pRB plays an essential role in cell cycle arrest induced by DNA damage (original) (raw)
Abstract
To maintain genome stability, cells with damaged DNA must arrest to allow repair of mutations before replication. Although several key components required to elicit this arrest have been discovered, much of the pathway remains elusive. Here we report that pRB acts as a central mediator of the proliferative block induced by a diverse range of DNA damaging stimuli. _Rb_−/− mouse embryo fibroblasts are defective in arrest after γ-irradiation, UV irradiation, and treatment with a variety of chemotherapeutic drugs. In contrast, the pRB related proteins p107 and p130 do not play an essential part in the DNA damage response. pRB is required specifically for the G1/S phase checkpoint induced by γ-irradiation. Despite a defect in G1/S phase arrest, levels of p53 and p21 are increased normally in _Rb_−/−cells in response to γ-irradiation. These results lead us to propose a model in which pRB acts as an essential downstream target of the DNA damage-induced arrest pathway. The ability of pRB to prevent replication of damaged DNA is likely to inhibit the propagation of carcinogenic mutations and may therefore contribute to its role as a tumor suppressor. Furthermore, because many cancer therapies act by damaging DNA, these findings also have implications for the treatment of tumors in which pRB is inactivated.
Integrity of the mammalian genome relies upon the repair of damaged DNA before its replication. Cell cycle arrest is essential to allow time to repair such damage before the start of DNA synthesis and thus prevent propagation of potentially deleterious mutations. Several central components of the DNA damage response have been elucidated, but many steps in the pathway await discovery.
One essential mediator in the DNA damage pathway is the tumor suppressor, p53. Cells lacking p53 fail to arrest in response to a wide variety of DNA-damaging agents (reviewed in refs. 1–3). Following such damage, the p53 protein is stabilized and its transcriptional activity is subsequently enhanced (4–7). This is thought to elicit cell cycle arrest at both the G1/S and G2/M phases of the cell cycle via transcriptional regulation of a subset of specific target genes. p53-dependent transactivation of 14-3-3 σ is proposed to play a role in inhibition of G2/M phase progression (8), whereas G1/S phase arrest after DNA damage is controlled, at least in part, by up-regulation of p21 (9–11).
Cell cycle progression is thought to be primarily controlled by the sequential activation of a series of cyclin-dependent protein kinases (CDKs) (reviewed in refs. 12 and 13). Although the actions of the CDKs are not fully understood, they are presumed to act by phosphorylating a variety of key cell cycle regulatory proteins (reviewed in refs. 14 and15). It is likely that p21 controls DNA damage-induced arrest by inhibiting the phosphorylation of one or more critical CDK targets (16,17). The identity of CDK substrates required for arrest after irradiation remains unclear. Knowledge of such targets is of central importance in understanding the DNA damage response.
Studies of the human papilloma virus-16 protein E7 have provided insight into the role of certain cellular proteins in the DNA damage pathway. E7 abrogates cell cycle arrest after DNA damage by γ-irradiation or actinomycin D treatment (18–20). A variety of cellular targets for the E7 protein have been documented (21). Foremost among these are pRB, p107, and p130—a family of structurally and functionally conserved cellular proteins that play a key role in negative regulation of cell cycle progression. Members of this family are among the most well characterized CDK substrates. During G1, phosphorylation by specific cyclin-CDK complexes inhibits the growth suppressive function of pRB family members (22). After DNA damage, it is likely that up-regulation of p21 prevents this inhibitory phosphorylation by the CDKs (16, 17) and hence maintains the active growth inhibitory state of the pRB family. Data from E7 experiments imply a central role for the pRB family in the DNA damage response because E7 mutants that are unable to bind this family can no longer abrogate arrest in response to DNA damage (20, 23). However, another class of E7 mutants that can bind to pRB also fail to overcome DNA damage-induced arrest (23). This result suggests that the ability of E7 to inactivate pRB family members may be necessary but not sufficient for abrogation of the proliferative block. The role of pRB family members in the DNA damage response has not been demonstrated directly. Moreover, studies of E7 are unable to dissect the contributions of individual members of this group. The question of which proteins within the pRB family play a role in arrest after DNA damage therefore remains unanswered.
In this study, we ask whether one or more members of the pRB family play an essential role in cell cycle arrest induced by DNA damage. Mouse embryo fibroblasts (MEFs) from_pRb_−/−,_p107_−/−, and_p130_−/− animals are ideal reagents with which to address this question. Here we show that pRB plays an essential role in DNA damage-induced arrest at the G1/S phase checkpoint but that p107 and p130 are not required for this cell cycle block. While arrest at G1/S phase is clearly defective in_Rb_−/− cells, levels of p53 and p21 are increased normally after γ-irradiation. These results lead us to propose a model in which pRB acts as an essential downstream target of the DNA damage-induced arrest pathway.
MATERIALS AND METHODS
Cell Culture and Retroviral Infections.
MEF cultures were prepared from 13.5-day embryos by using standard techniques as described (24). For all experiments, wild-type littermate control MEFs were isolated in parallel. The cells were maintained in DMEM containing 10% fetal bovine serum, 50 units/ml of penicillin, 50 μg/ml of streptomycin, and 2 mM l-glutamine. MEFs between passages 4 and 6 were used for these experiments.
The retroviral vectors pBabe and pBabe pRB (containing full length mouse pRB with an N-terminal hemagglutinin tag) were kindly provided by Bruno Amati, Swiss Institute for Experimental Cancer Research, Switzerland. These vectors were used to generate high titre retroviral supernatants as described (25). Infected, early passage MEFs were selected with 2 μg/ml puromycin (Sigma) 3 days before use.
BrdUrd Labeling, Immunofluorescence Staining, and Flow Cytometric Analysis.
MEFs at passage 4–6 were seeded onto glass coverslips in 24-well plates at a density of 1.5 × 104cells per well. Following treatment, cells were incubated with BrdUrd cell proliferation-labeling reagent for 8 hr (Amersham). Indirect immunofluorescence was performed on paraformaldehyde-fixed cells by using an anti-bromodeoxyuridine monoclonal antibody (Amersham). For each experiment, the percentage of BrdUrd positive cells in a field of at least 500 cells was calculated.
Flow cytometric analysis was carried out as described (26), and cell cycle analysis was performed on a Becton Dickinson FACScan.
DNA-Damaging Agents and Chemotherapeutic Drugs.
Early passage MEFs were irradiated with γ rays from a137cesium source at a dose rate of 5.66 Gy/min. Cells were UV irradiated with indicated doses of UV (UV-C) in a Stratalinker 1800 apparatus (Stratagene). Etoposide, cis-platinum (II) diammine dichloride, carboplatin, bleomycin sulfate, vincristine, and vinblastine were all purchased from Sigma and added to MEFs at the indicated doses for a 24-hr period.
Western Blot Analysis.
Western blots were performed on equal amounts of extracted proteins as described (27) by using the following reagents; pAb 421, a mouse mAb, which recognizes mouse p53 protein, 14001A (PharMingen), an anti-human pRB monoclonal antibody, which also recognizes the mouse protein, and a rabbit polyclonal antibody raised against amino acids 117–137 of the mouse p21 protein (28) (kindly provided by Claudio Schneider, Laboratorio Nazionale Consorzio Interuniversitario per le Biotecnologie, Trieste).
RESULTS
_Rb_−/− MEFs Are Defective in Cell Cycle Arrest After γ-Irradiation.
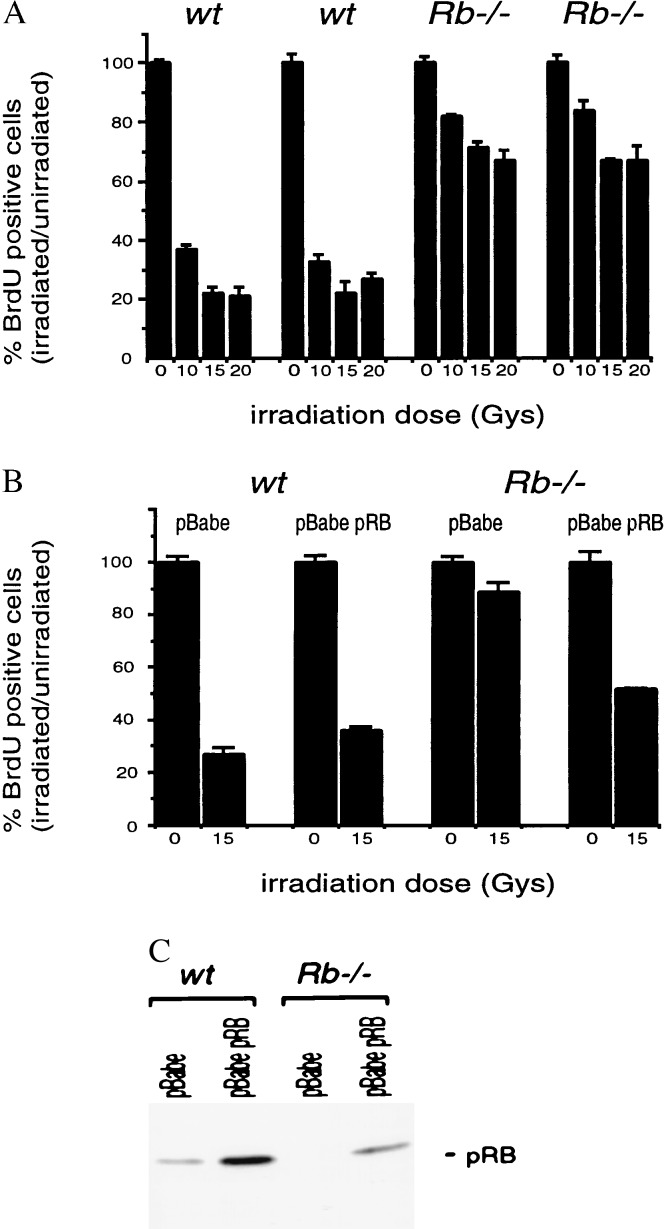
To address whether any of the pRB family members play a role in the DNA damage response, we generated MEFs from _pRb_−/−,_p107_−/−, and_p130_−/− embryos at 13.5 days of gestation. In the first series of experiments, we asked whether early passage, asynchronously growing_Rb_−/− MEFs arrest in response to γ-irradiation doses that do not induce appreciable cell death in MEFs (0–20 Gy). Twenty four hours after treatment, S phase entry was assessed by calculating the percentage of cells that incorporated BrdUrd over an 8-hr period. We found that_Rb_−/− cells were impaired severely in DNA damage-induced arrest compared with genetically matched wild-type embryos from the same litter (Fig.1A). These results are highly reproducible in embryos from at least three independent litters (Fig. 1A and data not shown) and demonstrate an essential role for pRB in the DNA damage response pathway.
Figure 1.
_Rb_−/− MEFs are defective in cell cycle arrest after γ-irradiation. (A) Asynchronous cultures of _Rb_−/− and wild-type littermate control MEFs were γ-irradiated at the indicated doses. Twenty-four hours after treatment, cells were labeled with BrdUrd for 8 hr. BrdUrd incorporation was then assessed by immunocytochemistry. Data from three independent samples are presented as the percentage of cells that incorporated BrdUrd in irradiated vs. unirradiated cultures. (B)Rb_−/− and wild-type MEFs infected with pBabe or pBabe pRB retroviruses were treated as in A. (C) Expression of pRB in infected cells from_B was confirmed by Western blotting.
Exogenous Expression of pRB Restores DNA Damage-Induced Arrest in_Rb_−/− MEFs.
To confirm that the arrest defect in Rb−/− cells is specifically due to loss of pRB, we asked whether DNA damage-induced arrest could be restored in _Rb_−/−cells by exogenously expressed pRB. Early passage_Rb_−/− and matched wild-type MEFs were infected with either a retrovirus directing low-level expression of full length pRB protein (pBabe pRB) or a control retrovirus (pBabe). Infected cells were selected for 3 days with puromycin before use. Western blot analysis confirmed moderate over-expression of pRB in those cells infected with the pBabe pRB retrovirus (Fig.1C). The infected cells were subjected to γ-irradiation (0 or 15 Gy), and monitored for S phase progression 24 hr later with an 8-hr BrdUrd pulse. Moderate over-expression of pRB does not alter the cell cycle profile of unirradiated cells (data not shown) but clearly restores the DNA damage-induced arrest in_Rb_−/− cells (Fig.1B).
p107 and p130 Do Not Play an Essential Role in Irradiation-Induced Arrest.
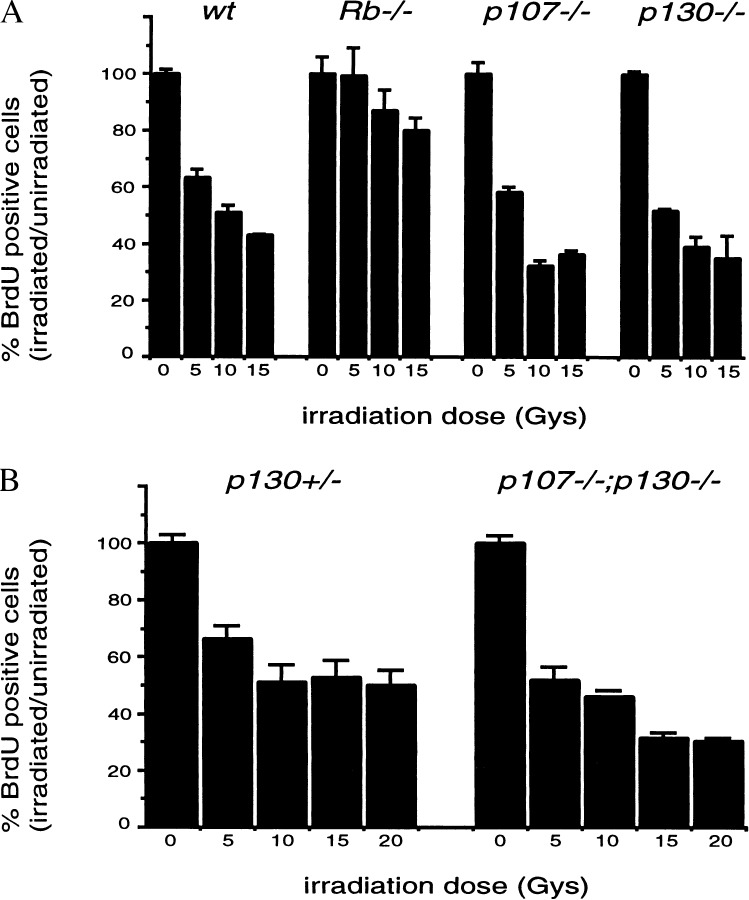
pRB is one member of a family of structurally and functionally conserved proteins that includes p107 and p130. To test the role of other family members in the DNA damage response, we asked whether _p107_−/− and _p130_−/− MEFs arrest in response to γ-irradiation. Twenty four hours after irradiation (0–15 Gy), entry into S phase was assessed by monitoring BrdUrd incorporation over the next 8 hr. While _Rb_−/− cells show a defect in arrest in response to DNA damage, the_p107_−/− and _p130_−/− MEFs showed an identical arrest profile to cells from wild-type embryos (Fig.2A).
Figure 2.
p107 and p130 do not play an essential role in γ-irradiation induced arrest. Asynchronous cultures of wild type,_Rb_−/−, p107−/− and p130−/− (A) or p107−/−; p130−/− (B) and littermate control MEFs were γ-irradiated at the indicated doses. Twenty-four hours after irradiation, cells were given an 8-hr BrdUrd pulse, and BrdUrd incorporation was assessed by immunocytochemistry. Data from three independent samples are presented as the percentage of cells that incorporated BrdUrd in irradiated vs. unirradiated cultures.
To address whether p107 and p130 play redundant roles in the irradiation response, we generated MEFs from embryos possessing homozygous deletions in both genes. Cell cycle arrest of these_p107_−/−_;p130_−/−cells after γ-irradiation was assessed as in the previous experiment. From Fig. 2B, it can be seen that_p107_−/−_;p130_−/−cells arrest in an identical fashion to wild-type cells. These results demonstrate that pRB plays a specific and unique role in mediating arrest after DNA damage, which is not shared by other highly homologous proteins within the same family.
_Rb_−/− Cells Show a Specific Defect at the G1/S Phase Checkpoint.
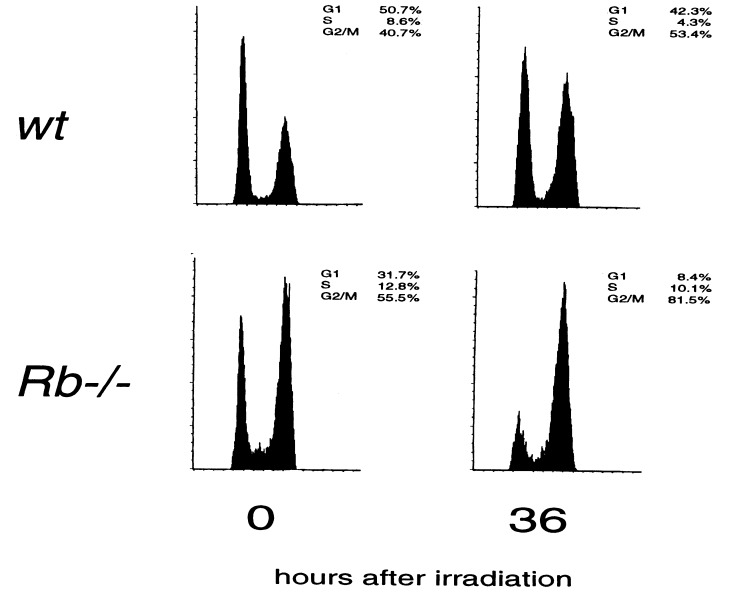
DNA damage is known to activate checkpoint responses that cause arrest at both the G1/S and G2/M phases of the cell cycle. To examine the role of pRB at these individual checkpoints, we used fluorescence-activated cell sorter analysis to monitor the cell cycle profiles of _Rb_−/− and wild-type MEFs after γ-irradiation. This investigation revealed a specific role for pRB at the G1/S phase checkpoint. Thirty-six hours after irradiation, the majority of_Rb_−/− cells accumulated at the G2/M phase of the cell cycle—demonstrating a failure to arrest at G1/S after DNA damage (Fig.3). Although this experiment does not directly address whether_Rb_−/− cells are arrested, such a dramatic accumulation of cells in the G2/M phase of the cell cycle suggests that the G2/M phase checkpoint remains intact in _Rb_−/− cells. In striking contrast, wild-type cells contain populations at both the G1/S and G2/M phases of the cell cycle after irradiation—as would be expected for cells that could respond to both checkpoints. From these results, we conclude that pRB plays a unique role in G1/S phase arrest after DNA damage.
Figure 3.
pRB is required for G1/S phase arrest after γ-irradiation. Zero or 36 hr after γ-irradiation the cell cycles profile of _Rb_−/− and wild-type MEFs were examined by FACs analysis.
_Rb_−/− MEFs Show Defective Responses to a Diverse Range of DNA-Damaging Stimuli.
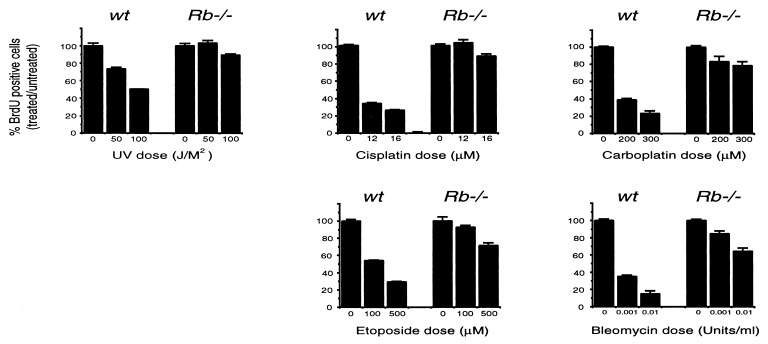
Different DNA-damaging stimuli are known to activate distinct signaling pathways. For example, γ-irradiation activates c-Abl and the tumor suppressor ATM, whereas the JNK pathway is specifically activated by UV treatment (29). We therefore sought to address whether the defect in_Rb_−/− cells is specific to γ-irradiation or is observed also in response to other DNA-damaging agents. To test this, asynchronously growing_Rb_−/− and wild-type MEFs were UV irradiated or treated with the chemotherapeutic drugs carboplatin, cisplatin, etoposide, and bleomycin. These drugs induce distinct types of DNA damage—cisplatin and carboplatin are alkylating agents, etoposide acts by inhibiting topoisomerase II, and bleomycin directly induces double strand breaks. In all cases these DNA-damaging agents induced very little cell death in MEFs of either genotype (data not shown). Twenty-four hours after treatment, cells were given an 8-hr BrdUrd pulse to monitor S phase entry._Rb_−/− cells are defective in arrest after treatment with all of these distinct DNA-damaging agents (Fig. 4). pRB therefore plays a role in mediating cell cycle arrest after treatment with a diverse range of DNA-damaging agents that activate differential sets of damage responsive genes.
Figure 4.
_Rb_−/− cells are impaired in cell cycle arrest after treatment with a diverse range of DNA-damaging agents. Twenty-four hours after treatment with the indicated DNA-damaging agents, BrdUrd incorporation in wild-type and_Rb_−/− MEFs was assessed by immunocytochemistry. The mean percentage of BrdUrd positive cells in treated vs. untreated cultures from three independent samples is presented.
Response to Chemotherapeutic Drugs that Block Mitosis.
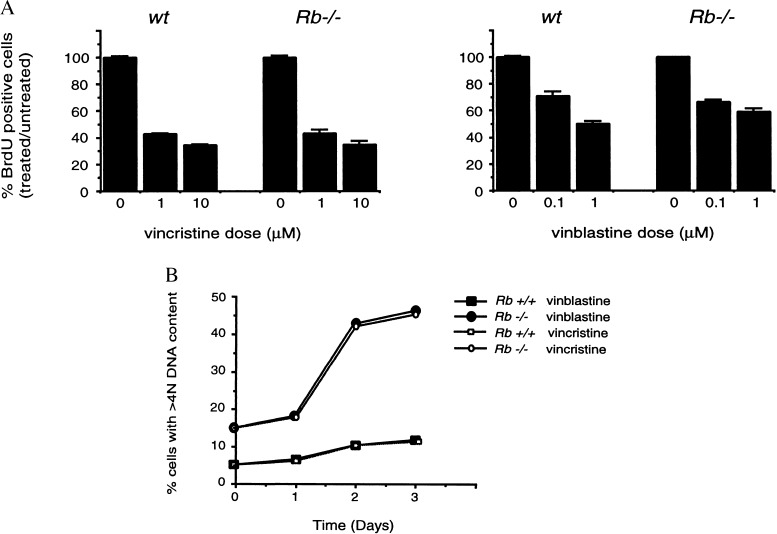
We next wished to address whether pRB plays a specific role in response to DNA-damaging agents or whether it also mediates arrest in response to drugs that block at other phases of the cell cycle. To examine this, we treated _Rb_−/− MEFs and wild-type controls with vinblastine or vincristine, chemotherapeutic drugs that interfere with microtubule function at mitosis. Twenty-four hours after treatment, _Rb_−/− cells arrested in an identical fashion to wild-type cells (Fig.5A). At later time points after drug treatment, we saw an increased proportion of cells with >4N DNA content in the _Rb_−/−cultures (Fig. 5B). These results are consistent with earlier studies that demonstrate DNA re-replication in_Rb_−/− MEFs and cells expressing human papilloma virus-16 protein E7 after treatment with microtubule inhibitors (30–32). However, it should be noted that although there is a dramatic difference in the overall percentage of cells with >4N DNA content between the two genotypes after drug treatment, the fold increase in this value between untreated and treated cells is similar for _Rb_−/− and wild-type cells.
Figure 5.
Response of _Rb_−/−cells to treatment with vincristine or vinblastine. (A) Asynchronous cultures of wild-type and_Rb_−/− MEFs were treated for 24 hr with the indicated doses of vincristine or vinblastine. BrdUrd incorporation over the next 8 hr was then assessed by immunocytochemistry. The percentage of BrdUrd positive cells in treated vs. untreated cultures from three independent samples is shown. (B) Asynchronous cultures of wild-type and _Rb_−/− MEFs at passage 4 were treated with 1 μM of vincristine or vinblastine. The percentage of cells with >4N DNA content was assessed by FACs analysis.
Levels of p53 and p21 Proteins Are Increased Normally in_Rb_−/− cells After Irradiation.
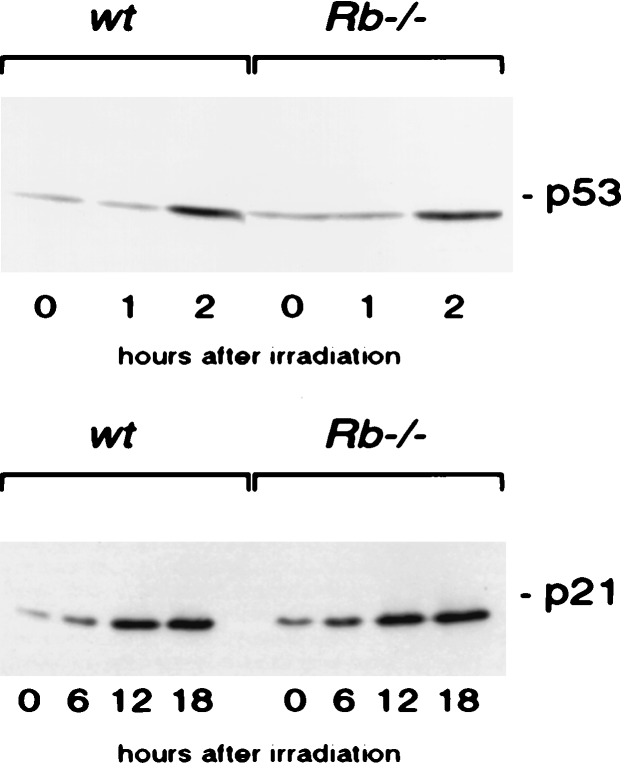
_Rb_−/− cells may be defective in irradiation-induced arrest because they fail to activate critical DNA damage signaling pathways or because they are unable to respond to such signals. p53 is a critical component of the arrest pathway activated by a multitude of distinct DNA-damaging agents. After treatment with these agents, p53 protein is stabilized and its transcriptional activity is enhanced. The ability of p53 to elicit G1/S phase arrest after DNA damage is, at least in part, attributed to its ability to up-regulate p21 expression. It is conceivable that_Rb_−/− cells are defective in arrest after DNA damage because they fail to activate these essential signaling events. To test whether p53 and p21 levels increase after DNA damage, _Rb_−/− and wild-type control MEFs were γ-irradiated with 15 Gy and lysates were made at specific time points after treatment. Western blot analysis revealed that p53 and p21 protein levels increase normally in_Rb_−/− cells after DNA damage (Fig.6). Because_Rb_−/− cells show a defect in G1/S phase arrest despite normal activation of p53, we propose that pRB acts either down stream of p53 or in a parallel, p53-independent checkpoint pathway.
Figure 6.
p53 and p21 levels are increased normally in_Rb_−/− cells after γ-irradiation. Lysates from _Rb_−/− and wild-type MEFs were made at the indicated time points after γ-irradiation (15 Gy). Levels of p53 (A) and p21 (B) were assessed by Western blotting.
DISCUSSION
In this study, we describe a previously unknown role for pRB in eliciting cell cycle arrest in response to DNA damage. We show that pRB acts as an essential component of the arrest pathway induced by a diverse range of DNA-damaging stimuli._Rb_−/− cells are defective in arrest after γ-irradiation, UV irradiation, and treatment with chemotherapeutic drugs. We consider it highly unlikely that this defect is caused by additional mutations in these early passage primary cells because an identical phenotype is observed in cells derived from multiple embryos from independent litters, and exogenous expression of pRB restores DNA damage-induced arrest in_Rb_−/− MEFs.
One previous study attempted to examine the role of pRB in cell cycle arrest after γ-irradiation. The results from this investigation were ambiguous and led the authors to suggest that the ability of E7 to abrogate irradiation-induced arrest may be due to inactivation of p107 and/or p130 in addition to pRB (19). Subsequent reviews hold contradictory theories about whether pRB plays a role in the DNA damage response (2, 33). We can now resolve this controversy at least in MEFs. Our data show that pRB plays a central role in cell cycle arrest after DNA damage, whereas p107 and p130 are dispensable for this process.
DNA damage elicits arrest at both the G1/S and G2/M phases of the cycle. Here we show that pRB is required for the G1/S checkpoint. pRB is not universally required for arrest in G1. It is essential to elicit G1 phase arrest in response to certain specific signals, such as over-expression of p16 (34–37), inactivation of ras (38), or repression of thymidylate synthesis by methotrexate (39), but many other stimuli arrest cells in an pRB-independent manner. The fact that_Rb_−/− embryos appear essentially normal at day 13 of gestation indicates that many cell types respond to physiological growth arrest signals in the absence of pRB (40–42). Indeed, in chimeric mice, _Rb_−/− cells can arrest to form normal components of the majority of adult tissues (43). Moreover, _Rb_−/− MEFs arrest in G1 in response to serum deprivation (44) and over-expression of p27 or dominant negative CDK3 (38). The DNA damage response therefore falls into a subset of signaling events that require pRB to elicit G1 phase arrest.
To play a part in irradiation-induced arrest, pRB must respond to upstream signals in the DNA damage pathway. p53 is a required component of this signaling pathway. Its ability to elicit G1/S phase arrest after irradiation is thought to be due, at least in part, to transcriptional activation of p21 (9–11). DNA damage-induced arrest is presumed to be enforced by the ability of p21 to inhibit phosphorylation of one or more critical CDK substrates, but the identity of targets involved in the irradiation response has so far remained elusive. Here we show that in_Rb_−/− cells, p53 and p21 levels are increased normally in response to γ-irradiation, yet the G1/S phase arrest is defective. We therefore suggest that pRB acts as a central downstream target of the p53-mediated arrest pathway. It is most likely that up-regulation of p21 acts to prevent CDK phosphorylation of pRB and therefore maintains pRB in the growth suppressive state. The theory that pRB acts downstream of p21 in the irradiation response is supported by the observation that the ability of p21 to elicit G1/S phase arrest correlates with the presence of functional pRB in a panel of cell lines (45). This study proposed that p21 also plays a role in G2/M phase arrest. It is also possible that pRB functions in an independent checkpoint pathway that acts in parallel to the p53-activated signaling cascade. UV irradiation engages a checkpoint pathway that inactivates CDK4 via tyrosine phosphorylation (46). It is likely that this serves to inhibit CDK4-mediated phosphorylation of pRB and therefore maintains pRB in an active, growth inhibitory state.
How does modulation of pRB activity by DNA damage signaling pathways lead to G1/S phase arrest? While pRB has been reported to bind to a multitude of cellular proteins, its actions in cell cycle progression are primarily attributed to binding of the E2F family of transcription factors. Binding of pRB to the E2Fs is thought to repress the transactivation of a set of genes required for cell cycle progression (47). Thus, although it is conceivable that pRB mediates DNA damage-induced arrest via modulation of other associated proteins, it seems most likely that its actions are primarily due to altered regulation of E2F target genes. With a few notable exceptions (44, 24), it is not yet possible to determine which E2F target genes are regulated by pRB in vivo. Knowledge of the set of regulated genes that contribute to arrest after DNA damage therefore remains elusive.
Although we consider it most likely that pRB acts as a central component of the DNA damage response pathway, it is also possible that the defect in _Rb_−/− cells reflects an indirect role for pRB. For example, lack of pRB may lead to deregulation of genes that influence the DNA damage response._Rb_−/− MEFs express higher levels of cyclin E during G0-G1 than their wild-type counterparts (44, 24). It is possible that_Rb_−/− cells are impaired in arrest after DNA damage because the levels of p21 induced by irradiation are not sufficient to inhibit the S phase-promoting activity of the higher levels of cyclin E-associated kinase activity in these cells.
pRB is one member of a family of structurally and functionally conserved proteins that play a key role in negative regulation of the cell cycle via binding to E2F transcription factors. Despite such similarity, pRB is the only member required for DNA damage-induced arrest. These results reveal a previously unknown biological difference between pRB family members that provides one possible explanation for the paradox of why Rb is the only tumor suppressor in this family of highly related genes. Mutation of Rb is a common event in the genesis of a wide variety of human tumors (48, 49). Moreover, it is estimated that deregulation or mutation of regulatory genes such as p16 results in the functional inactivation of pRB in the majority of neoplasias. In striking contrast, mutations of_p107_ and p130 have never been associated with human tumors. Here we show that following irradiation, p107−/−, p130−/−, and p107−/−;p130−/−cells arrest, whereas cells deficient in pRB continue to replicate the genome despite the presence of damaged DNA. Such a defect is likely to encourage the propagation of carcinogenic mutations in_Rb_−/− cells. We therefore believe that the ability of pRB to prevent replication of damaged DNA is likely to contribute to its role as a tumor suppressor.
The majority of therapies for human neoplasia act by damaging DNA. From these studies we suggest that after treatment, Rb_-negative cells might show an increased propensity to replicate damaged DNA and hence accumulate further mutations. It will be important to test the prediction that pRB plays a significant role in DNA damage-induced arrest in human cells. An ideal resource for such studies is the National Cancer Institute’s collection of cell lines derived from human cancers of many tissue types. One powerful study by O’Connor_et al. (50) examined the p53 status of these 60 cell lines and found a strong correlation between the presence of functional p53 and the ability to arrest in response to irradiation or drug treatment. Of the cell lines that contained wild-type p53, 19% showed minimal or no G1 arrest after low doses of γ-irradiation, 25% displayed an intermediate G1 arrest capacity, whereas 56% arrested strongly in G1. It would be of great interest to determine whether the ability of these cell lines to arrest in response to γ-irradiation correlates with Rb status. It will also be important to address whether the failure of_Rb_−/− cells to arrest appropriately in response to DNA damage results in altered sensitivity to the induction of apoptosis. Because a wide variety of tumors lack functional pRB, further studies into the role of pRB in the DNA damage response may increase the understanding of the genesis and treatment of many human neoplasias.
Acknowledgments
We thank Joshua LaBaer and Diane Fingar for critical reading of the manuscript and all other colleagues in the Molecular Oncology Laboratory for support, advice, and stimulating discussions. We are indebted to Robert Hurford for preparation of MEF cultures and Sofie Salama for help with FACs analysis. We thank Bruno Amati and Claudio Schneider for kindly supplying the pBabe pRB construct and polyclonal p21 antibody, respectively. E.A.H was supported by a postdoctoral fellowship from Boehringer Ingelheim Fonds. E.H. is an American Cancer Society Research Professor. This work was supported by grants from the National Institutes of Health to E.H. and N.D.
ABBREVIATIONS
CDK
cyclin-dependent protein kinase
MEF
mouse embryo fibroblast
References
- Cox L S, Lane D P. BioEssays. 1995;17:501–508. doi: 10.1002/bies.950170606. [DOI] [PubMed] [Google Scholar]
- 2.Levine A J. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 3.Morgan S E, Kastan M B. Adv Cancer Res. 1997;71:1–25. doi: 10.1016/s0065-230x(08)60095-0. [DOI] [PubMed] [Google Scholar]
- 4.Maltzman W, Czyzyk L. Mol Cell Biol. 1984;4:1689–1694. doi: 10.1128/mcb.4.9.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kastan M B, Onyekwere O, Sidransky D, Vogelstein B, Craig R W. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- 6.Kastan M B, Zhan Q, El-Deiry W S, Carrier F, Jacks T, Walsh W V, Plunkett B S, Vogelstein B, Fornace A J J. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 7.El-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 8.Hermeking H, Lengauer C, Polyak K, He T-C, Zhang L, Thiagalingam S, Kinzler K W, Vogelstein B. Mol Cell. 1997;1:3–11. doi: 10.1016/s1097-2765(00)80002-7. [DOI] [PubMed] [Google Scholar]
- 9.Brugarolas J, Chandrasekaran C, Gordon J I, Beach D, Jacks T, Hannon G J. Nature (London) 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 10.Deng C, Zhang P, Harper J W, Elledge S J, Leder P. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 11.Waldman T, Lengauer C, Kinzler K W, Vogelstein B. Nature (London) 1996;381:713–716. doi: 10.1038/381713a0. [DOI] [PubMed] [Google Scholar]
- 12.Sherr C J. Cell. 1994;79:551–556. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 13.Sherr C J. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 14.Nigg E A. Curr Opin Cell Biol. 1993;5:187–193. doi: 10.1016/0955-0674(93)90101-u. [DOI] [PubMed] [Google Scholar]
- 15.Nigg E A. BioEssays. 1995;17:471–480. doi: 10.1002/bies.950170603. [DOI] [PubMed] [Google Scholar]
- 16.Dulic V, Kaufmann W K, Wilson S J, Tlsty T D, Lees E, Harper J W, Elledge S J, Reed S I. Cell. 1994;76:1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 17.Poon R Y C, Jiang W, Toyoshima H, Hunter T. J Biol Chem. 1996;271:13283–13291. doi: 10.1074/jbc.271.22.13283. [DOI] [PubMed] [Google Scholar]
- 18.Demers G W, Foster S A, Halbert C L, Galloway D A. Proc Natl Acad Sci USA. 1994;91:4382–4386. doi: 10.1073/pnas.91.10.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slebos R J C, Lee M H, Plunkett B S, Kessis T, Williams B O, Jacks T, Hedrick L, Kastan M B, Cho K R. Proc Natl Acad Sci USA. 1994;91:5320–5324. doi: 10.1073/pnas.91.12.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song S, Gulliver G A, Lambert P F. Proc Natl Acad Sci USA. 1998;95:2290–2295. doi: 10.1073/pnas.95.5.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones D L, Munger K. Semin Cancer Biol. 1996;7:327–337. doi: 10.1006/scbi.1996.0042. [DOI] [PubMed] [Google Scholar]
- 22.Farnham P J, editor. Transcriptional Control of Cell Growth: The E2F Gene Family. New York: Springer; 1995. [Google Scholar]
- 23.Demers G W, Espling E, Harry J B, Etscheid B G, Galloway D A. J Virol. 1996;70:6862–6869. doi: 10.1128/jvi.70.10.6862-6869.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurford R, Cobrinik D, Lee M-H, Dyson N. Genes Dev. 1997;11:1447–1463. doi: 10.1101/gad.11.11.1447. [DOI] [PubMed] [Google Scholar]
- 25.Pear W S, Nolan G P, Scott M L, Baltimore D. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu L, van den Heuvel S, Helin K, Fattaey A, Ewen M, Livingston D, Dyson N, Harlow E. Genes Dev. 1993;7:1111–1125. doi: 10.1101/gad.7.7a.1111. [DOI] [PubMed] [Google Scholar]
- 27.Hu Q, Bautista C, Edwards G, Defeo-Jones D, Jones R, Harlow E. Mol Cell Biol. 1991;11:5792–5799. doi: 10.1128/mcb.11.11.5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sal G D, Ruaro E M, Utrera R, Cole C N, Levine A J, Schneider C. Mol Cell Biol. 1995;15:7152–7160. doi: 10.1128/mcb.15.12.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Z, Baskaran R, Lea-Chou E T, Wood L D, Chen Y, Karin M, Wang J Y J. Nature (London) 1996;384:273–276. doi: 10.1038/384273a0. [DOI] [PubMed] [Google Scholar]
- 30.Leonardo A D, Khan S H, Linke S P, Greco V, Seidita G, Wahl G M. Cancer Res. 1997;57:1013–1019. [PubMed] [Google Scholar]
- 31.Khan S H, Wahl G M. Cancer Res. 1998;58:396–401. [PubMed] [Google Scholar]
- 32.Thomas J T, Laimins L A. J Virol. 1998;72:1131–1137. doi: 10.1128/jvi.72.2.1131-1137.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J Y J. Curr Opin Genet Dev. 1997;7:39–45. doi: 10.1016/s0959-437x(97)80107-4. [DOI] [PubMed] [Google Scholar]
- 34.Guan K-L, Jenkins C W, Li Y, Nichols M A, Wu X, O’Keefe C L, Matera A G, Xiong Y. Genes Dev. 1994;8:2939–2952. doi: 10.1101/gad.8.24.2939. [DOI] [PubMed] [Google Scholar]
- 35.Koh J, Enders G H, Dynlacht B D, Harlow E. Nature (London) 1995;375:506–510. doi: 10.1038/375506a0. [DOI] [PubMed] [Google Scholar]
- 36.Lukas j, Parry D, Aagarrd L, Mann D J, Bartkova J, Strauss M, Peters G, Bartek J. Nature (London) 1995;375:503–506. doi: 10.1038/375503a0. [DOI] [PubMed] [Google Scholar]
- 37.Medema R H, Herrera R E, Lam F, Weinberg R A. Proc Natl Acad Sci USA. 1995;92:6289–6293. doi: 10.1073/pnas.92.14.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peeper D S, Upton T M, Ladha M H, Neuman E, Zalvide J, Bernards R, DeCaprio J A, Ewen M E. Nature (London) 1997;386:177–181. doi: 10.1038/386177a0. [DOI] [PubMed] [Google Scholar]
- 39.Almasan A, Yin Y, Kelly R E, Lee E Y, Bradley A, Li W, Bertino J R, Wahl G M. Proc Natl Acad Sci USA. 1995;92:5436–5440. doi: 10.1073/pnas.92.12.5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clarke A, Maandag E, van Roon M, van der Lugt N, van der Valk M, Hooper M, Berns A, te Riele H. Nature (London) 1992;359:328–330. doi: 10.1038/359328a0. [DOI] [PubMed] [Google Scholar]
- 41.Jacks T, Fazeli A, Schmitt E, Bronson R, Goodell M, Weinberg R. Nature (London) 1992;359:295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- 42.Lee E Y-H P, Chang C-Y, Hu N, Wang Y-C J, Lai C-C, Herrup K, Lee W-H, Bradley A. Nature (London) 1992;359:288–294. doi: 10.1038/359288a0. [DOI] [PubMed] [Google Scholar]
- 43.Williams B O, Schmitt E M, Remington L, Bronson R T, Albert D M, Weinbert R A, Jacks T. EMBO J. 1994;13:4251–4259. doi: 10.1002/j.1460-2075.1994.tb06745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herrera R E, Sah V P, Williams B O, Makela T P, Weinberg R A, Jacks T. Mol Cell Biol. 1996;16:2402–2407. doi: 10.1128/mcb.16.5.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niculescu III A B, Chen X, Smeets M, Hengst L, Prives C, Reed S I. Mol Cell Biol. 1998;18:629–643. doi: 10.1128/mcb.18.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terada Y, Tatsuka M, Jinno S, Okayama H. Nature (London) 1995;376:358–362. doi: 10.1038/376358a0. [DOI] [PubMed] [Google Scholar]
- 47.Cobrinik D. Curr Top Microbiol Immunol. 1996;208:31–61. doi: 10.1007/978-3-642-79910-5_2. [DOI] [PubMed] [Google Scholar]
- 48.Weinberg R A. In: Tumor Suppressor Genes: The Cell Cycle and Cancer. Levine A J, editor. Vol. 12. Plainview, NY: Cold Spring Harbor Lab. Press; 1992. pp. 43–57. [Google Scholar]
- 49.Riley D J, Lee E Y-H P, Lee W-H. Annu Rev Cell Biol. 1994;10:1–29. doi: 10.1146/annurev.cb.10.110194.000245. [DOI] [PubMed] [Google Scholar]
- 50.O’Connor P M, Jackman J, Bae I, Myers T G, Fan S, Mutoh M, Scudiero D A, Monks A, Sausville E A, Weinstein J N, et al. Cancer Res. 1997;57:4285–4300. [PubMed] [Google Scholar]