oocytes Kv8.1 seems to abolish the functional expression of K+channels of the Kv2 and Kv3 families. Hugnot and colleagues (1996) have shown that the interaction of Kv8.1 with the other α-subunits seems to occur through the N-terminal domains. These authors indicated in their article that, besides the original clone from hamster, they used a cDNA clone isolated from rat brain with similar electrophysiological results. Thus, the differences between their data and our observations described here are difficult to explain and could be ascribed to the fact that we used mammalian cells as the cDNA expression system instead of Xenopus oocytes.
On the basis of our experimental results, it is most tempting to propose that the normal function of Kv2.3r (or Kv8.1) is neither to produce channels by itself nor to abolish the expression of other channels but to coassemble with selected α-subunits to form heteromultimeric channels with modified electrophysiological properties. There are no precedents of this type of regulatory function in α-subunits of voltage-gated channels; however, it is a phenomenon well known in ligand-gated channels. For example, both NMDA (Monyer et al., 1992) and nicotinic (McGehee and Role, 1995) receptors contain membrane-spanning subunits without intrinsic channel activity that are functional only in heteromultimeric channels. Thus, Kv2.3r could be the first identified member of a family of regulatory α-subunits, possibly including other previously cloned K+ channel subunits without intrinsic channel activity (such as IK8 and K13; Drewe et al., 1992). Mammalian central neurons are known to exhibit a wide variety of characteristic electrical responses independent of their morphology or synaptology (see Llinás, 1988). Hence, the selective expression in individual neurons of regulatory K+channel α-subunits is a mechanism that could be used to finely tune their intrinsic electrophysiological properties.
Footnotes
This work was supported by grants from the Spanish Ministry of Science and Education. A.M. is a fellow of the Spanish Ministry of Science and Education. F.M. is recipient of a fellow/credit from Colciencias (Colombia). We thank Drs. P. de la Peña and A. Carrión, Mrs. P. Ortega, and Mr. R. Pardal for help with the experiments; we also thank Drs. J. Pascual, A. Brown, and R. Aldrich for providing us with the Kv2.1, Kv1.1, and Shaker Δ6–46 cDNAs. We are indebted to Drs. A. Franco-Obregón and L. Tabares for valuable comments on this manuscript.
Correspondence should be addressed to Dr. J. López-Barneo, Facultad de Medicina, Departamento de Fisiología Médica y Biofísica, Universidad de Sevilla, Avenida Sánchez Pizjuán 4, E-41009, Sevilla, Spain.
REFERENCES
- 1.Beckh S, Pongs O. Members of the RCK potassium channel family are differentially expressed in the rat nervous system. EMBO J. 1990;9:777–782. doi: 10.1002/j.1460-2075.1990.tb08173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castellano A, Wei X, Birnbaumer L, Pérez-Reyes E. Cloning and expression of a neuronal calcium channel β subunit. J Biol Chem. 1993;268:12359–12366. [PubMed] [Google Scholar]
- 3.Castellano A, Molina A, Mellström B, Naranjo JR, López-Barneo J. A neural K+ channel transcript encodes for a regulatory alfa subunit. Eur J Neurosci [Suppl] 1996;9:14. [Google Scholar]
- 4.Chandy KG, Gutman GA. Nomenclature for mammalian potassium channel genes. Trends Pharmacol Sci. 1993;14:434. doi: 10.1016/0165-6147(93)90181-i. [DOI] [PubMed] [Google Scholar]
- 5.Christie MJ, Adelman JP, Douglass J, North RA. Expression of a cloned rat brain potassium channel in Xenopus oocytes. Science. 1989;244:221–224. doi: 10.1126/science.2539643. [DOI] [PubMed] [Google Scholar]
- 6.Christie MJ, North RA, Osborne PB, Douglass J, Adelman JP. Heteropolymeric potassium channels expressed in Xenopus oocytes from cloned subunits. Neuron. 1990;2:405–411. doi: 10.1016/0896-6273(90)90052-h. [DOI] [PubMed] [Google Scholar]
- 7.Connor JA, Stevens CF. Voltage-clamp studies of a transient outward current in gastropod neural somata. J Physiol (Lond) 1971;213:21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Covarrubias M, Wei AA, Salkoff L. Shaker, Shal, Shab, and Shaw express independent K+ current systems. Neuron. 1991;7:763–773. doi: 10.1016/0896-6273(91)90279-9. [DOI] [PubMed] [Google Scholar]
