miR-155 Deletion in Mice Overcomes Neuron-Intrinsic and Neuron-Extrinsic Barriers to Spinal Cord Repair - PubMed (original) (raw)
miR-155 Deletion in Mice Overcomes Neuron-Intrinsic and Neuron-Extrinsic Barriers to Spinal Cord Repair
Andrew D Gaudet et al. J Neurosci. 2016.
Abstract
Axon regeneration after spinal cord injury (SCI) fails due to neuron-intrinsic mechanisms and extracellular barriers including inflammation. microRNA (miR)-155-5p is a small, noncoding RNA that negatively regulates mRNA translation. In macrophages, miR-155-5p is induced by inflammatory stimuli and elicits a response that could be toxic after SCI. miR-155 may also independently alter expression of genes that regulate axon growth in neurons. Here, we hypothesized that miR-155 deletion would simultaneously improve axon growth and reduce neuroinflammation after SCI by acting on both neurons and macrophages. New data show that miR-155 deletion attenuates inflammatory signaling in macrophages, reduces macrophage-mediated neuron toxicity, and increases macrophage-elicited axon growth by ∼40% relative to control conditions. In addition, miR-155 deletion increases spontaneous axon growth from neurons; adult miR-155 KO dorsal root ganglion (DRG) neurons extend 44% longer neurites than WT neurons. In vivo, miR-155 deletion augments conditioning lesion-induced intraneuronal expression of SPRR1A, a regeneration-associated gene; ∼50% more injured KO DRG neurons expressed SPRR1A versus WT neurons. After dorsal column SCI, miR-155 KO mouse spinal cord has reduced neuroinflammation and increased peripheral conditioning-lesion-enhanced axon regeneration beyond the epicenter. Finally, in a model of spinal contusion injury, miR-155 deletion improves locomotor function at postinjury times corresponding with the arrival and maximal appearance of activated intraspinal macrophages. In miR-155 KO mice, improved locomotor function is associated with smaller contusion lesions and decreased accumulation of inflammatory macrophages. Collectively, these data indicate that miR-155 is a novel therapeutic target capable of simultaneously overcoming neuron-intrinsic and neuron-extrinsic barriers to repair after SCI.
Significance statement: Axon regeneration after spinal cord injury (SCI) fails due to neuron-intrinsic mechanisms and extracellular barriers, including inflammation. Here, new data show that deleting microRNA-155 (miR-155) affects both mechanisms and improves repair and functional recovery after SCI. Macrophages lacking miR-155 have altered inflammatory capacity, which enhances neuron survival and axon growth of cocultured neurons. In addition, independent of macrophages, adult miR-155 KO neurons show enhanced spontaneous axon growth. Using either spinal cord dorsal column crush or contusion injury models, miR-155 deletion improves indices of repair and recovery. Therefore, miR-155 has a dual role in regulating spinal cord repair and may be a novel therapeutic target for SCI and other CNS pathologies.
Keywords: axon regeneration; microRNA; neuroinflammation; neuroprotection; spinal cord injury.
Copyright © 2016 the authors 0270-6474/16/368516-17$15.00/0.
Figures
Figure 1.
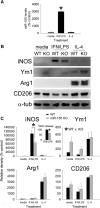
miR-155 is required for macrophages to acquire an inflammatory phenotype. A, BMDMs stimulated with IFN-γ + LPS increase their expression of miR-155 by ∼3000%. miR-155 expression was not increased by IL-4. B, C, WT and miR-155 KO macrophages were stimulated with media, IFN-γ + LPS, or IL-4 for 24 h (loading control: α-tubulin) and then cell lysates were collected to assess expression of canonical M1 (iNOS) and M2 (Ym1, Arg1, CD206) markers. Inset in C shows that, consistent with a significant reduction in iNOS, nitric oxide (NO) release was reduced (∼30%) in miR-155 KO macrophages after stimulation with IFN-γ + LPS. miR-155 KO macrophages also expressed higher levels of Ym1 (*p < 0.05; main effect of genotype). As expected, Arginase-1 and CD206 were significantly increased after activation with IL-4; however, expression of these M2 markers was not significantly different between genotypes. *p < 0.05 (ANOVA with Holm–Sidak post hoc test).
Figure 2.
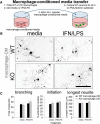
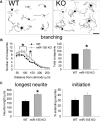
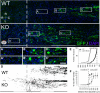
miR-155 KO macrophages enhance neurite outgrowth from adult DRG neurons in a coculture model. A, WT or miR-155 KO macrophages were stimulated with media (control) or IFN-γ + LPS for 5 h before adding WT DRG neurons (inset, GFP+ neurons growing on macrophages). B–D, At 48 h after initial culture, miR-155 KO macrophages were more supportive of neurite outgrowth. Sholl analysis showed that miR-155 KO macrophages enhanced neurite outgrowth, particularly in areas close to the cell body (C, overall total branching and individual media/IFN-γ + LPS Sholl analyses). D, miR-155 KO macrophages increased neurite initiation, the length of the longest neurite, and neuron survival. *p < 0.05 versus the appropriate WT control; †difference versus media-treated WT control. Scale bar, 200 μm.
Figure 3.
Secreted factors likely do not underlie KO-macrophage-enhanced neurite. Unlike cocultures, in which KO macrophages augment axon growth, transfer of macrophage-conditioned media (CM) from miR-155 KO macrophages does not enhance neurite outgrowth compared with WT macrophage-CM. A, DRG neurons were cultured in the presence of WT/miR-155 KO macrophage-CM for 48 h. B, C, miR-155 KO macrophage-CM (control or inflammatory) did not significantly enhance neurite branching, initiation, or length. Scale bar, 200 μm.
Figure 4.
miR-155 KO neurons have greater intrinsic growth potential. A, Representative WT and miR-155 KO DRG neurons at 24 h after culture. B, C, Neurite outgrowth from adult miR-155 KO neurons is enhanced compared with WT neurons. miR-155 KO neurons had 27% more total cross points (B) and 44% longer neurites (C). The percentage of extending neurites was not significantly different between strains. *p < 0.05 versus WT control. Scale bar, 100 μm.
Figure 5.
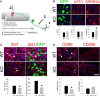
After conditioning lesion and dorsal column injury, miR-155 KO DRGs show improved intrinsic growth responses of sensory neurons and reduced intraganglionic macrophage activation. A, Experimental model and timeline for studying DRG responses and sensory axon regeneration. B, A similar proportion of neurons expressed GFP and ATF3 in the DRG of WT and miR-155 KO mice; however, 44% more miR-155 KO neurons increased expression of the RAG SPRR1A. C, In WT DRGs, large, dense clusters of activated macrophages (arrowheads) accumulated in the DRG and surround a subset of DRG neurons (e.g., GFP-labeled neurons; arrows; also see inset). In miR-155 KO DRGs, macrophage density was reduced 28% and 47% fewer KO DRGs contained inflammatory foci. D, Both pro-inflammatory (CD86) and anti-inflammatory (CD206) markers were expressed in these lumbar DRGs. miR-155 KO DRGs reduced CD86 density by 45%. CD206 density was not different between strains. In all images, blue is nuclear DAPI stain. *p < 0.05 versus WT. Scale bars, 100 μm; inset scale bar, 25 μm.
Figure 6.
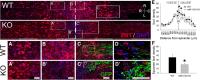
miR-155 deletion reduces intraspinal macrophage activation/accumulation in mice subjected to a peripheral conditioning lesion and spinal cord dorsal column crush injury. miR-155 KO spinal cord epicenters have reduced density of Iba1+ macrophages/microglia. Top, Overview of Iba1 immunoreactivity in horizontal sections of WT and miR-155 KO dorsal columns. A, A′, WT and miR-155 KO spinal cords had similar Iba1+ cell densities in rostral dorsal columns. Rostral is to the left; dashed line delineates injury epicenter. B, B′, graphs, miR-155 KO spinal cords had reduced Iba1+ macrophage/microglial density in the injury epicenter. C, C′, GFP+ axons and endbulbs localized near inflammatory Iba1+ cells. Note the more rostral location of activated macrophages that colocalize with regrowing axon tips in KO spinal cord. D, D′, Confocal images showing detail of Iba1+ cells and GFP+ axons at the rostral-most front of growing axons. E, In miR-155 KO mice, Iba1 density was significantly reduced compared with WT mice from 200 μm rostral to 400 μm caudal to injury epicenter. F, Compared with WT mice, Iba1+ cell density in the KO epicenter was reduced by 46% (quantified between 200 μm and −200 μm). *p < 0.05 versus WT control. R-C, Rostral–caudal axis; R-L, right–left (horizontal axis). Scale bars: top, alternating gray and white bars, each 400 μm; inset panels, 50 μm.
Figure 7.
miR-155 KO axons show enhanced plasticity and regrowth after peripheral conditioning lesion and spinal cord dorsal column crush injury. Top, Overview of GFP immunoreactivity in horizontal sections of WT and miR-155 KO dorsal columns. A, A′, Axons at the regenerative front were located further rostral in KO spinal cords. B, B′, Areas containing axon growth cones or end bulbs in WT and KO mice. C, C′, Axons located in more caudal regions of the injury epicenter. D, Representative thresholded images of GFP+ axons illustrating the enhanced growth of miR-155 KO axons up to and beyond the injury site (dashed line). E, Translesional extension of the longest-growing axons is increased in miR-155 KO spinal cords. F, In miR-155 KO mice, GFP+ axon density was higher than in WT mice between 0 and −400 μm from epicenter, suggesting that KOs have improved axon growth and/or reduced dieback. *p < 0.05 versus WT control. R-C, Rostral–caudal axis; R-L, right–left (horizontal axis). Rostral is to the left; dashed lines identify injury epicenter. Scale bars: Top and D, gray and white bars, each 400 μm; A–C, 50 μm.
Figure 8.
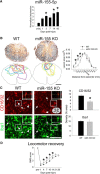
Contusion SCI increases expression of miR-155-5p in WT mice and miR-155 deletion promotes neuroprotection, reduces inflammatory macrophage accumulation, and improves spontaneous locomotor recovery. A, After SCI in WT mice, miR-155-5p expression increases over time after injury, reaching levels >300% above uninjured control by 42 dpi. B, Eriochrome and neurofilament were used to identify intact myelin and axons, respectively. miR-155 KO mice had significantly smaller lesions with the most notable changes evident rostral to injury epicenter: At 600 μm rostral to epicenter, miR-155 KO lesions were 64% smaller than WT lesions. Colored outlines represent lesion size in every animal examined for each genotype at 600 μm rostral to epicenter. C, Overall density of inflammatory CD16/32+ macrophages was reduced by 25% in rostral lesion extensions of miR-155 KO spinal cords, although overall Iba1+ macrophage density in this region was not different between KO and WT mice. D, Beginning at 4 dpi and extending until 14 dpi, spontaneous locomotor recovery was improved in miR-155 KO mice compared with WT mice. By 21 and 28 dpi, mice in both groups regained similar levels of locomotor function. Arrowheads, CD16/32+Iba1+ cells; arrows, CD16/32−Iba1+ cells. *p < 0.05 versus WT control. Scale bars: C, 100 μm; F, 50 μm; inset, 5 μm.
Similar articles
- Complement protein C1q modulates neurite outgrowth in vitro and spinal cord axon regeneration in vivo.
Peterson SL, Nguyen HX, Mendez OA, Anderson AJ. Peterson SL, et al. J Neurosci. 2015 Mar 11;35(10):4332-49. doi: 10.1523/JNEUROSCI.4473-12.2015. J Neurosci. 2015. PMID: 25762679 Free PMC article. - Deletion of the Fractalkine Receptor, CX3CR1, Improves Endogenous Repair, Axon Sprouting, and Synaptogenesis after Spinal Cord Injury in Mice.
Freria CM, Hall JC, Wei P, Guan Z, McTigue DM, Popovich PG. Freria CM, et al. J Neurosci. 2017 Mar 29;37(13):3568-3587. doi: 10.1523/JNEUROSCI.2841-16.2017. Epub 2017 Mar 6. J Neurosci. 2017. PMID: 28264978 Free PMC article. - CCL2 Mediates Neuron-Macrophage Interactions to Drive Proregenerative Macrophage Activation Following Preconditioning Injury.
Kwon MJ, Shin HY, Cui Y, Kim H, Thi AH, Choi JY, Kim EY, Hwang DH, Kim BG. Kwon MJ, et al. J Neurosci. 2015 Dec 2;35(48):15934-47. doi: 10.1523/JNEUROSCI.1924-15.2015. J Neurosci. 2015. PMID: 26631474 Free PMC article. - The Dorsal Column Lesion Model of Spinal Cord Injury and Its Use in Deciphering the Neuron-Intrinsic Injury Response.
Attwell CL, van Zwieten M, Verhaagen J, Mason MRJ. Attwell CL, et al. Dev Neurobiol. 2018 Oct;78(10):926-951. doi: 10.1002/dneu.22601. Epub 2018 May 11. Dev Neurobiol. 2018. PMID: 29717546 Free PMC article. Review. - The age factor in axonal repair after spinal cord injury: A focus on neuron-intrinsic mechanisms.
Geoffroy CG, Meves JM, Zheng B. Geoffroy CG, et al. Neurosci Lett. 2017 Jun 23;652:41-49. doi: 10.1016/j.neulet.2016.11.003. Epub 2016 Nov 3. Neurosci Lett. 2017. PMID: 27818358 Free PMC article. Review.
Cited by
- Inhibiting tau protein improves the recovery of spinal cord injury in rats by alleviating neuroinflammation and oxidative stress.
Chen GL, Sun K, Liu XZ, Tong KL, Chen ZJ, Yu L, Chen NN, Liu SY. Chen GL, et al. Neural Regen Res. 2023 Aug;18(8):1834-1840. doi: 10.4103/1673-5374.363183. Neural Regen Res. 2023. PMID: 36751813 Free PMC article. - SPRR1A is a key downstream effector of MiR-150 during both maladaptive cardiac remodeling in mice and human cardiac fibroblast activation.
Kawaguchi S, Moukette B, Sepúlveda MN, Hayasaka T, Aonuma T, Haskell AK, Mah J, Liangpunsakul S, Tang Y, Conway SJ, Kim IM. Kawaguchi S, et al. Cell Death Dis. 2023 Jul 19;14(7):446. doi: 10.1038/s41419-023-05982-y. Cell Death Dis. 2023. PMID: 37468478 Free PMC article. - Neuronal Dynamics and miRNA Signaling Differ between SH-SY5Y APPSwe and PSEN1 Mutant iPSC-Derived AD Models upon Modulation with miR-124 Mimic and Inhibitor.
Garcia G, Pinto S, Cunha M, Fernandes A, Koistinaho J, Brites D. Garcia G, et al. Cells. 2021 Sep 14;10(9):2424. doi: 10.3390/cells10092424. Cells. 2021. PMID: 34572073 Free PMC article. - Axon regeneration induced by environmental enrichment- epigenetic mechanisms.
Tang BL. Tang BL. Neural Regen Res. 2020 Jan;15(1):10-15. doi: 10.4103/1673-5374.264440. Neural Regen Res. 2020. PMID: 31535635 Free PMC article. Review. - Amelioration of visual deficits and visual system pathology after mild TBI with the cannabinoid type-2 receptor inverse agonist SMM-189.
Guley NM, Del Mar NA, Ragsdale T, Li C, Perry AM, Moore BM, Honig MG, Reiner A. Guley NM, et al. Exp Eye Res. 2019 May;182:109-124. doi: 10.1016/j.exer.2019.03.013. Epub 2019 Mar 26. Exp Eye Res. 2019. PMID: 30922891 Free PMC article.
References
- Bartus K, James ND, Didangelos A, Bosch KD, Verhaagen J, Yáñez-Muñoz RJ, Rogers JH, Schneider BL, Muir EM, Bradbury EJ. Large-scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J Neurosci. 2014;34:4822–4836. doi: 10.1523/JNEUROSCI.4369-13.2014. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials