Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells - PubMed (original) (raw)
. 2010 Mar 5;6(3):251-64.
doi: 10.1016/j.stem.2010.02.001.
Daniel J Nolan, Eva L Vertes, Barbara Varnum-Finney, Hideki Kobayashi, Andrea T Hooper, Marco Seandel, Koji Shido, Ian A White, Mariko Kobayashi, Larry Witte, Chad May, Carrie Shawber, Yuki Kimura, Jan Kitajewski, Zev Rosenwaks, Irwin D Bernstein, Shahin Rafii
Affiliations
- PMID: 20207228
- PMCID: PMC2866527
- DOI: 10.1016/j.stem.2010.02.001
Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells
Jason M Butler et al. Cell Stem Cell. 2010.
Abstract
Bone marrow endothelial cells (ECs) are essential for reconstitution of hematopoiesis, but their role in self-renewal of long-term hematopoietic stem cells (LT-HSCs) is unknown. We have developed angiogenic models to demonstrate that EC-derived angiocrine growth factors support in vitro self-renewal and in vivo repopulation of authentic LT-HSCs. In serum/cytokine-free cocultures, ECs, through direct cellular contact, stimulated incremental expansion of repopulating CD34(-)Flt3(-)cKit(+)Lineage(-)Sca1(+) LT-HSCs, which retained their self-renewal ability, as determined by single-cell and serial transplantation assays. Angiocrine expression of Notch ligands by ECs promoted proliferation and prevented exhaustion of LT-HSCs derived from wild-type, but not Notch1/Notch2-deficient, mice. In transgenic notch-reporter (TNR.Gfp) mice, regenerating TNR.Gfp(+) LT-HSCs were detected in cellular contact with sinusoidal ECs. Interference with angiocrine, but not perfusion, function of SECs impaired repopulation of TNR.Gfp(+) LT-HSCs. ECs establish an instructive vascular niche for clinical-scale expansion of LT-HSCs and a cellular platform to identify stem cell-active trophogens.
2010 Elsevier Inc. All rights reserved.
Figures
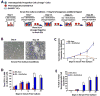
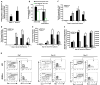
Figure 1. Serum- and cytokine- free E4ORF1+ EC co-cultures expand phenotypically marked HSCs
A) Schematic representing assay for serial ex vivo expansion of hematopoietic cells. B) Representative phase contrast micrographs of proliferating hematopoietic cells co-cultured with E4ORF1+ EC + sKitL at Day 0 and Day 28. C) Comparison of total lineage negative hematopoietic cell expansion from culture systems that included the conditions E4ORF1+ EC + sKitL or sKitL alone. D) Five thousand enriched Sca1+Lineage− cells from both culture conditions and were mixed with methylcellulose containing cytokines to generate total colony forming units (CFU). E) Total lineage negative hematopoietic cells were compared from culture conditions that included E4ORF1+ EC + sKitL or sKitL alone for HSC phenotypic expression of KLS. Error Bars represent SD based on three to four independent experiments. (*p<0.05, n = 10)
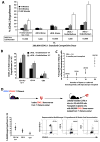
Figure 2. Hematopoietic cells co-cultured with E4ORF1+ ECs and sKitL give rise to long-term multi-lineage engraftment and maintain self-renewal capacity
A) Comparison of engraftment efficiency between 10,000 freshly isolated Sca1+Lineage− (Control) (n=10) vs. hematopoietic cells co-cultured for 3 weeks with E4ORF1+ ECs + sKitL (n=10) vs hematopoietic cells cultured for 3 weeks with sKitL alone (n=10), using a two dose competitive repopulation assay. As little as 1,000 and 10,000 hematopoietic cells co-cultured with E4ORF1+ ECs + sKitL gave rise to long-term multi-lineage engraftment and out competed the standard competitive dose of 200,000 freshly isolated CD45.1 whole BM. B) The percentage of long-term multi-lineage engraftment in both test cell numbers in the hematopoietic cells cocultured with E4ORF1+ ECs and sKitL. C) Limiting dilution assay to determine the frequency of LT-HSC in day 21 co-cultured hematopoietic cells. CRU of 1 in 4 was determined using Poisson statistics by L-calc software (Stem Cell Technologies). D) Whole BM was isolated from mice that had been long-term engrafted (Figure 2A) and CD45.2 cells were sorted and were transplanted into lethaly irradiated (950 Rads) secondary recipients (n=10), as depicted in schematic. The primary cells that were co-cultured with E4ORF1+ ECs and sKitL were able to sustain self-renewal, as is shown in the representative FACS dot plot showing long-term multi-lineage engraftment in all secondary recipients. Error bars represent SD.
Figure 3. Notch signaling is activated in hematopoietic cells co-cultured with E4ORF1+ ECs and sKitL
A) One thousand freshly isolated KLS hematopoietic cells from the BM of TNR.Gfp mice were co-cultured with E4ORF1+ ECs and sKitL. The degree of Notch signaling in the culture increased over time as indicated by enhanced Gfp expression (TNR.Gfp+ cells) in the expanding hematopoietic cells. B) Hematopoietic colonies that attach to ECs increase Notch signaling over time. C) Quantification of total number of TNR.Gfp+ Lin− versus Lin+ hematopoietic cell expansion. TNR.Gfp+ hematopoietic cells cultured in the absence of endothelium died by three weeks in culture. D) Phenotypic expression comparing the expansion of total KLS versus TNR.Gfp+ KLS cells expanded in the presence of E4ORF1+ ECs and sKitL. E,F) TNR.Gfp hematopoietic cells cultured in direct cellular contact with E4ORF1+ EC + sKitL or placed on the upper chamber of the transwells, physically separated from E4ORF1+ ECs. Lack of cellular contact of the TNR.Gfp hematopoietic cells with the E4ORF1+ EC monolayers, even in the presence of sKitL, results in a severe impairment of survival and diminished proliferation of the Lin− TNR.Gfp hematopoietic cells (E) and impaired expansion of TNR.Gfp+ KLS cells (F). By contrast, TNR.Gfp hematopoietic cells that were cultured in direct cellular contact with E4ORF1+ ECs and sKitL underwent significant expansion over a 28 day period, generating large numbers of Lin− TNR.Gfp (E) and TNR.Gfp+ KLS cells (F). Error bars represent SD. (*p<0.05, n = 6)
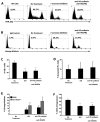
Figure 4. Blocking angiogenic pathways in E4ORF1+ ECs decreases Notch signaling favoring hematopoietic cell differentiation
A) Representative FACS histographs demonstrating a rapid decrease in Notch activity as reflected in a reduction in Gfp expression in TNR.Gfp hematopoietic cell population, when treated with the γ-secretase inhibitor or the vascular targeting antibodies. B) Representative FACS histograms showing a rapid increase in hematopoietic lineage markers when treated with the γ-secretase inhibitor or the vascular targeting antibodies. C) Quantification of Figure 4A (n=3 wells, 3 independent experiments). D) Annexin V/Propidium Iodide staining to determine if the treatment with the γ-secretase inhibitor or the vascular targeting antibodies caused a decrease in Gfp expression due to hematopoietic cell death. There was no change in cell viability after treatment. E) Quantification of Figure 4B (n=3, 3 independent experiments). F) Quantification of percent engraftment of 200,000 CD45.2+ test cells co-cultured with E4ORF1+ ECs and sKitL with or without treatment with the γ-secretase inhibitor or the vascular targeting antibodies against 200,000 standard competitive dose of CD45.1+ freshly isolated whole BM (*p<0.05, n=10 mice per treatment group).
Figure 5. E4ORF1+ ECs prevent attrition of KLS cells through Notch activation
A) Quantitative RT-PCR for assessing Notch 1 and Notch 2 expression on day 21 co-cultured hematopoietic cells. B) E4ORF1+ ECs were infected with adenovirus that overexpress a Notch decoy construct or an empty adenovirus. The Notch decoy significantly decreased the ability of E4ORF1+ ECs to expand total hematopoietic cells and total TNR.Gfp+KLS cells. C-F) Notch1-/-Notch2-/- lineage− (N1N2-/-) BM cells (25,000 cells) were co-cultured with E4ORF1+ ECs. BM cells were analyzed on weekly time points and were demi-depopulated each week. C) Total hematopoietic cell expansion was significantly decreased by day 21 in co-culture with Notch1-/-Notch2-/- versus WT BM cells. D) Total lineage− hematopoietic cell expansion was significantly decreased by day 21 in co-culture of Notch1-/- Notch2-/- versus WT BM cells with E4ORF1+ ECs. E) Total lineage+ hematopoietic cell expansion was significantly decreased by day 21 in co-culture of Notch1-/-Notch2-/- BM cells versus WT cells E4ORF1+ ECs. F) Total CD34-Flt3-KLS cells (containing a large number of phenotypically marked HSCs) were significantly decreased by day 14 in co-cultures of E4ORF1+ ECs with Notch1-/-Notch2-/-versus WT BM cells. G) Representative contour plots of phenotypic analysis of Notch1-/-Notch2-/-CD34− Flt3−KLS versus WT BM CD34−Flt3−KLS cells co-cultured for 7, 14, and 21 days with E4ORF1+ ECs. (*p<0.05, n = 6).
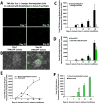
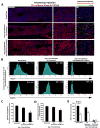
Figure 6. Selective vascular targeting diminishes the expression of Notch-ligands on the functional patent SECs
A) Full length montage Z-stacks of femurs of mice under steady state conditions and 650 irradiated mice with and without vascular targeting that received intravenous injection of low dose VE-cadherin Alexa 647 (Red fluorescence) and Isolectin GS-IB4 Alexa 488 (Green fluorescence) to identify functional perfused vessels. B) Mean fluorescence intensity of Jagged-1 and Jagged-2 expression on the functional Isolectin+VE-cadherin+CD45−Ter119− BM endothelium at Steady State and Day 7 Post 650 irradiation with and without treatment with blocking mAbs to VEGFR2 and VE-cadherin. Note the dramatic decrease in mean fluorescence intensity of the Jagged-1 and Jagged-2 expression on the perfused patent Isolectin+VE-cadherin+ vessels in the cohort of mice that were treated with the neutralizing mAbs to VEGFR2 and VE-cadherin (as compared to Steady State and Day 7 650 irradiated No Treatment cohorts). C) Quantification of functional patent SECs. D) Mean fluorescent intensity of Isolectin GS-IB4 gated on VE-cadherin+ BM endothelium. Error bars represent SD. p≤0.05 as compared to No Treatment controls. E) Quantification of the mean fluorescence intensity of the Notch-ligands Jagged-1 and Jagged-2 expression. Error bars represent SD. (*p≤0.05 as compared to No Treatment controls). Scale Bars: Left and Middle Panels 500μm; Right Panels 50μm.
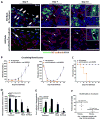
Figure 7. Selective disruption of SECs angiocrine function inhibits reconstitution of TNR.Gfp+ LT-HSCs
A) Analysis of TNR.Gfp+ hematopoietic cells following 650 sublethally irradiation with or without administration of vascular targeting agents. There is a profound inhibition of TNR.Gfp+ hematopoietic cell regeneration and the impaired remodeling of BM ECs manifesting as disrupted and dilated ECs (red stain) in the vascular-targeted cohort (white arrowheads). (Scale Bar 50mm). TNR.Gfp+ cells reside in close proximity to the VE-cadherin+ SECs, and are often cellularly positioned in between the bone (osteoblastic cells) and vascular cells (white arrows and inserts in right panel of 650 rads treated alone mice). VE-cadherin+ ECs could be detected in the close proximity of the GFP+ osteoblasts in the Col2.3GFP mice (Insets), in which the expression of the GFP is restricted to the osteoblasts. B) Circulating blood counts in the control cohort vs. the vascular-targeted cohort. C) Survival curve of both cohorts. D) Comparison of total hematopoietic cell numbers and populations between the control cohort and the vascular targeted cohort at day 7 post sublethal irradiation. E) Total TNR.Gfp+ and TNR.Gfp_− KLS cells per femur. Targeting the SECs inhibits the regeneration of Notch-dependent phenotypically marked LT-HSC. F) TNR.Gfp mice were sublethally irradiated with 650 Rads and at day 10 post sublethal irradiated BM was isolated and TNR.Gfp+Sca-1+Lineage- (TNR.Gfp_+) and TNR._Gfp_−Sca-1+Lineage− (TNR._Gfp_−) were sorted. One hundred cells isolated from each of the sorted hematopoietic population were transplanted along with 300,000 NOD-SCID whole BM into irradiated NOD-SCID recipients (n=5). TNR.Gfp+Sca-1+Lineage− hematopoietic cells are highly enriched for LT-HSC. Note the majority of the TNR._Gfp_-Sca-1+Lineage− hematopoietic cells were unable to give rise to long-term engraftment. Only one out of 5 mice showed long-term engraftment. Error bars represent SD. (*p<0.05, n = 10).
Similar articles
- Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells.
Kobayashi H, Butler JM, O'Donnell R, Kobayashi M, Ding BS, Bonner B, Chiu VK, Nolan DJ, Shido K, Benjamin L, Rafii S. Kobayashi H, et al. Nat Cell Biol. 2010 Nov;12(11):1046-56. doi: 10.1038/ncb2108. Epub 2010 Oct 24. Nat Cell Biol. 2010. PMID: 20972423 Free PMC article. - Notch2 blockade enhances hematopoietic stem cell mobilization and homing.
Wang W, Yu S, Myers J, Wang Y, Xin WW, Albakri M, Xin AW, Li M, Huang AY, Xin W, Siebel CW, Lazarus HM, Zhou L. Wang W, et al. Haematologica. 2017 Oct;102(10):1785-1795. doi: 10.3324/haematol.2017.168674. Epub 2017 Jul 20. Haematologica. 2017. PMID: 28729299 Free PMC article. - Notch2 governs the rate of generation of mouse long- and short-term repopulating stem cells.
Varnum-Finney B, Halasz LM, Sun M, Gridley T, Radtke F, Bernstein ID. Varnum-Finney B, et al. J Clin Invest. 2011 Mar;121(3):1207-16. doi: 10.1172/JCI43868. J Clin Invest. 2011. PMID: 21285514 Free PMC article. - Regulation of the hematopoietic stem cell lifecycle by the endothelial niche.
Ramalingam P, Poulos MG, Butler JM. Ramalingam P, et al. Curr Opin Hematol. 2017 Jul;24(4):289-299. doi: 10.1097/MOH.0000000000000350. Curr Opin Hematol. 2017. PMID: 28594660 Free PMC article. Review. - Quantitative assessment of the stem cell self-renewal capacity.
Nakauchi H, Sudo K, Ema H. Nakauchi H, et al. Ann N Y Acad Sci. 2001 Jun;938:18-24; discussion 24-5. doi: 10.1111/j.1749-6632.2001.tb03570.x. Ann N Y Acad Sci. 2001. PMID: 11458506 Review.
Cited by
- Context-specific roles for paracrine IL-6 in lymphomagenesis.
Gilbert LA, Hemann MT. Gilbert LA, et al. Genes Dev. 2012 Aug 1;26(15):1758-68. doi: 10.1101/gad.197590.112. Genes Dev. 2012. PMID: 22855834 Free PMC article. - Thorny ground, rocky soil: Tissue-specific mechanisms of tumor dormancy and relapse.
Lim AR, Ghajar CM. Lim AR, et al. Semin Cancer Biol. 2022 Jan;78:104-123. doi: 10.1016/j.semcancer.2021.05.007. Epub 2021 May 9. Semin Cancer Biol. 2022. PMID: 33979673 Free PMC article. Review. - Mesenchymal Stem and Progenitor Cells in Normal and Dysplastic Hematopoiesis-Masters of Survival and Clonality?
Pleyer L, Valent P, Greil R. Pleyer L, et al. Int J Mol Sci. 2016 Jun 27;17(7):1009. doi: 10.3390/ijms17071009. Int J Mol Sci. 2016. PMID: 27355944 Free PMC article. Review. - The vascular stem cell niche.
Gómez-Gaviro MV, Lovell-Badge R, Fernández-Avilés F, Lara-Pezzi E. Gómez-Gaviro MV, et al. J Cardiovasc Transl Res. 2012 Oct;5(5):618-30. doi: 10.1007/s12265-012-9371-x. Epub 2012 May 30. J Cardiovasc Transl Res. 2012. PMID: 22644724 Review. - The bone marrow at the crossroads of blood and immunity.
Mercier FE, Ragu C, Scadden DT. Mercier FE, et al. Nat Rev Immunol. 2011 Dec 23;12(1):49-60. doi: 10.1038/nri3132. Nat Rev Immunol. 2011. PMID: 22193770 Free PMC article. Review.
References
- Antonchuk J, Sauvageau G, Humphries RK. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell. 2002;109:39–45. - PubMed
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. - PubMed
- Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, Jin DK, Dias S, Zhang F, Hartman TE, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. - PubMed
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RC1 AI080309-01/AI/NIAID NIH HHS/United States
- P01 HL059312-090006/HL/NHLBI NIH HHS/United States
- R01 HL097797-03/HL/NHLBI NIH HHS/United States
- P50 HL084936/HL/NHLBI NIH HHS/United States
- P01 HL059312-100006/HL/NHLBI NIH HHS/United States
- R01 HL097797-01/HL/NHLBI NIH HHS/United States
- U01 HL066952-020002/HL/NHLBI NIH HHS/United States
- RC1 AI080309/AI/NIAID NIH HHS/United States
- P01 HL059312/HL/NHLBI NIH HHS/United States
- U01 HL066952-040002/HL/NHLBI NIH HHS/United States
- P01 HL059312-080006/HL/NHLBI NIH HHS/United States
- HL075234/HL/NHLBI NIH HHS/United States
- R21 HL083222-01/HL/NHLBI NIH HHS/United States
- U01 HL066952/HL/NHLBI NIH HHS/United States
- U01 HL066952-030002/HL/NHLBI NIH HHS/United States
- P50 HL084936-010003/HL/NHLBI NIH HHS/United States
- R21 HL083222-02/HL/NHLBI NIH HHS/United States
- P01 HL067839/HL/NHLBI NIH HHS/United States
- P50 HL084936-030003/HL/NHLBI NIH HHS/United States
- R01 HL097797-02/HL/NHLBI NIH HHS/United States
- R01 HL097797/HL/NHLBI NIH HHS/United States
- P50 HL084936-020003/HL/NHLBI NIH HHS/United States
- P01 HL059312-060006/HL/NHLBI NIH HHS/United States
- P01 HL084205/HL/NHLBI NIH HHS/United States
- HL097797/HL/NHLBI NIH HHS/United States
- P01 HL067839-050004/HL/NHLBI NIH HHS/United States
- P01 HL059312-070006/HL/NHLBI NIH HHS/United States
- P50 HL084936-040003/HL/NHLBI NIH HHS/United States
- U01 HL066952-050002/HL/NHLBI NIH HHS/United States
- U01 HL066952-010002/HL/NHLBI NIH HHS/United States
- R01 CA136673/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- P01 HL067839-040004/HL/NHLBI NIH HHS/United States
- R21 HL083222/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous