Src Inhibition Attenuates Neuroinflammation and Protects Dopaminergic Neurons in Parkinson's Disease Models - PubMed (original) (raw)
Src Inhibition Attenuates Neuroinflammation and Protects Dopaminergic Neurons in Parkinson's Disease Models
Hanyu Yang et al. Front Neurosci. 2020.
Abstract
Chronic neuroinflammation is of great importance in the pathogenesis of Parkinson's disease (PD). During the process of neuroinflammation, overactivated microglia release many proinflammatory factors, which eventually induce neurodegeneration. Inhibition of excessive microglial activation is regarded as a promising strategy for PD treatment. Src is a non-receptor tyrosine kinase that is closely related to tumors. Recently, some reports indicated that Src is a central mediator in multiple signaling pathways including neuroinflammation. The aim of our study was to demonstrate the role of Src in microglial regulation and neuroinflammation. The lipopolysaccharide (LPS)-stimulated BV2 microglia model and the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD model were applied in this study. The results showed that inhibition of Src could significantly relieve microgliosis and decrease levels of inflammatory factors. Besides, inhibition of Src function reduced the loss of dopaminergic neurons and improved the motor behavior of the MPTP-treated mice. Thus, this study not only verified the critical role of Src tyrosine kinase in neuroinflammation but also further proved that interfering neuroinflammation is beneficial for PD treatment. More importantly, this study shed a light on the hypothesis that Src tyrosine kinase might be a potential therapeutic target for PD and other neuroinflammation-related diseases.
Keywords: Parkinson’s disease; Src; microglia; neuroinflammation; neuroprotection.
Copyright © 2020 Yang, Wang, Zang, Wang, Shang, Zhang, Liu, Bao, Wang and Zhang.
Figures
FIGURE 1
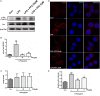
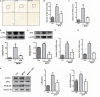
The efficiency of Src inhibitor PP2 was confirmed in lipopolysaccharide (LPS)-induced BV2 activation. Cultured BV2 microglia were treated with two concentrations of PP2 (2 and 20 μM) in the presence of LPS (1 μg/ml) for 24 h. (A–C) The protein levels of p-Src and Src were determined by western blot analysis. β-Actin was used as an internal loading control. Each bar represents the mean ± SEM. n = 4. ##P < 0.01 vs. control group,**P < 0.01, and *P < 0.05 vs. LPS group. (D) The cells were stained with anti-p-Src antibody (red) and DAPI stain (scale bar: 8 μm). (E) The fluorescence intensity of the p-Src staining of was provided in a histogram. Each bar represents the mean ± SEM. n = 3. ###P < 0.001 vs. control group, **P < 0.01 vs. LPS group.
FIGURE 2
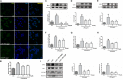
Src inhibition prevented the activation of BV2 microglia and the production of neuroinflammatory molecules subjected to lipopolysaccharide (LPS). (A) Cultured BV2 cells were treated with two concentrations of PP2 (2 and 20 μM) in the presence of LPS (1 μg/ml) for 24 h, and then the cells were stained with anti-IBA1 antibody (green) and DAPI stain (scale bar: 8 μm). (B) Quantification of the IBA1 staining was provided in a histogram. Each bar represents the mean ± SEM. n = 4. ###P < 0.001 vs. control group, *P < 0.05 vs. LPS group. (C) BV2 cells were treated with two concentrations of PP2 (2 and 20 μM) in the presence of LPS (1 μg/ml) for 24 h. The protein level of IBA1 was analyzed by western blot with anti-IBA1 antibody. β-Actin was used as an internal loading control. Each bar represents the mean ± SEM. n = 4. #P < 0.05 vs. control group,**P < 0.01 vs. LPS group. (D,E) The protein level of cyclooxygenase-2 (COX2) and iNOS were examined by western blot. Each bar represents the mean ± SEM. n = 4. ###P < 0.001 and ##P < 0.01 vs. control group, **P < 0.01 and *P < 0.05 vs. LPS group. (F,G) The mRNA levels of IL-6 and TNF-α were analyzed by quantitative reverse transcription (qRT)-PCR. Each bar represents the mean ± SEM. n = 4. ##P < 0.01 and ###P < 0.001 vs. control group, *P < 0.05 and ***P < 0.001 vs. LPS group. (H) The level of NO production was determined using the Griess reaction. Each bar represents the mean ± SEM. n = 5. ###P < 0.001 vs. control group, *P < 0.05 and **P < 0.01 vs. LPS group. (I) The protein expression level of p-IKKα, IKKα, NF-κB p65, and histone H3 were measured by western blot. Each bar represents the mean ± SEM. n = 4. ###P < 0.001 vs. control group, **P < 0.01 vs. LPS group.
FIGURE 3
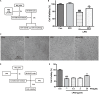
Src inhibition decreased neurotoxicity mediated by lipopolysaccharide (LPS)-stimulated microglia. (A) The experimental design. (B) The cell viability of SH-SY5Y cells was assessed by MTT assay. Each bar represents the mean ± SEM. n = 6. ##P < 0.01 vs. control group, **P < 0.01 vs. LPS group. (C) The morphology of SH-SY5Y cells was observed by the phase-contrast microscope (scale bar: 20 μm). (D) The experimental design. (E) The cell viability of SH-SY5Y cells was assessed by MTT assay. Each bar represents the mean ± SEM. n = 5. ##P < 0.01 vs. control group, **P < 0.01 vs. LPS group.
FIGURE 4
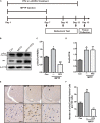
The efficiency of Src inhibitor PP2 was confirmed in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice. (A) The experimental arrangement. (B–D) The protein level of p-Src and Src in SNpc of MPTP-treated mice was analyzed by western blot with anti-p-Src and anti-Src antibodies. Data are expressed as means ± SEM. n = 4. #P < 0.05 vs. control group,***P < 0.001 vs. lipopolysaccharide (LPS) group. (E) The brown stain represented p-Src-immunoreactive cells in SNpc (scale bar: top, 600 μm; bottom, 25 μm). (F) The number of p-Src-positive cells per 100 cells in SNpc was counted and provided in a histogram. Data are expressed as means ± SEM. n = 4. #P < 0.05 and ##P < 0.01 vs. control group, **P < 0.01 and ***P < 0.001 vs. MPTP group.
FIGURE 5
Src inhibition reduced activation of microglial cells and neuroinflammation in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice. (A) The activation of microglia in SNpc showed by IBA1 immunostaining (scale bar: top, 200 μm; bottom, 50 μm). (B) The number of IBA1-positive cells per 100 cells was counted and provided in a histogram. Data are expressed as means ± SEM. n = 4. ##P < 0.01 vs. control group,*P < 0.05 vs. lipopolysaccharide (LPS) group. (C) The mRNA expression level of IBA1 in SNpc was determined by quantitative reverse transcription (qRT)-PCR. Data are expressed as means ± SEM. n = 4. ##P < 0.01 vs. control group,*P < 0.05 vs. LPS group. (D,E) The protein expression level of cyclooxygenase-2 (COX2) and iNOS were determined by western blot with anti-COX2 and anti-iNOS antibodies. Data are expressed as means ± SEM. n = 4. #P < 0.05 vs. control group,*P < 0.05 and **P < 0.01 vs. LPS group. (F,G) The mRNA expression level of IL-6 and TNF-α was determined by qRT-PCR. Each bar represents the mean ± SEM. n = 4. #P < 0.05 and ##P < 0.01 vs. control group, *P < 0.05 vs. MPTP group. (H) The protein expression level of p-IKKα, IKKα, NF-κB p65, and histone H3 were measured by western blot. Each bar represents the mean ± SEM. n = 4. #P < 0.05 and ##P < 0.01 vs. control group, *P < 0.05 vs. MPTP group.
FIGURE 6
Src inhibition enhanced the survival of dopaminergic neurons of the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice. (A) Representative images showed tyrosine hydroxylase (TH)-immunoreactive neurons in the SNpc (scale bar: top, 500 μm; bottom, 250 μm). (B) The number of TH-positive neurons per slide in SNpc was counted for each section and provided in a histogram. Data are expressed as means ± SEM. n = 4. #P < 0.05 vs. control group,*P < 0.05 vs. lipopolysaccharide (LPS) group. (C) The protein expression level of TH in SNpc of MPTP-treated mice was analyzed by western blot. Data are expressed as means ± SEM. n = 4. #P < 0.05 vs. control group,*P < 0.05 vs. LPS group. (D) The mRNA expression level of TH in SNpc of MPTP-treated mice was determined by quantitative reverse transcription (qRT)-PCR. Data are expressed as means ± SEM. n = 4. ###P < 0.001 vs. control group, *P < 0.05 vs. MPTP group.
FIGURE 7
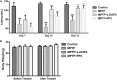
Src inhibition improved the motor behavior of the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice. Evaluation of mice motor performance using the rotarod test. (A) Latency represent time of mice staying on the rod on days 7, 10, and 12. (B) Body weight was measured before and after the experiment (at days 1 and 13). Each bar represents the mean ± SEM. ###P < 0.001 vs. control group, *P < 0.05 and ***P < 0.001 vs. MPTP group.
Similar articles
- Inhibition of Src tyrosine kinase activity by squamosamide derivative FLZ attenuates neuroinflammation in both in vivo and in vitro Parkinson's disease models.
Tai W, Ye X, Bao X, Zhao B, Wang X, Zhang D. Tai W, et al. Neuropharmacology. 2013 Dec;75:201-12. doi: 10.1016/j.neuropharm.2013.07.020. Epub 2013 Aug 2. Neuropharmacology. 2013. PMID: 23916477 - Protein kinase Cδ upregulation in microglia drives neuroinflammatory responses and dopaminergic neurodegeneration in experimental models of Parkinson's disease.