- 9.De Biasi M, Drewe JA, Kirsch GE, Brown AM. Histidine substitution identifies a surface position and confers Cs+ selectivity on a K+ pore. Biophys J. 1993;65:1235–1242. doi: 10.1016/S0006-3495(93)81154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deal KK, Lovinger DM, Tamkun MM. The brain Kv1.1 potassium channel: in vitro and in vivo studies on subunit assembly and post-translational processing. J Neurosci. 1994;14:1666–1676. doi: 10.1523/JNEUROSCI.14-03-01666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drewe JA, Verma S, Frech G, Joho RH. Distinct spatial and temporal expression patterns of K+ channel mRNAs from different subfamilies. J Neurosci. 1992;12:538–548. doi: 10.1523/JNEUROSCI.12-02-00538.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frech G, VanDongen AMJ, Schuster G, Brown AM, Joho RH. A novel potassium channel with delayed rectifier properties isolated from rat brain by expression cloning. Nature. 1989;340:642–645. doi: 10.1038/340642a0. [DOI] [PubMed] [Google Scholar]
- 13.Gilly FW, Armstrong CM. Divalent cations and the activation kinetics of potassium channels in squid axons. J Gen Physiol. 1982;79:965–996. doi: 10.1085/jgp.79.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth F. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 15.Hille B. Ionic channels of excitable membranes. Sinauer; Sunderland, MA: 1992. [Google Scholar]
- 16.Hugnot JP, Salinas M, Lesage F, Guillemare E, de Weille J, Heurteaux C, Mattei MG, Lazdunski M. Kv8.1, a new neuronal potassium channel subunit with specific inhibitory properties towards Shab and Shaw channels. EMBO J. 1996;15:3322–3331. [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang PM, Glatt CE, Bredt DS, Yellen G, Snyder SH. A novel K+ channel with unique localizations in mammalian brain: molecular cloning and characterization. Neuron. 1992;8:473–481. doi: 10.1016/0896-6273(92)90275-i. [DOI] [PubMed] [Google Scholar]
- 18.Isacoff EY, Jan YN, Jan LY. Evidence for the formation of heteromultimeric potassium channels in Xenopus oocytes. Nature. 1990;345:530–534. doi: 10.1038/345530a0. [DOI] [PubMed] [Google Scholar]
- 19.Jan LY, Jan YN. Potassium channels and their evolving gates. Nature. 1994;371:119–122. doi: 10.1038/371119a0. [DOI] [PubMed] [Google Scholar]
- 20.Kamb A, Iverson LE, Tanouye MA. Molecular characterization of Shaker, a Drosophila gene that encodes a potassium channel. Cell. 1987;50:405–413. doi: 10.1016/0092-8674(87)90494-6. [DOI] [PubMed] [Google Scholar]
- 21.Kozak M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15:8125–8132. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee TE, Philipson LH, Kuznetsov A, Nelson DJ. Structural determinant for assembly of mammalian K+ channels. Biophys J. 1994;66:667–673. doi: 10.1016/s0006-3495(94)80840-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li M, Jan YN, Jan LY. Specification of subunit assembly by the hydrophilic amino-terminal domain of the Shaker potassium channel. Science. 1992;257:1225–1230. doi: 10.1126/science.1519059. [DOI] [PubMed] [Google Scholar]
- 24.Llinás R. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242:1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- 25.López-Barneo J, Hoshi T, Heinemann SH, Aldrich RW. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels. 1993;1:61–71. [PubMed] [Google Scholar]
- 26.Lucas JJ, Mellström B, Colado MI, Naranjo JR. Molecular mechanisms of pain: serotonin1A receptor agonists trigger transactivation by c-fos of the prodynorphin gene in spinal cord neurons. Neuron. 1993;10:599–561. doi: 10.1016/0896-6273(93)90163-l. [DOI] [PubMed] [Google Scholar]
- 27.MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991;350:232–235. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- 28.Marshall J, Molloy R, Moss GWJ, Howe JR, Hughes TE. The jellyfish green fluorescent protein: a new tool for studying ion channel expression and function. Neuron. 1995;14:211–215. doi: 10.1016/0896-6273(95)90279-1. [DOI] [PubMed] [Google Scholar]
- 29.McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- 30.Mellström B, Naranjo JR, Foulkes NS, Lafarga M, Sassone-Corsi P. Transcriptional response to cAMP in brain: specific distribution and induction of CREM agonists. Neuron. 1993;10:655–665. doi: 10.1016/0896-6273(93)90167-p. [DOI] [PubMed] [Google Scholar]
- 31.Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- 32.Papazian DM, Schwartz TL, Tempel BL, Timpe LC, Jan LY, Yan YN. Cloning of genomic and complementary DNA from Shaker, a putative potassium channel from Drosophila. Science. 1987;237:749–753. doi: 10.1126/science.2441470. [DOI] [PubMed] [Google Scholar]
- 33.Pardo LA, Heinemann SH, Terlau H, Ludewig U, Lorra C, Pongs O, Stühmer W. Extracellular K+ specifically modulates a rat brain K+ channel. Proc Natl Acad Sci USA. 1992;89:2466–2470. doi: 10.1073/pnas.89.6.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pongs O, Kecskemethy N, Muller R, Krah-Jentgens I, Baumann A, Kiltz HH, Canal I, Llamazares S, Ferrús A. Shaker encodes a family of putative potassium channel proteins in the nervous system of Drosophila. EMBO J. 1988;7:1087–1096. doi: 10.1002/j.1460-2075.1988.tb02917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rettig J, Heinemann SH, Wunder F, Lorra C, Parcej DN, Dolly JO, Pongs O. Inactivation properties of voltage-gated K+ channels altered by the presence of β-subunit. Nature. 1994;345:535–537. doi: 10.1038/369289a0. [DOI] [PubMed] [Google Scholar]
- 36.Rogawski MA. The A-current: how ubiquitous a feature of excitable cells is it? Trends Neurosci. 1985;8:214–219. [Google Scholar]
- 37.Rudy B. Diversity and ubiquity of K+ channels. Neuroscience. 1988;25:729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- 38.Ruppersberg JP, Schröter KH, Sakmann B, Stocker M, Sewing S, Pongs O. Heteromultimeric channels formed by rat brain potassium channel proteins. Nature. 1990;345:535–537. doi: 10.1038/345535a0. [DOI] [PubMed] [Google Scholar]
- 39.Salkoff L, Baker K, Buttler A, Covarrubias M, Park MD, Wei A. An essential set of K+ channels conserved in flies, mice, and humans. Trends Neurosci. 1992;15:161–166. doi: 10.1016/0166-2236(92)90165-5. [DOI] [PubMed] [Google Scholar]
- 40.Séquier JM, Brennand J, Bathanin J, Lazdunski M. Regional expression of a MCD peptide and dendrotoxin I-sensitive voltage-dependent potassium channels in rat brain. FEBS Lett. 1990;263:163–165. doi: 10.1016/0014-5793(90)80729-3. [DOI] [PubMed] [Google Scholar]
- 41.Sheng M, Liao YJ, Jan YN, Jan LY. Presynaptic A-current based on heteromultimeric K+ channels detected in vivo. Nature. 1993;365:72–75. doi: 10.1038/365072a0. [DOI] [PubMed] [Google Scholar]
- 42.Stühmer W, Ruppersberg JP, Schröter KH, Sakmann B, Stocker M, Giese KP, Perschke A, Baumann A, Pongs O. Molecular basis of functional diversity of voltage-gated potassium channels in mammalian brain. EMBO J. 1989;8:3235–3244. doi: 10.1002/j.1460-2075.1989.tb08483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tempel BL, Papazian DM, Schwartz TL, Jan YN, Jan LY. Sequence of a probable potassium channel component encoded at the Shaker locus of Drosophila. Science. 1987;237:770–775. doi: 10.1126/science.2441471. [DOI] [PubMed] [Google Scholar]
- 44.VanDongen AMJ, Frech GC, Drewe JA, Joho RH, Brown AM. Alteration and restoration of K+ channel function by deletions at the N- and C-termini. Neuron. 1990;5:433–443. doi: 10.1016/0896-6273(90)90082-q. [DOI] [PubMed] [Google Scholar]
- 45.Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature. 1993;365:75–79. doi: 10.1038/365075a0. [DOI] [PubMed] [Google Scholar]
- 46.Wei A, Covarrubias M, Butler A, Baker K, Pak M, Salkoff L. K+ current diversity is produced by extended gene family conserved in Drosophila and mouse. Science. 1990;248:599–603. doi: 10.1126/science.2333511. [DOI] [PubMed] [Google Scholar]
- 47.Weiser M, Vega-Sáenz de Miera E, Kentros C, Moreno H, Franzen L, Hillman D, Baker H, Rudy B. Differential expression of Shaw-related K+ channels in the rat central nervous system. J Neurosci. 1994;14:949–972. doi: 10.1523/JNEUROSCI.14-03-00949.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu J, Yu W, Jan YN, Jan LY, Li M. Assembly of voltage-gated potassium channels: conserved hydrophilic motifs determine subfamily-specific interactions between the α-subunits. J Biol Chem. 1995;270:24761–24768. doi: 10.1074/jbc.270.42.24761. [DOI] [PubMed] [Google Scholar]
- 49.Yu W, Xu J, Li M. NAB domain is essential for the subunit assembly of both α–α and α–β complexes of Shaker-like potassium channels. Neuron. 1996;16:441–453. doi: 10.1016/s0896-6273(00)80062-8. [DOI] [PubMed] [Google Scholar]
- 50.Zhao B, Rassendren F, Kaang BK, Furukawa Y, Kubo T, Kandel ER. A new class of noninactivating K+ channels from Aplysia capable of contributing to the resting potential and firing patterns of neurons. Neuron. 1994;13:1205–1213. doi: 10.1016/0896-6273(94)90058-2. [DOI] [PubMed] [Google Scholar]