Ablation of lysozyme M-positive cells prevents aircraft noise-induced vascular damage without improving cerebral side effects - PubMed (original) (raw)
doi: 10.1007/s00395-021-00869-5.
Johanna Helmstädter 1, Yue Ruan 2, Eva Schramm 3, Sanela Kalinovic 1, Swenja Kröller-Schön 1, Maria Teresa Bayo Jimenez 1, Omar Hahad 1 4, Matthias Oelze 1, Subao Jiang 2, Philip Wenzel 1 4 5, Clemens J Sommer 6, Katrin B M Frauenknecht 6, Ari Waisman 3, Adrian Gericke 2, Andreas Daiber 7 8, Thomas Münzel # 9 10, Sebastian Steven # 1 5
Affiliations
- PMID: 33929610
- PMCID: PMC8087569
- DOI: 10.1007/s00395-021-00869-5
Ablation of lysozyme M-positive cells prevents aircraft noise-induced vascular damage without improving cerebral side effects
Katie Frenis et al. Basic Res Cardiol. 2021.
Abstract
Aircraft noise induces vascular and cerebral inflammation and oxidative stress causing hypertension and cardiovascular/cerebral dysfunction. With the present studies, we sought to determine the role of myeloid cells in the vascular vs. cerebral consequences of exposure to aircraft noise. Toxin-mediated ablation of lysozyme M+ (LysM+) myeloid cells was performed in LysMCreiDTR mice carrying a cre-inducible diphtheria toxin receptor. In the last 4d of toxin treatment, the animals were exposed to noise at maximum and mean sound pressure levels of 85 and 72 dB(A), respectively. Flow cytometry analysis revealed accumulation of CD45+, CD11b+, F4/80+, and Ly6G-Ly6C+ cells in the aortas of noise-exposed mice, which was prevented by LysM+ cell ablation in the periphery, whereas brain infiltrates were even exacerbated upon ablation. Aircraft noise-induced increases in blood pressure and endothelial dysfunction of the aorta and retinal/mesenteric arterioles were almost completely normalized by ablation. Correspondingly, reactive oxygen species in the aorta, heart, and retinal/mesenteric vessels were attenuated in ablated noise-exposed mice, while microglial activation and abundance in the brain was greatly increased. Expression of phagocytic NADPH oxidase (NOX-2) and vascular cell adhesion molecule-1 (VCAM-1) mRNA in the aorta was reduced, while NFκB signaling appeared to be activated in the brain upon ablation. In sum, we show dissociation of cerebral and peripheral inflammatory reactions in response to aircraft noise after LysM+ cell ablation, wherein peripheral myeloid inflammatory cells represent a dominant part of the pathomechanism for noise stress-induced cardiovascular effects and their central nervous counterparts, microglia, as key mediators in stress responses.