Gordon R, Singh N, Lawana V, Ghosh A, Harischandra DS, Jin H, Hogan C, Sarkar S, Rokad D, Panicker N, Anantharam V, Kanthasamy AG, Kanthasamy A. Gordon R, et al. Neurobiol Dis. 2016 Sep;93:96-114. doi: 10.1016/j.nbd.2016.04.008. Epub 2016 May 2. Neurobiol Dis. 2016. PMID: 27151770 Free PMC article. - The circadian clock protein Rev-erbα provides neuroprotection and attenuates neuroinflammation against Parkinson's disease via the microglial NLRP3 inflammasome.
Kou L, Chi X, Sun Y, Han C, Wan F, Hu J, Yin S, Wu J, Li Y, Zhou Q, Zou W, Xiong N, Huang J, Xia Y, Wang T. Kou L, et al. J Neuroinflammation. 2022 Jun 6;19(1):133. doi: 10.1186/s12974-022-02494-y. J Neuroinflammation. 2022. PMID: 35668454 Free PMC article. - Role of microgliosis, oxidative stress and associated neuroinflammation in the pathogenesis of Parkinson's disease: The therapeutic role of Nrf2 activators.
Jayaram S, Krishnamurthy PT. Jayaram S, et al. Neurochem Int. 2021 May;145:105014. doi: 10.1016/j.neuint.2021.105014. Epub 2021 Mar 8. Neurochem Int. 2021. PMID: 33689805 Review. - Neuroinflammation in Parkinson's disease: focus on the relationship between miRNAs and microglia.
Xu K, Li Y, Zhou Y, Zhang Y, Shi Y, Zhang C, Bai Y, Wang S. Xu K, et al. Front Cell Neurosci. 2024 Jul 26;18:1429977. doi: 10.3389/fncel.2024.1429977. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39131043 Free PMC article. Review.
Cited by
- The FGF/FGFR system in the microglial neuroinflammation with Borrelia burgdorferi: likely intersectionality with other neurological conditions.
Parthasarathy G, Pattison MB, Midkiff CC. Parthasarathy G, et al. J Neuroinflammation. 2023 Jan 17;20(1):10. doi: 10.1186/s12974-022-02681-x. J Neuroinflammation. 2023. PMID: 36650549 Free PMC article. - The Neglected Sibling: NLRP2 Inflammasome in the Nervous System.
Ducza L, Gaál B. Ducza L, et al. Aging Dis. 2024 May 7;15(3):1006-1028. doi: 10.14336/AD.2023.0926-1. Aging Dis. 2024. PMID: 38722788 Free PMC article. Review. - Saracatinib, a Src Tyrosine Kinase Inhibitor, as a Disease Modifier in the Rat DFP Model: Sex Differences, Neurobehavior, Gliosis, Neurodegeneration, and Nitro-Oxidative Stress.
Gage M, Putra M, Wachter L, Dishman K, Gard M, Gomez-Estrada C, Thippeswamy T. Gage M, et al. Antioxidants (Basel). 2021 Dec 28;11(1):61. doi: 10.3390/antiox11010061. Antioxidants (Basel). 2021. PMID: 35052568 Free PMC article. - Integrative Organelle-Based Functional Proteomics: In Silico Prediction of Impaired Functional Annotations in SACS KO Cell Model.
Morani F, Doccini S, Galatolo D, Pezzini F, Soliymani R, Simonati A, Lalowski MM, Gemignani F, Santorelli FM. Morani F, et al. Biomolecules. 2022 Jul 24;12(8):1024. doi: 10.3390/biom12081024. Biomolecules. 2022. PMID: 35892334 Free PMC article. - Differential Impact of Severity and Duration of Status Epilepticus, Medical Countermeasures, and a Disease-Modifier, Saracatinib, on Brain Regions in the Rat Diisopropylfluorophosphate Model.
Gage M, Putra M, Gomez-Estrada C, Golden M, Wachter L, Gard M, Thippeswamy T. Gage M, et al. Front Cell Neurosci. 2021 Oct 15;15:772868. doi: 10.3389/fncel.2021.772868. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34720886 Free PMC article.
References
- Calvello R., Cianciulli A., Nicolardi G., De Nuccio F., Giannotti L., Salvatore R., et al. (2017). Treatment attenuates neuroinflammation and dopaminergic neurodegeneration in an animal model of Parkinson’s disease, shifting M1 to M2 Microglia responses. J. Neuroimmune Pharmacol. 12 327–339. 10.1007/s11481-016-9720-7 - DOI - PubMed
- Chen H., Zhang S. M., Hernán M. A., Schwarzschild M. A., Willett W. C., Colditz G. A., et al. (2005). Nonsteroidal antiinflammatory drug use and the risk for Parkinson’s disease. Ann. Neurol. 58 963–967. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous