HSP90 Inhibitor, 17-DMAG, Alone and in Combination with Lapatinib Attenuates Acquired Lapatinib-Resistance in ER-positive, HER2-Overexpressing Breast Cancer Cell Line - PubMed (original) (raw)
HSP90 Inhibitor, 17-DMAG, Alone and in Combination with Lapatinib Attenuates Acquired Lapatinib-Resistance in ER-positive, HER2-Overexpressing Breast Cancer Cell Line
Hye Jin Lee et al. Cancers (Basel). 2020.
Abstract
Lapatinib, a Human Epidermal growth factor Receptor 2 (HER2)-targeting therapy in HER2-overexpressing breast cancer, has been widely used clinically, but the prognosis is still poor because most patients acquire resistance. Therefore, we investigated mechanisms related to lapatinib resistance to evaluate new therapeutic targets that may overcome resistance. Lapatinib-resistant cell lines were established using SKBR3 and BT474 cells. We evaluated cell viability and cell signal changes, gene expression and protein changes. In the xenograft mouse model, anti-tumor effects were evaluated using drugs. Analysis of the protein interaction network in two resistant cell lines with different lapatinib resistance mechanisms showed that HSP90 protein was commonly increased. When Heat Shock Protein 90 (HSP90) inhibitors were administered alone to both resistant cell lines, cell proliferation and protein expression were effectively inhibited. However, inhibition of cell proliferation and protein expression with a combination of lapatinib and HSP90 inhibitors showed a more synergistic effect in the LR-BT474 cell line than the LR-SKBR3 cell line, and the same result was exhibited with the xenograft model. These results suggest that HSP90 inhibitors in patients with lapatinib-resistant Estrogen Receptor (ER) (+) HER2 (+) breast cancer are promising therapeutics for future clinical trials.
Keywords: ER (+) HER2 (+); HSP90; breast cancer; lapatinib resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Figure 1
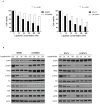
Establishment of lapatinib-acquired-resistant Human Epidermal growth factor Receptor 2 (HER2) (+) cell lines and biologic changes in HER2 downstream signaling. (A) Lapatinib was administered for 48 and 72 h at different concentrations in SKBR3, BT474, LR-SKBR3, and LR-BT474 cell lines and measured using MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium) analysis. Results are expressed as percentage of viable cells from three independent experiments (mean ± SD) (***, p < 0.001). (B) Parental and lapatinib-resistant cell lines were treated with lapatinib at 20, 50, or 100 nM concentrations for 24 h. Western blot was performed using same amounts of protein. Protein activation was analyzed by evaluating phosphorylation status using corresponding p-HER2, p-EGFR, p-Akt, and p-Erk antibodies. Results were obtained from two independent experiments.
Figure 2
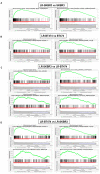
Gene set enrichment analysis (GSEA) enrichment plot in lapatinib-resistant cell lines. (A) GSEA plot shows G2M checkpoint and E2F target in LR-SKBR3 and SKBR3 cells. (B) GSEA plot shows early and late estrogen responses in LR-BT474 and BT474 cells. (C) GSEA analysis represents gene expression enrichment in LR-SKBR3 cell compared with LR-BT474 cell. (D) GSEA analysis represents gene expression enrichment in LR-BT474 cell compared with LR-SKBR3 cell.
Figure 3
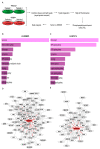
Phosphoproteomic profiling of lapatinib-resistant and sensitive cell lines. (A) Experimental schematic outline of SILAC experiment. (B) Enriched cellular pathways in LR-SKBR3. (C) Enriched cellular pathways in LR-BT474. (D) Major cluster by GLay clustering in LR-SKBR3. (E) Major cluster by GLay clustering in LR-BT474.
Figure 4
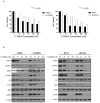
17DMAG induces inhibition of cell proliferation and affects HER2 downstream signaling in lapatinib-resistant cell lines. (A) 17DMAG was administered for 48 and 72 h at different concentrations in parent and lapatinib-resistant cell lines and measured using MTT analysis. Results are expressed as percentage of viable cells from three independent experiments (mean ± SD) (***, p < 0.001). (B) Parental and lapatinib-resistant cell lines were treated with 17DMAG at 20, 50, or 100 nM concentrations for 24 h. Western blot was performed using same amounts of protein. Protein activation was analyzed using corresponding p-HER2, p-EGFR, p-Akt, and p-ERK antibodies. Results represent 2 independent experiments.
Figure 5
Combination of lapatinib and 17DMAG in lapatinib-resistant cell lines. (A) Isobologram plot shows combination treatment of lapatinib and 17-DMAG in LR-SKBR3 and LR-BT474 cell lines. (B) All cell lines were treated with lapatinib, 17DMAG, or combination lapatinib and 17DMAG for 24 h, lysed, and analyzed using western blot with indicated antibodies.
Figure 6
Antitumor efficacy of 17DMAG and lapatinib combination in BT474 and LR-BT474 xenograft models. (A,B) Mice were injected with BT474 and LR-BT474 cells on the flanks. After tumor formation, randomly grouped nude mice were treated with control, lapatinib (75 mg/kg), 17DMAG (5 mg/kg), or combination (lapatinib ,17DMAG) for 5 weeks. Tumor measurements were obtained twice a week. Average tumor volumes in control, lapatinib, 17-DMAG, and combination treatment groups are presented as mean ± SEM (*, p < 0.05; **, p < 0.01). Scale bars, 1 cm. (C) Representative image of immunohistochemical staining in BT474 and LR-BT474 tumors of xenograft models. Tumor sections from vehicle and combination groups were stained for HER2 and ER. Scale bars, 100 μm. Scatter plot of ER Allred scores in BT474 and LR-BT474 xenograft tumors measured by IHC. Data are presented as mean ± SEM (n = 4 in each group).
Similar articles
- HSP90 inhibitor, AUY922, debilitates intrinsic and acquired lapatinib-resistant HER2-positive gastric cancer cells.
Park KS, Hong YS, Choi J, Yoon S, Kang J, Kim D, Lee KP, Im HS, Lee CH, Seo S, Kim SW, Lee DH, Park SR. Park KS, et al. BMB Rep. 2018 Dec;51(12):660-665. doi: 10.5483/BMBRep.2018.51.12.259. BMB Rep. 2018. PMID: 30591093 Free PMC article. - A preclinical evaluation of the MEK inhibitor refametinib in HER2-positive breast cancer cell lines including those with acquired resistance to trastuzumab or lapatinib.
O'Shea J, Cremona M, Morgan C, Milewska M, Holmes F, Espina V, Liotta L, O'Shaughnessy J, Toomey S, Madden SF, Carr A, Elster N, Hennessy BT, Eustace AJ. O'Shea J, et al. Oncotarget. 2017 Jul 22;8(49):85120-85135. doi: 10.18632/oncotarget.19461. eCollection 2017 Oct 17. Oncotarget. 2017. PMID: 29156708 Free PMC article. - Activated estrogen receptor-mitogen-activated protein kinases cross talk confer acquired resistance to lapatinib.
Li Z, Yang SS, Yin PH, Chang T, Shi LX, Fang L, Fang GE. Li Z, et al. Thorac Cancer. 2015 Nov;6(6):695-703. doi: 10.1111/1759-7714.12239. Epub 2015 Feb 13. Thorac Cancer. 2015. PMID: 26557906 Free PMC article. - Lapatinib.
Voigtlaender M, Schneider-Merck T, Trepel M. Voigtlaender M, et al. Recent Results Cancer Res. 2018;211:19-44. doi: 10.1007/978-3-319-91442-8_2. Recent Results Cancer Res. 2018. PMID: 30069757 Review.
Cited by
- HSP90 inhibitors and cancer: Prospects for use in targeted therapies (Review).
Li ZN, Luo Y. Li ZN, et al. Oncol Rep. 2023 Jan;49(1):6. doi: 10.3892/or.2022.8443. Epub 2022 Nov 11. Oncol Rep. 2023. PMID: 36367182 Free PMC article. Review. - Heat shock proteins as hallmarks of cancer: insights from molecular mechanisms to therapeutic strategies.
Zuo WF, Pang Q, Zhu X, Yang QQ, Zhao Q, He G, Han B, Huang W. Zuo WF, et al. J Hematol Oncol. 2024 Sep 4;17(1):81. doi: 10.1186/s13045-024-01601-1. J Hematol Oncol. 2024. PMID: 39232809 Free PMC article. Review. - Alvespimycin Inhibits Heat Shock Protein 90 and Overcomes Imatinib Resistance in Chronic Myeloid Leukemia Cell Lines.
Alves R, Santos D, Jorge J, Gonçalves AC, Catarino S, Girão H, Melo JB, Sarmento-Ribeiro AB. Alves R, et al. Molecules. 2023 Jan 26;28(3):1210. doi: 10.3390/molecules28031210. Molecules. 2023. PMID: 36770876 Free PMC article. - Protein tyrosine kinase inhibitor resistance in malignant tumors: molecular mechanisms and future perspective.
Yang Y, Li S, Wang Y, Zhao Y, Li Q. Yang Y, et al. Signal Transduct Target Ther. 2022 Sep 17;7(1):329. doi: 10.1038/s41392-022-01168-8. Signal Transduct Target Ther. 2022. PMID: 36115852 Free PMC article. Review. - Targeting Heat-Shock Protein 90 in Cancer: An Update on Combination Therapy.
Ren X, Li T, Zhang W, Yang X. Ren X, et al. Cells. 2022 Aug 17;11(16):2556. doi: 10.3390/cells11162556. Cells. 2022. PMID: 36010632 Free PMC article. Review.
References
- Press M.F., Bernstein L., Thomas P.A., Meisner L.F., Zhou J.Y., Ma Y., Hung G., Robinson R.A., Harris C., El-Naggar A., et al. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: Poor prognosis in node-negative breast carcinomas. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1997;15:2894–2904. doi: 10.1200/JCO.1997.15.8.2894. - DOI - PubMed
- DiGiovanna M.P., Stern D.F., Edgerton S.M., Whalen S.G., Moore D., 2nd, Thor A.D. Relationship of epidermal growth factor receptor expression to ErbB-2 signaling activity and prognosis in breast cancer patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005;23:1152–1160. doi: 10.1200/JCO.2005.09.055. - DOI - PubMed
- Nieto Y., Nawaz F., Jones R.B., Shpall E.J., Nawaz S. Prognostic significance of overexpression and phosphorylation of epidermal growth factor receptor (EGFR) and the presence of truncated EGFRvIII in locoregionally advanced breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007;25:4405–4413. doi: 10.1200/JCO.2006.09.8822. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous