Pressure Injuries (Pressure Ulcers) and Wound Care: Practice Essentials, Background, Anatomy (original) (raw)
Practice Essentials
Although the terms decubitus ulcer, pressure sore, and pressure ulcer have often been used interchangeably, the National Pressure Injury Advisory Panel (NPIAP; formerly the National Pressure Ulcer Advisory Panel [NPUAP]) has stated that pressure injury the best term to use, given that open ulceration does not always occur. [1] According to the NPIAP, a pressure injury is localized damage to the skin and underlying soft tissue, usually over a bony prominence or related to a medical or other device. It can present as intact skin or an open ulcer and may be painful. It occurs as a result of intense or prolonged pressure or pressure in combination with shear.
See the image below.
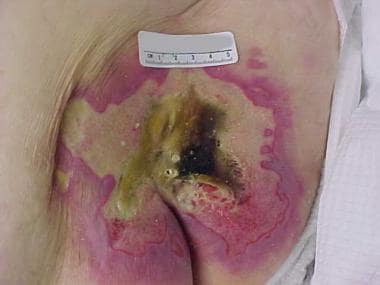
Advanced sacral pressure ulcer shows effects of pressure, shearing, and moisture.
Signs and symptoms
The following important information should be obtained from the history:
- Overall physical and mental health, including life expectancy
- Previous hospitalizations, operations, or ulcerations
- Diet and recent weight changes
- Bowel habits and continence status
- Presence of spasticity or flexion contractures
- Medications and allergies to medications
- Tobacco, alcohol, and recreational drug use
- Place of residence and the support surface used in bed or while sitting
- level of independence, mobility, and ability to comprehend and cooperate with care
- Underlying social and financial support structure
- Presence of specific cultural, religious, or ethnic issues
- Presence of advanced directives, power of attorney, or specific preferences regarding care
- Information related to the current ulceration - Pain, foul odor or discharge, natural history of the present ulcer, and associated medical cause of the ulcer
A thorough physical examination is necessary to evaluate the patient’s overall state of health, comorbidities, nutritional status, and mental status. After the general physical examination, attention should be turned to the wound.
For the purposes of workup and treatment, it is helpful to stage the pressure injury according to the system promulgated by the NPIAP, [2] as follows:
- Stage 1 pressure injury - Nonblanchable erythema of intact skin
- Stage 2 pressure injury - Partial-thickness skin loss with exposed dermis
- Stage 3 pressure injury - Full-thickness skin loss
- Stage 4 pressure injury - Full-thickness skin and tissue loss
- Unstageable pressure injury - Obscured full-thickness skin and tissue loss
- Deep pressure injury - Persistent nonblanchable deep red, maroon or purple discoloration
Complications of ulceration include the following:
- Malignant transformation
- Autonomic dysreflexia
- Osteomyelitis
- Pyarthrosis
- Sepsis
- Urethral fistula
- Amyloidosis
See Presentation for more detail.
Diagnosis
Laboratory studies that may be helpful include the following:
- Complete blood count (CBC) with differential
- Erythrocyte sedimentation rate (ESR)
- Albumin and prealbumin
- Transferrin
- Serum protein
When indicated by the specific clinical situation, the following should be obtained:
- Urinalysis and culture in the presence of urinary incontinence
- Stool examination for fecal WBCs and Clostridium difficile toxin when pseudomembranous colitis may be the cause of fecal incontinence
- Blood cultures if bacteremia or sepsis is suggested
Additional studies that may be considered include the following:
- Plain radiography
- Bone scan
- Magnetic resonance imaging
- Tissue or bone biopsy
See Workup for more detail.
Management
General principles of wound assessment and treatment are as follows:
- Wound care may be broadly divided into nonoperative and operative methods
- For stage 1 and 2 pressure injuries, wound care is usually conservative (ie, nonoperative)
- For stage 3 and 4 lesions, surgical intervention (eg, flap reconstruction) may be required, though some of these lesions must be treated conservatively because of coexisting medical problems
- Approximately 70%-90% of pressure injuries are superficial and heal by second intention
Successful medical management of pressure injuries relies on the following key principles:
- Reduction of pressure
- Adequate débridement of necrotic and devitalized tissue
- Control of infection
- Meticulous wound care
If surgical reconstruction of a pressure injury is indicated, medical status must be optimized before reconstruction is attempted. General measures for optimizing medical status include the following:
- Control of spasticity
- Nutritional support as appropriate
- Cessation of smoking
- Adequate pain control
- Maintenance of adequate blood volume
- Correction of anemia
- Maintenance of the cleanliness of the wound and surrounding intact skin
- Management of urinary or fecal incontinence as appropriate
- Management of bacterial contamination or infection
Additional nonsurgical treatment measures include the following:
- Pressure reduction - Repositioning and use of support surfaces
- Wound management - Débridement, cleansing agents, dressings, and antimicrobials
- Newer approaches still being studied - Growth factors (eg, becaplermin), negative-pressure wound therapy, and electrotherapy
Surgical interventions that may be warranted include the following:
- Surgical débridement
- Diversion of the urinary or fecal stream
- Release of flexion contractures
- Wound closure
- Amputation
Options available for surgical management of pressure injuries are as follows:
- Direct closure (rarely usable for pressure injuries being considered for surgical treatment)
- Skin grafts
- Skin flaps
- Myocutaneous (musculocutaneous) flaps
- Free flaps
The choice of reconstruction approach depends on the location of the pressure injury (eg, ischial, sacral, or trochanteric).
Prevention, if achievable, is optimal. Prevention of pressure injuries has two main components:
- Identification of patients at risk
- Interventions designed to reduce the risk
See Treatment and Medication for more detail.

Background
The terms decubitus ulcer (from Latin decumbere, “to lie down”), pressure sore, and pressure ulcer have often been used interchangeably in the medical community. However, as the name suggests, decubitus ulcer occurs at sites overlying bony structures that are prominent when a person is recumbent. Hence, it is not an accurate term for ulcers occurring in other positions, such as prolonged sitting (eg, ischial tuberosity ulcer). Because the common denominator of all such ulcerations is pressure, pressure ulcer came to be considered the best term to use.
The National Pressure Ulcer Advisory Panel (NPUAP) was an independent nonprofit organization formed in 1987 and dedicated to the prevention, management, treatment, and research of pressure ulcers. In April 2016, the NPUAP announced that it was changing its preferred terminology from pressure ulcer to pressure injury, on the grounds that the latter term better described this injury process in both intact and ulcerated skin. [1] In November 2019, the NPUAP changed its name to the National Pressure Injury Advisory Panel (NPIAP).
Currently, the NPIAP defines a pressure injury as localized damage to the skin and underlying soft tissue, usually over a bony prominence or related to a medical or other device. [1] Such injury can present either as intact skin or an open ulcer and may be painful. It results from intense or prolonged pressure or pressure combined with shear. The NPIAP also notes that the tolerance of soft tissue for pressure and shear may be affected by microclimate, nutrition, perfusion, comorbid conditions, and the condition of the soft tissue.
Pressure is exerted on the skin, soft tissue, muscle, and bone by the weight of an individual against a surface beneath. These pressures often exceed capillary filling pressure (~32 mm Hg). In patients with normal sensitivity, mobility, and mental faculty, pressure injuries do not occur. Feedback, conscious and unconscious, from the areas of compression leads them to change their body position, and these changes shift the pressure before any irreversible tissue damage develops. (See Pathophysiology and Etiology.)
Those who cannot avoid long-term uninterrupted pressure over bony prominences (eg, persons who are elderly, have neurologic impairment, or are undergoing acute hospitalization) are at increased risk for pressure injuries. They cannot protect themselves from the pressure unless they consciously change position or are helped to do so. Even a highly conscientious patient with an extensive support group and unlimited financial resources may develop such injuries as a result of a brief lapse in avoidance of the ill effects of pressure. [3, 4]
Addressing the overall management of pressure injuries is now a prominent national healthcare issue. Despite current interest and advances in medicine, surgery, nursing care, and self-care education, pressure injuries remain a major cause of morbidity and mortality, and patients with pressure injuries are important users of medical resources. [5, 6]
Many factors are involved in the management of pressure injuries. Nursing plays a pivotal role in this challenging and complex process, using a multifaceted approach that includes skin care, pressure relief, and nutritional support. Prevention is the key to managing pressure injuries, and it begins with a complete medical and nursing history, a risk assessment, and skin examination when the patient is admitted. (See Treatment.)
Factors that subject the tissue at risk to potential skin breakdown should receive particular attention. Patients should be kept clean and dry and should be repositioned frequently. For patients at risk, adequate pressure relief must be provided, along with adequate nutritional support.
For patients who develop pressure injuries, these preventive measures must be used in conjunction with the techniques of general wound care. Nonoperative wound care may involve simple topical therapy, as for pressure injuries with unbroken skin or superficial lesions with nondraining, noninfected granulation tissue. For draining necrotic or infected lesions, treatment also may include absorption agents, calcium alginate dressings, wound coverings, debridement, and antimicrobial therapy.
Other therapeutic modalities, such as whirlpool, physical therapy, and specialty beds, may also be added to the treatment regimen.
Research in the area of pressure injuries—specifically, in the characterization, prevention, and treatment of these lesions—is important for preventing secondary complications in persons with disabilities. As the standards of acute, posttraumatic, and rehabilitation care improve, the population of persons with lifelong functional impairments continues to grow. Consequently, the prevention of secondary complications has become an increasingly prominent concern.
To date, clinical studies of pressure injuries have been difficult to assess because they have often been qualitatively based on random observation and uncontrolled studies. To arrive at more reliable conclusions, more fundamental approaches to these injuries must be considered. Questions that might be asked include the following:
- What are the basic histologic, pathologic, and biochemical markers in an evolving pressure injury?
- Is it ethical to take a biopsy specimen of a human pressure injury for purposes of research?
- What are the multiple variables in the formation of pressure injuries in the human environment?
A monograph prepared by the Research Committee of the NPUAP (now the NPIAP) suggested the following research priorities [7] :
- Outcome-focused research
- Intervention and product efficacy studies
- Basic research related to staging of ulcers
- Refinement of risk assessment methods
- Risk-based, multi-interventional trials
Additional issues requiring investigation included cost issues, ethical decision making, guideline dissemination, public policy, and national outcome evaluations. Methodologic issues, such as research design, study population, and control group use, also were considered to warrant further investigation.

Anatomy
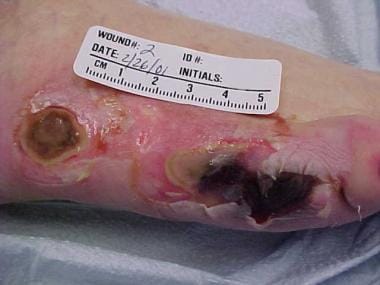
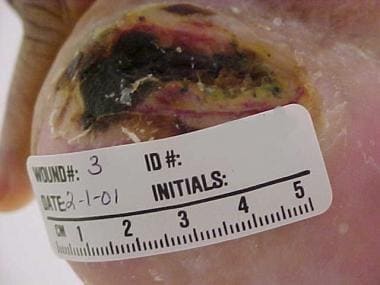
Pressure injuries are typically described in terms of location and depth of involvement. The hip and buttock regions account for up to 70% of all pressure injuries, with ischial tuberosity, trochanteric, and sacral locations being most common. [8] The lower extremities account for an additional 15-25% of all pressure injuries, with malleolar, heel, patellar, and pretibial locations being most common (see the images below).
Pressure ulcers of lateral aspect of right foot.
Heel pressure ulcer.
The remaining small percentage of pressure injuries may occur in any location that experiences long periods of uninterrupted pressure. [8] The nose, chin, forehead, occiput, chest, back, and elbow are among the more common of the infrequent sites for pressure injuries. No surface of the body can be considered immune to the effects of pressure.
Pressure injuriescan involve different levels of tissue. Muscle has been proved to be most susceptible to pressure. However, Daniel and Faibisoff found that muscle rarely was interposed between bone and skin in normal weightbearing positions in cadaver and clinical dissections. [9]

Pathophysiology
In 1873, Sir James Paget described the production of pressure ulcers remarkably well, and his description is still quite accurate today. Many factors contribute to the development of pressure injuries, but pressure leading to ischemia and necrosis is the final common pathway.
In this view, pressure injuries result from constant pressure sufficient to impair local blood flow to soft tissue for an extended period. This external pressure must be greater than the arterial capillary pressure (32 mm Hg) to impair inflow and greater than the venous capillary closing pressure (8-12 mm Hg) to impede the return of flow for an extended time.
Tissues are capable withstanding enormous pressures for brief periods, but prolonged exposure to pressures just slightly above capillary filling pressure initiates a downward spiral toward tissue necrosis and ulceration. [10, 11] The inciting event is compression of the tissues against an external object such as a mattress, wheelchair pad, bed rail, or other surface.
Lindan et al documented ranges of pressure applied to various anatomic points in certain positions. [12] The points of highest pressure with the patient supine included the sacrum, heel, and occiput (40-60 mm Hg). With the patient prone, the chest and knees absorbed the highest pressure (50 mm Hg). When the patient is sitting, the ischial tuberosities were under the most pressure (100 mm Hg). Obviously, these pressures are greater than the end capillary pressure, which is why these are the areas where pressure injuries are most common.
Shear forces and friction aggravate the effects of pressure and are important components of the mechanism of injury (see the image below). Maceration may occur in a patient who has incontinence, predisposing the skin to injury. Pressure, shear forces, and friction cause microcirculatory occlusion and consequent ischemia, which leads to inflammation and tissue anoxia. Tissue anoxia leads to cell death, necrosis, and ulceration.
Advanced sacral pressure ulcer shows effects of pressure, shearing, and moisture.
Of the various tissues at risk for death due to pressure, muscle tissue is damaged first, before skin and subcutaneous tissue, probably because of its increased need for oxygen and higher metabolic requirements. Irreversible changes may occur during as little as 2 hours of uninterrupted pressure. Skin can withstand ischemia from direct pressure for up to 12 hours. By the time ulceration is present through the skin level, significant damage of underlying muscle may already have occurred, making the overall shape of the ulcer an inverted cone.
Reperfusion has been suggested as a cause of additional damage to the ulcerated area, inducing an ulcer to enlarge or become more chronic—as, for example, when a paraplegic or quadriplegic patient is turned from one side to the other in an attempt to combat prolonged pressure on a given side. The exact mechanism of ischemia-reperfusion injury is yet to be fully understood. Continued production of inflammatory mediators and reactive oxygen species during ischemia-reperfusion may contribute to the chronicity of pressure ulcers.

Etiology
Impaired mobility is probably the most common reason why patients are exposed to the prolonged uninterrupted pressure that causes pressure injuries. This situation may be present in patients who are neurologically impaired, heavily sedated or anesthetized, restrained, demented, or recovering from a traumatic injury. These patients cannot alter their position far enough or often enough to relieve the pressure. Prolonged immobility may lead to muscle and soft tissue atrophy, decreasing the bulk over which bony prominences are supported.
Contractures and spasticity often contribute to ulcer formation by repeatedly exposing tissues to trauma through flexion of a joint. Contractures rigidly hold a joint in flexion, whereas spasticity subjects tissues to repeated friction and shear forces. Skin breakdown and pressure injuries may frequently be found under and between toes and on the palm of the hand.
Inability to perceive pain, whether from neurologic impairment or from medication, contributes to pressure injuries by removing one of the most important stimuli for repositioning and pressure relief. Conversely, pain from surgical incisions, fracture sites, or other sources may make the patient unwilling or unable to change position.
The quality of the skin also influences whether pressure leads to ulceration. Paralysis, insensibility, and aging lead to atrophy of the skin with thinning of this protective barrier. A decrease in epidermal turnover, a flattening of the dermal-epidermal junction, and a loss of vascularity occur with advanced age.
In addition, the skin becomes more susceptible to minor traumatic forces, such as the friction and shear forces typically exerted during the moving of a patient. Trauma that causes deepithelialization or skin tears removes the barrier to bacterial contamination and leads to transdermal water loss, creating maceration and causing the skin to adhere to clothing and bedding.
Incontinence or the presence of a fistula contributes to ulceration in several ways. These conditions cause the skin to be continually moist, thus leading to maceration. In addition, frequent soiling has the effect of regularly introducing bacteria into an open wound.
Bacterial contamination, though not truly an etiologic factor, must be considered in the treatment of pressure injuries, in that it can delay or prevent wound healing. These lesions are warm, moist reservoirs for bacterial overgrowth, where antibiotic resistance may develop. A pressure injury may progress from simple contamination (as in any open wound) to gross infection (indicating bacterial tissue invasion). This may lead to uncommon but life-threatening complications (eg, bacteremia, sepsis, myonecrosis, gangrene, or necrotizing fasciitis).
Malnutrition, hypoproteinemia, and anemia reflect the overall status of the patient and can contribute to tissue vulnerability to trauma as well as cause delayed wound healing. Poor nutritional status certainly contributes to the chronicity often seen in these lesions and inhibits the ability of the immune system to prevent infections. Anemia indicates poor oxygen-carrying capacity of the blood. Vascular disease and hypovolemia also may impair blood flow to the region of ulceration.
In patients with normal sensitivity, mobility, and mental faculty, pressure injuries are unlikely. Conscious or unconscious feedback from the areas of compression leads them to change position, thereby shifting the pressure from one area to another long before any irreversible ischemic damage occurs. In individuals who cannot avoid long periods of uninterrupted pressure, the risk of necrosis and ulceration is increased. These individuals cannot protect themselves from the pressure unless they consciously change position or are helped to do so.

Epidemiology
United States statistics
Pressure injuries are common among patients hospitalized in acute- and chronic-care facilities. It has been estimated that about 1 million pressure injuries occur in the United States; however, definitive information on the epidemiology and natural history of this condition is still limited. Unfortunately, studies to date have been encumbered by methodologic issues and variability in describing the lesions. [7]
Reported incidences of pressure injuries in hospitalized patients range from 2.7% to 29%, and reported prevalences in hospitalized patients range from 3.5% to 69%. [13, 14, 15, 16, 17] Patients in critical care units have an increased risk of pressure injuries, as evidenced by a 33% incidence and a 41% prevalence. [18, 19]
The fifth National Pressure Ulcer Prevalence Survey, conducted in 1999 among patients in acute care hospitals, showed an overall prevalence of 14.8%, with 7.1% of ulcers having occurred during that hospital visit. [20] Of the various hospital settings, intensive care units (ICUs) had the highest prevalence, at 21.5%. The largest single age group of patients with pressure injuries consisted of patients aged 71-80 years (29%).
Elderly patients admitted to acute care hospitals for nonelective orthopedic procedures are at even greater risk for pressure injuries than other hospitalized patients are, with a 66% incidence. [21, 22] In a study of 658 patients aged 65 years or older who underwent surgery for hip fracture, Baumgarten et al found that 36.1% developed an acquired pressure injury within 32 days after hospital admission. [23]
In nursing homes, the prevalence of pressure injuries is 2.6-24% [24] ; the incidence is 25% in residents admitted from an acute care hospital. [24] Patients with preexisting pressure injuries show a 26% incidence of additional pressure injury formation over a 6-month period. The incidence in chronic care hospitals is reported to be 10.8%, [25] whereas 33% of those admitted to a chronic care hospital have pressure injuries. [26] Long-term follow-up demonstrates that most ulcers heal within 1 year. [27]
Among patients with neurologic impairments, pressure injuries occur with an incidence of 7-8% annually, [28] with a lifetime risk estimated to be 25-85%. [29] Moreover, pressure injuries are listed as the direct cause of death in 7-8% of all individuals with paraplegia; these individuals also have the highest recurrence rate (80%). [30] In persons with spinal cord injury (SCI) and associated comorbidity, the incidence of pressure injuries is in the range of 25-66%. [31, 32, 33, 34]
A study of the prevalence of pressure injuries in community residents with SCI demonstrated that those with higher-level SCI lesions carry a greater risk of developing pressure injuries than those with lower-level lesions do. [31] Of 100 patients with pressure injuries, 33 had injuries that were classified as stage 2 or greater. Black patients had more severe injuries than other racial groups did.
Some authors have speculated that detecting erythema can be more difficult with skin that has darker pigmentation. [35] Because prolonged nonblanching erythema is typically an early warning sign of pressure injury risk and development, difficulty in detecting erythema can result in failure to recognize grade I pressure injuries.
International statistics
In a study from Germany that reviewed the prevalence of pressure injuries in more than 18,000 patients residing in long-term care facilities, the prevalence was found to have decreased from 12.5% in 2002 to 5% in 2008. [36] This decrease is thought to be due to more effective management strategies and better prevention.
Age-related demographics
The prevalence of pressure injuries appears to have a bimodal age distribution. A small peak occurs during the third decade of life, reflecting ulceration in those with traumatic neurologic injury. Immobility and lack of sensation make these patients susceptible to developing pressure injuries. Treatment of these lesions in this patient population represents a financial challenge, with one hospital reporting an average cost of $78,000 for each admission of a patient with a pressure injury.
As patients move from the age category of 40-58 years to the age category of 75 years or older, a larger increase in the incidence of pressure injuries occurs. [37] Two thirds of pressure injuries occur in patients older than 70 years. [25] As elderly individuals become the fastest-growing segment of the population, with an estimated 1.5 million people living in extended-care facilities, the problem of pressure injuries will have an even more profound influence on the American economy.
Sex-related demographics
Most younger individuals suffering from pressure injuries are males. The higher incidence in males reflects the greater number of men suffering traumatic SCIs. In the older population, most patients with pressure injuries are women, as a consequence of their survival advantage over men.
Race-related demographics
A study by Howard and Taylor found the incidence of pressure injuries in nursing home residents in the southeastern United States to be higher in Black patients than in White ones. [38] The authors examined data from 113,869 nursing home residents, none of whom had pressure injuries at nursing home admission. They determined that 4.7% of Black residents developed postadmission ulcerations, compared with 3.4% of White residents.
In addition, the racial differences in pressure injury incidence displayed a sex predilection based on patient characteristics. [38] The variation in incidence between Black and White males was noted in residents who were dependent in mobility, whereas the difference in incidence between Black and White females was noted in residents who were bedfast and living in nursing homes with fewer than 200 beds.

Prognosis
Pressure injuries are listed as the direct cause of death in 7-8% of all patients with paraplegia. [39, 28] As many as one third of hospitalized patients with pressure injuries die during their hospitalization. More than half of those who develop a pressure injury in the hospital will die within the next 12 months. As a rule, these patients die of their primary disease process rather than of pressure ulceration, but the pressure injury may be a contributing factor in some instances.
Each year, approximately 60,000 people die of complications of pressure injuries. [40] Individuals with pressure ulcers have a 4.5 times greater risk of death than persons with the same risk factors but without pressure injuries. A secondary complication, wound-related bacteremia, can increase the risk of mortality to 55%. [40, 41, 42, 43]
The most common causes of fatality for patients with chronic pressure injuries are renal failure and amyloidosis. In general, mortality is higher for patients who develop a new pressure injury and in whom the injury fails to heal.
Infection is the most common major complication of pressure injuries. The offending pathologic organisms can be either anaerobic or aerobic. Aerobic pathogens commonly are present in all pressure injuries, whereas anaerobes tend to be present more often in larger wounds (65% in grade 3 and above). [44]
The organisms most commonly isolated from pressure injuries are as follows:
- Proteus mirabilis
- Group D streptococci
- Escherichia coli
- Staphylococcus
- Pseudomonas
- Corynebacterium
Patients with bacteremia are more likely to have Bacteroides species in their pressure injuries. [44] These wounds need not be cultured routinely unless systemic signs of infection are present (eg, malodorous drainage, leukocytosis, fever, hypotension, increased heart rate, changes in mental status).
Clinical alertness is vital because the signs commonly associated with impeding or fulminating infection are frequently absent in elderly or immunocompromised patients. In geriatric patients with pressure injuries, bacteremia is reported to occur at a rate of 3.5 per 10,000 hospital discharges.
In view of the high mortality in this population (nearly 50%), [42] it is important that antibiotic treatment of wound infection or secondary bacteremia provide the appropriate spectrum of coverage specific to the offending organisms. Because indiscriminate use of antibiotics leads to resistant organisms and because the specific drugs of choice and antimicrobial agents change rapidly, management of these complex problems may be facilitated by consulting an infectious disease specialist.
Sepsis also can occur secondary to osteomyelitis, which has been reported to occur in 26% of nonhealing ulcers. A prospective study demonstrated that osteomyelitis was associated with nonhealing grade 4 pressure injuries in 86% of the study population. [45] This study utilized three-phase technetium methyl diphosphate radionuclide flow to detect early osteomyelitis.
Various tests can be used to diagnose osteomyelitis in patients with pressure injuries. Plain radiographs have a sensitivity of 78% and a specificity of 50%, but radiographic findings often are not present in the early stages of infection. Bone scans are more sensitive, but their specificity is low (50%). Bone biopsy has the highest specificity (96%) and sensitivity (73%). [45]
A combination of diagnostic tests (eg, white blood cell [WBC] count, erythrocyte sedimentation rate [ESR], and plain radiography) provides a sensitivity of 89% and a specificity of 88%. If all three test results are positive, the positive predictive value of this combination is 69%. If all three test results are negative, the negative predictive value is 96%. [45]
Osteomyelitis should be considered whenever an ulcer does not heal, especially if the ulcer is over a bony prominence. Clinicians also should rule out other conditions associated with nonhealing ulcers, such as heterotopic calcification or ossification. Most findings indicate that antibiotic treatment for osteomyelitis should last 6-8 weeks. Surgery is needed for some cases of chronic osteomyelitis.
Systemic amyloidosis can result from chronic suppurative pressure injuries. Additional complications of pressure injuries include spreading cellulitis, a sinus tract abscess, septic arthritis, squamous cell carcinoma in the ulcer, a periurethral fistula, and heterotopic ossification. Because some of the secondary complications of pressure injuries can preclude wound healing, they should be aggressively prevented and treated. Complications may include infection, pain, depression, and even death.

Patient Education
Patients and their support system must realize that it is their responsibility to avoid recurrent and new ulceration and that this is a lifelong process. Education on the proper avoidance of pressure should begin in the hospital and continue into the home.
For patient education resources, see the Skin, Hair, and Nails Center and Diabetes Center, as well as Wound Care and Diabetic Foot Care.

- Edsberg LE, Black JM, Goldberg M, McNichol L, Moore L, Sieggreen M. Revised National Pressure Ulcer Advisory Panel Pressure Injury Staging System: Revised Pressure Injury Staging System. J Wound Ostomy Continence Nurs. 2016 Nov/Dec. 43 (6):585-597. [QxMD MEDLINE Link]. [Full Text].
- NPIAP pressure injury stages. National Pressure Injury Advisory Panel. Available at https://cdn.ymaws.com/npiap.com/resource/resmgr/online_store/npiap_pressure_injury_stages.pdf. Accessed: January 31, 2024.
- Schweinberger MH, Roukis TS. Effectiveness of instituting a specific bed protocol in reducing complications associated with bed rest. J Foot Ankle Surg. 2010 Jul-Aug. 49(4):340-7. [QxMD MEDLINE Link].
- Zhao G, Hiltabidel E, Liu Y, Chen L, Liao Y. A cross-sectional descriptive study of pressure ulcer prevalence in a teaching hospital in China. Ostomy Wound Manage. 2010 Feb 1. 56(2):38-42. [QxMD MEDLINE Link].
- Pham B, Stern A, Chen W, Sander B, John-Baptiste A, Thein HH, et al. Preventing pressure ulcers in long-term care: a cost-effectiveness analysis. Arch Intern Med. 2011 Nov 14. 171(20):1839-47. [QxMD MEDLINE Link].
- Pham B, Teague L, Mahoney J, Goodman L, Paulden M, Poss J, et al. Early prevention of pressure ulcers among elderly patients admitted through emergency departments: a cost-effectiveness analysis. Ann Emerg Med. 2011 Nov. 58(5):468-78.e3. [QxMD MEDLINE Link].
- Pieper B, ed. Pressure Ulcers: Prevalence, Incidence, and Implications for the Future. Washington, DC: National Pressure Ulcer Advisory Panel; 2013.
- Leblebici B, Turhan N, Adam M, Akman MN. Clinical and epidemiologic evaluation of pressure ulcers in patients at a university hospital in Turkey. J Wound Ostomy Continence Nurs. 2007 Jul-Aug. 34(4):407-11. [QxMD MEDLINE Link].
- Daniel RK, Faibisoff B. Muscle coverage of pressure points--the role of myocutaneous flaps. Ann Plast Surg. 1982 Jun. 8(6):446-52. [QxMD MEDLINE Link].
- Gefen A. Reswick and Rogers pressure-time curve for pressure ulcer risk. Part 1. Nurs Stand. 2009 Jul 15-21. 23(45):64, 66, 68 passim. [QxMD MEDLINE Link].
- Gefen A. Reswick and Rogers pressure-time curve for pressure ulcer risk. Part 2. Nurs Stand. 2009 Jul 22-28. 23(46):40-4. [QxMD MEDLINE Link].
- LINDAN O, GREENWAY RM, PIAZZA JM. PRESSURE DISTRIBUTION ON THE SURFACE OF THE HUMAN BODY. I. EVALUATION IN LYING AND SITTING POSITIONS USING A "BED OF SPRINGS AND NAILS". Arch Phys Med Rehabil. 1965 May. 46:378-85. [QxMD MEDLINE Link].
- Fogerty M, Guy J, Barbul A, Nanney LB, Abumrad NN. African Americans show increased risk for pressure ulcers: a retrospective analysis of acute care hospitals in America. Wound Repair Regen. 2009 Sep-Oct. 17(5):678-84. [QxMD MEDLINE Link].
- Manley MT. Incidence, contributory factors and costs of pressure sores. S Afr Med J. 1978 Feb 11. 53(6):217-22. [QxMD MEDLINE Link].
- Gerson LW. The incidence of pressure sores in active treatment hospitals. Int J Nurs Stud. 1975. 12(4):201-4. [QxMD MEDLINE Link].
- Shannon ML, Skorga P. Pressure ulcer prevalence in two general hospitals. Decubitus. 1989 Nov. 2(4):38-43. [QxMD MEDLINE Link].
- Meehan M. Multisite pressure ulcer prevalence survey. Decubitus. 1990 Nov. 3(4):14-7. [QxMD MEDLINE Link].
- Bergstrom N, Demuth PJ, Braden BJ. A clinical trial of the Braden Scale for Predicting Pressure Sore Risk. Nurs Clin North Am. 1987 Jun. 22 (2):417-28. [QxMD MEDLINE Link].
- Robnett MK. The incidence of skin breakdown in a surgical intensive care unit. J Nurs Qual Assur. 1986 Nov. 1(1):77-81. [QxMD MEDLINE Link].
- Amlung SR, Miller WL, Bosley LM. The 1999 National Pressure Ulcer Prevalence Survey: a benchmarking approach. Adv Skin Wound Care. 2001 Nov-Dec. 14 (6):297-301. [QxMD MEDLINE Link].
- Roberts BV, Goldstone LA. A survey of pressure sores in the over sixties on two orthopaedic wards. Int J Nurs Stud. 1979. 16(4):355-64. [QxMD MEDLINE Link].
- Versluysen M. How elderly patients with femoral fracture develop pressure sores in hospital. Br Med J (Clin Res Ed). 1986 May 17. 292(6531):1311-3. [QxMD MEDLINE Link]. [Full Text].
- Baumgarten M, Margolis DJ, Orwig DL, Shardell MD, Hawkes WG, Langenberg P, et al. Pressure ulcers in elderly patients with hip fracture across the continuum of care. J Am Geriatr Soc. 2009 May. 57(5):863-70. [QxMD MEDLINE Link]. [Full Text].
- Reed JW. Pressure ulcers in the elderly: prevention and treatment utilizing the team approach. Md State Med J. 1981 Nov. 30(11):45-50. [QxMD MEDLINE Link].
- Barbenel JC, Jordan MM, Nicol SM, Clark MO. Incidence of pressure-sores in the Greater Glasgow Health Board area. Lancet. 1977 Sep 10. 2(8037):548-50. [QxMD MEDLINE Link].
- Schols JM, Heyman H, Meijer EP. Nutritional support in the treatment and prevention of pressure ulcers: an overview of studies with an arginine enriched oral nutritional supplement. J Tissue Viability. 2009 Aug. 18(3):72-9. [QxMD MEDLINE Link].
- Berlowitz DR, Wilking SV. Risk factors for pressure sores. A comparison of cross-sectional and cohort-derived data. J Am Geriatr Soc. 1989 Nov. 37(11):1043-50. [QxMD MEDLINE Link].
- Kenkel JM. Pressure Sores (overview). Kenkel JM. Selected Read Plast Surg. Texas: Baylor University Medical Center; 1998. Vol 8, No 39: 1-29.
- Klitzman B, Kalinowski C, Glasofer SL, Rugani L. Pressure ulcers and pressure relief surfaces. Clin Plast Surg. 1998 Jul. 25(3):443-50. [QxMD MEDLINE Link].
- Evans GR, Dufresne CR, Manson PN. Surgical correction of pressure ulcers in an urban center: is it efficacious?. Adv Wound Care. 1994 Jan. 7 (1):40-6. [QxMD MEDLINE Link].
- Fuhrer MJ, Garber SL, Rintala DH, Clearman R, Hart KA. Pressure ulcers in community-resident persons with spinal cord injury: prevalence and risk factors. Arch Phys Med Rehabil. 1993 Nov. 74(11):1172-7. [QxMD MEDLINE Link].
- Basson MD, Burney RE. Defective wound healing in patients with paraplegia and quadriplegia. Surg Gynecol Obstet. 1982 Jul. 155(1):9-12. [QxMD MEDLINE Link].
- Regan MA, Teasell RW, Wolfe DL, Keast D, Mortenson WB, Aubut JA. A systematic review of therapeutic interventions for pressure ulcers after spinal cord injury. Arch Phys Med Rehabil. 2009 Feb. 90(2):213-31. [QxMD MEDLINE Link]. [Full Text].
- Okamoto GA, Lamers JV, Shurtleff DB. Skin breakdown in patients with myelomeningocele. Arch Phys Med Rehabil. 1983 Jan. 64(1):20-3. [QxMD MEDLINE Link].
- Basset A, Liautaud B, Ndiaye B. Dermatology of Black Skin. Oxford: Oxford University Press; 1986.
- Lahmann NA, Dassen T, Poehler A, Kottner J. Pressure ulcer prevalence rates from 2002 to 2008 in German long-term care facilities. Aging Clin Exp Res. 2010 Apr. 22(2):152-6. [QxMD MEDLINE Link].
- Fogerty MD, Abumrad NN, Nanney L, Arbogast PG, Poulose B, Barbul A. Risk factors for pressure ulcers in acute care hospitals. Wound Repair Regen. 2008 Jan-Feb. 16(1):11-8. [QxMD MEDLINE Link].
- Howard DL, Taylor YJ. Racial and gender differences in pressure ulcer development among nursing home residents in the Southeastern United States. J Women Aging. 2009. 21(4):266-78. [QxMD MEDLINE Link].
- Dinsdale SM. Decubitus ulcers: role of pressure and friction in causation. Arch Phys Med Rehabil. 1974 Apr. 55(4):147-52. [QxMD MEDLINE Link].
- Allman RM. Pressure ulcers among the elderly. N Engl J Med. 1989 Mar 30. 320(13):850-3. [QxMD MEDLINE Link].
- Allman RM, Walker JM, Hart MK, Laprade CA, Noel LB, Smith CR. Air-fluidized beds or conventional therapy for pressure sores. A randomized trial. Ann Intern Med. 1987 Nov. 107(5):641-8. [QxMD MEDLINE Link].
- Maklebust J. Pressure ulcers: etiology and prevention. Nurs Clin North Am. 1987 Jun. 22(2):359-77. [QxMD MEDLINE Link].
- Doughty D. The process of wound healing: a nursing perspective. Prog Develop Ostomy Wound Care. 1990. 2:3-12.
- Peromet M, Labbe M, Yourassowsky E, Schoutens E. Anaerobic bacteria isolated from decubitus ulcers. Infection. 1973. 1(4):205-7. [QxMD MEDLINE Link].
- Deloach ED, DiBenedetto RJ, Womble L, Gilley JD. The treatment of osteomyelitis underlying pressure ulcers. Decubitus. 1992 Nov. 5(6):32-41. [QxMD MEDLINE Link].
- Stausberg J, Kiefer E. Classification of pressure ulcers: a systematic literature review. Stud Health Technol Inform. 2009. 146:511-5. [QxMD MEDLINE Link].
- Black J, Baharestani M, Cuddigan J, Dorner B, Edsberg L, Langemo D, et al. National Pressure Ulcer Advisory Panel's updated pressure ulcer staging system. Dermatol Nurs. 2007 Aug. 19(4):343-9; quiz 350. [QxMD MEDLINE Link].
- Copcu E. Marjolin's ulcer: a preventable complication of burns?. Plast Reconstr Surg. 2009 Jul. 124(1):156e-64e. [QxMD MEDLINE Link].
- James GA, Swogger E, Wolcott R, Pulcini Ed, Secor P, Sestrich J, et al. Biofilms in chronic wounds. Wound Repair Regen. 2008 Jan-Feb. 16(1):37-44. [QxMD MEDLINE Link].
- Kirker KR, Secor PR, James GA, Fleckman P, Olerud JE, Stewart PS. Loss of viability and induction of apoptosis in human keratinocytes exposed to Staphylococcus aureus biofilms in vitro. Wound Repair Regen. 2009 Sep-Oct. 17(5):690-9. [QxMD MEDLINE Link]. [Full Text].
- Schierle CF, De la Garza M, Mustoe TA, Galiano RD. Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair Regen. 2009 May-Jun. 17(3):354-9. [QxMD MEDLINE Link].
- Rhoads DD, Wolcott RD, Percival SL. Biofilms in wounds: management strategies. J Wound Care. 2008 Nov. 17 (11):502-8. [QxMD MEDLINE Link].
- Woolsey RM, McGarry JD. The cause, prevention, and treatment of pressure sores. Neurol Clin. 1991 Aug. 9 (3):797-808. [QxMD MEDLINE Link].
- Gorecki C, Nixon J, Madill A, Firth J, Brown JM. What influences the impact of pressure ulcers on health-related quality of life? A qualitative patient-focused exploration of contributory factors. J Tissue Viability. 2012 Feb. 21(1):3-12. [QxMD MEDLINE Link].
- [Guideline] Qaseem A, Mir TP, Starkey M, Denberg TD. Risk assessment and prevention of pressure ulcers: a clinical practice guideline from the american college of physicians. Ann Intern Med. 2015 Mar 3. 162(5):359-69. [QxMD MEDLINE Link].
- [Guideline] Qaseem A, Humphrey LL, Forciea MA, Starkey M, Denberg TD. Treatment of pressure ulcers: a clinical practice guideline from the american college of physicians. Ann Intern Med. 2015 Mar 3. 162(5):370-9. [QxMD MEDLINE Link].
- Banks MD, Graves N, Bauer JD, Ash S. Cost effectiveness of nutrition support in the prevention of pressure ulcer in hospitals. Eur J Clin Nutr. 2013 Jan. 67(1):42-6. [QxMD MEDLINE Link].
- Cereda E, Gini A, Pedrolli C, Vanotti A. Disease-specific, versus standard, nutritional support for the treatment of pressure ulcers in institutionalized older adults: a randomized controlled trial. J Am Geriatr Soc. 2009 Aug. 57(8):1395-402. [QxMD MEDLINE Link].
- Benbow M, Bateman S. Working towards clinical excellence Pressure ulcer prevention and management in primary and secondary care. J Wound Care. 2012 Sep. 21(9):S25-40. [QxMD MEDLINE Link].
- Heyneman A, Vanderwee K, Grypdonck M, Defloor T. Effectiveness of two cushions in the prevention of heel pressure ulcers. Worldviews Evid Based Nurs. 2009. 6(2):114-20. [QxMD MEDLINE Link].
- McInnes E, Dumville JC, Jammali-Blasi A, Bell-Syer SE. Support surfaces for treating pressure ulcers. Cochrane Database Syst Rev. 2011 Dec 7. CD009490. [QxMD MEDLINE Link].
- Cullum N, McInnes E, Bell-Syer SE, Legood R. Support surfaces for pressure ulcer prevention. Cochrane Database Syst Rev. 2004. CD001735:
- McInnes E, Jammali-Blasi A, Bell-Syer SE, Dumville JC, Cullum N. Support surfaces for pressure ulcer prevention. Cochrane Database Syst Rev. 2011 Apr 13. CD001735. [QxMD MEDLINE Link].
- McInnes E, Jammali-Blasi A, Bell-Syer SE, Dumville JC, Middleton V, Cullum N. Support surfaces for pressure ulcer prevention. Cochrane Database Syst Rev. 2015 Sep 3. CD001735. [QxMD MEDLINE Link]. [Full Text].
- Clark M, Hiskett G, Russell L. Evidence-based practice and support surfaces: are we throwing the baby out with the bath water?. J Wound Care. 2005 Nov. 14(10):455-8. [QxMD MEDLINE Link].
- Bruce TA, Shever LL, Tschannen D, Gombert J. Reliability of pressure ulcer staging: a review of literature and 1 institution's strategy. Crit Care Nurs Q. 2012 Jan-Mar. 35(1):85-101. [QxMD MEDLINE Link].
- Bergstrom N, Bennett MA, Carlson CE, et al. Treatment of Pressure Ulcers. Clinical Practice Guideline Number 14. Agency for Health Care Policy and Research, Public Health Service. Rockville, MD: US Department of Health and Human Services; 1994. AHCPR Publication No. 95-0642.:
- Ho CH, Bensitel T, Wang X, Bogie KM. Pulsatile lavage for the enhancement of pressure ulcer healing: a randomized controlled trial. Phys Ther. 2012 Jan. 92(1):38-48. [QxMD MEDLINE Link].
- Werdin F, Tennenhaus M, Schaller HE, Rennekampff HO. Evidence-based management strategies for treatment of chronic wounds. Eplasty. 2009 Jun 4. 9:e19. [QxMD MEDLINE Link]. [Full Text].
- Flemming K, Cullum N. Electromagnetic therapy for the treatment of pressure sores. Cochrane Database Syst Rev. 2001. CD002930. [QxMD MEDLINE Link].
- Landi F, Aloe L, Russo A, Cesari M, Onder G, Bonini S, et al. Topical treatment of pressure ulcers with nerve growth factor: a randomized clinical trial. Ann Intern Med. 2003 Oct 21. 139 (8):635-41. [QxMD MEDLINE Link].
- Salcido R, Carney J, Fisher S, et al. A reliable animal model of pressure sore development: The role of free radicals. J Am Paraplegia Soc. 1993. 16:61.
- Salcido R, Fisher SB, Donofrio JC, Bieschke M, Knapp C, Liang R, et al. An animal model and computer-controlled surface pressure delivery system for the production of pressure ulcers. J Rehabil Res Dev. 1995 May. 32(2):149-61. [QxMD MEDLINE Link].
- Ford CN, Reinhard ER, Yeh D, Syrek D, De Las Morenas A, Bergman SB, et al. Interim analysis of a prospective, randomized trial of vacuum-assisted closure versus the healthpoint system in the management of pressure ulcers. Ann Plast Surg. 2002 Jul. 49(1):55-61; discussion 61. [QxMD MEDLINE Link].
- Evans D, Land L. Topical negative pressure for treating chronic wounds. Cochrane Database Syst Rev. 2001. CD001898. [QxMD MEDLINE Link].
- Gupta S, Baharestani M, Baranoski S, de Leon J, Engel SJ, Mendez-Eastman S, et al. Guidelines for managing pressure ulcers with negative pressure wound therapy. Adv Skin Wound Care. 2004 Nov-Dec. 17 Suppl 2:1-16. [QxMD MEDLINE Link].
- Niezgoda JA. Combining negative pressure wound therapy with other wound management modalities. Ostomy Wound Manage. 2005 Feb. 51 (2A Suppl):36S-38S. [QxMD MEDLINE Link].
- Mendez-Eastman S. Guidelines for using negative pressure wound therapy. Adv Skin Wound Care. 2001 Nov-Dec. 14(6):314-22; quiz 324-5. [QxMD MEDLINE Link].
- He J, Xu H, Wang T, Ma S, Dong J. Treatment of complex ischial pressure sores with free partial lateral latissimus dorsi musculocutaneous flaps in paraplegic patients. J Plast Reconstr Aesthet Surg. 2012 May. 65(5):634-9. [QxMD MEDLINE Link].
- Lin H, Hou C, Chen A, Xu Z. Treatment of ischial pressure sores using a modified gracilis myofasciocutaneous flap. J Reconstr Microsurg. 2010 Apr. 26(3):153-7. [QxMD MEDLINE Link].
- Wound, Ostomy and Continence Nurses Society. Guideline for Prevention and Management of Pressure Ulcers (Injuries). Mt Laurel, NJ: Wound, Ostomy and Continence Nurses Society; 2016.
- Schmitt S, Andries MK, Ashmore PM, Brunette G, Judge K, Bonham PA. WOCN Society Position Paper: Avoidable Versus Unavoidable Pressure Ulcers/Injuries. J Wound Ostomy Continence Nurs. 2017 Sep/Oct. 44 (5):458-468. [QxMD MEDLINE Link]. [Full Text].
- Schoonhoven L, Grobbee DE, Donders AR, Algra A, Grypdonck MH, Bousema MT, et al. Prediction of pressure ulcer development in hospitalized patients: a tool for risk assessment. Qual Saf Health Care. 2006 Feb. 15 (1):65-70. [QxMD MEDLINE Link]. [Full Text].
- Walton-Geer PS. Prevention of pressure ulcers in the surgical patient. AORN J. 2009 Mar. 89(3):538-48; quiz 549-51. [QxMD MEDLINE Link].
- Saleh M, Anthony D, Parboteeah S. The impact of pressure ulcer risk assessment on patient outcomes among hospitalised patients. J Clin Nurs. 2009 Jul. 18(13):1923-9. [QxMD MEDLINE Link].
- Bergstrom N. Panel for the Prediction and Prevention of Pressure Ulcers in Adults. Prediction and Prevention Clinical Practice Guideline, Number 3. Agency for Health Care Policy and Research, Public Health Service. AHCPR Publication No. 920047. Rockville, MD: US Department of Health and Human Services; 1992.
- Milne CT, Trigilia D, Houle TL, Delong S, Rosenblum D. Reducing pressure ulcer prevalence rates in the long-term acute care setting. Ostomy Wound Manage. 2009 Apr. 55(4):50-9. [QxMD MEDLINE Link].
- Crewe R. Problems of rubber ring nursing cushions and a clinical survey of alternative cushions for ill patients. Care Sci Pract. 1987. 5:9-11.
- Bergstrom N, Braden BJ, Laguzza A, Holman V. The Braden Scale for Predicting Pressure Sore Risk. Nurs Res. 1987 Jul-Aug. 36(4):205-10. [QxMD MEDLINE Link].
- Norton D. Calculating the risk: reflections on the Norton Scale. Decubitus. 1989 Aug. 2(3):24-31. [QxMD MEDLINE Link].
- Norton D, McLaren R, Exton-Smith A. An Investigation of Geriatric Nursing Problems in Hospital. London: Churchill Livingstone; 1975.
- Watanabe S, Yamada K, Ono S, Ishibashi Y. Skin changes in patients with amyotrophic lateral sclerosis: light and electron microscopic observations. J Am Acad Dermatol. 1987 Dec. 17(6):1006-12. [QxMD MEDLINE Link].
- Conine TA, Daechsel D, Lau MS. The role of alternating air and Silicore overlays in preventing decubitus ulcers. Int J Rehabil Res. 1990. 13(1):57-65. [QxMD MEDLINE Link].
- Warner DJ. A clinical comparison of two pressure-reducing surfaces in the management of pressure ulcers. Decubitus. 1992 May. 5(3):52-5, 58-60, 62-4. [QxMD MEDLINE Link].
- Smoot EC 3rd. Clinitron bed therapy hazards. Plast Reconstr Surg. 1986 Jan. 77(1):165. [QxMD MEDLINE Link].
- Nimit K. Guidelines for home air-fluidized bed therapy, 1989. Health Technol Assess Rep. 1989. 1-11. [QxMD MEDLINE Link].
Author
Specialty Editor Board
Disclosure: Nothing to disclose.
Chief Editor
John Geibel, MD, MSc, DSc, AGAF Vice Chair and Professor, Department of Surgery, Section of Gastrointestinal Medicine, Professor, Department of Cellular and Molecular Physiology, Yale University School of Medicine; Director of Surgical Research, Department of Surgery, Yale-New Haven Hospital; American Gastroenterological Association Fellow; Fellow of the Royal Society of Medicine
John Geibel, MD, MSc, DSc, AGAF is a member of the following medical societies: American Gastroenterological Association, American Physiological Society, American Society of Nephrology, Association for Academic Surgery, International Society of Nephrology, New York Academy of Sciences, Society for Surgery of the Alimentary Tract
Disclosure: Nothing to disclose.
Acknowledgements
Kat Kolaski, MD Assistant Professor, Departments of Orthopedic Surgery and Pediatrics, Wake Forest University School of Medicine
Kat Kolaski, MD is a member of the following medical societies: American Academy for Cerebral Palsy and Developmental Medicine and American Academy of Physical Medicine and Rehabilitation
Disclosure: Nothing to disclose.
Consuelo T Lorenzo, MD Physiatrist, Department of Physical Medicine and Rehabilitation, Alegent Health Immanuel Rehabilitation Center
Consuelo T Lorenzo, MD is a member of the following medical societies: American Academy of Physical Medicine and Rehabilitation
Disclosure: Nothing to disclose.
Joseph A Molnar, MD, PhD, FACS Director, Wound Care Center, Associate Director of Burn Unit, Associate Professor, Department of Plastic and Reconstructive Surgery, Wake Forest University School of Medicine
Joseph A Molnar, MD, PhD, FACS is a member of the following medical societies: American Association of Plastic Surgeons, American Burn Association, American College of Surgeons, American Medical Association, American Society for Parenteral and Enteral Nutrition, American Society of Plastic Surgeons, North Carolina Medical Society, Peripheral Nerve Society, Undersea and Hyperbaric Medical Society, and Wound Healing Society
Disclosure: Abbott Laboratories Honoraria Speaking and teaching; Clincal Cell Culture Grant/research funds Co-investigator; KCI, Inc Wake Forest University receives royalties Other
Michael Neumeister, MD, FRCSC, FRCSC, FACS Chairman, Professor, Division of Plastic Surgery, Director of Hand/Microsurgery Fellowship Program, Chief of Microsurgery and Research, Institute of Plastic and Reconstructive Surgery, Southern Illinois University School of Medicine
Michael Neumeister, MD, FRCSC, FRCSC, FACS is a member of the following medical societies: American Association for Hand Surgery, American Association of Plastic Surgeons, American Burn Association, American College of Surgeons, American Medical Association, American Society for Reconstructive Microsurgery, American Society for Surgery of the Hand, American Society of Plastic Surgeons, Association of Academic Chairmen of Plastic Surgery, CanadianSocietyofPlastic Surgeons, Illinois State Medical Society, Illinois State Medical Society, Ontario Medical Association, Plastic Surgery Research Council, Royal College of Physicians and Surgeons of Canada, and Society of University Surgeons
Disclosure: Nothing to disclose.
Adrian Popescu, MD Research Fellow, Department of Physical Medicine and Rehabilitation, University of Pennsylvania School of Medicine
Disclosure: Nothing to disclose.
Patrick J Potter, MD, FRCP(C) Associate Professor, Department of Physical Medicine and Rehabilitation, University of Western Ontario School of Medicine; Consulting Staff, Department of Physical Medicine and Rehabilitation, St Joseph's Health Care Centre
Patrick J Potter, MD, FRCP(C) is a member of the following medical societies: American Paraplegia Society, Canadian Association of Physical Medicine and Rehabilitation, Canadian Medical Association, College of Physicians and Surgeons of Ontario, Ontario Medical Association, and Royal College of Physicians and Surgeons of Canada
Disclosure: Nothing to disclose.
Don R Revis Jr, MD Consulting Staff, Department of Surgery, Division of Plastic and Reconstructive Surgery, University of Florida College of Medicine
Don R Revis Jr, MD is a member of the following medical societies: American College of Surgeons, American Medical Association, American Society for Aesthetic Plastic Surgery, and American Society of Plastic Surgeons
Disclosure: Nothing to disclose.
Richard Salcido, MD Chairman, Erdman Professor of Rehabilitation, Department of Physical Medicine and Rehabilitation, University of Pennsylvania School of Medicine
Richard Salcido, MD is a member of the following medical societies: American Academy of Pain Medicine, American Academy of Physical Medicine and Rehabilitation, American College of Physician Executives, American Medical Association, and American Paraplegia Society
Disclosure: Nothing to disclose.
Wayne Karl Stadelmann, MD Stadelmann Plastic Surgery, PC
Wayne Karl Stadelmann, MD is a member of the following medical societies: Alpha Omega Alpha, American College of Surgeons, American Society of Plastic Surgeons, New Hampshire Medical Society, Northeastern Society of Plastic Surgeons, and Phi Beta Kappa
Disclosure: Nothing to disclose.
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Medscape Salary Employment
Bradon J Wilhelmi, MD Professor and Endowed Leonard J Weiner, MD, Chair of Plastic Surgery, Residency Program Director, University of Louisville School of Medicine
Bradon J Wilhelmi, MD is a member of the following medical societies: Alpha Omega Alpha, American Association for Hand Surgery, American Association of Clinical Anatomists, American Association of Plastic Surgeons, American Burn Association, American College of Surgeons, American Society for Aesthetic Plastic Surgery, American Society for Reconstructive Microsurgery, American Society for Surgery of the Hand, American Society of Plastic Surgeons,Association for Surgical Education, Plastic Surgery Research Council, and Wound Healing Society
Disclosure: Nothing to disclose.
Acknowledgments
The authors and editors of Medscape Reference gratefully acknowledge the contributions of Steve Jenkins in the Department of Physical Medicine and Rehabilitation at the University of Kentucky for his significant editorial assistance in preparing this article.
Dr Richard Salcido acknowledges that his studies cited in this article are supported by the National Heart, Lung and Blood Institute, the National Institutes of Health grant P01HL36552-07, the National Center for Medical Rehabilitation Research grant R01HD31426-01, the Paralyzed