Deficiency of ITGAM Attenuates Experimental Abdominal Aortic Aneurysm in Mice - PubMed (original) (raw)
Deficiency of ITGAM Attenuates Experimental Abdominal Aortic Aneurysm in Mice
Min Zhou et al. J Am Heart Assoc. 2021.
Abstract
Background Integrin αM (CD11b), which is encoded by the Integrin Subunit Alpha M (ITGAM) gene, is not only a surface marker of monocytes but also an essential adhesion molecule. In this study, we investigated the effect of CD11b on experimental abdominal aortic aneurysm and the potential underlying mechanisms. Methods and Results The incidence of abdominal aortic aneurysm was not significantly lower in ITGAM(-/-) mice than in control mice. Nevertheless, knockout of CD11b reduced the maximum abdominal aortic diameter, macrophage infiltration, matrix metalloproteinase-9 expression, and elastin and collagen degradation. Additionally, lower expression of IL-6 was found in both the peripheral blood and abdominal aortas of ITGAM(-/-) mice, indicating a biological correlation between CD11b and the inflammatory response in abdominal aortic aneurysm. In vitro, the number of ITGAM(-/-) bone marrow-derived macrophages (BMDMs) that adhered to endothelial cells was significantly lower than the number of wild-type BMDMs. Moreover, the CD11b monoclonal antibody and CD11b agonist leukadherin-1 decreased and increased the number of adherent wild-type BMDMs, respectively. Through RNA sequencing, genes associated with leukocyte transendothelial migration were found to be downregulated in ITGAM(-/-) BMDMs. Furthermore, immunoprecipitation-mass spectrometry analysis predicted that the Akt pathway might be responsible for the impaired transmigratory ability of ITGAM(-/-) BMDMs. The reduced activation of Akt was then confirmed, and the Akt agonist SC79 partially rescued the transendothelial migratory function of ITGAM(-/-) BMDMs. Conclusions CD11b might promote the development and progression of abdominal aortic aneurysm by mediating the endothelial cells adhesion and transendothelial migration of circulating monocytes/macrophages.
Keywords: abdominal aortic aneurysm; adhesion molecule; inflammation; integrin αM; migration.
Conflict of interest statement
None.
Figures
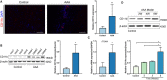
Figure 1. CD11b is upregulated in human AAA tissues and a CaCl2‐induced AAA model.
A, Representative confocal immunofluorescence staining of CD11b and DAPI in human AAA tissues and control tissues. Scale bars=100 μm. Quantification of CD11b based on the integral optical density in AAA (n=4) and control (n=4) tissues, **P<0.01 vs control. B, WB and densitometric analysis of CD11b protein expression in human AAA samples (n=4) and control samples (n=4), *P<0.05 vs control. C, Quantitative real‐time polymerase chain reaction for CD11b mRNA expression in human AAA tissues (n=4) and nonaneurysmal abdominal aortic tissues (n=4). *P<0.05 vs control. D, WB and densitometric analyses of CD11b protein expression in abdominal aortic tissues from control wild‐type mice, and from mice 2 weeks, 4 weeks, and 6 weeks after AAA establishment, *P<0.05 vs control or 2 weeks. AAA indicates abdominal aortic aneurysm; DAPI, 4',6‐diamidino‐2‐phenylindole; ITGAM, integrin subunit alpha M; and WB, Western blot.
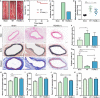
Figure 2. ITGAM deficiency attenuates the development of experimental AAA, vascular structural injury, and the inflammatory response.
A, Representative photographs of the infrarenal abdominal aortas in saline‐ or CaCl2‐treated WT and ITGAM(‐/‐) male mice. The black dotted lines indicate the edge of abdominal aortas. B, Kaplan–Meier survival curves of WT (n=15) and ITGAM(‐/‐) mice (n=15). No significant difference was found regarding the survival rate. C, Incidence of AAA formation in WT and ITGAM(‐/‐) mice in response to CaCl2. D, Maximal abdominal aortic diameter of WT (n=15) and ITGAM(‐/‐) mice (n=13) 6 weeks after being exposed to CaCl2 and the sham‐treated mice (n=15). The data represent the mean±SEM. **P<0.01, ***P<0.001. E, Representative H&E, EVG, and Masson trichrome staining of the abdominal aortic tissues from the sham‐treated, WT, and ITGAM(‐/‐) mice. Scale bar=100 μm. F and G, Quantification of elastin fragmentation and collagen deposition in the experimental groups (n=6), *P<0.05, **P<0.01, ****P<0.0001. H through K, Serum CCL2, TNF‐α, IL‐6, and IL‐1β levels in the experimental groups (n=6 for each group) detected by ELISA, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. AAA indicates abdominal aortic aneurysm; EVG, Elastica Van Gieson; H&E, hematoxylin and eosin; ITGAM, integrin subunit alpha M; and WT, wild‐type.
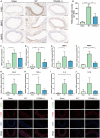
Figure 3. ITGAM deficiency ameliorates macrophage infiltration and polarization.
A, Representative images of immunohistochemical staining for F4/80, MMP2, and MMP9 in the aortic tissues of saline‐ or CaCl2‐treated WT or ITGAM(‐/‐) mice. Scale bar=100 μm. B, Quantification of F4/80‐positive macrophages in the infrarenal abdominal aortas of the mice from the 3 experimental groups under a microscope (n=6 for each group), **P<0.01, ***P<0.001. C and D, Quantification of MMP2 and MMP9 immunostaining in the abdominal aortic walls of the experimental mice (n=6), **P<0.01, ***P<0.001. E through J, Relative MMP2, MMP9, CCL2, TNF‐α, IL‐6, and IL‐1β mRNA expression in the abdominal aortic walls of the experimental mice (n=3), *P<0.05, **P<0.01. K and L, Representative images of dual immunofluorescence staining of iNOS (green), CD206 (green), F4/80 (red), and DAPI (blue) in the abdominal aortas of WT and ITGAM(‐/‐) mice 6 weeks after exposure to CaCl2. Scale bars=50 μm. DAPI indicates 4',6‐diamidino‐2‐phenylindole; IL‐6, interleukin 6; IL‐1β, interleukin 1β; iNOS, inducible nitric oxide synthase; ITGAM, integrin subunit alpha M; MMP, matrix metalloproteinase; TNF‐α, tumor necrosis factor α; and WT, wild‐type.
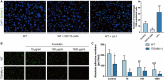
Figure 4. CD11b induces macrophage adhesion.
A, Representative images and quantification of WT BMDM adhesion to primary mouse aortic ECs in the absence or presence of CD11b monoclonal antibody or the CD11b agonist LA‐1. The ECs were stimulated with 1 μmol/L angiotensin II overnight before coculture. n=6, *P<0.05 vs control, ***P<0.001 vs control. B, Representative images of WT and ITGAM(‐/‐) BMDM adhesion to primary ECs, which were activated and incubated with 10 μg/mL, 100 μg/mL, or 1000 μg/mL fucoidan or vehicle before coculture. C, Quantification of WT and ITGAM(‐/‐) BMDM adhesion to activated primary ECs under different conditions. n=5, *P<0.05 vs WT BMDMs under the same conditions, ##P<0.01 vs control wild type BMDMs, ###P<0.001 vs control WT BMDMs. BMDMs indicates bone marrow–derived macrophages; DAPI, 4',6‐diamidino‐2‐phenylindole; ECs, endothelial cells; ITGAM, integrin subunit alpha M; LA‐1, leukadherin‐1; MOMA, monocyte/macrophage and WT, wild‐type.
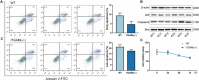
Figure 5. CD11b does not affect macrophage survival.
A, Primary BMDMs from WT and ITGAM(‐/‐) mice were collected and stained with FITC‐Annexin V and PI. The early and late cell apoptosis rates were measured by flow cytometry. No significant difference in the apoptosis rate was observed between the 2 groups (n=3). B, The expression of apoptosis‐related proteins, including p53, caspase3, and Bax, in WT and ITGAM(‐/‐) BMDMs was measured by Western blot. C, A cell viability assay was performed in WT and ITGAM(‐/‐) BMDMs using the Cell Counting Kit‐8. No significant difference in the absorbance (OD 450 nm) was found between the 2 groups (n=3). BMDMs indicates bone marrow–derived macrophages; ITGAM, integrin subunit alpha M; FITC, fluorescein isothiocyanate conjugated; PI, propidium iodide and WT, wild‐type.
Figure 6. CD11b regulates macrophage transendothelial migration.
A, Volcano plots show the ‐lg (adjusted P value) against the log2 (fold change) for each gene. The black horizontal and vertical dashed lines indicate the filtering criteria (|log2 (fold change)| >1.5 and P <0.05). The red dots indicate upregulated DEGs, and the blue dots indicate downregulated DEGs. B, Heat maps of the top 10 upregulated and downregulated DEGs between WT and ITGAM(‐/‐) BMDMs. C, Pathway enrichment analysis of downregulated DEGs in ITGAM(‐/‐) BMDMs, P<0.05. D, Pathway enrichment analysis of upregulated DEGs in ITGAM(‐/‐) BMDMs, P<0.05. E, Quantification of CCL3, CCL4, CCR5, GNB4, CYBB, CCL2, IL‐1β, IL‐6, and TNF‐α mRNA expression in WT and ITGAM(‐/‐) BMDMs, *P<0.05 vs WT BMDMs, **P<0.01 vs WT BMDMs. F, Schematic experimental design of BMDM transendothelial migration, BMDMs seeded in the Transwell insert transmigrated across the endothelial cells and adhered to the lower side of the upper chamber. G, Representative images and quantification of WT and ITGAM(‐/‐) BMDMs that had migrated through the endothelial cell layer. The data represent the mean±SEM of 3 independent experiments, *P<0.05 vs WT BMDMs. H, MS assays of CD11b‐interacting proteins. Whole‐cell lysates of WT BMDMs were immunoprecipitated with anti‐flag protein A/G or control IgG. The immunoprecipitated products were separated and silver stained. MS identified an interaction between CD11b and RACK1 in WT BMDMs via the differential bands. I, The protein–protein interaction network showed that CD18 (ITGB2) was a bridging protein between CD11b (ITGAM) and RACK1 (GNB2L1), and Akt was a common protein downstream of RACK1 and CD11b. J, Western blot of CD11b, Akt, and phosphorylated Akt expression in WT and ITGAM(‐/‐) BMDMs (n=3). K, Representative images and quantification of WT and ITGAM(‐/‐) BMDMs that had migrated through the endothelial cell layer in the absence or presence of the Akt agonist SC79. The data represent the mean±SEM of 3 independent experiments, *P<0.05 vs WT BMDMs. BMDMs indicates bone marrow–derived macrophages; CCL, C‐C motif chemokine ligand; DEGs, differentially expressed genes; ITGAM, integrin subunit alpha M; MS, mass spectrometry; TNF‐α, tumor necrosis factor α; and WT, wild‐type.
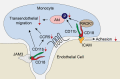
Figure 7. Proposed mechanism of CD11b‐mediated macrophage adhesion and transendothelial migration.
(1) Monocyte‐derived CD11b specifically recognizes ICAM on endothelial cells, helping monocytes adhere to endothelial cells and further transmigrate into the subendothelial space. (2) ITGAM deficiency impairs the macrophage transendothelial migratory function by limiting the expression of CCR5, inhibiting the phosphorylation of Akt via RACK1, and reducing the interaction with JAM3. ICAM indicates intercellular adhesion molecule; ITGAM, integrin subunit alpha M; and RACK1, receptor for activated C kinase 1.
Similar articles
- CD95-ligand contributes to abdominal aortic aneurysm progression by modulating inflammation.
Liu Z, Fitzgerald M, Meisinger T, Batra R, Suh M, Greene H, Penrice AJ, Sun L, Baxter BT, Xiong W. Liu Z, et al. Cardiovasc Res. 2019 Mar 15;115(4):807-818. doi: 10.1093/cvr/cvy264. Cardiovasc Res. 2019. PMID: 30428004 Free PMC article. - Pharmacological inhibitor of notch signaling stabilizes the progression of small abdominal aortic aneurysm in a mouse model.
Cheng J, Koenig SN, Kuivaniemi HS, Garg V, Hans CP. Cheng J, et al. J Am Heart Assoc. 2014 Oct 27;3(6):e001064. doi: 10.1161/JAHA.114.001064. J Am Heart Assoc. 2014. PMID: 25349182 Free PMC article. - Membrane-Bound Thrombomodulin Regulates Macrophage Inflammation in Abdominal Aortic Aneurysm.
Wang KC, Li YH, Shi GY, Tsai HW, Luo CY, Cheng MH, Ma CY, Hsu YY, Cheng TL, Chang BI, Lai CH, Wu HL. Wang KC, et al. Arterioscler Thromb Vasc Biol. 2015 Nov;35(11):2412-22. doi: 10.1161/ATVBAHA.115.305529. Epub 2015 Sep 3. Arterioscler Thromb Vasc Biol. 2015. PMID: 26338301 - Lipocalin-2 deficiency or blockade protects against aortic abdominal aneurysm development in mice.
Tarín C, Fernandez-Garcia CE, Burillo E, Pastor-Vargas C, Llamas-Granda P, Castejón B, Ramos-Mozo P, Torres-Fonseca MM, Berger T, Mak TW, Egido J, Blanco-Colio LM, Martín-Ventura JL. Tarín C, et al. Cardiovasc Res. 2016 Aug 1;111(3):262-73. doi: 10.1093/cvr/cvw112. Epub 2016 May 26. Cardiovasc Res. 2016. PMID: 27229458 - Elevated Wall Tension Initiates Interleukin-6 Expression and Abdominal Aortic Dilation.
Akerman AW, Stroud RE, Barrs RW, Grespin RT, McDonald LT, LaRue RAC, Mukherjee R, Ikonomidis JS, Jones JA, Ruddy JM. Akerman AW, et al. Ann Vasc Surg. 2018 Jan;46:193-204. doi: 10.1016/j.avsg.2017.10.001. Epub 2017 Oct 26. Ann Vasc Surg. 2018. PMID: 29107003 Free PMC article.
Cited by
- ITGAM-mediated macrophages contribute to basement membrane damage in diabetic nephropathy and atherosclerosis.
Lou Y, Li PH, Liu XQ, Wang TX, Liu YL, Chen CC, Ma KL. Lou Y, et al. BMC Nephrol. 2024 Feb 27;25(1):72. doi: 10.1186/s12882-024-03505-1. BMC Nephrol. 2024. PMID: 38413872 Free PMC article. - Deciphering the Intercellular Communication Between Immune Cells and Altered Vascular Smooth Muscle Cell Phenotypes in Aortic Aneurysm From Single-Cell Transcriptome Data.
Cao G, Lu Z, Gu R, Xuan X, Zhang R, Hu J, Dong H. Cao G, et al. Front Cardiovasc Med. 2022 Jun 28;9:936287. doi: 10.3389/fcvm.2022.936287. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35837612 Free PMC article. - Identification of important genes related to HVSMC proliferation and migration in graft restenosis based on WGCNA.
Liu X, Qin M, Chen Q, Jiang N, Wang L, Bai Y, Guo Z. Liu X, et al. Sci Rep. 2024 Jan 12;14(1):1237. doi: 10.1038/s41598-024-51564-z. Sci Rep. 2024. PMID: 38216708 Free PMC article. - Differential Gene Expression and Immune Cell Infiltration in Carotid Intraplaque Hemorrhage Identified Using Integrated Bioinformatics Analysis.
Lv X, Wang F, Sun M, Sun C, Fan X, Ma B, Yang Y, Ye Z, Liu P, Wen J. Lv X, et al. Front Cardiovasc Med. 2022 May 17;9:818585. doi: 10.3389/fcvm.2022.818585. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35656397 Free PMC article. - Development of genomic phenotype and immunophenotype of acute respiratory distress syndrome using autophagy and metabolism-related genes.
Xia F, Chen H, Liu Y, Huang L, Meng S, Xu J, Xie J, Wang G, Guo F. Xia F, et al. Front Immunol. 2023 Oct 23;14:1209959. doi: 10.3389/fimmu.2023.1209959. eCollection 2023. Front Immunol. 2023. PMID: 37936685 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials