Clinical phenotype and prevalence of hereditary nonpolyposis colorectal cancer syndrome in Chinese population (original) (raw)
Colorectal Cancer Open Access
Copyright ©2005 Baishideng Publishing Group Inc. All rights reserved.
World J Gastroenterol. Mar 14, 2005; 11(10): 1481-1488
Published online Mar 14, 2005. doi: 10.3748/wjg.v11.i10.1481
Clinical phenotype and prevalence of hereditary nonpolyposis colorectal cancer syndrome in Chinese population
Yuan-Zhi Zhang, Department of Gastroenterology, General Hospital of Perking Military Area and Third Military Medical College, Beijing 10070, China
Jian-Qiu Sheng, Shi-Rong Li, Hong Zhang, Department of Gastroenterology, General Hospital of Perking Military Area, Beijing 10070, China
ORCID number: $[AuthorORCIDs]
Author contributions: All authors contributed equally to the work.
Correspondence to: Dr. Yuan-Zhi Zhang, Department of Gastroenterology, General Hospital of Perking Military Area, Beijing 10070, China. jianming2000@163.com
Telephone: +86-01-86357923
Received: August 18, 2004
Revised: August 19, 2004
Accepted: September 18, 2004
Published online: March 14, 2005
Abstract
AIM: To describe systematically the clinical characteristics and phenotype of HNPCC families and the prevalence of HNPCC in the general population of CRC patients in China.
METHODS: HNPCC kindreds and CRC patients were from two sources. One was that we consecutively investigated kindreds and patients by ourselves. And the other was the published Chinese and foreign literature related to Chinese HNPCC syndrome. There were 142 HNPCC families fulfilling AC I and/or AC II including 57 families with detailed data, and 3874 general primary CRC patients in all. All statistical tests were two-sided.
RESULTS: In AC I families, the number of Lynch syndrome I and II families were 25 (47.2%) and 28 (52.8%) respectively. There were 215 patients (82.4%) with CRC, 67 patients (25.7%) with extracolonic cancer and 50 patients (19.2%) with multiple primary cancers. In all CRC patients, multiple primary CRC were in 41 patients (19.1%), and the first-CRC was right-sided colorectal cancer in 143 patients (66.5%) and rectal cancer in 44 patients (20.5%). 8.8% and 19.2% of the first cancer were CRC and extracolonic cancers. Among those patients whose first cancer was CRC, 66.8% and 19.9% were right-sided colorectal cancer and rectal cancer, respectively. The similar results were found in AC II families. Normal distribution was only found in the distribution of the age of diagnosis of the first cancer in both AC I families (coefficient of skewness: u = 0.81, 0.20<0.40<_P_<0.50; coefficient of kurtosis: u = 1.13, 0.20<_P_<0.40, α = 0.20) and AC II families (coefficient of skewness: u = 0.63, _P_>0.5>0.20; coefficient of kurtosis: u = 0.84, 0.20<0.40<P<0.50, α = 0.20), but not found in the distribution of the age of diagnosis of the first CRC. When patients with HNPCC-associated cancer suffered from the first malignant tumor in HNPCC families diagnosed by AC I and AC II, the mean age and median age were 45.1±12.7 years and 44.0 years, 45.2±12.7 years and 44.5 years, respectively. The median age of diagnosis of the first tumor of the patients in the later generation was younger than that in the previous generation. Many extracolonic cancers were found to be associated with HNPCC syndrome. Gastric cancer was the most frequent extracolonic cancer followed by endometrial cancer and hepatocarcinoma. In general population of CRC patients, the prevalence of HNPCC diagnosed by AC I and AC II were 1.3% and 2.2%, respectively.
CONCLUSION: The clinical phenotype and prevalence of Chinese HNPCC syndrome are similar to those of Europeans and Americans. Gastric cancer is the most common extracolonic malignant tumor. The age of diagnosis of the first malignant tumor tends to be increasingly younger in patients with HNPCC-related tumors.
- Citation: Zhang YZ, Sheng JQ, Li SR, Zhang H. Clinical phenotype and prevalence of hereditary nonpolyposis colorectal cancer syndrome in Chinese population. World J Gastroenterol 2005; 11(10): 1481-1488
- URL: https://www.wjgnet.com/1007-9327/full/v11/i10/1481.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i10.1481
INTRODUCTION
Colorectal cancer (CRC) is the second most frequent cancers in Western countries[1], and the third leading cause of cancer deaths in the United States[2]. In China, the mortality rate of CRC is the fourth to sixth leading cause of cancer deaths[3]. And its mortality and prevalence are inclined to increase in the coming years. Patients with a familial risk, who have two or more first- or second-degree relatives (or both) with CRC, make up approximately 20 percent of all patients with CRC, whereas approximately 5 to 10 percent of the total annual burden of CRC is Mendelian in nature, that is, it is inherited in an autosomal dominant manner. Hereditary nonpolyposis colorectal cancer (HNPCC) syndrome, also referred to as the Lynch syndrome, is the most common form of hereditary CRC in an autosomal dominant manner[4]. In addition to CRC, tumors also occur in the endometrium, small bowel, pancreas, biliary tract, stomach, ovary, and urinary tract. Clinically, this disorder is mainly defined by the Amsterdam criteria (AC), which was established by the International Collaborative Group on HNPCC (ICG-HNPCC) at its meeting in Amsterdam in 1990[5]. The AC includes the following: (1) at least three relatives with histologically verified CRC, one of them a first-degree relative of the other two (familial adenomatous polyposis excluded); (2) at least two successive generations affected; (3) in one of the individuals, diagnosis of CRC before the age of 50. These criteria were pivotal in identifying kindreds that eventually led to the association of the HNPCC syndrome with germline mismatch repair gene mutations (MMR). However, the criteria are strict and do not account for extracolonic cancers or for small kindreds. In 1998, the set of new clinical criteria for collaborative studies were proposed that included the extracolonic cancers associated with HNPCC and accepted by the majority (85%) of the group[6]. In addition, it was proposed to keep the classical criteria, which are referred to as Amsterdam Criteria I (AC I). And the revised ICG-HNPCC criteria are referred to as Amsterdam Criteria II (AC II). The AC II includes the following: (1) At least 3 relatives with an HNPCC-associated cancer (CRC, cancer of the endometrium, small bowel, ureter or renal pelvis); (2) One should be a first-degree relative of the other 2; (3) At least 2 successive generations should be affected; (4) At least 1 should be diagnosed before age 50; (5) Familial adenomatous polyposis should be excluded in the CRC case(s) if any and (6) Tumors should be verified by pathological examination. Although there had been many reports on characteristics of HNPCC kindreds in China in the past decade, their samples on fulfilled AC I or AC II kindreds were very small, and most of theirs were only case reports. So there is no systematic and all round clinical research on HNPCC until now. Therefore, it is necessary and urgent to find out the phenotype and prevalence of HNPCC fulfilled AC I or AC II in a larger sample in Chinese populations and the differences between the Chinese patients and the Euro-Americans.
In this study, we on the one hand enrolled kindreds fulfilled AC I or AC II and investigated CRC patients who on our own. On the other hand, in order to provide the overall information on Chinese HNPCC, we reviewed and synthesized the literature on Chinese HNPCC. Based on clinical data, we hoped to find out clinical phenotype and characteristics of HNPCC families in a larger sample and the prevalence of HNPCC in a larger population of CRC patients and to evaluate whether the two sets of criteria for HNPCC are useful in clinical diagnosis and management of HNPCC in modern Chinese society.
MATERIALS AND METHODS
Materials
All subjects could be divided into two groups: HNPCC kindreds and general CRC patients. They were from two sources. One was that we consecutively investigated kindreds and patients by ourselves. During the period from October 2001 to May 2004, we consecutively investigated 25 Chinese families that fulfilled the AC I or AC II. Although there are only four HNPCC-associated cancers (CRC, cancer of the endometrium, small bowel, ureter or renal pelvis) in AC II, ICG definition of HNPCC-associated cancers also included cancers of the stomach, ovary, brain, hepatobiliary tract, and skin (sebaceous tumors)[6]. So when we clinically diagnosed HNPCC families that fulfilled AC II, those tumors also were included in the criteria. These families were from Beijing, Henan Province, Inner Mongolia Autonomous Region, Hebei Province, Yunnan Province and Liaoning Province. From January 2002 to December 2002, we also consecutively investigated 594 CRC inpatients with detailed family history in the general hospital of Perking military area. The other source was the published Chinese and foreign literature related to Chinese HNPCC from 1994 to 2004, which were collected by bibliographic searches through Index of Chinese Science, Technology Data and PUBMED. The selection criteria of literature were as follows: the independent kindreds and prevalence of HNPCC were published in Chinese or English magazines, each report should have the detailed kindred data or extracolonic tumor records. Duplicated, poor quality reports or those with little information on each kindred were discarded. Thus, 24 papers were chosen by screening (Table 1), 117 families that fulfilled AC I or AC II and 3280 general CRC patients were accumulated from 11 districts in China. In Table 1, all or partially reported families have complete data from number 1 to 14; there were detailed data on extracolonic cancer associated with HNPCC from 15 to 21, and HNPCC prevalence data from 20 to 24. In the present study, there were 142 HNPCC families and 3874 general CRC patients. They were from 15 regions in China, including Beijing, Tianjin, Shanghai, Zhejiang Province, Guangdong Province, Jiangsu Province, Shandong Province, Hebei Province, Henan Province, Hunan Province, Sichuan Province, Yunnan Province, Liaoning Province, Inner Mongolia and Taiwan. Of the HNPCC families, there were 57 families with complete data.
Table 1 Condition of all chosen papers and their studied districts.
| Number | First authors | District | Source of references |
|---|---|---|---|
| 1 | Zhen-Jun Wang | Beijing | Journal of Coloproctological Surgery,2001, 7(3): 3-4 |
| 2 | Ding-Cun Luo | Zhejiang | China Oncology, 2000, 10(2): 145-146, 152 |
| 3 | Shi-Lin Wang | Beijing | Journal of General Hospital of Air Force, 2000, 16(1):33-34,37 |
| 4 | Yan-Ting Jian | Guangdong | J First Mil Med Univ, 2001, 21(1): 15-16 |
| 5 | Shi-Xin Xu | Jiangsu | China Oncology, 1998, 8(1): 56-58 |
| 6 | Shan-Jing Mo | Shanghai | Chin J Dig, 1996, 16(6): 326-328 |
| 7 | Jian-Sheng Li | Zhejiang | Journal of Practical Oncology, 2000, 15(1): 46-47 |
| 8 | Meng-Hong Sun | Shanghai | Natl Med J China, 2001, 81(20): 1268-1269 |
| 9 | Zhi-Zhuang Li | Shandong | Acta Academiae Medicinae Nantong, 2001,21(4):437 |
| 10 | Chen-Yi Wang | Hunan | Hunan Medical Journal, 1998, 15(1): 64 |
| 11 | Gang Ye | Zhejiang | Medical Journal of Ningbo, 1997, 9(5): 226 |
| 12 | Liang Xu | Sichuan | The Practical Journal of Cancer, 2002,17(4): 395-397 |
| 13 | Zhen-Jun Wang | Beijing | Chin J Gastrointest Surg, 2000, 3(4): 217-219 |
| 14 | Yi-Tao Jia | Hebei | Chin J Med Genet, 1999, 16(3): 200 |
| 15 | Hei-Ying Jin | Shanghai | J Clin Surg, 2001, 9(6): 356-357 |
| 16 | Shi-Lin Wang | Beijing | Journal of Oncology, 2002, 8(1): 24-26 |
| 17 | Guo-Fu Chen | Zhejiang | China Oncology, 2003, 13(3): 240-242 |
| 18 | Li-Xin Liu | Beijing | Chinese Journal of Coal Industry Medicine, 2002, 5(7):643-644 |
| 19 | SC Wei | Taiwan | J Formos Med Assoc, 2002, 101(3): 206-209 |
| 20 | San-Jun Cai | Shanghai | World J Gastroenterol, 2003, 9(2): 284-287 |
| 21 | Gang Chen | Tianjin | Chinese Journal of Clinical Oncology, 2003, 30(4):247-249 |
| 22 | Hua-Wei Jin | Guangdong | Chinese Journal of Practical Surgery, 2000, 20(4): 231-232 |
| 23 | Zhi-Qiang Xue | Guangdong | Guangdong Medical Journal, 2003, 24(7): 752-753 |
| 24 | Shi-Lin Wang | Beijing | Chin J Gen Surg, 2000, 15(11): 667-668 |
Methods
In 57 fulfilled AC I or AC II families with complete data, common characteristics of HNPCC kindreds were analyzed. In the different criteria, the following were to be analyzed: Distribution of the age of diagnosis of the first tumor and the first CRC, proportion of CRC patients, multiple primary CRC and tumors, proximal cancer to the splenic flexure (or right-sided CRC) and rectal cancer. Extracolonic tumor spectrum associated with HNPCC was analyzed in 142 families, and the prevalence of HNPCC was done in 3874 general CRC patients.
Statistical analysis
All data were descriptively analyzed by software SPSS 10.0. Test of normality was done by method of movement, and size of the test was α = 0.20. When the two values of P on the coefficient of skewness and coefficient of kurtosis are both _P_>0.20, the data were considered to be normal distribution. All statistical tests were two-sided.
RESULTS
Characterization of HNPCC families
In 57 HNPCC kindreds, there were 53 families that fulfilled AC I and 4 families that only fulfilled AC II. In 53 HNPCC families that fulfilled AC I, the number of Lynch syndrome I and II families were 25 (47.2%) and 28 (52.8%), respectively. There were 261 cancer patients and 343 primary cancers in all. In 261 cancer patients, there were 215 patients (82.4%) with CRC, 67 patients (25.7%) with extracolonic cancer and 50 patients (19.2%) with multiple primary cancers. In 215 CRC patients, there were 41 patients (19.1%) with synchronous (multiple colorectal cancers at or within six months after surgical resection for CRC) or metachronous (CRC occurring more than six months after surgery) CRC and 20 patients (9.3%) with both extracolonic cancer and CRC. In all CRC patients, the first CRC was right-sided CRC in 143 patients (66.5%) and rectal cancer in 44 patients (20.5%). The first cancer was CRC in 211 patients (80.8%) and extracolonic cancer in 50 patients (19.2%). In patients whose first cancer was CRC, 141 patients (66.8%) had right-sided CRC and 42 patients (19.9%) had rectal cancer. There were 270 CRCs (78.7%) and 73 extracolonic cancers (21.3%) in all primary cancers. The proportion of right-sided colorectal cancer and rectal cancer were 62.6% and 21.1% in primary CRCs respectively.
If HNPCC families were diagnosed by AC II, 25 families (43.9%) were Lynch syndrome I and 32 families (56.1%) were Lynch syndrome II. There were 276 cancer patients and 364 primary cancers in all. In all cancer patients, there were 223 CRC patients (80.8%), 75 patients (27.2%) with extracolonic cancer and 52 patients (18.8%) with multiple primary cancers. In 223 CRC patients, there were 43 patients (19.3%) with synchronous or metachronous CRC and 22 patients (9.9%) with both extracolonic cancer and CRC. The first cancer was CRC in 219 patients (79.3%) and extracolonic cancer in 57 patients (20.7%). In patients whose first cancer was CRC, 144 (65.8%) patients had right-sided CRC and 46 patients (21%) had rectal cancer. In 223 CRC patients, proportions of right-sided CRC and rectal cancer of the first CRCs were 65.5% and 21.5% respectively. In 364 primary cancers, there were 282 CRCs (77.5%) and 82 extracolonic cancers (22.5%). In 282 CRCs, the proportion of right-sided CRC and rectal cancer were 63.1% and 21.6% respectively.
Distribution of the age of diagnosis of the first malignant tumor in HNPCC-associated cancer patients and general primary CRC patients
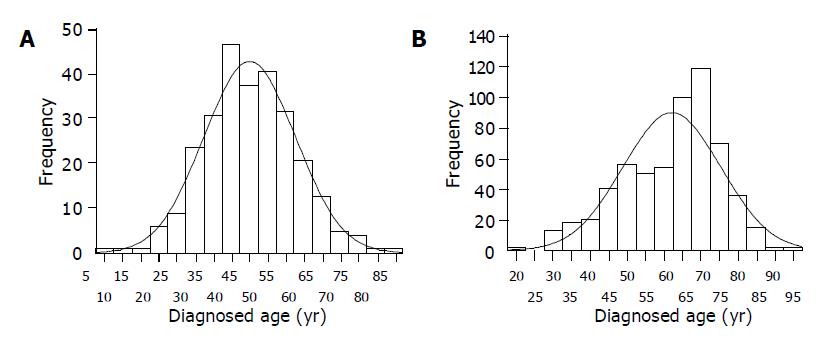
In 57 AC II HNPCC families, the distribution of the age of diagnosis of the first cancer was normal distribution (coefficient of skewness: u = 0.63, _P_>0.5; coefficient of kurtosis: u = 0.84, 0.40<P<0.50) (Figure 1A). The mean age and median age were 45.2±12.7 and 44.5 years (range from 5 to 84 years) respectively. The distribution of the age of diagnosis of the first CRC was not normal distribution (coefficient of skewness: u = 1.85, 0.05<P<0.10; coefficient of kurtosis: u = 0.81, 0.40<P<0.50) in CRC patients in these HNPCC families. Its median age was 43.0 years (range from 12 to 84 years).
Figure 1 Distribution of the age. A: Distribution of the ages of diagnosis of the first cancer of HNPCC-associated cancer patients in AC II families; B: Distribution of the ages of diagnosis of CRC of 594 general primary CRC patients.
In 53 AC I families, the distribution of the age of diagnosis of the first cancer were normal distribution (coefficient of skewness: u = 0.81, 0.40<P<0.50; coefficient of kurtosis: u = 1.13, 0.20<P<0.40). The mean age and median age of diagnosis of the first cancer were 45.1±12.7 years and 44.0 years (range from 5 to 84 years) respectively. But the distribution of the age diagnosed the first CRC in AC I families was the same as that in the AC II families. It also was not normal distribution (coefficient of skewness: u = 1.85, 0.05<P<0.10; coefficient of kurtosis: u = 0.96, 0.20<P<0.40). Its median age was 43.0 years (range from 12 to 84 years).
In 594 primary CRC inpatients consecutively investigated, the distribution of the diagnosed age was not normal distribution (coefficient of skewness: u = 5.03, P<0.001; coefficient of kurtosis: u = 1.04, 0.20<P<0.40) (Figure 1B). The median age was 65 years (range from 21 to 95 years). There was a statistical significance on the difference of the mean or median age between HNPCC patients and general primary CRC patients (P<0.05).
Age of diagnosis of the first tumor of HNPCC-associated cancer patients in different generations
Because 4 were only fulfilled AC II in 57 AC II HNPCC families, here we only discuss the age of diagnosis of the first tumor of HNPCC-associated cancer patients in different generations in AC II families. At least 2 and at most 5 successive generations should be affected. The numbers of patients in the first, second, third, fourth and fifth generation were 93, 134, 43, 5 and 1 respectively. The distribution of the age of diagnosis of the first tumor of the patients both in the first (coefficient of skewness: u = 0.97, 0.20<_P_<0.40; coefficient of kurtosis: _u_ = 1.08, 0.20<_P_<0.40) and second generation (coefficient of skewness: _u_ = 0.50, _P_>0.50; coefficient of kurtosis: u = 0.59, _P_>0.50) were normal distribution, but that in third generation was not normal distribution (coefficient of skewness: u = 1.94, 0.05 <P<0.10; coefficient of kurtosis: u = 1.80, 0.05<P<0.10). Because the number of patients both in the fourth and fifth generation was too small, their distributions were not analyzed. The mean and median age in the first generation and second generation were 52.7±11.7 and 52.0, 43.1±11.7 and 43.0 years respectively. The median age in the third generation and fourth generation were 38 years and 31 years respectively. The median age of diagnosis of the first tumor of the patients in the later generation was younger than that in the previous generation.
Spectrum of extracolonic cancer associated with HNPCC and HNPCC prevalence in general primary CRC
In 142 HNPCC families fulfilled AC II, there were 105 families that definitely fulfilled AC I. All these families had the detailed spectrum of extracolonic cancers. There were 125 extracolonic cancers in AC I families and 174 extracolonic cancers in AC II families (Table 2). The spectrum of extracolonic cancers were almost the same in AC I families as in AC II families. Gastric cancer was the most frequent extracolonic cancers in both AC I and AC II families, followed by endometrial cancer, hepatocarcinoma and the other extracolonic cancers.
Table 2 Extracolonic tumors found in HNPCC families.
| Extracolonic tumor | Number of AC (%) | Number of AC II (%) |
|---|---|---|
| Gastric cancer | 36 (28.8) | 49 (28.2) |
| Endometrial cancer | 28 (22.4) | 36 (20.7) |
| Ovarian cancer | 6 (4.8) | 9 (5.2) |
| Small intestinal cancer | 3 (2.4) | 9 (5.2) |
| Esophageal cancer | 6 (4.8) | 9 (5.2) |
| Pancreatic cancer | 5 (4.0) | 6 (3.4) |
| Breast cancer | 5 (4.0) | 7 (4.0) |
| Cancer of nasopharynx/larynx | 2 (1.6) | 3 (1.7) |
| Malignant lymphoma | 2 (1.6) | 2 (1.2) |
| Leukemia | 1 (0.8) | 1 (0.6) |
| Thyroid cancer | 5 (4.0) | 5 (2.9) |
| Lung cancer | 4 (3.2) | 8 (4.6) |
| Hepatocarcinoma | 10 (8.0) | 11 (6.3) |
| Bladder carcinoma | 1 (0.8) | 3 (1.7) |
| Cancer of brain | 3 (2.4) | 5 (2.9) |
| Ureter/renal pelvis cancer | 0 (0.0) | 1 (0.6) |
| Carcinoma of kidney | 2 (1.6) | 2 (1.2) |
| Skin cancer | 2 (1.6) | 2 (1.2) |
| Osteosarcoma | 0 (0.0) | 2 (1.2) |
| Carcinoma of prostate | 1 (0.8) | 1 (0.6) |
| Malignant teratoma | 1 (0.8) | 1 (0.6) |
| Cancer of parotid gland | 1 (0.8) | 1 (0.6) |
| Liposarcoma | 1 (0.8) | 1 (0.6) |
| Total | 125 (100.0) | 174 (100.0) |
Among 3874 primary CRC patients, the condition of HNPCC patients whose families fulfilled AC I were only analyzed in 942 patients, and the condition of HNPCC patients whose families fulfilled AC II were only analyzed in 1058 patients. Both of them were analyzed in the other 1874 patients. So in 2816 patients who were analyzed the condition of HNPCC patients whose families fulfilled AC I, there were 35 HNPCC patients (1.2%; 95% confidence interval [CI] = 0.8-1.7%). And in 2932 patients who were analyzed the condition of HNPCC patients whose families fulfilled AC II there were 63 HNPCC patients (2.2%; 95% confidence interval [CI] = 1.6-2.7%).
DISCUSSION
Aldred Warthin first described what is now recognized as the HNPCC or Lynch syndrome in 1913. HNPCC is an autosomal dominantly inherited disorder of cancer susceptibility with high penetrance (80-85%)[6], and accounts for approximately 1% to 3% of all cases of colorectal cancer[7]. This disorder is often characterized by multiple generations being affected and the development of colorectal cancer that is often diagnosed at an early age (mean age, approximately 45 years) and is mostly right-sided colorectal cancer (approximately 70% proximal to the splenic flexure)[4,6,7]. There is an excess of synchronous CRC and metachronous CRC in 35% of patients[6]. In addition, there is an excess of extracolonic cancers[4,6]. The cancer of the endometrium, stomach, ovaries, small bowel, ureter, renal pelvis, brain, and hepatobiliary tract are all clinically associated with HNPCC. But in AC II established in 1998, only CRC, cancer of the endometrium, small bowel, ureter or renal pelvis were thought as HNPCC-associated cancers[6]. Stomach cancer has a high prevalence in some Asian countries. Occasionally, it results in the chance of familial aggregation of CRC and stomach cancer. But in HNPCC families reported in the Western countries, stomach cancer is uncommon and is observed mainly in patients from older generations. Though stomach cancer was frequently reported in HNPCC families in Asia, there were no studies in these countries revealing the relative risk of developing stomach cancer in carriers of a mutated MMR gene. Therefore, stomach cancer was not included in the spectrum of HNPCC-associated cancers in AC II established by ICG-HNPCC[6].
HNPCC families can be classically subdivided into Lynch syndrome I and II. Lynch syndrome I only displays colorectal cancer, whereas Lynch syndrome II also exhibits extracolonic tumors. In China, our study showed that the proportion of Lynch syndrome I and II were 43.9% and 56.1% by AC II, 47.2% and 52.8% by AC I respectively. It suggested there were extracolonic cancers in most of Chinese HNPCC families. In AC I families, the overall proportion of CRC, extracolonic cancer, multiple primary cancers and multiple primary CRC patients were 82.4%, 25.7%, 19.2% and 19.1% respectively. The first CRC was right-sided CRC in 66.5% CRC patients. In addition, the first cancer was CRC in 80.8% patients. Among those patients whose first cancer was CRC, 66.8% and 19.9% were right-sided CRC and rectal cancer respectively. At the same time, there were also the similar results in AC II families. It showed that most of HNPCC associated-cancer patients were susceptible to CRC. Moreover, CRC was mainly located in the proximal rather than the distal part of the colon in two-thirds of CRC patients where CRC was the first cancer. It was an important characteristic of HNPCC syndrome. Extracolonic cancer was the first cancer in one-fifth or so of HNPCC associated-cancer patients diagnosed by both AC and AC II. It implied that extracolonic cancers were associated with HNPCC, and many similar results were also reported from other countries[8-11]. So extracolonic cancers should be included in clinical criteria of HNPCC families. In addition, the proportions of multiple CRC and right-sided CRC patients in the Chinese HNPCC families were similar to those in the Western HNPCC families[6,12-15].
One of the most important characteristics is that HNPCC-associated cancer is diagnosed at an early age. The mean or median age at cancer diagnosis is approximately from 41 to 50 years[4,6-7,12-15]. Mean and median are two most important parameters which statistically describe the central tendency of quantitative data, but there are some differences in their applications. Mean applies to quantitative data of normal distribution. If quantitative data are not normal distribution, mean cannot really describe the central tendency and average level of data. Median applies to all types of quantitative data. If quantitative data are normal distribution, median is theoretically equal to mean. So when we describe the central tendency of some quantitative data, normal distribution should be tested. In HNPCC-associated cancer patients, our study showed that the age distribution of patients diagnosed with the first malignant tumor was normal distribution. The same results were also observed in HNPCC-associated cancer patients of the first and second generations. But the distribution of the age of diagnosis of the first CRC was not normal distribution in all CRC patients. So both mean age and median age could be used to describe the central tendency of the age distribution of patients diagnosed with the first malignant tumor, and median age is the only parameter which can accurately describe the central tendency of the age distribution of patients diagnosed with the first CRC. When patients with HNPCC-associated cancer suffered from the first malignant tumor in Chinese HNPCC families diagnosed by AC and AC II, the mean and median age were 45.1 and 44.0, 45.2 and 44.5 years respectively. When they suffered from the first CRC, the median ages were both 43.0 years. These results were similar to those from other countries[4,6,7,12-15]. In addition, our findings showed a tendency that HNPCC-associated cancer patients can be diagnosed the first malignancy at an earlier age in later generation than in previous generation. The median ages in the first, second, third and fourth generations were 52, 43, 38 and 31 years respectively.
The other important feature of HNPCC syndrome is frequent occurrence of extracolonic cancers. Many evidences suggest that extracolonic cancers are associated with HNPCC syndrome in clinic and (or) genetics[4,6,7,9-11,15,16-19]. Our study revealed that about one-fourth of HNPCC-associated cancer patients had extracolonic cancer in China. Its proportion was smaller than in American HNPCC families[15]. The spectrum of extracolonic cancers included gastric cancer, endometrial cancer, hepatocarcinoma, ovary cancer, esophageal cancer, pancreatic cancer, breast cancer, thyroid cancer, lung cancer, small intestinal caner, cancer of brain, cancer of nasopharynx/larynx, malignant lymphoma, carcinoma of kidney, skin cancer, leukemia, bladder carcinoma, carcinoma of prostate, malignant teratoma, cancer of parotid gland, liposarcoma, ureter or renal pelvis cancer and osteosarcoma. Ureter or renal pelvis cancer and osteosarcoma were only found in AC II HNPCC families. Gastric cancer, endometrial cancer and hepatocarcinoma were the first, second and third most frequent extracolonic cancers in both AC I HNPCC families and AC II HNPCC families, respectively. They were followed by ovary cancer, esophageal cancer, pancreatic cancer, breast cancer, thyroid cancer, lung cancer, small intestinal caner, cancer of brain, cancer of nasopharynx/larynx, malignant lymphoma, carcinoma of kidney, skin cancer, leukemia, bladder carcinoma, carcinoma of prostate, malignant teratoma, cancer of parotid gland and liposarcoma in AC HNPCC families, and gastric cancer, endometrial cancer, hepatocarcinoma, ovary cancer, esophageal cancer, small intestinal cancer, lung cancer, breast cancer, pancreatic cancer, thyroid cancer, cancer of brain, cancer of nasopharynx/larynx, bladder carcinoma, malignant lymphoma, carcinoma of kidney, skin cancer, osteosarcoma, leukemia, ureter/renal pelvis cancer, carcinoma of prostate, malignant teratoma, cancer of parotid gland, and liposarcoma in AC II HNPCC families. Gastric cancer was more common than endometrial cancer in Chinese HNPCC families if difference of sex was not considered. And similar results were reported in Korea and Brazil[18,19]. It was significantly different from most European and American HNPCC families whose most frequent extracolonic cancer was endometrial cancer[4,6,7,11]. However, the extracolonic cancers of AC II only included endometrial cancer, small intestinal cancer and ureter or renal pelvis cancer, because gastric cancer was only frequently reported in Asian HNPCC families, and is uncommon and is observed mainly in patients from older generations in western countries. Moreover, there were no studies in these countries revealing the relative risk of developing stomach cancer in carriers of a mutated MMR gene at that time[6]. In fact, some studies from Europe and America also supported that gastric cancer was associated with HNPCC syndrome[19,20]. Recently, Kim _et al_[18] reported gastric cancer was the most common extracolonic malignancy in HNPCC and suspected HNPCC families, and five germline mutations in the hMLH1 and six germline mutations in the hMSH2 were exclusively found in families with gastric cancer. It suggested that gastric cancer was also genetically associated with HNPCC syndrome. Endometrial cancer was the most common extracolonic cancer in Chinese female patients with HNPCC-associated cancer, it is the same in other countries[4,6,7,11,19]. At the same time, ovarian cancer and breast cancer were also more frequent extracolonic cancer in Chinese HNPCC-associated cancer patients. They were the second and third most frequent extracolonic cancer in female patients. Although they were not included in AC II, they often occurred in HNPCC-associated cancer patients in both China and other countries[19,21,22]. Moreover, it had been reported that carriers of an hMSH2 mutation developed ovarian cancer[21]. Compared to the European and American HNPCC families, hepatocarcinoma and esophageal caner were also more common in Chinese HNPCC families. In addition, Soravia _et al_[16] reported that prostate cancer was found in HNPCC families, and the MSI and IHC analysis of the prostatic cancer clearly linked its etiology to the germline mutation of mismatch repair genes. The suggestion of other countries, including ours that all extracolonic cancers, which were clinically found in HNPCC families should be included in the malignant tumor spectrum of HNPCC syndrome, though only some of them were genetically confirmed to be HNPCC-associated cancers. First, the selection criteria for HNPCC is that it should be clinical because genetic analyses (MSI and mutation analyses) are not accessible to all families, the techniques are not available in all countries, and the significance of the findings is not completely understood[6]. Secondly, it is generally accepted that genetic pathogenesis of HNPCC is associated with dysfunction of DNA mismatch repair system resulting from germline mutation of mismatch repair genes[4,7,23-26]. Germline mutation of mismatch repair genes was detected in about two third to ninety-two percent in all HNPCC families fulfilled AC I[25-28], and plays an important role in revealing genetic pathogenesis of HNPCC syndrome and early detection and treatment of HNPCC-associated cancer patients in HNPCC families[23,29-32]. But definite association between genotype and phenotype has not been found in HNPCC until now[24-26,28,33]. Thirdly, since AC II was established by ICG-HNPCC in 1998, more extracolonic cancers, which were clinically found to be associated with HNPCC syndrome, have proved genetically to be associated with HNPCC syndrome than before[16-18,21,34]. Moreover, some of them were rare both in HNPCC-associated tumors and in sporadic cancers[33,34]. Finally, HNPCC in fact is a syndrome. By analogy with several other genetic disorders, HNPCC encompasses a wide spectrum of different clinical presentations, including Muir-Torre syndrome, Turcot syndrome, and the neurofibromatosis-hematological malignancy association[33,35]. So, it is improper only to define the extracolonic cancers, which have been genetically ascertained to be associated with HNPCC as the spectrum of HNPCC-associated cancers until genetic pathogenesis of HNPCC syndrome is completely understood.
Members of HNPCC families are high-risk individuals who are more easily predisposed to malignant tumors than members of families with sporadic CRC patients especially CRC. Estimates of the frequency of HNPCC are generally based on clinical criteria including AC I and AC II, and have varied from country to country. In the general population of CRC patients, the prevalence of HNPCC fulfilled AC I vary from 0.3% to 3% in European and American[36-39]. In China, our study showed that the prevalence of HNPCC fulfilled AC I was 1.2% in general CRC patients, which was similar to that in Europeans and Americans. In addition, the prevalence of HNPCC fulfilled AC II was 2.2% in Chinese general CRC patients.
On the basis of our study, we realize that current AC II has some defects and may result in failure to screen some HNPCC families. So we suggest that the AC II should be revised according to the development of clinical and genetic findings for HNPCC families, and the HNPCC-associated cancers include CRC and extracolonic cancers. Extracolonic cancer is referred to any malignant tumor outside the colorectum. In addition, the other important factors should also be thought when the HNPCC families are screened. With the development of socioeconomics and carrying through Planned Parenthood policy in China, the size of most of Chinese families is growing smaller than before. Under this condition, it is difficult to seek three relatives that one is a first degree relative of the others in a family these days and in future. Therefore, we propose a set of new clinical criteria to diagnose the HNPCC families. It includes the following: 1) at least 3 relatives with an HNPCC-associated cancer (CRC, extracolonic cancer, which can be in any site), at least one must be CRC; 2) One should be a first-degree or second relative of the other 2, at least 2 are first-degree relatives of each other; 3) At least 2 successive generations should be affected; 4) At least 1 should be diagnosed before age 50; 5) Familial adenomatous polyposis should be excluded in the CRC case(s) if any and 6) Tumors should be verified by pathological examination.
To our knowledge, this report is a first systematic description of HNPCC families and the prevalence of HNPCC in China. As such, it provides a broad and detailed description of general characteristics of HNPCC families in a larger sample, and the prevalence of HNPCC diagnosed by both AC I and AC II in a larger general population of CRC patients in China. We acknowledge that this is not the final answer for characteristics of HNPCC families and HNPCC prevalence figures in the general CRC population in China; however, it is necessary as a first step and provides general data for the scientific community. It will accelerate genetic pathogenesis of HNPCC syndrome and the prevention of HNPCC-associated cancers in members of HNPCC families in China.
Footnotes
Edited by Guo SY Language Editor Elsevier HK
References
| 12. | Bernstein IT, Bisgaard ML, Myrhøj T. Registration of hereditary non-polyposis colorectal cancer. Ugeskr Laeger. 1999;161:6174-6178. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 18. | Kim JC, Kim HC, Roh SA, Koo KH, Lee DH, Yu CS, Lee JH, Kim TW, Lee HL, Beck NE. hMLH1 and hMSH2 mutations in families with familial clustering of gastric cancer and hereditary non-polyposis colorectal cancer. Cancer Detect Prev. 2001;25:503-510. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 19. | Oliveira Ferreira F, Napoli Ferreira CC, Rossi BM, Toshihiko Nakagawa W, Aguilar S, Monteiro Santos EM, Vierira Costa ML, Lopes A. Frequency of extra-colonic tumors in hereditary nonpolyposis colorectal cancer (HNPCC) and familial colorectal cancer (FCC) Brazilian families: An analysis by a Brazilian Hereditary Colorectal Cancer Institutional Registry. Fam Cancer. 2004;3:41-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
|---|
| 25. | Wagner A, Barrows A, Wijnen JT, van der Klift H, Franken PF, Verkuijlen P, Nakagawa H, Geugien M, Jaghmohan-Changur S, Breukel C. Molecular analysis of hereditary nonpolyposis colorectal cancer in the United States: high mutation detection rate among clinically selected families and characterization of an American founder genomic deletion of the MSH2 gene. Am J Hum Genet. 2003;72:1088-1100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 160] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
|---|
| 31. | Caldés T, Godino J, Sanchez A, Corbacho C, De la Hoya M, Lopez Asenjo J, Saez C, Sanz J, Benito M, Ramon Y Cajal S. Immunohistochemistry and microsatellite instability testing for selecting MLH1, MSH2 and MSH6 mutation carriers in hereditary non-polyposis colorectal cancer. Oncol Rep. 2004;12:621-629. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 35. | Lucci-Cordisco E, Zito I, Gensini F, Genuardi M. Hereditary nonpolyposis colorectal cancer and related conditions. Am J Med Genet A. 2003;122A:325-334. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 38. | Katballe N, Christensen M, Wikman FP, Ørntoft TF, Laurberg S. Frequency of hereditary non-polyposis colorectal cancer in Danish colorectal cancer patients. Gut. 2002;50:43-51. [PubMed] [DOI] [Cited in This Article: ] |
|---|