Unfolded-protein response-associated stabilization of p27(Cdkn1b) interferes with lens fiber cell denucleation, leading to cataract - PubMed (original) (raw)
. 2016 Mar;30(3):1087-95.
doi: 10.1096/fj.15-278036. Epub 2015 Nov 20.
Elizabeth A Whitcomb 1, Shuhong Jiang 1, Min-Lee Chang 1, Yumei Gu 1, Melinda K Duncan 1, Ales Cvekl 1, Wei-Lin Wang 1, Saima Limi 1, Lixing W Reneker 1, Fu Shang 1, Linfang Du 2, Allen Taylor 2
Affiliations
- PMID: 26590164
- PMCID: PMC4750420
- DOI: 10.1096/fj.15-278036
Unfolded-protein response-associated stabilization of p27(Cdkn1b) interferes with lens fiber cell denucleation, leading to cataract
Lei Lyu et al. FASEB J. 2016 Mar.
Abstract
Failure of lens fiber cell denucleation (LFCD) is associated with congenital cataracts, but the pathobiology awaits elucidation. Recent work has suggested that mechanisms that direct the unidirectional process of LFCD are analogous to the cyclic processes associated with mitosis. We found that lens-specific mutations that elicit an unfolded-protein response (UPR) in vivo accumulate p27(Cdkn1b), show cyclin-dependent kinase (Cdk)-1 inhibition, retain their LFC nuclei, and are cataractous. Although a UPR was not detected in lenses expressing K6W-Ub, they also accumulated p27 and showed failed LFCD. Induction of a UPR in human lens epithelial cells (HLECs) also induced accumulation of p27 associated with decreased levels of S-phase kinase-associated protein (Skp)-2, a ubiquitin ligase that regulates mitosis. These cells also showed decreased lamin A/C phosphorylation and metaphase arrest. The suppression of lamin A/C phosphorylation and metaphase transition induced by the UPR was rescued by knockdown of p27. Taken together, these data indicate that accumulation of p27, whether related to the UPR or not, prevents the phosphorylation of lamin A/C and LFCD in maturing LFCs in vivo, as well as in dividing HLECs. The former leads to cataract and the latter to metaphase arrest. These results suggest that accumulation of p27 is a common mechanism underlying retention of LFC nuclei.
Keywords: cell cycle; endoplasmic reticulum stress; nuclear disassembly; ubiquitin.
© FASEB.
Figures
Figure 1.
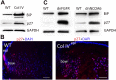
Expression of cataract-inducing proteins induces a UPR and sustained p27 expression_. A_) Lenses from Col IV transgenic mice (P1) were lysed with SDS-gel loading buffer. Levels of BiP, p27, and GAPDH were determined by Western blot analysis. GAPDH was used as a loading control_. B_) P1 lenses from WT (left) and mutant Col IV (right) transgenic mice were cryosectioned and immunostained with anti-p27 (red) and counterstained with DAPI (blue). Note that p27 staining diminishes more peripherally in the WT than in the Col IV lens. Bow, bow region; epi, epithelium_. C_) Lens lysates from dn-FGFR (P0) and dnNCOA6 (P1) mice were also examined for levels of BiP and p27 by Western blot analysis, as described in (A). Scale bars, 50 μm.
Figure 2.
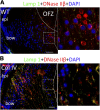
Phosphorylation of lamin A/C in lens fiber cells is inhibited by the UPR. P1 lenses from WT (A) and mutant Col IV transgenic mice (B) were cryosectioned and immunostained with anti-phospho-lamin A/C (green) and counterstained with DAPI (blue). Right: higher magnifications of boxed areas. Scale bars, 50 μm.
Figure 3.
UPR blocks the nuclear translocation of DNase IIβ in lens fiber cells. P1 lenses from WT (A) and mutant Col IV transgenic mice (B) were cryosectioned and immunostained with anti- DNAse IIβ (red) and counterstained with DAPI (blue). Lysosomes are stained with anti-Lamp1 (green). Right: higher magnification of boxed areas. Scale bars, 50 μm.
Figure 4.
Induction of a UPR in HLECs increases p27 through reduced degradation and increased mRNA expression_. A_) Cells were treated with 1 μg/ml TM and collected at 16, 20, and 24 h. Levels of BiP and p27 were determined by Western blot. β-Actin was used as a loading control_. B_) Cells were treated with TM or left untreated for 16 h, and CHX was then added to stop protein synthesis. The p27 level was determined by Western blot. MG132 was used to inhibit proteasome-dependent degradation_. C_) The effect of UPR induction on the levels of Ub ligases, Skp2, DDB1, Kpc1, and Pirh2, which are related to p27 stability, was determined by Western blot analysis. Cells were treated with 1 μg/ml TM and collected at 16, 20, and 24 h_. D_) mRNA levels of p27 and BiP in the presence or absence of TM were determined by real-time quantitative PCR, with GAPDH as the control. *P < 0.001, vs. 0 h after TM treatment.
Figure 5.
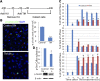
UPR induction prevents nuclear lamin phosphorylation and leads to cell cycle arrest at the G2/M phase_. A_) Experimental scheme. Cells were synchronized by HU treatment. TM was added when most of the cells were in the S phase_. B_) Cellular localization of phosphorylated lamin A/C was determined with immunostaining after 8 h of TM treatment. The bar graph shows the percentage of cells with phosphorylated lamin_. C_) Cell cycle profiles were determined with propidium iodide staining and FACS analysis_. D_) The level of multiple Cdk substrates indicated as phospho-Ser substrates (top) and the level of specific Cdk1 product phospho-NuMA (T2055) (middle) were blotted after 24 h, in the presence or absence of TM, as the indicator of Cdk1 activation. *P < 0.01; ** P < 0.001 vs. the control cells at the specified time points.
Figure 6.
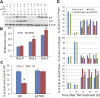
p27KD abolishes the inhibition of phospho-lamin A/C and G2/M arrest caused by the UPR_. A_) p27 was knocked down in HLECs using lentivirus-mediated shRNA. The NC and p27KD HLECs were synchronized by HU and treated with TM (Fig. 5_A_). The levels of p27 and BiP, in the absence or presence of TM, were detected at the indicated time points with Western Blot_. B_) The growth rates of NC and p27KD cells were determined by the MTS assay, as described in experimental procedures_. C_) The percentages of phospho-lamin A/C+ cells in the synchronized NC, and p27KD cells were detected by immunostaining 8 h after TM treatment_. D_) The synchronized NC and p27KD cells were treated with TM or left untreated (Fig. 5_A_). The cell cycle progressions were determined by FACS analysis. *P < 0.001 vs. TM-treated NC cells at the indicated time points.
Similar articles
- Disassembly of the lens fiber cell nucleus to create a clear lens: The p27 descent.
Rowan S, Chang ML, Reznikov N, Taylor A. Rowan S, et al. Exp Eye Res. 2017 Mar;156:72-78. doi: 10.1016/j.exer.2016.02.011. Epub 2016 Mar 3. Exp Eye Res. 2017. PMID: 26946072 Free PMC article. Review. - Endoplasmic reticulum stress inhibits cell cycle progression via induction of p27 in melanoma cells.
Han C, Jin L, Mei Y, Wu M. Han C, et al. Cell Signal. 2013 Jan;25(1):144-9. doi: 10.1016/j.cellsig.2012.09.023. Epub 2012 Sep 23. Cell Signal. 2013. PMID: 23010535 - Phosphorylation of p27(KIP1) in lens epithelial cells after extraction of fiber cells.
Kase S, Yoshida K, Jin XH, Koyama Y, Kitaichi N, Ohgami K, Shiratori K, Ilieva I, Ohno S. Kase S, et al. Int J Mol Med. 2006 Dec;18(6):1187-91. Int J Mol Med. 2006. PMID: 17089025 - Selenite cataracts: activation of endoplasmic reticulum stress and loss of Nrf2/Keap1-dependent stress protection.
Palsamy P, Bidasee KR, Shinohara T. Palsamy P, et al. Biochim Biophys Acta. 2014 Sep;1842(9):1794-805. doi: 10.1016/j.bbadis.2014.06.028. Epub 2014 Jul 2. Biochim Biophys Acta. 2014. PMID: 24997453 Free PMC article. - [Proliferative regulation in the cornea and lens].
Yoshida K, Harada T, Harada C, Kase S, Ikeda H, Sakai M, Nishi S, Imaki J, Nakayama K, Nagahama H, Nakayama KI, Ohno S. Yoshida K, et al. Nippon Ganka Gakkai Zasshi. 2003 Nov;107(11):678-86. Nippon Ganka Gakkai Zasshi. 2003. PMID: 14661541 Review. Japanese.
Cited by
- The involvement of caspases in the process of nuclear removal during lens fiber cell differentiation.
Gheyas R, Menko AS. Gheyas R, et al. Cell Death Discov. 2023 Oct 21;9(1):386. doi: 10.1038/s41420-023-01680-y. Cell Death Discov. 2023. PMID: 37865680 Free PMC article. - Aged Nrf2-Null Mice Develop All Major Types of Age-Related Cataracts.
Rowan S, Jiang S, Francisco SG, Pomatto LCD, Ma Z, Jiao X, Campos MM, Aryal S, Patel SD, Mahaling B, Riazuddin SA, Duh EJ, Lachke SA, Hejtmancik JF, de Cabo R, FitzGerald PG, Taylor A. Rowan S, et al. Invest Ophthalmol Vis Sci. 2021 Dec 1;62(15):10. doi: 10.1167/iovs.62.15.10. Invest Ophthalmol Vis Sci. 2021. PMID: 34882206 Free PMC article. - Age-related cataracts: Role of unfolded protein response, Ca2+ mobilization, epigenetic DNA modifications, and loss of Nrf2/Keap1 dependent cytoprotection.
Periyasamy P, Shinohara T. Periyasamy P, et al. Prog Retin Eye Res. 2017 Sep;60:1-19. doi: 10.1016/j.preteyeres.2017.08.003. Epub 2017 Aug 31. Prog Retin Eye Res. 2017. PMID: 28864287 Free PMC article. Review. - Inherited cataracts: Genetic mechanisms and pathways new and old.
Shiels A, Hejtmancik JF. Shiels A, et al. Exp Eye Res. 2021 Aug;209:108662. doi: 10.1016/j.exer.2021.108662. Epub 2021 Jun 12. Exp Eye Res. 2021. PMID: 34126080 Free PMC article. Review. - N-myc regulates growth and fiber cell differentiation in lens development.
Cavalheiro GR, Matos-Rodrigues GE, Zhao Y, Gomes AL, Anand D, Predes D, de Lima S, Abreu JG, Zheng D, Lachke SA, Cvekl A, Martins RAP. Cavalheiro GR, et al. Dev Biol. 2017 Sep 1;429(1):105-117. doi: 10.1016/j.ydbio.2017.07.002. Epub 2017 Jul 14. Dev Biol. 2017. PMID: 28716713 Free PMC article.
References
- Pascolini D., Mariotti S. P. (2012) Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 96, 614–618 - PubMed
- Francis P. J., Moore A. T. (2004) Genetics of childhood cataract. Curr. Opin. Ophthalmol. 15, 10–15 - PubMed
- Bloemendal H. (1977) The vertebrate eye lens. Science 197, 127–138 - PubMed
- Fukushi S., Spiro R. G. (1969) The lens capsule. Sugar and amino acid composition. J. Biol. Chem. 244, 2041–2048 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY013250/EY/NEI NIH HHS/United States
- R01 EY011717/EY/NEI NIH HHS/United States
- R01 EY026979/EY/NEI NIH HHS/United States
- EY11717/EY/NEI NIH HHS/United States
- R01 EY015279/EY/NEI NIH HHS/United States
- R29 EY011717/EY/NEI NIH HHS/United States
- EY13250/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous