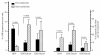Effect of lenalidomide therapy on hematopoiesis of patients with myelodysplastic syndrome associated with chromosome 5q deletion - PubMed (original) (raw)
doi: 10.3324/haematol.2009.010876. Epub 2009 Sep 22.
Athanasios Galanopoulos, Mirjam Klaus, Agapi Parcharidou, Krinio Giannikou, Maria Psyllaki, Argyrios Symeonidis, Vasiliki Pappa, Zafiris Kartasis, Dimitra Liapi, Eleftheria Hatzimichael, Styliani Kokoris, Penelope Korkolopoulou, Constantina Sambani, Charalampos Pontikoglou, Helen A Papadaki; Hellenic MDS Study Group
Affiliations
- PMID: 19773257
- PMCID: PMC2833070
- DOI: 10.3324/haematol.2009.010876
Effect of lenalidomide therapy on hematopoiesis of patients with myelodysplastic syndrome associated with chromosome 5q deletion
Maria Ximeri et al. Haematologica. 2010 Mar.
Abstract
Background: Lenalidomide improves erythropoiesis in patients with low/intermediate-1 risk myelodysplastic syndrome and interstitial deletion of the long arm of chromosome 5 [del(5q)]. The aim of this study was to explore the effect of lenalidomide treatment on the reserves and functional characteristics of bone marrow hematopoietic progenitor/precursor cells, bone marrow stromal cells and peripheral blood lymphocytes in patients with low/intermediate-1 risk myelodysplastic syndrome with del(5q).
Design and methods: We evaluated the number and clonogenic potential of bone marrow erythroid/myeloid/megakaryocytic progenitor cells using clonogenic assays, the apoptotic characteristics and adhesion molecule expression of CD34(+) cells by flow cytometry, the hematopoiesis-supporting capacity of bone marrow stromal cells using long-term bone marrow cultures and the number and activation status of peripheral blood lymphocytes in ten patients with low/intermediate-1 risk myelodysplastic syndrome with del(5q) receiving lenalidomide.
Results: Compared to baseline, lenalidomide treatment significantly decreased the proportion of bone marrow CD34+ cells, increased the proportion of CD36(+)/GlycoA(+) and CD36(-)/GlycoA(+) erythroid cells and the percentage of apoptotic cells within these cell compartments. Treatment significantly improved the clonogenic potential of bone marrow erythroid, myeloid, megakaryocytic colony-forming cells and increased the proportion of CD34(+) cells expressing the adhesion molecules CD11a, CD49d, CD54, CXCR4 and the SLAM antigen CD48. The hematopoiesis-supporting capacity of bone marrow stroma improved significantly following treatment, as demonstrated by the number of colony-forming cells and the level of stromal-derived factor-1 alpha and intercellular adhesion molecule-1 in long-term bone marrow culture supernatants. Lenalidomide treatment also increased the proportion of activated peripheral blood T lymphocytes.
Conclusions: The beneficial effect of lenalidomide in patients with lower risk myelodysplastic syndrome with del(5q) is associated with significant increases in the proportion of bone marrow erythroid precursor cells and in the frequency of clonogenic progenitor cells, a substantial improvement in the hematopoiesis-supporting potential of bone marrow stroma and significant alterations in the adhesion profile of bone marrow CD34(+) cells.
Figures
Figure 1.
Flow-cytometric analysis of BM cells. (A) Scattergram of forward scatter (FSC) versus side scatter (SSC) to allow gating on cells with low FSC and low SSC properties (R1). (B) Scattergram of anti-CD34 fluorescence versus SSC gated on R1, to allow gating on CD34+ cells (R2). (C) Scattergram of FSC versus 7AAD fluorescence gated on the CD34+ cells (R2) showing the live (R3), early apoptotic (R4) and late apoptotic/dead (R5) cells. Apoptosis was similarly studied within the CD34+/CD71+ erythroid progenitor cells (R6) (plot D) and the CD36+/GlycoA+ (R7) and CD36−/GlycoA+ (R8) erythroid precursor cells (plot E). An example of apoptosis in the ungated cells is depicted in scattergram (F) to show the live (R9), early (R10) and late apoptotic/dead (R11) cells.
Figure 2.
Flow-cytometric evaluation of the reserves and survival characteristics of BM progenitor and erythroid precursor cells before and after lenalidomide therapy. The left bars represent the mean proportion (± SEM) of BM CD34+ progenitor and CD36+/GlycoA+ and CD36−/GlycoA+ erythroid precursor cells before and after treatment. The right bars represent the mean proportion (± SEM) of apoptotic (7AADdim) cells within the CD34+, CD36+/GlycoA+ and CD36−/GlycoA+ cell compartments before and after therapy. Results were compared using the Student’s t test for paired samples and P values are indicated. SEM: standard error of the mean.
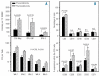
Figure 3.
BM clonogenic progenitor cells and peripheral blood lymphocyte subsets before and after lenalidomide therapy. (A) The bars in the upper graph represent the mean number (±SEM) of CFU-Meg, CFU-GM and BFU-E colonies obtained from 107 BMMC. The bars in the lower graph represent the mean CFC numbers (±SEM) in LTBMC supernatants over 5 weeks of culture, before and after therapy. Results were compared using Student’s t test for paired samples (upper graph) and 2-way ANOVA (lower graph) and the P and F values are indicated. (B) The bars in graph B represent the mean proportion (±SEM) of PB lymphocyte subpopulations (upper panel) and the mean percentage of cells expressing markers of activation within the CD3+ cell fraction (lower panel), before and after therapy. Results were compared using Student’s t test for paired samples and P values are shown. SEM; standard error of the mean.
Similar articles
- Persistent malignant stem cells in del(5q) myelodysplasia in remission.
Tehranchi R, Woll PS, Anderson K, Buza-Vidas N, Mizukami T, Mead AJ, Astrand-Grundström I, Strömbeck B, Horvat A, Ferry H, Dhanda RS, Hast R, Rydén T, Vyas P, Göhring G, Schlegelberger B, Johansson B, Hellström-Lindberg E, List A, Nilsson L, Jacobsen SE. Tehranchi R, et al. N Engl J Med. 2010 Sep 9;363(11):1025-37. doi: 10.1056/NEJMoa0912228. N Engl J Med. 2010. PMID: 20825315 - Deep sequencing of bone marrow microenvironments of patients with del(5q) myelodysplastic syndrome reveals imprints of antigenic selection as well as generation of novel T-cell clusters as a response pattern to lenalidomide.
Mährle T, Akyüz N, Fuchs P, Bonzanni N, Simnica D, Germing U, Asemissen AM, Jann JC, Nolte F, Hofmann WK, Nowak D, Binder M. Mährle T, et al. Haematologica. 2019 Jul;104(7):1355-1364. doi: 10.3324/haematol.2018.208223. Epub 2019 Jan 17. Haematologica. 2019. PMID: 30655375 Free PMC article. Clinical Trial. - Correlation of clinical response and response duration with miR-145 induction by lenalidomide in CD34(+) cells from patients with del(5q) myelodysplastic syndrome.
Venner CP, Woltosz JW, Nevill TJ, Deeg HJ, Caceres G, Platzbecker U, Scott BL, Sokol L, Sung S, List AF, Karsan A. Venner CP, et al. Haematologica. 2013 Mar;98(3):409-13. doi: 10.3324/haematol.2012.066068. Epub 2012 Aug 28. Haematologica. 2013. PMID: 22929976 Free PMC article. - Pleiotropic mechanisms of action of lenalidomide efficacy in del(5q) myelodysplastic syndromes.
Heise C, Carter T, Schafer P, Chopra R. Heise C, et al. Expert Rev Anticancer Ther. 2010 Oct;10(10):1663-72. doi: 10.1586/era.10.135. Expert Rev Anticancer Ther. 2010. PMID: 20942636 Review. - Lenalidomide: targeted anemia therapy for myelodysplastic syndromes.
List AF, Baker AF, Green S, Bellamy W. List AF, et al. Cancer Control. 2006 Dec;13 Suppl:4-11. doi: 10.1177/107327480601304s02. Cancer Control. 2006. PMID: 17242661 Review.
Cited by
- In vitro effects of perifosine, bortezomib and lenalidomide against hematopoietic progenitor cells from healthy donors.
Schmidt-Hieber M, Dabrowski R, Aicher B, Lohneis P, Busse A, Tietze-Buerger C, Reufi B, Thiel E, Blau IW. Schmidt-Hieber M, et al. Invest New Drugs. 2012 Aug;30(4):1396-403. doi: 10.1007/s10637-011-9705-6. Epub 2011 Jul 13. Invest New Drugs. 2012. PMID: 21750922 - Cell intrinsic and extrinsic factors synergize in mice with haploinsufficiency for Tp53, and two human del(5q) genes, Egr1 and Apc.
Stoddart A, Wang J, Fernald AA, Karrison T, Anastasi J, Le Beau MM. Stoddart A, et al. Blood. 2014 Jan 9;123(2):228-38. doi: 10.1182/blood-2013-05-506568. Epub 2013 Nov 21. Blood. 2014. PMID: 24264229 Free PMC article. - Important genes in the pathogenesis of 5q- syndrome and their connection with ribosomal stress and the innate immune system pathway.
Fuchs O. Fuchs O. Leuk Res Treatment. 2012;2012:179402. doi: 10.1155/2012/179402. Epub 2012 Feb 13. Leuk Res Treatment. 2012. PMID: 23213547 Free PMC article. - The Apc(min) mouse has altered hematopoietic stem cell function and provides a model for MPD/MDS.
Lane SW, Sykes SM, Al-Shahrour F, Shterental S, Paktinat M, Lo Celso C, Jesneck JL, Ebert BL, Williams DA, Gilliland DG. Lane SW, et al. Blood. 2010 Apr 29;115(17):3489-97. doi: 10.1182/blood-2009-11-251728. Epub 2010 Mar 2. Blood. 2010. PMID: 20197553 Free PMC article. - Pathophysiology and treatment of the myelodysplastic syndrome with isolated 5q deletion.
Jädersten M. Jädersten M. Haematologica. 2010 Mar;95(3):348-51. doi: 10.3324/haematol.2009.019141. Haematologica. 2010. PMID: 20207839 Free PMC article. No abstract available.
References
- Mufti GJ. Pathobiology, classification, and diagnosis of myelodysplastic syndrome. Best Pract Res Clin Haematol. 2004;17(4):543–57. - PubMed
- Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17(12):3835–49. - PubMed
- Galili N, Cerny J, Raza A. Current treatment options: impact of cytogenetics on the course of myelodysplasia. Curr Treat Options Oncol. 2007;8(2):117–28. - PubMed
- Giagounidis AA, Germing U, Aul C. Biological and prognostic significance of chromosome 5q deletions in myeloid malignancies. Clin Cancer Res. 2006;12(1):5–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous