Estrogen receptor α in cancer associated fibroblasts suppresses prostate cancer invasion via reducing CCL5, IL6 and macrophage infiltration in the tumor microenvironment - PubMed (original) (raw)
Estrogen receptor α in cancer associated fibroblasts suppresses prostate cancer invasion via reducing CCL5, IL6 and macrophage infiltration in the tumor microenvironment
Chiuan-Ren Yeh et al. Mol Cancer. 2016.
Abstract
Background: Cancer associated fibroblasts (CAF) play important roles in tumor growth that involves inflammation and epithelial cell differentiation. Early studies suggested that estrogen receptor alpha (ERα) was expressed in stromal cells in normal prostates and prostate cancer (PCa), but the detailed functions of stromal ERα in the PCa remain to be further elucidated.
Methods: Migration and invasion assays demonstrated the presence of high levels of ERα in CAF cells (CAF.ERα(+)) suppressed PCa invasion via influencing the infiltration of tumor associated macrophages. ERα decreased CAF CCL5 secretion via suppressing the CCL5 promoter activity was examined by luciferase assay. ERα decreased CCL5 and IL-6 expression in conditioned media that was collected from CAF cell only or CAF cell co-cultured with macrophages as measured by ELISA assay.
Results: Both in vitro and in vivo studies demonstrated CAF.ERα(+) led to a reduced macrophage migration toward PCa via inhibiting CAF cells secreted chemokine CCL5. This CAF.ERα(+) suppressed macrophage infiltration affected the neighboring PCa cells invasion and the reduced invasiveness of PCa cells are at least partly due to reduced IL6 expression in the macrophages and CAF.
Conclusion: Our data suggest that CAF ERα could be applied as a prognostic marker to predict cancer progression, and targeting CCL5 and IL6 may be applied as an alternative therapeutic approach to reduce M2 type macrophages and PCa invasion in PCa patients with low or little ERα expression in CAF cells.
Figures
Fig. 1
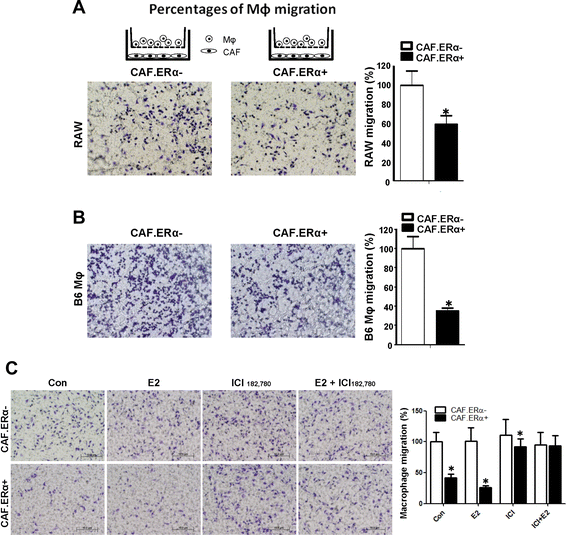
ERα expression in CAF reduced the macrophage (Mφ) migration. a and b CAF ERα reduces the migration of macrophages. CAF.ERα(+) or ERα(−) cells were cultured in 24-well plates for 24 h. We then added macrophages in inserted upper transwells (5 μm pore size) for 24 h and then compared macrophages migration rate toward CAF.ERα(−) vs. CAF.ERα(+) cells. c 17β-estradiol (E2) treatment can reduce macrophage migration in CAF.ERα(+) cells and ICI182,780 (ICI) treatment can reverse E2 effects. CAF.ERα(−) or CAF.ERα(+) cells were first cultured in media with 5 % CD FBS for 2 days, then seeded in 24-well plates, and incubated with vehicle, E2 (10 nM) or/and ICI (10 μM) for 24 h. We then added macrophages into inserted upper transwells (5 μm pore size) for 24 h and compared macrophage migration rates toward CAF.ERα(−) vs. CAF.ERα(+). Migrated macrophages were fixed and stained by 1 % toluidine blue in PBS. Quantifications are in right panels. *, P < 0.05 vs. CAF.ERα(−) cells
Fig. 2
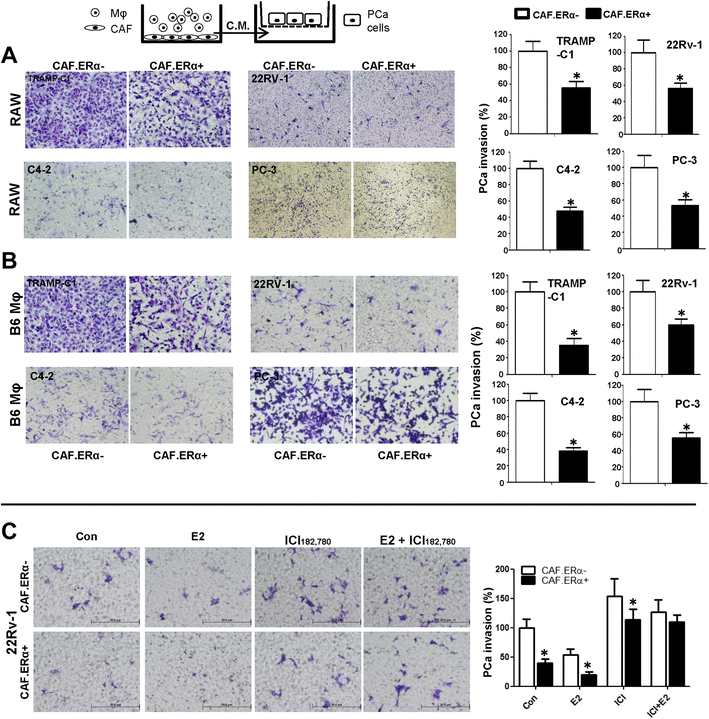
Effects of CM from co-cultured CAF.ERα(+)/macrophages or CAF.ERα(−) /macrophages (Mφ) on PCa invasion. The carton illustrates the PCa invasion transwell system. CM was collected from 48 h co-culture of CAF.ERα(+) or CAF.ERα(−) and RAW264.7 (RAW) cells (a) or B6 primary macrophages (Mφ) (b), co-cultured CM was added to 24-well plates and the PCa cells TRAMP-C1, CWR22Rv-1 (22Rv1), C4-2, or PC-3, were seeded into inserted transwells pre-coated with matrigel. After 24 to 48 h incubation (TRAMPC-1 and PC-3 for 24 h; CWR22Rv-1 and C4-2 for 48 h), invaded PCa cells were counted and compared between CM of CAF.ERα(−)/macrophages and CAF.ERα(+)/macrophages. c Estrogen treatment further triggers CAF.ERα(+) reduced PCa invasion. CAF.ERα(−) or ERα(+) cells were treated with vehicle, E2 (10 nM) or/and ICI (10 μM) and co-cultured with macrophages for 48 h. CMs were collected and added to 24-well plates and the PCa cells (CWR22Rv-1) were seeded onto inserted transwells pre-coated with matrigel. After 48 h incubation, invaded PCa cells were counted and compared, and quantitation data is shown at right. *, P < 0.05 vs. CAF.ERα(−)/macrophages CM treatment group
Fig. 3
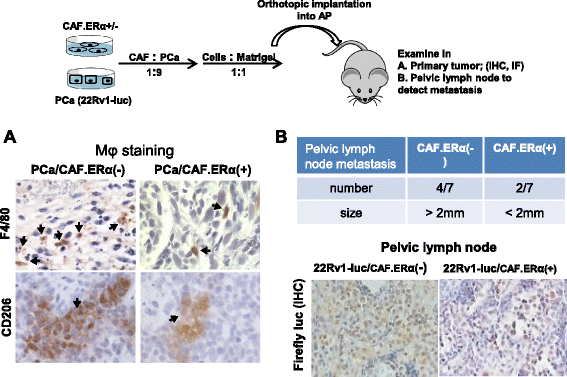
In vivo tumor model demonstrated CAF ERα reduced macrophages infiltration and PCa cell invasion. Nude mice were orthotopically implanted with CAF.ERα(+) or CAF.ERα(−) mixed with CWR22Rv1 cells that were stably transfected with firefly luciferase (22Rv1-Luc). a Tumors were collected 12 weeks after implantation, and macrophage infiltration was examined by IHC staining of F4/80 (M1 macrophage, upper panel) and CD206 (M2 macrophage, lower panel) [30]. b Pelvic lymph nodes were collected from CAF.ERα(−)/22Rv1-Luc and CAF.ERα(+)/22Rv1-Luc co-cultured groups to determine metastasis by measuring sizes of lymph nodes. We confirmed, by IHC staining of luciferase, the presence of 22Rv1-Luc cells in the lymph nodes (lower panel). There were seven mice in each group. We defined pelvic lymph nodes as metastatic when the diameter was over 2 mm (upper panel) *, P < 0.05 vs. CAF.ERα(−)/22Rv1-Luc tumors
Fig. 4
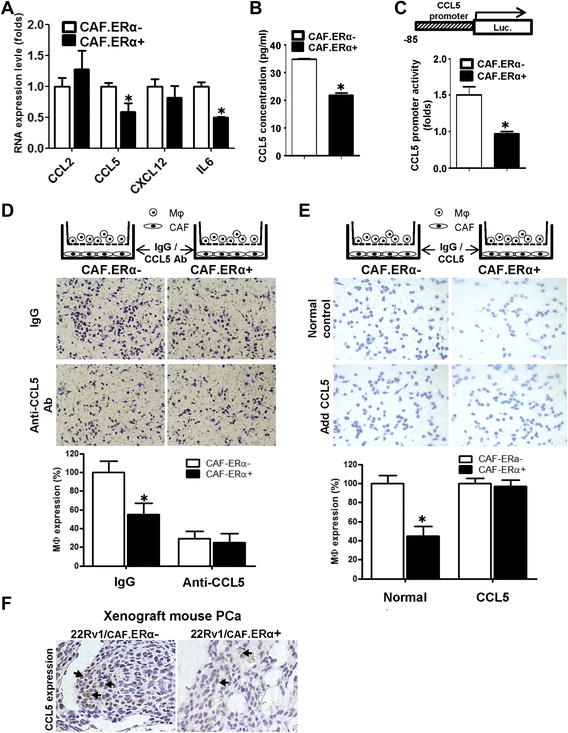
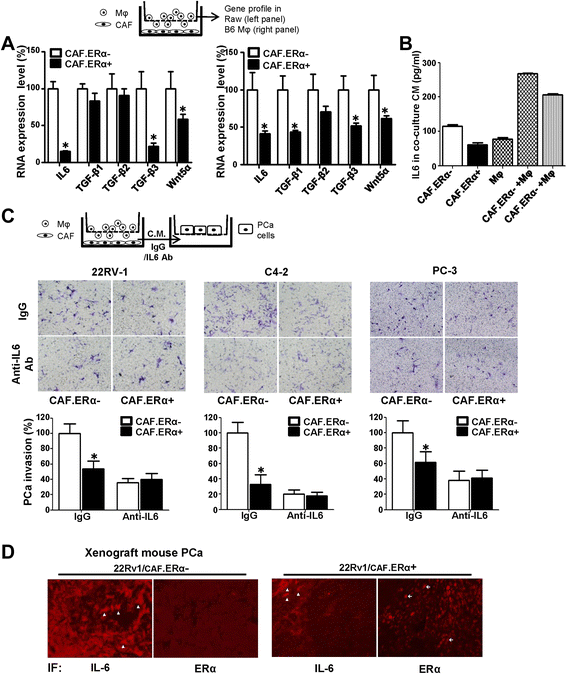
ERα reduced the CCL5 expression in CAF cells and consequently decreased macrophages infiltration. a We compared gene profiles of macrophage attraction related chemokines/cytokines between CAF.ERα(+) and CAF.ERα(−) by QPCR. b The comparison of CCL5 production from CAF.ERα(+) and CAF.ERα(−) was determined by ELISA through measuring CCL5 concentration in the CM. c CCL5 promoter luciferase activity was used to determine whether ERα regulates CCL5 expression. CCL5 promoter (−83 bp)-luciferase reporter was transfected into CAF.ERα(+) and CAF.ERα(−) and cultured in CD-FBS media. 10 nM E2 was added and CCL5 promoter luciferase activity was analyzed using a dual luciferase kit. d CAF were cultured in bottom wells and incubated with CCL5 neutralizing Ab or IgG (control). After 24 h, macrophages were added into inserted upper transwells that also contained CCL5 neutralizing Ab or IgG (control). Migrated macrophages were counted and compared to CAF.ERα(−) with IgG as control. Quantification is in lower panel. e Adding recombinant CCL5 protein reversed CAF.ER(+) reduced macrophage migration. CAF cells were incubated with recombinant CCL5 protein or control for 24 h, and then macrophages with recombinant CCL5 protein or control were added to the inserted transwells for migration assay. All migrated macrophages were compared to CAF.ERα(−) with control protein. Quantification is in lower panel. f CCL5 expression was confirmed by IHC staining in the in vivo mouse PCa tumors co-implanted with/without both CAF/22Rv1-Luc cells. Arrows show positive CCL5 staining. *, P < 0.05 vs. CAF.ERα(−)/22Rv1 tumors
Fig. 5
CAF.ERα(+) CM treated macrophages have a reduced capability of producing IL6, which could consequently reduce PCa invasion. a We compared metastatic-related gene profile expressions by QPCR in macrophages after co-culture with CAF.ERα(+) or CAF.ERα(−) cells. Macrophages were seeded in bottom wells, then CAF.ERα(+) or CAF.ERα(−) cells were seeded onto inserted transwells (0.4 μm) and co-cultured for 24 h. Macrophage RNA was collected and converted to cDNA. Selected metastatic related genes expressions in macrophage were measured by QPCR, RAW cells in left panel and Mφ in right panel. b IL6 concentration in CM from CAF/macrophage co-inoculation was measured by ELISA. c IL6 neutralizing antibody blocks macrophages promoted PCa invasion. The next experiment compared macrophages that were incubated with CM either from CAF.ERα(+) or CAF.ERα(−). PCa cells (CWR22Rv1, C4-2, or PC3) were seeded onto matrigel pre-coated transwells for 48 h to demonstrate invasive ability. d There is a lower IL6 staining in CAF.ERα(+)/CWR22Rv1 co-implanted tumors. Arrowheads show the cells that express IL6 protein. Arrows indicate cells positive for the ERα expression. IL6 expression is reversely correlative to CAF ERα expression using IF staining. *, P < 0.05 vs. CAF.ERα-/ 22Rv1 tumors
Fig. 6
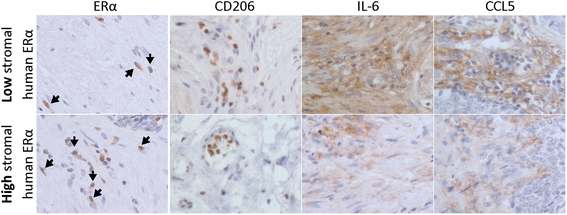
M2 macrophages, CCL5 and IL6 expression increase in prostate cancer patients with high stromal ERα expression. To confirm our findings in human prostate tumors, we also examined ERα, CD206 (M2 macrophage marker), CCL5 and IL6 in human samples by IHC staining. Samples were provides by Department of Pathology, University of Rochester Medical center
Fig. 7
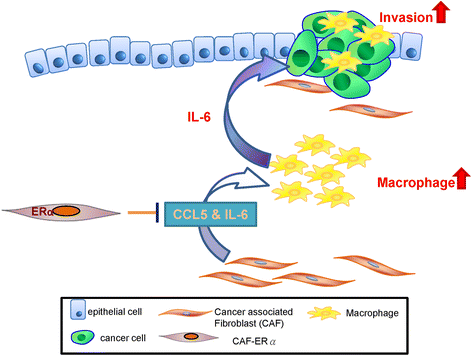
CAF.ERα(+) decreases prostate cancer invasion via diminishing tumor associated macrophage infiltration and IL6 secretion. Schematic diagram shows that CAF.ERα(+) cells diminishes macrophage recruited toward to PCa via reducing CAF CCL5 secretion. These CAF.ERα(+) cells reduced invasion of PCa cells are at least partly due to reduced IL6 expression in the macrophages and CAFs
Similar articles
- Estrogen receptor α in cancer-associated fibroblasts suppresses prostate cancer invasion via modulation of thrombospondin 2 and matrix metalloproteinase 3.
Slavin S, Yeh CR, Da J, Yu S, Miyamoto H, Messing EM, Guancial E, Yeh S. Slavin S, et al. Carcinogenesis. 2014 Jun;35(6):1301-9. doi: 10.1093/carcin/bgt488. Epub 2013 Dec 28. Carcinogenesis. 2014. PMID: 24374826 Free PMC article. - Estrogen Receptor Alpha (ERα)-Associated Fibroblasts Promote Cell Growth in Prostate Cancer.
Da J, Lu M, Wang Z. Da J, et al. Cell Biochem Biophys. 2015 Dec;73(3):793-8. doi: 10.1007/s12013-015-0700-y. Cell Biochem Biophys. 2015. PMID: 27259327 - Fu-Zheng-Yi-Liu Formula inhibits the stem cells and metastasis of prostate cancer via tumor-associated macrophages/C-C motif chemokine ligand 5 pathway in tumor microenvironment.
Chen C, Huang R, Wang N, Zheng Y, Zhou J, Yang B, Wang X, Zhang J, Pan B, Chen Z, Wang S, Wang Z, Xiang S. Chen C, et al. Chin J Nat Med. 2024 Jun;22(6):501-514. doi: 10.1016/S1875-5364(24)60653-9. Chin J Nat Med. 2024. PMID: 38906598 - Fibroblast heterogeneity in prostate carcinogenesis.
ChallaSivaKanaka S, Vickman RE, Kakarla M, Hayward SW, Franco OE. ChallaSivaKanaka S, et al. Cancer Lett. 2022 Jan 28;525:76-83. doi: 10.1016/j.canlet.2021.10.028. Epub 2021 Oct 29. Cancer Lett. 2022. PMID: 34715252 Free PMC article. Review. - Macrophage dynamics in prostate cancer: Molecular to therapeutic insights.
Gu Q, Qi A, Wang N, Zhou Z, Zhou X. Gu Q, et al. Biomed Pharmacother. 2024 Aug;177:117002. doi: 10.1016/j.biopha.2024.117002. Epub 2024 Jul 2. Biomed Pharmacother. 2024. PMID: 38960836 Review.
Cited by
- Estrogen receptors α and β and aromatase as independent predictors for prostate cancer outcome.
Grindstad T, Skjefstad K, Andersen S, Ness N, Nordby Y, Al-Saad S, Fismen S, Donnem T, Khanehkenari MR, Busund LT, Bremnes RM, Richardsen E. Grindstad T, et al. Sci Rep. 2016 Sep 9;6:33114. doi: 10.1038/srep33114. Sci Rep. 2016. PMID: 27610593 Free PMC article. Clinical Trial. - LNRRIL6, a novel long noncoding RNA, protects colorectal cancer cells by activating the IL-6-STAT3 pathway.
Wang J, Zhou J, Jiang C, Zheng J, Namba H, Chi P, Asakawa T. Wang J, et al. Mol Oncol. 2019 Nov;13(11):2344-2360. doi: 10.1002/1878-0261.12538. Epub 2019 Sep 30. Mol Oncol. 2019. PMID: 31246342 Free PMC article. - Influence of Estrogen on the NSCLC Microenvironment: A Comprehensive Picture and Clinical Implications.
Smida T, Bruno TC, Stabile LP. Smida T, et al. Front Oncol. 2020 Feb 18;10:137. doi: 10.3389/fonc.2020.00137. eCollection 2020. Front Oncol. 2020. PMID: 32133288 Free PMC article. Review. - ERα36-High Cancer-Associated Fibroblasts as an Unfavorable Factor in Triple-Negative Breast Cancer.
Nagel A, Popeda M, Muchlinska A, Sadej R, Szade J, Zielinski J, Skokowski J, Niemira M, Kretowski A, Markiewicz A, Zaczek AJ. Nagel A, et al. Cancers (Basel). 2022 Apr 15;14(8):2005. doi: 10.3390/cancers14082005. Cancers (Basel). 2022. PMID: 35454913 Free PMC article. - Estrogen receptor α promotes lung cancer cell invasion via increase of and cross-talk with infiltrated macrophages through the CCL2/CCR2/MMP9 and CXCL12/CXCR4 signaling pathways.
He M, Yu W, Chang C, Miyamoto H, Liu X, Jiang K, Yeh S. He M, et al. Mol Oncol. 2020 Aug;14(8):1779-1799. doi: 10.1002/1878-0261.12701. Epub 2020 Jun 28. Mol Oncol. 2020. PMID: 32356397 Free PMC article.
References
- Nunez C, Cansino JR, Bethencourt F, Perez-Utrilla M, Fraile B, Martinez-Onsurbe P, et al. TNF/IL-1/NIK/NF-kappa B transduction pathway: a comparative study in normal and pathological human prostate (benign hyperplasia and carcinoma) Histopathology. 2008;53:166–176. doi: 10.1111/j.1365-2559.2008.03092.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous