Effects of glucagon-like peptide-1 receptor agonists on non-alcoholic fatty liver disease and inflammation (original) (raw)
Review Open Access
Copyright ©2014 Baishideng Publishing Group Inc. All rights reserved.
World J Gastroenterol. Oct 28, 2014; 20(40): 14821-14830
Published online Oct 28, 2014. doi: 10.3748/wjg.v20.i40.14821
Effects of glucagon-like peptide-1 receptor agonists on non-alcoholic fatty liver disease and inflammation
Xing-Chun Wang, Shen Qu, Department of Endocrinology and Metabolism, Shanghai 10th People’s Hospital, Tongji University, Shanghai 200072, China
Aaron M Gusdon, Department of Neurology and Neuroscience, Weill Cornell Medical College, New York, NY 10065, United States
Xing-Chun Wang, Huan Liu, Shen Qu, Nanjing Medical University, Nanjing 210029, Jiangsu Province, China
ORCID number: $[AuthorORCIDs]
Author contributions: Wang XC identified the concept and wrote the manuscript; Gusdon AM and Liu H were involved in editing the manuscript; Qu S organized the paper and approved the final version.
Correspondence to: Shen Qu, Professor, Department of Endocrinology and Metabolism, Shanghai 10th People’s Hospital, Tongji University, 301 Middle Yanchang Road, Shanghai 200072, China. qushencn@hotmail.com
Telephone: +86-21-66302531 Fax: +86-21-66302531
Received: March 25, 2014
Revised: May 14, 2014
Accepted: July 15, 2014
Published online: October 28, 2014
Processing time: 218 Days and 6.4 Hours
Abstract
Glucagon-like peptide1 (GLP-1) is secreted from Langerhans cells in response to oral nutrient intake. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are a new class of incretin-based anti-diabetic drugs. They function to stimulate insulin secretion while suppressing glucagon secretion. GLP-1-based therapies are now well established in the management of type 2 diabetes mellitus (T2DM), and recent literature has suggested potential applications of these drugs in the treatment of obesity and for protection against cardiovascular and neurological diseases. As we know, along with change in lifestyles, the prevalence of non-alcoholic fatty liver disease (NAFLD) in China is rising more than that of viral hepatitis and alcoholic fatty liver disease, and NAFLD has become the most common chronic liver disease in recent years. Recent studies further suggest that GLP-1RAs can reduce transaminase levels to improve NAFLD by improving blood lipid levels, cutting down the fat content to promote fat redistribution, directly decreasing fatty degeneration of the liver, reducing the degree of liver fibrosis and improving inflammation. This review shows the NAFLD-associated effects of GLP-1RAs in animal models and in patients with T2DM or obesity who are participants in clinical trials.
Core tip: The findings showed that glucagon-like peptide-1 receptor agonists (GLP-1RAs) may improve liver function, fat content and distribution, lipid metabolism and reduce the activity of inflammatory cytokines and their associated signal transduction pathways. Thus, we review here that GLP-1RAs are a potential method for pharmacologic treatment that can benefit patients with non-alcoholic fatty liver disease and chronic inflammation.
- Citation: Wang XC, Gusdon AM, Liu H, Qu S. Effects of glucagon-like peptide-1 receptor agonists on non-alcoholic fatty liver disease and inflammation. World J Gastroenterol 2014; 20(40): 14821-14830
- URL: https://www.wjgnet.com/1007-9327/full/v20/i40/14821.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i40.14821
INTRODUCTION
Glucagon-like peptide-1 (GLP-1) is a 30-amino acid peptide secreted from Langerhans cells and is a naturally existing hormone which reduces blood glucose during hyperglycemia by stimulating insulin secretion and reducing glucose-dependent glucagon secretion[1,2]. GLP-1 is rapidly degraded by the enzyme dipeptidy1 peptidase-4 (DPP-4)[1]. However, modified forms of GLP-1, also called glucagon-like peptide-1 receptor agonists (GLP-1RAs), have a prolonged half-life and can be administered once daily[3]. GLP-1RAs have the effects of decreasing glucose levels and stimulating weight loss and have been used for the treatment of type 2 diabetes mellitus (T2DM). Expression of the GLP-l receptor (GLP-1R) has been found in islet cells, the lung, brain, kidney, liver, and adipose tissues of animals[4]. The extensive distribution of GLP-1R suggests that GLP-1RAs may have a variety of actions. In recent years, the prevalence of non-alcoholic fatty liver disease (NAFLD) has continued to rise, and approximately 10%-25% of NAFLD cases will progress to non-alcoholic steatohepatitis (NASH), while 10%-15% of NASH cases will develop into hepatocellular carcinoma[5]. There has been increasing interest in the role of GLP-1RAs in NAFLD. In fact, recent studies have found that GLP-1RAs can regulate lipid metabolism in the liver under pro-inflammatory conditions. Therefore, we review the effects of GLP-1RAs on NAFLD and inflammation with the aim of extending the use of GLP-1RAs to the treatment of NAFLD.
IMPROVEMENT OF NAFLD WITH GLP-1RAs
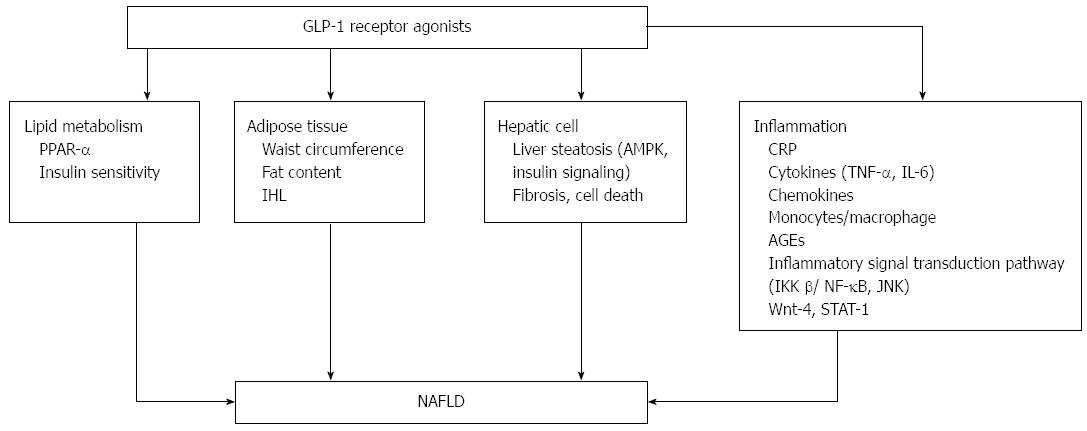
Serum transaminases are mildly or moderately elevated in patients with NAFLD, and GLP-1RA treatment can reduce serum transaminase levels, thereby improving liver function in patients with NAFLD. A meta-analysis included 12 trials that studied changes in alanine aminotransferase (ALT) after at least 20 wk of treatment with liraglutide in patients with T2DM, and the results showed that ALT levels were decreased[6]. Exenatide treatment for 52 wk contributed to significant reductions in ALT (P < 0.0001) in patients with T2DM[7]. Ohki _et al_[8] studied the effectiveness of liraglutide in patients with NAFLD and T2DM, and the results showed that the levels of ALT and AST (aspartate transaminase) were decreased (P < 0.01). Treatment for 6 mo with GLP-1RAs in obese patients with T2DM was also associated with significant reductions in ALT and glutamyl transpeptidase (GGT)[9]. Patients with T2DM with elevated serum ALT who were treated with exenatide for at least 3 years had reduced ALT levels (P < 0.0001), and 41% achieved normalization of ALT[10]. Buse _et al_[11] also demonstrated that exenatide treatment for 2 years was associated with a significant improvement in abnormal liver transaminases; ALT was decreased in patients with elevated ALT at baseline (P < 0.05), and 39% of patients achieved normal levels of ALT[11]. Patients who had abnormal levels of ALT at baseline and were treated with liraglutide showed reduced levels of ALT compared to those treated with the placebo (P = 0.003)[12]. The mechanisms are shown in Figure 1.
Figure 1 Effects of glucagon-like peptide-1 receptor agonist on non-alcoholic fatty liver disease and inflammation. PPAR-α: Peroxisome proliferator-activated receptor; IHL: intrahepatic lipids; AMPK: AMP-activated protein kinase; CRP: C reactive protein; AGEs: Advanced glycation and end products; JNK: c-Jun NH2-terminal kinase; GLP-1RA: Glucagon-like peptide-1 receptor agonist; NAFLD: Non-alcoholic fatty liver disease.
MECHANISM OF ACTION OF GLP-1RAs ON NAFLD
GLP-1RAs and lipid metabolism
Obesity, T2DM and dyslipidemia are the most common predisposing factors for NAFLD. GLP-1RAs can reduce fatty liver through an improvement in lipid metabolism. Hepatic triglycerides were reduced in diet-induced model of obesity (DIO) mice after exendin-4 treatment for 4 wk[13]. Kelly _et al_[14] compared the effects of exenatide with metformin and found that triglycerides were reduced more in the group that received exenatide treatment (P = 0.032). Exenatide treatment for 3 or more years in patients with T2DM resulted in improvements in the following components of the lipid profile: triglycerides decreased by 12% (P = 0.0003), total cholesterol decreased by 5% (P = 0.0007), low density lipoprotein-cholesterol decreased by 6% (P < 0.0001), and high density lipoprotein-cholesterol increased by 24% (P < 0.0001)[10]. The possible mechanism by which GLP-1RAs improve lipid metabolism may involve the activation of peroxisome proliferator-activated receptor (PPAR-α) on the hepatic cell surface, which reduces the synthesis of apolipoprotein C, degrades fat in plasma, and removes triglycerides; it may also be associated with delayed gastric emptying[15]. In addition, GLP-1RAs can enhance insulin sensitivity, promote insulin secretion, and reduce lipid metabolism indirectly[16-18].
GLP-1RAs and adipose tissue
GLP-1RAs and waist circumference: Waist circumference (WC), a risk factor for metabolic syndrome (MS), is associated with insulin resistance and NAFLD. Increased WC can lead to insulin resistance and NAFLD, and thus, may be an important risk factor for the development of NAFLD[19,20]. WC is significantly higher in patients with NAFLD[21]. However, WC was decreased significantly in obese women after 35 wk of exenatide treatment[22]. There was a significant endpoint difference in WC between patients who were treated with exenatide and those who were treated with insulin glargine, and the reduction in WC after exenatide treatment was statistically significant[23]. WC was also significantly reduced with liraglutide treatment[24-26].
GLP-1RAs and fat content: A significant positive correlation exists between visceral fat and transaminases in patients with NALFD. Additionally, total fat and visceral fat are independent predictors of NAFLD[27]. GLP-1RA treatment changed body composition, as patients who were treated with liraglutide showed a decrease in total body mass, and the majority of the total body mass that was lost was fat mass[28]. Weight loss in the patients treated with GLP-1RAs is associated with a reduction in adipose tissue[29]. The results of a randomized, double-blind, placebo-controlled 20-wk study with 2-year extension showed that body fat was decreased by 15.4% in patients treated with liraglutide[30]. A month treatment with exenatide significantly decreased subcutaneous fat deposition by 4.4%[31]. Additionally, abdominal fat is closely related to the components of MS such as hyperinsulinemia, T2DM, and NAFLD, as intra-abdominal fat accumulation will increase insulin resistance[32]. GLP-1RAs can decrease weight and reduce abdominal visceral fat content. Liraglutide significantly reduced fat mass and fat percentage in patients with T2DM in the 26-wk LEAD-2 (Liraglutide Effect and Action in Diabetes-2) and in the 52-wk LEAD-3 trials[29]. Visceral and subcutaneous adipose tissues were significantly reduced after treatment with liraglutide compared to treatment with glimepiride (P < 0.05) in the LEAD-2 trial. Inoue _et al_[33] found that treatment with liraglutide (dose range: 0.3-0.9 mg/d) for 20.0 ± 6.4 d significantly reduced the estimated visceral fat area (eVFA). GLP-1RA treatment in severely obese adolescents also led to a significant decrease in the eVFA[34].
GLP-1RAs and intrahepatic lipids: The reduction of abdominal visceral adipose by GLP-1RAs results in a reduction in liver fat content and can alleviate NAFLD. Cuthbertson _et al_[9] determined the impact on intrahepatic lipids (IHL) of a 6-month-long treatment with GLP-1RAs in obese patients with T2DM and demonstrated that this resulted in significant reductions in IHL; in addition, the median relative reduction in IHL was 42%. Tushuizen _et al_[35] directly examined the effect of exenatide therapy on hepatic steatosis, and the results showed that 44 wk of treatment led to a reduction in liver fat from 16.0% to 5.4%. Samson _et al_[13] found that combined pioglitazone and exenatide therapy was associated with a greater decrease in hepatic fat than that achieved with pioglitazone therapy alone in patients with T2DM. Sathyanarayana _et al_[36] also concluded that combined pioglitazone and exenatide therapy was associated with a greater reduction in hepatic fat content compared to the addition of pioglitazone therapy (P < 0.05) in patients with T2DM. Hopkins _et al_[37] treated patients with T2DM with a GLP-1RA for 6 mo, and found that the liver fat content was reduced from 21.3% ± 19.3% to 12.7% ± 10.6%. The ability of GLP-1 to reduce fat is due to binding with a specific GLP-1R in adipose tissue. Vendrell _et al_[38] confirmed the expression of GLP-1R in mature adipose cells by the detection of the mRNA and protein. The GLP-1R also exists in T3-L1 adipocytes, and GLP-1RAs can promote lipolysis in 3T3-L1 adipocytes[38]. A possible mechanism of lipolysis by GLP-1RAs may involve the increase in the phosphorylation of perilipin and hormone-sensitive lipase (HSL) with subsequent dissolution of fat molecules. When perilipin is phosphorylated, HSL has access to fat droplets, which results in fat degradation[38]. Gupta _et al_[39] treated fat cells with a GLP-1RA and detected phosphorylated perilipin (Ser 497) and HSL (Ser 563) by confocal microscopy. The results illustrated that the levels of phosphorylated perilipin were increased in patients in the GLP-1RA treatment group compared to patients in the control group. In addition, GLP-1RA treatment resulted in HSL accumulation within fat droplets, which allows for the initiation of fat dissolution.
Direct effects of GLP-1RAs on hepatic cells
GLP-1RAs and liver steatosis: NAFLD is characterized by diffuse macrovesicular steatosis in hepatocytes as the main clinical pathological syndrome caused by factors other than alcohol and other types of liver damage. GLP-1RAs can reduce hepatic steatosis, and thus relieve the symptoms of NAFLD. GLP-1R is present on human hepatocytes and has a direct role in the decrease of hepatic steatosis in vitro by modulating elements of the insulin signaling pathway[40]. GLP-1RAs reduce hepatocyte steatosis and improve survival by enhancing the unfolded protein response and by promoting macroautophagy. GLP-1RAs appear to protect hepatocytes from fatty acid-related death by inhibition of a dysfunctional endoplasmic reticulum (ER) stress response and by reduction of fatty acid accumulation by activation of both macro-autophagy and chaperone-mediated autophagy. GLP-1RAs have a role in halting the progression of underlying steatosis to more aggressive lesions in patients with NAFLD[41]. In the LEAD-2 trial, the liver-to-spleen attenuation ratio increased after treatment with 1.8 mg of liraglutide, which may indicate reduced hepatic steatosis[29]. Ding _et al_[42] showed that exendin-4 appears to reverse hepatic steatosis in ob/ob mice by effectively improving insulin sensitivity. Their data also suggested that GLP-1 proteins in the liver have a novel direct effect on hepatocyte fat metabolism. Gupta _et al_[39] treated HuH7 cells that were exposed to an ischemic insult with a GLP-1RA and also demonstrated that liver steatosis was significantly reduced (P < 0.006). The effect of GLP-1RAs on fatty degeneration in the liver is directly mediated by key signaling pathways, including the AMP-activated protein kinase (AMPK) and insulin signaling pathways[40,43]. GLP-1RAs can directly activate signal transduction pathways within liver cells. For example, GLP-1RAs can activate the phosphorylation of AMPK in cultured liver cells _in vitro_[43]. Samson _et al_[13] found that exendin-4 treatment for 4 wk enhances hepatic AMPK phosphorylation in DIO mice. Liraglutide also induced phosphorylation of AMPK through a signaling pathway independent of cyclic AMP[44]. In addition, the main pathophysiological characteristic in patients with NAFLD is insulin resistance. Improvement in insulin resistance and sensitivity will decrease hepatic steatosis and prevent liver cell injury. GLP-1RAs can improve insulin resistance and insulin sensitivity to prevent the progression of NAFLD. Another study[42] showed that exendin-4 treatment improves insulin resistance in ob/ob mice, as measured by the glucose and HOMA fractions.
Effects of GLP-1RAs on fibrosis and cell death: NAFLD can progress from simple fatty liver to hepatitis and liver cirrhosis. Protein and RNA levels of fibroblast growth factor 21 (FGF21) in the plasma and in the liver are higher in patients who are obese and have NAFLD. In one study, treatment with GLP-1 RAs reduced the level of FGF21[45]. Samson _et al_[13] examined the effects of exenatide on FGF21 in patients with T2DM and in DIO mice. Their results suggest that combined pioglitazone and exenatide therapy is associated with a reduction in plasma FGF21 levels than that achieved with pioglitazone therapy alone. In addition, the AST-to-platelet count ratio index (APRI) can reflect the degree of liver fibrosis, and in this case, the APRI index was decreased (P < 0.01) in the liraglutide treatment group[8]. NAFLD is associated with cell death and fibrosis and ultimately progresses to cirrhosis[46]. The receptors of GLP-1RAs exist in human hepatocytes, and the administration of GLP-1RAs have been reported to directly reduce fibrosis _in vivo_[13,40]. Out of 8 patients with T2DM and biopsy-proven NAFLD who underwent liver biopsies before and after 28 wk of exenatide therapy, 3 patients demonstrated improvement, as verified by the liver histology[47]. GLP-1RAs can also reduce hepatocyte cell death, thereby preventing the progression of NAFLD. Liver cell death mainly includes two forms: necrosis and apoptosis, and Gupta _et al_[39] showed that a GLP-1RA significantly reduced cell necrosis. In addition, histological evidence showed that a GLP-1RA protected liver cells from cell death caused by fat deposition by the inhibition of cell apoptosis. The possible mechanism of its effect may be due to its ability to reduce hepatic ER stress, because hepatocytes are known to have a high content of ER and are particularly susceptible to ER stress[48]. The C/EBP homolog (CHOP) was decreased, which leads to ER stress-related cell necrosis after Ex-4 treatment; reduced CHOP protein expression can significantly decrease the fatty degeneration of liver cells[41].
GLP-1RAs AND INFLAMMATION
NAFLD includes a disease spectrum that ranges from simple steatosis to NASH, with varying degrees of inflammation, and progresses to liver cirrhosis[49,50]. GLP-1RAs have modest anti-inflammatory effects in addition to hypoglycemic effects. Moreover, GLP-1RAs have anti-inflammatory effects in different cell types including human umbilical vein endothelial cells, glomerular endothelial cells, monocytes and macrophages[51,52]. Lipopolysaccharide-induced inflammation was reduced by exendin-4 in 3T3-L1 adipocytes and ATM[53]. GLP-1RAs improve the inflammatory response by reducing the release of inflammatory cytokines and by improving inflammatory pathways.
GLP-1RAs and C-reactive protein
C-reactive protein (CRP) is an acute phase protein that is produced by the liver when body tissues are subjected to various types of injuries or inflammation. Cytokines such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) secreted by fat cells are the major contributors to the hepatic synthesis of CRP. CRP is a sensitive marker of inflammation and is often used to monitor the degree of inflammation. GLP-1RAs can reduce CRP levels as well as the inflammatory response in patients with diabetes. Varanasi _et al_[54] performed a retrospective analysis of 110 obese patients with T2DM who were treated with liraglutide and found that the mean CRP levels decreased for a mean duration of 7.5 mo (P < 0.05). High sensitivity C-reactive protein (hs-CRP) was reduced with both the 2 mg once-weekly and 10 μg twice-daily exenatide regimens (P < 0.05)[55]; exenatide decreased the concentration of hs-CRP whereas insulin glargine did not[56]. Exenatide plus metformin treatment also reduced concentrations of hs-CRP[57].
GLP-1RAs and cytokines: Adipose tissue, especially the excessive accumulation of fat tissue in the abdomen, leads to the synthesis and release of large amounts of inflammatory cytokines such as TNF-α and IL-6. GLP-1RAs have anti-inflammatory effects and function to down-regulate the levels of certain cytokines. The expression and production of IL-6, TNF-α and monocyte chemotactic protein 1 (MCP-1) were significantly reduced in adipose tissue of recombinant adenovirus (rAd)-producing GLP-1 (rAd-GLP-1)-treated ob/ob mice[53].
GLP-1RAs and TNF-α
GLP-1RAs could exert beneficial effects through their anti-inflammatory properties. Exendin-4 inhibited the increase in expression of TNF-α in myocardial cells exposed to high glucose[58]. Chen _et al_[59] injected female SD rats with exendin-4 for 9 wk and found that the level of TNF-α mRNA was significantly reduced in the liver of the mice. Exendin-4 also suppressed MPTP (1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine)-induced expression of pro-inflammatory molecules and TNF-α[60]. Real-time quantitative PCR analysis showed that the expression of TNF-α mRNA was significantly reduced in the exendin-4 treatment group[60]. Exendin-4 also inhibited TNF-α production by peritoneal macrophages in response to inflammatory stimulation[61], and exendin-4 changed the expression of inflammatory cytokines via the c-AMP signaling pathway _in vitro_[61].
GLP-1RAs and IL-6: IL-6 is a key inflammatory mediator that is strongly linked to the development of obesity and T2DM-related insulin resistance. IL-6 levels are increased in obese and insulin-resistant subjects[62]. GLP-1 infusion in patients with T2DM was associated with a significant reduction in circulating IL-6 at 120 min and at 180 min (P = 0.0001 and P = 0.001)[63]. Ex-4 decreased the mRNA levels of inflammatory adipokines[64]. Chen[59] treated female SD rats with exendin-4 for 9 wk, and the results revealed that the expression level of IL-6 mRNA was reduced in the liver of the mice. To explore the effect of exendin-4 on inflammatory adipokines, the expression of IL-6 in 3T3-L1 adipocytes was examined by quantitative real-time RT-PCR. The results illustrated that exendin-4 decreased the level of IL-6 mRNA in 3T3-L1 adipocytes[64]. The levels of the pro-inflammatory cytokines IL-6 (P < 0.01), IL-12p70 (P < 0.01), and IL-1β (P < 0.05) were reduced in the brains of animals treated with liraglutide for 30 d[65]. Exendin-4 also suppressed MPTP-induced expression of pro-inflammatory molecules and IL-1β[60].
GLP-1RAs and chemokines
Chemokines are chemo-attractant proteins that attract leukocytes to migrate to sites of infection and play an important role in the inflammatory response. MCP-1 induces insulin resistance[66]. Ojima _et al_[67] perfused mice with exendin-4 continuously for 2 wk and showed that the expression of MCP-1 was decreased in the glomeruli. This effect of GLP-1RAs is due to an increased level of adiponectin mRNA through the GLP-1R. Exendin-4 decreased MCP-1 mRNA levels in 3T3-L1 adipocytes[60,64]. In vitro treatment of mouse macrophages with exendin-4 suppressed lipopolysaccharide-induced mRNA expression of MCP-1[52]. Additionally, liraglutide reduced TNFα-induced MCP-1 (also known as CCL2), VCAM1, ICAM1 and E-selectin mRNA expression[44]. Exendin-4 also decreased IFN-γ-induced gene encoding chemokine CXCL10 [(C-X-C motif) ligand (CXCL)10] expression in human islets and in MIN6 cells (a mouse beta cell line)[68].
GLP-1RAs and monocytes/macrophages
With the occurrence of obesity, monocytes in the blood migrate into adipose tissue, where they differentiate into resident macrophages. They reside in adipose tissue as two phenotypes: M1 macrophages (classically activated, pro-inflammatory) and M2 macrophages (alternatively activated, anti-inflammatory). The expression of GLP-1R in monocytes/macrophages is abundant. GLP-1 can bind to GLP-1R and directly influence anti-inflammatory activity. In vitro exendin-4 acts directly on the GLP-1R and attenuates the release of pro-inflammatory cytokines from macrophages in glomerular endothelial cells[51]. In adipose tissue, cytokines and chemokines are secreted either by adipocytes or by macrophages that have infiltrated the adipose tissue, which leads to a chronic sub-inflammatory state that could play a central role in the development of insulin resistance and type 2 diabetes[69]. However, GLP-1 has anti-inflammatory effects on adipose tissue, including adipocytes and adipose tissue macrophages (ATM). Macrophage populations [F4/80(+) and F4/80(+)CD11b(+)CD11c(+) cells] were significantly reduced in adipose tissue of rAd-GLP-1-treated ob/ob mice[53]. The expression of M1-specific mRNAs was significantly reduced, but that of M2-specific mRNAs was unchanged in rAd-GLP-1-treated ob/ob mice. GLP-1 reduces macrophage infiltration and directly inhibits inflammatory pathways in adipocytes and in ATM, which may contribute to the improvement of insulin sensitivity.
GLP-1RAs and advanced glycation end products
Advanced glycation end products (AGEs) accumulate in the circulation and in tissues gradually with aging, diabetes and renal failure. AGEs interact with their receptors (RAGE) and induce vascular inflammation, and inhibition of the AGE-RAGE axis can reduce vascular inflammation. GLP-1 acts directly on HUVECs via GLP-1R, and may function as an anti-inflammatory agent against AGEs by reducing RAGE expression via the activation of cyclic AMP pathways[70]. GLP-1RAs may inhibit AGE-RAGE-mediated asymmetric dimethylarginine generation by suppressing protein arginine methyltransfetase-1 expression via the inhibition of ROS (reactive oxygen species) generation[67].
GLP-1RAs and inflammatory signal transduction pathways
There is considerable evidence in support of the hypothesis that inflammation plays an important role in metabolic dysregulation, as the inhibition of inflammatory signaling pathways is correlated with improved insulin sensitivity. Chronic inflammation in adipose tissue involves the IκB kinase beta (IKK β)/nuclear factor-κB (NF-κB) pathway and the c-Jun NH2-terminal kinase (JNK) pathway. Activation of these pathways leads to the expression of pro-inflammatory cytokines, inflammatory chemokines and cell adhesion factors and to the recruitment and infiltration of mononuclear macrophages[71]. The activation of the NF-κB and JNK pathways was significantly reduced in adipose tissue of rAd-GLP-1-treated ob/ob mice[53]. Dose-dependent liraglutide treatment inhibited NF-κB activation and TNFα-induced IκB degradation[44].
GLP-1RAs and IKKβ**/NF-κB:** IKKβ/NF-κB signaling pathways are important regulators of chronic inflammation. Nuclear transcription factor NF-κB exists in various cells and can regulate the expression of cytokines, chemokines and adhesion factors to effect an inflammatory reaction in the body[72]. In one study, exendin-4 decreased NF-κB activation in kidney tissue[51]. Mice were given 10 μg/kg exendin-4, and after 48 wk of treatment, results showed that activation of NF-κB was reduced; exendin-4 also significantly inhibited the binding activity of NF-κBp65 in patients with diabetes mellitus[45]. Liraglutide significantly inhibited NF-κB activation and up-regulated the IκB family of proteins while phosphorylation of IKK-α/β, which is upstream of NF-κB signaling, was also down-regulated after 15 min of TNF-α treatment[73]. ERK 1/2 and PI3-K/AKT are involved in LPS-induced NF-κB activation[74,75]. Lee _et al_[53] found that exendin-4 treatment could inhibit the phosphorylation of ERK1/2 and Akt in LPS-induced 3T3-L1 adipocytes. NF-κB binding activity was decreased after exendin-4 treatment for 2 wk in LPS-induced 3T3-L1 adipocytes, and a Western blot assay showed that exendin-4 treatment inhibited LPS-induced NF-κB nuclear translocation in 3T3-L1 adipocytes. Liraglutide also inhibited NF-κB phosphorylation and its translocation from the cytoplasm to the nucleus[76]. A dose-dependent treatment with liraglutide increased nitric oxide production in HUVECs. It also caused eNOS phosphorylation, potentiated eNOS activity and restored the cytokine-induced down-regulation of eNOS (also known as NOS3) mRNA levels, which are dependent on NF-κB activation[44].
GLP-1RAs and JNK: JNK belongs to the mitogen-activated protein kinase family and mediates inflammatory stress within cells and between different cells. The JNK inflammatory pathway plays an important role in the occurrence and development of cell differentiation, apoptosis, stress response and a variety of human diseases. GLP-1RAs have protective effects on cytokine-induced apoptosis in beta cells by interfering with the JNK pathway. Exendin-4 also protects beta cells from interleukin-1 beta-induced apoptosis by interfering with the JNK pathway[77]. Zhang _et al_[78] treated mice on a high-fat diet with 1 mg/kg liraglutide twice a day, and after 8 wk, the results showed that JNK phosphorylation was significantly reduced. Liraglutide modulated inflammation by mitogen-activated protein kinase 4/JNK signaling, which inhibited the activation and relieved the effect of low-grade inflammatory stress.
GLP-1RAs and other aspects of inflammation
Wnt-4 modulates canonical Wnt signaling and acts as a regulator of β-cell proliferation and the release of inflammatory cytokines. Stimulation with exendin-4 increases the expression of Wnt-4 in β-cells[79]. In addition, the mechanism of the anti-inflammatory action of exendin-4 involved a decrease in signal transducer and activator of transcription-1 levels[68].
CONCLUSION
The rising incidence of NAFLD brings with it a series of health problems. There is a clear association between NAFLD and MS which causes T2DM, obesity, hypertension, and dyslipidemia[80]. Overall, NAFLD has paralleled the rise in obesity and MS[81]. The current treatment of NAFLD is aimed at weight loss through lifestyle interventions that involve diet and exercise[82-85]. GLP-1RAs are a new class of pharmacological agents that improve glucose homeostasis in many ways. Their effects include enhance of glucose-stimulated insulin secretion, glucose-dependent inhibition of glucagon secretion, and a reduction in gastric emptying, appetite, food intake and body weight[86]. The effects of GLP-1RAs on body weight appear to be due to a reduction in food intake, mainly determined by a direct central (hypothalamic) effect of the hormone[87]. Additionally, it has been found to have numerous anti-diabetic effects, and numerous studies have shown that GLP-1RAs can improve lipid metabolism, promote fat redistribution, reduce insulin resistance, and decrease intrahepatic fat deposition. While its mechanism of action is just beginning to become understood, further studies are needed to fully elucidate its actions at the cellular level. In summary, GLP-1RAs may be effective drugs for the treatment of NAFLD, and their clinical applications and mechanisms of action remain to be explored in depth.
Footnotes
P- Reviewer: Ahboucha S, Fujiwara T, Leitman M S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Ma S
References
| 9. | Cuthbertson DJ, Irwin A, Gardner CJ, Daousi C, Purewal T, Furlong N, Goenka N, Thomas EL, Adams VL, Pushpakom SP. Improved glycaemia correlates with liver fat reduction in obese, type 2 diabetes, patients given glucagon-like peptide-1 (GLP-1) receptor agonists. PLoS One. 2012;7:e50117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
|---|
| 11. | Buse JB, Klonoff DC, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, Maggs DG, Wintle ME. Metabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: an interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trials. Clin Ther. 2007;29:139-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 213] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
|---|
| 29. | Jendle J, Nauck MA, Matthews DR, Frid A, Hermansen K, Düring M, Zdravkovic M, Strauss BJ, Garber AJ. Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes Metab. 2009;11:1163-1172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 207] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
|---|
| 30. | Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean ME, Niskanen L, Rasmussen MF, Rissanen A, Rössner S. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond). 2012;36:843-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 444] [Article Influence: 34.2] [Reference Citation Analysis (1)] |
|---|
| 32. | Khunti K, Gray LJ, Skinner T, Carey ME, Realf K, Dallosso H, Fisher H, Campbell M, Heller S, Davies MJ. Effectiveness of a diabetes education and self management programme (DESMOND) for people with newly diagnosed type 2 diabetes mellitus: three year follow-up of a cluster randomised controlled trial in primary care. BMJ. 2012;344:e2333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 219] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
|---|
| 33. | Inoue K, Maeda N, Kashine S, Fujishima Y, Kozawa J, Hiuge-Shimizu A, Okita K, Imagawa A, Funahashi T, Shimomura I. Short-term effects of liraglutide on visceral fat adiposity, appetite, and food preference: a pilot study of obese Japanese patients with type 2 diabetes. Cardiovasc Diabetol. 2011;10:109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
|---|
| 34. | Kelly AS, Rudser KD, Nathan BM, Fox CK, Metzig AM, Coombes BJ, Fitch AK, Bomberg EM, Abuzzahab MJ. The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity: a randomized, placebo-controlled, clinical trial. JAMA Pediatr. 2013;167:355-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
|---|
| 52. | Arakawa M, Mita T, Azuma K, Ebato C, Goto H, Nomiyama T, Fujitani Y, Hirose T, Kawamori R, Watada H. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes. 2010;59:1030-1037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 382] [Cited by in F6Publishing: 395] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
|---|
| 61. | Hirata Y, Kurobe H, Nishio C, Tanaka K, Fukuda D, Uematsu E, Nishimoto S, Soeki T, Harada N, Sakaue H. Exendin-4, a glucagon-like peptide-1 receptor agonist, attenuates neointimal hyperplasia after vascular injury. Eur J Pharmacol. 2013;699:106-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
|---|
| 62. | Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745-E751. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 66. | Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494-1505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1843] [Cited by in F6Publishing: 1959] [Article Influence: 108.8] [Reference Citation Analysis (0)] |
|---|
| 74. | Rodríguez-Calvo R, Serrano L, Coll T, Moullan N, Sánchez RM, Merlos M, Palomer X, Laguna JC, Michalik L, Wahli W. Activation of peroxisome proliferator-activated receptor beta/delta inhibits lipopolysaccharide-induced cytokine production in adipocytes by lowering nuclear factor-kappaB activity via extracellular signal-related kinase 1/2. Diabetes. 2008;57:2149-2157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
|---|
| 80. | Angelico F, Del Ben M, Conti R, Francioso S, Feole K, Fiorello S, Cavallo MG, Zalunardo B, Lirussi F, Alessandri C. Insulin resistance, the metabolic syndrome, and nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2005;90:1578-1582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 191] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
|---|
| 84. | Bugianesi E, Pagotto U, Manini R, Vanni E, Gastaldelli A, de Iasio R, Gentilcore E, Natale S, Cassader M, Rizzetto M. Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity. J Clin Endocrinol Metab. 2005;90:3498-3504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 308] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
|---|