Keywords: Aircraft noise exposure; Cerebral inflammation; Diphtheria toxin; Endothelial dysfunction; Environmental risk factor; Myeloid cell ablation; Oxidative stress.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Fig. 1
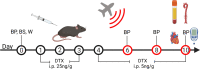
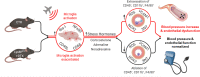
Scheme for ablation of LysM+ cells and noise exposure. Male LysMCre+/− iDTR+/− (LysMCreiDTR) mice were acclimatized to blood pressure measurement and baseline measurements were taken prior to treatment with diphtheria toxin. DTX was administered daily via i.p. injection for 10 days, first at a dose of 25 ng/g and then reduced to a dose of 5 ng/g. On day 6, after sufficient ablation, mice were exposed to aircraft noise for 4 days. Created with BioRender.com
Fig. 2
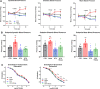
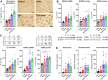
Effects of ablation of LysM+ cells on blood pressure and endothelial function of aircraft noise-exposed animals. a Aircraft noise increased systolic, diastolic, and mean blood pressure, all of which was prevented by treatment with DTX. Noise was applied on day 6. b Systolic, diastolic, and mean blood pressure on the final day of the exposure regimen. Ablation of LysM+ cells completely abolished noise-induced increases in blood pressure. c Noise caused a significant degree of endothelial dysfunction (ACh-response) that was completely prevented by ablation therapy, whereas endothelium-independent relaxation (NTG-response) was not changed in any group. Data points are measurements from individual animals (a, b) and data in (c) are the mean of n = 19–27 independent measurements; two-way ANOVA with Bonferroni’s multiple comparison test (a, c) or one-way ANOVA with Tukey’s multiple comparison test (b). *P < 0.05 vs. Control; $P < 0.05 vs. DTX, +P < 0.05 vs. DTX + Noise
Fig. 3
Noise-induced increases of eNOS uncoupling as well as vascular and cardiac oxidative stress are successfully corrected by ablation of LysM+ cells. a In control vessels, the eNOS inhibitor
l
-NAME increased vascular superoxide levels while decreasing it in noise-exposed animals, findings compatible with eNOS uncoupling. In contrast, after ablation of LysM+ cells,
l
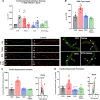
-NAME increased the vascular DHE signal indicating that eNOS uncoupling was prevented. b DHE staining of aortic cryo-sections revealed an increase in ROS production to noise that was prevented by ablation. Red fluorescence indicates the signal corresponding to oxidation product and green indicates the autofluorescence of aortic laminae. The scale bar reflects 100 µm. c, d Likewise, quantitative HPLC analysis of the superoxide-specific DHE product, 2-hydroxyethidium, established increases in response to noise in the aorta and heart that were normalized by ablation. Representative chromatograms are shown besides the quantification. Data points are measurements from individual animals; one-way ANOVA with Tukey’s multiple comparison test (a–d). *P < 0.05 vs. Control; $P < 0.05 vs. DTX, +P < 0.05 vs. DTX + Noise
Fig. 4
Flow cytometry of aortic lysates and whole blood demonstrates leukocyte extravasation into the aortic endothelium by noise and prevention by LysM+ cell ablation. Flow cytometry and representative plots in aortic lysates show an increase in count following noise exposure and a reduction in count in all leukocytes (a) as well as innate immune leukocytes (b). Specifically, monocytes (c) and macrophages (d) were reduced below baseline control counts and no increase was found in the aorta following both noise exposure and LysM+ cell ablation. e All immune cell subsets were found decreased in whole blood of noise-exposed CTR mice or in the DTX-treated groups. Data points are measurements from individual animals; one-way ANOVA with Tukey’s multiple comparison test (a–e). *P < 0.05 vs. Control; #P < 0.05 vs. Noise; $P < 0.05 vs. DTX, +P < 0.05 vs. DTX + Noise
Fig. 5
Ablation protects from noise-induced increases in mRNA expression of endothelial nitric oxide synthase (eNOS), oxidative stress and inflammatory parameters and plasma oxidative stress markers. Noise increased mRNA expression of eNOS, VCAM-1, NOX-2 expression (a–c). Ablation of LysM+ cells reduced eNOS and NOX-2 mRNA (a, c) below control levels, while the expression of VCAM-1 (b) remained at baseline. d, e Protein expression of NOX-2 and abundance of 3-nitrotyrosine (3-NT)-positive proteins in the aorta as determined by immunohistochemistry were increased by noise and partially normalized by DTX treatment. Representative immunohistochemical images are shown below the quantification and the scale bars reflect 50 µm. f Oxidative stress marker, malondialdehyde assessed by dot blot analysis, was increased by noise and normalized by LysM+ cell ablation. Data points are measurements from pools of 3–4 aortas (a–c), number of animals (d, e) or pools of plasma from 2–4 animals per data point (f); one-way ANOVA with Tukey’s multiple comparison test (a–f). *P < 0.05 vs. Control; #P < 0.05 vs. Noise; $P < 0.05 vs. DTX, +P < 0.05 vs. DTX + Noise
Fig. 6
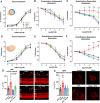
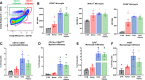
Noise-induced impairment of vasodilation and increase of oxidative stress in the retinal and mesenteric microvasculature is mostly prevented by ablation LysM+ cells. (a–c and d–f) In retinal and mesenteric arterioles, noise caused a marked degree of endothelial dysfunction (impaired ACh-response), while responses to the endothelium independent vasodilator nitroprusside (NP) and to the vasoconstrictor U46619 (thromboxane A2 agonist) remained unchanged. Ablation of LysM+ cells partially normalized the ACh-dose response relationship in retinal vessels and completely normalized it in mesenteric vessels. (g, h) DHE staining revealed an increase in ROS production in retinal and mesenteric vessels upon exposure to noise, with no effect by DTX treatment alone and partial or total prevention of the increase by DTX treatment prior to noise. Representative images of DHE-stained retinal and mesenteric cryosections are shown besides the densitometric quantification. The white arrows point to retinal vascular cross-sections. GCL ganglion cell layer; IPL inner plexiform layer; INL inner nuclear layer; OPL outer plexiform layer; ONL outer nuclear layer. Data points are measurements from individual animals (g, h) or n = 6 for (a–c) or n = 3–4 for (d–f); one-way ANOVA with Tukey’s multiple comparison test (a–c). two-way ANOVA with Bonferroni’s multiple comparison test (d–f). *P < 0.05 vs. Control; #P < 0.05 vs. Noise; $P < 0.05 vs. DTX, +P < 0.05 vs. DTX + Noise
Fig. 7
Diphtheria toxin treatment of LysMCreiDTR mice fails to prevent neuroinflammation in the brain and stress responses by noise. a Iba-1 staining revealed no ablation of microglia in LysMCreiDTR mice. Interestingly, Iba-1+ cells and % of Iba-1+ area were even increased in both DTX-treated groups and further aggravated by noise exposure. Representative immunohistochemical images are shown besides the quantification and the scale bar reflects 50 µm. b Presence of a neuroinflammatory phenotype was supported by higher levels of NFkB mRNA as well as NLRP3 and TXNIP protein expression in brains of LysMCreiDTR mice with DTX treatment, which was exacerbated by noise exposure. Representative western blot images are shown below the densitometric quantification. c IL-6 and CD68 protein as well as CD40L mRNA expression were slightly increased by noise and further exacerbated in the DTX groups. Representative dot blot images are shown above the densitometric quantification. d Neuronal stress response and release of stress hormones adrenaline, noradrenaline and corticosterone were also higher in the LysMCreiDTR mice with DTX treatment. Data points are measurements from individual animals; one-way ANOVA with Tukey’s multiple comparison test or respective non-parametric test (a–d). *P < 0.05 vs. Control; #P < 0.05 vs. Noise; $P < 0.05 vs. DTX
Fig. 8
Both noise and DTX treatment induce microglial activation and DTX also causes peripheral immune infiltration into the brain. a Microglia are moderate expressors of both CD11b and CD45, whereas peripheral immune cells express high levels of CD45. Using flow cytometry with this gating strategy, we were able to evaluate the activation of microglia and identify CD11b+ and CD11b− infiltrates from the periphery in the brains of LysMCre+/+iDTR+/+ mice. b CD68, CD86, and MHC-II are markers of microglial activation, demonstrating that both noise and DTX activate central immune responses. c CD45hi CD11b− (e.g., lymphoid) cells showed increased infiltration into the brain upon DTX treatment. d Myeloid infiltrates were detected by their expression of CD11b and their high expression of CD45. Despite ablation of these cells in the periphery, there was considerable migration of myeloid cells into the brain following DTX treatment, which also comprised monocyte- (e) and macrophage- (f) containing subpopulations. Original flow cytometry plots are shown for all cell types. Data points are measurements from individual animals; one-way ANOVA with Tukey’s multiple comparison test or respective non-parametric test (b–f). *P < 0.05 vs. control; #P < 0.05 vs. noise
Fig. 9
Summary scheme. Noise-induced stress responses were not prevented by DTX treatment as the central immune cells, microglia, were unablated and activated under these conditions. Stress hormone release was also exacerbated in LysMCreiDTR mice with DTX treatment. Nevertheless, LysM+ cell ablation prevented aircraft noise-induced endothelial dysfunction, oxidative stress, inflammation and blood pressure elevation. These data demonstrate that infiltration of the vasculature by LysM+ cells is crucial for noise-induced cardiovascular damage and that central immune responses via microglia are important mediators in these actions. Created with BioRender.com
Similar articles
- Mitigation of aircraft noise-induced vascular dysfunction and oxidative stress by exercise, fasting, and pharmacological α1AMPK activation: molecular proof of a protective key role of endothelial α1AMPK against environmental noise exposure.
Kvandová M, Rajlic S, Stamm P, Schmal I, Mihaliková D, Kuntic M, Bayo Jimenez MT, Hahad O, Kollárová M, Ubbens H, Strohm L, Frenis K, Duerr GD, Foretz M, Viollet B, Ruan Y, Jiang S, Tang Q, Kleinert H, Rapp S, Gericke A, Schulz E, Oelze M, Keaney JF Jr, Daiber A, Kröller-Schön S, Jansen T, Münzel T. Kvandová M, et al. Eur J Prev Cardiol. 2023 Oct 26;30(15):1554-1568. doi: 10.1093/eurjpc/zwad075. Eur J Prev Cardiol. 2023. PMID: 37185661 - Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction.
Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A, Münzel T. Wenzel P, et al. Circulation. 2011 Sep 20;124(12):1370-81. doi: 10.1161/CIRCULATIONAHA.111.034470. Epub 2011 Aug 29. Circulation. 2011. PMID: 21875910 - Effects of noise on vascular function, oxidative stress, and inflammation: mechanistic insight from studies in mice.
Münzel T, Daiber A, Steven S, Tran LP, Ullmann E, Kossmann S, Schmidt FP, Oelze M, Xia N, Li H, Pinto A, Wild P, Pies K, Schmidt ER, Rapp S, Kröller-Schön S. Münzel T, et al. Eur Heart J. 2017 Oct 1;38(37):2838-2849. doi: 10.1093/eurheartj/ehx081. Eur Heart J. 2017. PMID: 28329261 Free PMC article. - Exposure-response relationship of the association between aircraft noise and the risk of hypertension.
Babisch W, Kamp Iv. Babisch W, et al. Noise Health. 2009 Jul-Sep;11(44):161-8. doi: 10.4103/1463-1741.53363. Noise Health. 2009. PMID: 19602770 Review. - Environmental Noise and the Cardiovascular System.
Münzel T, Schmidt FP, Steven S, Herzog J, Daiber A, Sørensen M. Münzel T, et al. J Am Coll Cardiol. 2018 Feb 13;71(6):688-697. doi: 10.1016/j.jacc.2017.12.015. J Am Coll Cardiol. 2018. PMID: 29420965 Review.
Cited by
- In vivo analysis of noise dependent activation of white blood cells and microvascular dysfunction in mice.
Eckrich J, Ruan Y, Jiang S, Frenis K, Rodriguez-Blanco G, Maas AP, Jimenez MTB, Kuntic M, Oelze M, Hahad O, Li H, Steven S, Strieth S, von Kriegsheim A, Münzel T, Daiber A, Gericke A, Ernst BP. Eckrich J, et al. MethodsX. 2021 Oct 8;8:101540. doi: 10.1016/j.mex.2021.101540. eCollection 2021. MethodsX. 2021. PMID: 34754808 Free PMC article. - Environmental risk factors and cardiovascular diseases: a comprehensive expert review.
Münzel T, Hahad O, Sørensen M, Lelieveld J, Duerr GD, Nieuwenhuijsen M, Daiber A. Münzel T, et al. Cardiovasc Res. 2022 Nov 10;118(14):2880-2902. doi: 10.1093/cvr/cvab316. Cardiovasc Res. 2022. PMID: 34609502 Free PMC article. - Vascular Redox Signaling, Endothelial Nitric Oxide Synthase Uncoupling, and Endothelial Dysfunction in the Setting of Transportation Noise Exposure or Chronic Treatment with Organic Nitrates.
Münzel T, Daiber A. Münzel T, et al. Antioxid Redox Signal. 2023 May;38(13-15):1001-1021. doi: 10.1089/ars.2023.0006. Epub 2023 Apr 6. Antioxid Redox Signal. 2023. PMID: 36719770 Free PMC article. Review. - Long-Term Effects of Aircraft Noise Exposure on Vascular Oxidative Stress, Endothelial Function and Blood Pressure: No Evidence for Adaptation or Tolerance Development.
Frenis K, Kalinovic S, Ernst BP, Kvandova M, Al Zuabi A, Kuntic M, Oelze M, Stamm P, Bayo Jimenez MT, Kij A, Keppeler K, Klein V, Strohm L, Ubbens H, Daub S, Hahad O, Kröller-Schön S, Schmeisser MJ, Chlopicki S, Eckrich J, Strieth S, Daiber A, Steven S, Münzel T. Frenis K, et al. Front Mol Biosci. 2022 Jan 31;8:814921. doi: 10.3389/fmolb.2021.814921. eCollection 2021. Front Mol Biosci. 2022. PMID: 35174211 Free PMC article. - Methods to measure blood flow and vascular reactivity in the retina.
Böhm EW, Pfeiffer N, Wagner FM, Gericke A. Böhm EW, et al. Front Med (Lausanne). 2023 Jan 12;9:1069449. doi: 10.3389/fmed.2022.1069449. eCollection 2022. Front Med (Lausanne). 2023. PMID: 36714119 Free PMC article. Review.
References
- Agha G, Mendelson MM, Ward-Caviness CK, Joehanes R, Huan T, Gondalia R, Salfati E, Brody JA, Fiorito G, Bressler J, Chen BH, Ligthart S, Guarrera S, Colicino E, Just AC, Wahl S, Gieger C, Vandiver AR, Tanaka T, Hernandez DG, Pilling LC, Singleton AB, Sacerdote C, Krogh V, Panico S, Tumino R, Li Y, Zhang G, Stewart JD, Floyd JS, Wiggins KL, Rotter JI, Multhaup M, Bakulski K, Horvath S, Tsao PS, Absher DM, Vokonas P, Hirschhorn J, Fallin MD, Liu C, Bandinelli S, Boerwinkle E, Dehghan A, Schwartz JD, Psaty BM, Feinberg AP, Hou L, Ferrucci L, Sotoodehnia N, Matullo G, Peters A, Fornage M, Assimes TL, Whitsel EA, Levy D, Baccarelli AA. Blood leukocyte DNA methylation predicts risk of future myocardial infarction and coronary heart disease. Circulation. 2019;140:645–657. doi: 10.1161/CIRCULATIONAHA.118.039357. - DOI - PMC - PubMed
- Babisch W. The noise/stress concept, risk assessment and research needs. Noise Health. 2002;4:1–11. - PubMed
- Babisch W. Stress hormones in the research on cardiovascular effects of noise. Noise Health. 2003;5:1–11. - PubMed
- Baldwin AL, Bell IR. Effect of noise on microvascular integrity in laboratory rats. J Am Assoc Lab Anim Sci. 2007;46:58–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2017_A106/Else Kröner-Fresenius-Stiftung
- collaborative research group 'Novel and neglected cardiovascular risk factors: molecular mechanisms and therapeutic implications'/Boehringer Ingelheim Stiftung
- Partner Site Rhine-Main, Mainz/Deutsches Zentrum für Herz-Kreislaufforschung
- career development grant/Stavros Niarchos Foundation
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous