MLKL forms disulfide bond-dependent amyloid-like polymers to induce necroptosis - PubMed (original) (raw)
MLKL forms disulfide bond-dependent amyloid-like polymers to induce necroptosis
Shuzhen Liu et al. Proc Natl Acad Sci U S A. 2017.
Abstract
Mixed-lineage kinase domain-like protein (MLKL) is essential for TNF-α-induced necroptosis. How MLKL promotes cell death is still under debate. Here we report that MLKL forms SDS-resistant, disulfide bond-dependent polymers during necroptosis in both human and mouse cells. MLKL polymers are independent of receptor-interacting protein kinase 1 and 3 (RIPK1/RIPK3) fibers. Large MLKL polymers are more than 2 million Da and are resistant to proteinase K digestion. MLKL polymers are fibers 5 nm in diameter under electron microscopy. Furthermore, the recombinant N-terminal domain of MLKL forms amyloid-like fibers and binds Congo red dye. MLKL mutants that cannot form polymers also fail to induce necroptosis efficiently. Finally, the compound necrosulfonamide conjugates cysteine 86 of human MLKL and blocks MLKL polymer formation and subsequent cell death. These results demonstrate that disulfide bond-dependent, amyloid-like MLKL polymers are necessary and sufficient to induce necroptosis.
Keywords: MLKL; amyloid-like; disulfide bond; necroptosis; polymer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
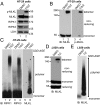
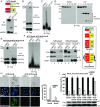
MLKL forms large polymers during necroptosis in human and mouse cells. (A) HT-29 cells were treated with DMSO or 20 ng/mL TNF (T), 100 nM Smac-mimetic (S), and 20 μM Z-VAD-FMK (Z). Cell lysates were subjected to Western blotting with the indicated antibodies. P-MLKL antibody detects phospho-S358 of human MLKL. (B) HT-29 cell lysates were separated by nonreducing SDS/PAGE and detected with antibodies against MLKL or p-MLKL. (C) HT-29 cell lysates were separated by SDD-AGE and detected with RIPK1, RIPK3, or MLKL antibodies. (D) L929 cells were treated with DMSO or 2 ng/mL TNF (T) and 20 μM Z-VAD-FMK (Z), and cell lysates were subjected to nonreducing or reducing SDS/PAGE and detected with MLKL antibody. (E) L929 cell lysates were separated by SDD-AGE and detected with MLKL antibody. IB, immunoblot.
Fig. 2.
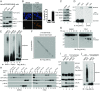
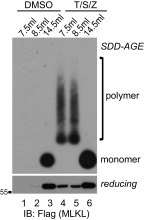
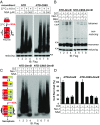
MLKL polymers are proteinase K-resistant, high molecular weight complexes. (A) Establishment of the HeLa:GFP-RIPK3:MLKL cell line. Endogenous MLKL was knocked out with CRISPR-Cas9 (lane 3), and C-terminal HA and Flag-tagged MLKL, as well as GFP-RIPK3, were stably expressed. HeLa cells do not express endogenous RIPK3. The asterisk denotes a nonspecific band. (B) HeLa:GFP-RIPK3:MLKL cells were stained with DAPI and PI. (Scale bar, 100 μm.) Quantification is shown on the right. Data are presented as mean ± SD. (C) Cell lysates were separated on SDS/PAGE and detected with the indicated antibodies. (D) Cell lysates were separated by nonreducing SDS/PAGE and detected with antibodies against Flag or p-MLKL. (E) Cell lysates were separated by SDD-AGE and detected with antibodies against RIPK1, RIPK3, or Flag. (F) Lysates from T/S/Z-treated cells were separated by 2D SDD-AGE and detected with Flag antibody. (G) DMSO- or T/S/Z-treated cell lysates (20 μg) were incubated with different amounts of proteinase K at 30 °C for 10 min and subjected to Western blotting with Flag antibody. (H) DMSO- or T/S/Z-treated cell lysates were subjected to Superdex 200 gel filtration fractionation. Fractions were analyzed by Western blotting with the indicated antibodies. The elution positions of the molecular mass markers are identified at the top. LDH was used as a control. (I and J) C-terminal HA- and Flag-tagged wild-type or T357A/S358A mutant MLKL was expressed at low level in MLK-knockout HeLa cells that were then treated with DMSO or T/S/Z. Cell lysates were subjected to nonreducing or reducing SDS/PAGE (I) or SDD-AGE (J) and detected with the indicated antibodies. IB, immunoblot.
Fig. S1.
MLKL polymers are high molecular weight complexes. DMSO- or T/S/Z-treated cell lysates were subjected to Superdex 200 gel filtration fractionation. Fractions eluted at 7.5 mL, 8.5 mL, and 14.5 mL were analyzed by SDD-AGE and SDS/PAGE and detected with Flag antibody. IB, immunoblot.
Fig. 3.
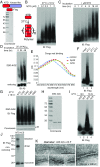
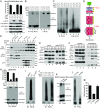
MLKL polymers are disulfide bond-dependent, amyloid-like fibers that are distinct from RIPK3 polymers. (A) DMSO- or T/S/Z-treated lysates or T/S/Z-treated lysates incubated with 10 mM DTT for 30 min were subjected to SDD-AGE and detected with RIPK3 or Flag antibody. (B and C) DMSO- or T/S/Z-treated cell lysates were depleted with a control antibody or an anti-GFP antibody and subjected to nonreducing or reducing SDS/PAGE (B) or SDD-AGE (C) and were detected with Flag or RIPK3 antibody. (D) Cell lysates were subjected to immunoprecipitation (IP) with anti-Flag M2 beads, eluted with pH 3 buffer, and analyzed by Western blotting. (E) The eluate from immunoprecipitation of the T/S/Z-treated lysates was analyzed by SDD-AGE. (F) The eluate from immunoprecipitation of the T/S/Z-treated lysates was subjected to negative staining and imaged by EM. At least 10 fibers were measured to calculate the average diameter. (G) Working model. MLKL is recruited to the necrosome to be phosphorylated and then forms disulfide bond-dependent tetramers. MLKL tetramers further polymerize to form amyloid-like polymers separated from the RIPK1/RIPK3 fibers to promote necroptosis. C–C, disulfide bond. IB, immunoblot.
Fig. 4.
The recombinant MLKL NTD forms disulfide bond-dependent amyloid-like polymers in vitro. (A) Coomassie staining of recombinant C-terminal Flag-tagged MLKL NTD. (B) Different concentrations of the MLKL NTD were incubated at 37 °C for 16 h. For each condition, 50 ng of protein was loaded for SDD-AGE. (C) Recombinant NTD (1 μM) was incubated at 37 °C for different durations. At each time point, 50 ng of protein was loaded for SDD-AGE. (D) Aβ42 peptide (25 μM) was incubated at 37 °C for 24 or 48 h. At each time point, 100 ng protein was loaded for SDD-AGE and SDS/PAGE. (E) Aβ42 polymers (10 μM) or NTD polymers (2.5 μM) were incubated with 50 μM Congo red for 10 min at room temperature, and absorptions were measured at the indicated wavelengths. (F) NTD (1 μM) was incubated at 37 °C for 16 h (lane 2), and half of the sample was further incubated with 10 mM DTT for 30 min (lane 3). For each lane, 50 ng of protein was loaded for SDD-AGE. (G) Recombinant C18S, C24S, C28S, and C86S proteins were purified (Coomassie staining, Lower), and 1 μM of each was incubated at 37 °C for 16 h. Then 50 ng of each was loaded for SDD-AGE (Upper). (H) Coomassie staining of recombinant NTD and NTD with all four cysteines mutated to serines (4CS). (I) Recombinant NTD or 4CS (1 μM) was incubated at 37 °C for 0 h or 16 h. Then 50 ng of each sample was loaded for SDD-AGE. (J) Purified NTD or 4CS (50 ng) was analyzed by nonreducing SDS/PAGE. (K) Recombinant NTD polymers were subjected to negative staining and imaged by EM. At least 10 fibers were measured to calculate the average diameter. IB, immunoblot.
Fig. 5.
Disulfide bonds are necessary for NTD-DmrB polymer formation and necroptosis induction in vivo. (A) Establishment of the NTD-DmrB cell line. C-terminal Flag-tagged NTD-DmrB fusion protein was stably expressed in MLKL-knockout HeLa cells. Cell lysates were subjected to Western blotting and detected with MLKL or HMGB1 antibody. (B) NTD-DmrB cells were treated with DMSO or 20 nM dimerizer (D) and 20 μM Z-VAD-FMK (Z). Cell lysates were separated by nonreducing or reducing SDS/PAGE. (C) DMSO- or D/Z-treated lysates or D/Z-treated lysates incubated with 10 mM DTT were subjected to SDD-AGE. (D) Cell lysates (20 μg) were incubated with different amounts of proteinase K at 30 °C for 10 min and then subjected to Western blotting. (E and F) NTD-4CS-DmrB was stably expressed in MLKL-knockout HeLa cells. DMSO- or D/Z-treated lysates were subjected to nonreducing or reducing SDS/PAGE (E) or SDD-AGE (F). (G) DMSO- or D/Z-treated cells were fractionated into cytosol and crude membrane fractions. (Left) The samples were then subjected to Western blotting with the indicated antibodies. LDH is a cytosol marker, and LAMP1 is a membrane marker. (Right) Working model. Dimerizer induces the formation of disulfide bond-dependent NTD-DmrB tetramers that further polymerize to induce necroptosis. (H) NTD-DmrB or NTD-4CS-DmrB cells were treated with DMSO or D/Z and were stained with DAPI and SYTOX Green. (Scale bar, 100 μm.) Quantification is shown on the right. Data are presented as mean ± SD. (I, Upper) NTD-DmrB cysteine mutants were expressed in MLKL-knockout HeLa cells, and cell survival was determined by CellTiter-Glo assay. Data are presented as mean ± SD. (Lower) Western blotting with Flag or LDH antibodies was performed to assess protein expression. IB, immunoblot.
Fig. 6.
NSA blocks NTD polymer formation in a cysteine 86-dependent manner to block necroptosis. (A) Recombinant NTD or NTD-C86S (1 μM) was incubated at 37 °C with or without NSA, and 50 ng protein from each treatment was loaded for SDD-AGE. (B and C) NTD-DmrB or NTD-C86S-DmrB cells were treated as indicated. Cell lysates were subjected to nonreducing or reducing SDS/PAGE (B) or SDD-AGE (C). (D) NTD-DmrB or NTD-C86S-DmrB cells were subjected to the indicated treatments, and cell survival was determined by CellTiter-Glo assay. Data are presented as mean ± SD. IB, immunoblot.
Fig. 7.
NSA blocks MLKL polymer formation in human cells, and N-terminal–tagged MLKL fails to form polymers to promote necroptosis. (A, Upper) HeLa:GFP-RIPK3:MLKL cells were subjected to the indicated treatments, and cell survival was determined by CellTiter-Glo assay. Data are presented as mean ± SD. (Lower) Cell lysates were subjected to Western blotting with the indicated antibodies. (B) Cells were treated as indicated, and cell lysates were subjected to nonreducing SDS/PAGE and detected with Flag or p-MLKL antibody. (C) Cells lysates were subjected to SDD-AGE and detected with RIPK3 or Flag antibody. (D) Cells were treated with DMSO, T/S/Z, or T/S/Z plus NSA, and cell lysates were separated by gel filtration. The fractions were subjected to Western blotting with the indicated antibodies. (E) Cell lysates were subjected to immunoprecipitation with an anti-GFP antibody and were analyzed by Western blotting with the indicated antibodies. (F) Cells were separated into cytosol and crude membrane fractions and were analyzed by Western blotting with the indicated antibodies. LDH is a cytosol marker, and EGF receptor (EGFR) is a membrane marker. (G) Working model. NSA does not block necrosome formation. Instead, it blocks the polymerization of the tetramers to inhibit necroptosis. (H, Upper) L929 cells were subjected to the indicated treatments, and cell survival was determined by CellTiter-Glo assay. Data are presented as mean ± SD. (Lower) Cell lysates were subjected to nonreducing or reducing SDS/PAGE and were detected with mouse MLKL antibody. (I) DMSO- or T/Z-treated lysates or T/Z-treated lysates incubated with 10 mM DTT were separated by SDD-AGE and detected with mouse MLKL antibody. (J) L929 cells were treated as indicated, and cell lysates were subjected to SDD-AGE and detected with mouse MLKL antibody. (K and L) C-terminal Flag-tagged MLKL or N-terminal Flag-tagged MLKL was overexpressed in MLKL-knockout HeLa cells. Cell lysates were subjected to nonreducing or reducing SDS/PAGE (K) or SDD-AGE (L). (M) C-Flag or N-Flag MLKL was overexpressed in MLKL-knockout HeLa cells, and cell survival was determined by CellTiter-Glo assay. Data are presented as mean ± SD. IB, immunoblot.
Fig. S2.
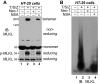
NSA blocks MLKL polymer formation in HT-29 cells. (A) HT-29 cells were treated as indicated, and cell lysates were subjected to nonreducing or reducing SDS/PAGE and detected with the indicated antibodies. (B) HT-29 cells were treated as indicated, and cell lysates were subjected to SDD-AGE and detected with MLKL antibody. IB, immunoblot.
Similar articles
- Discovery of potent necroptosis inhibitors targeting RIPK1 kinase activity for the treatment of inflammatory disorder and cancer metastasis.
Hou J, Ju J, Zhang Z, Zhao C, Li Z, Zheng J, Sheng T, Zhang H, Hu L, Yu X, Zhang W, Li Y, Wu M, Ma H, Zhang X, He S. Hou J, et al. Cell Death Dis. 2019 Jun 24;10(7):493. doi: 10.1038/s41419-019-1735-6. Cell Death Dis. 2019. PMID: 31235688 Free PMC article. - Thioredoxin-1 actively maintains the pseudokinase MLKL in a reduced state to suppress disulfide bond-dependent MLKL polymer formation and necroptosis.
Reynoso E, Liu H, Li L, Yuan AL, Chen S, Wang Z. Reynoso E, et al. J Biol Chem. 2017 Oct 20;292(42):17514-17524. doi: 10.1074/jbc.M117.799353. Epub 2017 Sep 6. J Biol Chem. 2017. PMID: 28878015 Free PMC article. - CAMK2/CaMKII activates MLKL in short-term starvation to facilitate autophagic flux.
Zhan Q, Jeon J, Li Y, Huang Y, Xiong J, Wang Q, Xu TL, Li Y, Ji FH, Du G, Zhu MX. Zhan Q, et al. Autophagy. 2022 Apr;18(4):726-744. doi: 10.1080/15548627.2021.1954348. Epub 2021 Jul 20. Autophagy. 2022. PMID: 34282994 Free PMC article. - Viral-induced neuronal necroptosis: Detrimental to brain function and regulation by necroptosis inhibitors.
Prasad Panda S, Kesharwani A, Prasanna Mallick S, Prasanth D, Kumar Pasala P, Bharadwaj Tatipamula V. Prasad Panda S, et al. Biochem Pharmacol. 2023 Jul;213:115591. doi: 10.1016/j.bcp.2023.115591. Epub 2023 May 16. Biochem Pharmacol. 2023. PMID: 37196683 Review. - Novel drug discovery strategies for atherosclerosis that target necrosis and necroptosis.
Coornaert I, Hofmans S, Devisscher L, Augustyns K, Van Der Veken P, De Meyer GRY, Martinet W. Coornaert I, et al. Expert Opin Drug Discov. 2018 Jun;13(6):477-488. doi: 10.1080/17460441.2018.1457644. Epub 2018 Mar 29. Expert Opin Drug Discov. 2018. PMID: 29598451 Review.
Cited by
- Mitochondrial Reactive Oxygen Species and Lytic Programmed Cell Death in Acute Inflammation.
Rius-Pérez S, Pérez S, Toledano MB, Sastre J. Rius-Pérez S, et al. Antioxid Redox Signal. 2023 Oct;39(10-12):708-727. doi: 10.1089/ars.2022.0209. Epub 2023 Aug 31. Antioxid Redox Signal. 2023. PMID: 37450339 Free PMC article. Review. - Discovery of potent necroptosis inhibitors targeting RIPK1 kinase activity for the treatment of inflammatory disorder and cancer metastasis.
Hou J, Ju J, Zhang Z, Zhao C, Li Z, Zheng J, Sheng T, Zhang H, Hu L, Yu X, Zhang W, Li Y, Wu M, Ma H, Zhang X, He S. Hou J, et al. Cell Death Dis. 2019 Jun 24;10(7):493. doi: 10.1038/s41419-019-1735-6. Cell Death Dis. 2019. PMID: 31235688 Free PMC article. - Regulated cell death and its role in Alzheimer's disease and amyotrophic lateral sclerosis.
Thal DR, Gawor K, Moonen S. Thal DR, et al. Acta Neuropathol. 2024 Apr 7;147(1):69. doi: 10.1007/s00401-024-02722-0. Acta Neuropathol. 2024. PMID: 38583129 Review. - MLKL in cancer: more than a necroptosis regulator.
Martens S, Bridelance J, Roelandt R, Vandenabeele P, Takahashi N. Martens S, et al. Cell Death Differ. 2021 Jun;28(6):1757-1772. doi: 10.1038/s41418-021-00785-0. Epub 2021 May 5. Cell Death Differ. 2021. PMID: 33953348 Free PMC article. Review. - RIP1/RIP3/MLKL-mediated necroptosis contributes to vinblastine-induced myocardial damage.
Zhou H, Liu L, Ma X, Wang J, Yang J, Zhou X, Yang Y, Liu H. Zhou H, et al. Mol Cell Biochem. 2021 Feb;476(2):1233-1243. doi: 10.1007/s11010-020-03985-3. Epub 2020 Nov 28. Mol Cell Biochem. 2021. PMID: 33247805 Free PMC article.
References
- Linkermann A, et al. Necroptosis in immunity and ischemia-reperfusion injury. Am J Transplant. 2013;13:2797–2804. - PubMed
- Sun L, Wang X. A new kind of cell suicide: Mechanisms and functions of programmed necrosis. Trends Biochem Sci. 2014;39:587–593. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous