Eosinophilia: Practice Essentials, Pathophysiology, Etiology (original) (raw)
Overview
Practice Essentials
Increased levels of eosinophilic leukocytes in the blood can be idiopathic, or may result from a variety of conditions, including connective tissue diseases, helminthic infections, neoplasias, and allergic disorders. In this article, the term eosinophilia is defined as an increase in peripheral blood eosinophils to more than 600 cells per microliter (μL) of blood. Hypereosinophilia has generally been defined as a peripheral blood eosinophil count greater than 1500/μL. [1]
Although emphasis is placed on the number of eosinophils circulating in the peripheral blood, an increase in eosinophils can be observed in other body fluids (eg, cerebrospinal fluid [CSF], urine) and many body tissues (eg, skin, lung, heart, liver, intestine, bladder, bone marrow, muscle, nerve). See the images below.
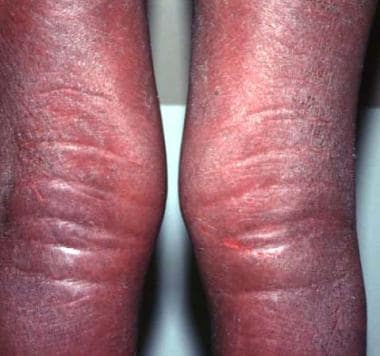
Indurated edematous plaques of hypereosinophilic syndrome on a patient's legs.
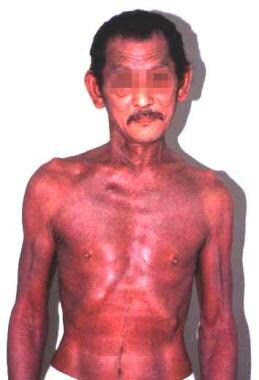
Erythroderma in a patient with hypereosinophilic syndrome.
Eosinophils are derived from hematopoietic stem cells initially committed to the myeloid line and then to the basophil-eosinophil granulocyte lineage. Nonpathologic functions of eosinophils and the cationic enzymes of their granules include mediating parasite defense reactions, allergic response, tissue inflammation, and immune modulation. [2, 3]
Tissues of the pulmonary and gastrointestinal systems are the normal residence for eosinophils, but peripheral, or blood, eosinophilia (absolute eosinophil count [AEC] >600 cells/µL) indicates an eosinophilic disorder. [4] Untreated, the eosinophilia can be categorized as mild (AEC 600-1500 cells/µL), moderate (AEC 1500-5000 cells/µL) or severe (AEC >5000 cells/µL). An increase in tissue eosinophilia may be seen with or without concurrent peripheral eosinophilia.
A secondary or reactive increase in blood eosinophils, tissue eosinophils, or both is associated with a wide variety of conditions, as follows [5, 6] :
- Infections (especially helminthic parasites)
- Allergic responses
- Neoplasms
- Connective tissue disorders
- Medications
- Endocrinopathies
Primary eosinophilia is not a reactive phenomenon and can be described as either clonal or idiopathic in nature. If an underlying molecular or cytogenetic abnormality can be identified, the eosinophilia can be designated as a clonal disorder. If reactive causes are ruled out and no underlying clonal origin is proven, the eosinophilia is described as idiopathic. [7]
Given the broad spectrum of conditions linked to eosinophilia, this article emphasizes the diagnostic considerations that clinicians may want to focus on in patients with eosinophilia. The individual disease manifestations and therapies for the dozens of diseases associated with eosinophilia are not described in detail; other Medscape articles specifically address these conditions, such as the following:

Pathophysiology
Over the past 2 decades, substantial progress has been made in understanding the mechanisms of eosinophil production, eosinophil programmed cell death (apoptosis), and how eosinophil immunology contributes to both host defenses against infections and to tissue damage within the host in cases of allergic and autoimmune diseases.
The primary stimuli for eosinophil production are interleukin (IL)-5, IL-3, and the granulocyte-macrophage colony-stimulating factor (GM-CSF). These cytokines are also the primary signals that inhibit eosinophil programmed cell death. Thus, eosinophilia can be triggered via these 3 eosinophilopoietic cytokines by increased eosinophil production, by eosinophil longevity, or by a combination of these. [2, 3]
In addition, an evolving number of chemotactic cytokines (ie, chemokines) have been established as causing eosinophils to migrate from their site of production in the bone marrow into the blood and then into peripheral tissues. These chemokines include eotaxin-1, eotaxin-2, and RANTES (regulated on activation normal T cell expressed and secreted).
Eosinophils are the source of a large number of cytokines, including the following:
- IL-2, IL-3, IL-4, IL-5, IL-7, IL-13, and IL-16
- Tumor necrosis factor–alpha (TNF-alpha),
- Transforming growth factor–beta (TGF-beta)
- RANTES
In addition to these cytokines, eosinophils are a source of several cationic proteins that also contribute to the immunologic responses against infectious disease agents and to tissue damage in allergic and autoimmune diseases. These cationic proteins include the following:
- Eosinophil cationic protein (ECP)
- Eosinophil peroxidase (EPO)
- Charcot-Leyden crystal lysophospholipase
- Major basic protein (MBP)
- Eosinophil-derived neurotoxin (EDN)
Secondary eosinophilia is a reactive phenomenon driven by eosinophilopoietic cytokine release by nonmyeloid cells. Eosinophilic differentiation occurs in the bone marrow from myeloid progenitors through the actions of GM-CSF, IL-3, and IL-5. Mature eosinophils are released into the bloodstream where they migrate quickly to peripheral tissues of the bronchial and gastrointestinal mucosa and skin. Their survival is short, unless apoptosis is blocked by cytokines (GM-CSF, IL-3, and IL-5).
Dysregulated production of these cytokines by various cell populations account for secondary hypereosinophilia such as seen in nonmyeloid malignancies (eg, Hodgkin lymphoma; transitional cell carcinoma [TCC] of the bladder; adenocarcinomas of the stomach, colon, and uterus; large cell undifferentiated lung carcinomas; and large cell cervical tumors), allergic reactions, parasitic infections, and other conditions.
Eosinophilia is a feature of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, a rare delayed hypersensitivity reaction that typically develops 2 to 8 weeks after starting a drug. Many drugs have been linked to DRESS, including anticonvulsants, antibiotics, and allopurinol. The clinical features include skin rash (widely variable, but often maculopapular or erythematous), fever, lymphadenopathy, and inflammation of one or more organs (eg, liver, lung, brain, kidney, heart). Other hematologic abnormalities may include thrombocytopenia and atypical lymphocytes. DRESS may be fatal, with mortality rates depending on the severity of the organ involvement. [8, 9]
Primary eosinophilias include both clonal and idiopathic hypereosinophilic syndrome (HES). These disorders have very heterogeneous underlying pathophysiologies, not all of which are well-defined. They are by definition eosinophilia for longer than 6 months, without evidence of reactive cause and with signs and symptoms of organ involvement. [10]
In some neoplastic disorders, the hypereosinophilia is part of neoplastic clonal expansion affecting the myeloid lineage. This pathophysiology would describe the eosinophilia in the following disorders:
A number of hypereosinophilic syndrome (HES) cases exhibit clonal expansion of abnormal lymphocytes. Immunophenotypically, they are characterized by aberrant and immature T cells, which exhibit abnormal cytokine production. T-cell receptor gene rearrangements are demonstrated in many. These T cells produce high levels of IL-5, thought to cause the hypereosinophilia.
Eosinophilia is further classified as clonal or idiopathic, both clinically and pathologically. The World Health Organization (WHO) proposed criteria to distinguish idiopathic hypereosinophilic syndrome (HES) from chronic eosinophilic leukemia–not otherwise specified (CEL-NOS). [1] WHO diagnostic criteria for CEL-NOS are as follows:
- Absence of the Philadelphia chromosome or a rearrangement involving PDGFRA/B and FGFR1
- Exclusion of other acute or chronic primary marrow neoplasms associated with eosinophilia (eg, AML, myelodysplastic syndrome, systemic mastocytosis)
- Blasts of more than 2% in peripheral blood, or bone marrow blasts of more than 5% but less than 20% (the upper threshold is to exclude acute leukemia as a diagnosis)
- Evidence for a clonal marker
The underlying chromosomal abnormalities leading to CEL have been described in some cases. A deletion on chromosome band 4q12 resulting in the FIP1L1-PDGFRA (FIR1- like-1–platelet-derived growth factor receptor–alpha) fusion gene causes an abnormal constitutively activated tyrosine kinase. These patients demonstrate CHIC2 gene deletion in peripheral blood mononuclear cells as a result of this fusion gene.
Another fusion gene involving BCR-PDGFRA has been seen in CML with marked eosinophilia. Mutations involving PDGFRB rearrangements have been described, as well as FGFR1 (fibroblast growth factor receptor–1) fusions. [3, 11, 12, 13] Clinical features of eosinophil leukemia result from accumulation of leukemic cells in bone marrow, liver, and spleen. [14] Inflammatory mediators from the eosinophils themselves cause tissue damage to the pericardium, myocardium, endocardium, and nervous system.
In 38 patients with chronic eosinophilia studied by array comparative genomic hybridization (aCGH), Arefi et al found that aCGH revealed clonality in eosinophils in most patients with myeloproliferative neoplasias. These authors suggested that aCGH could be a useful technique for defining clonality in these diseases. [15]
Finally, idiopathic hypereosinophilic syndrome (HES) is the diagnosis of exclusion in patients with marked prolonged (> 6 mo) eosinophilia with multiple organ involvement but without identifiable cytogenetic or molecular abnormalities. Organ damage occurs from release of the contents of eosinophilic granules. Some of these cases transform into identifiable entities. Because of the increasing ability to identify clonal markers, few cases now meet the classical definition of idiopathic HES; WHO considers HES to be a provisional diagnosis until a primary or secondary cause of eosinophilia is established. [16]

Etiology
The mnemonic CHINA (ie, _C_onnective tissue diseases, _H_elminthic infections, _I_diopathic hypereosinophilic syndrome [HES], _N_eoplasia, _A_llergies) describes the categories of diseases that sometimes are associated with blood eosinophilia.
Connective tissue diseases include the following:
- Eosinophilic granulomatosis with polyangiitis (Churg-Strauss Syndrome) (See the images below.)
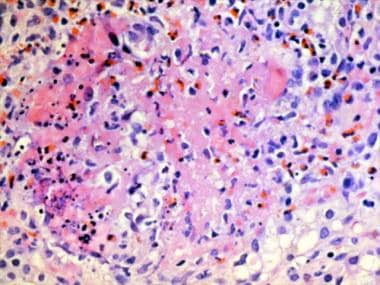
Granuloma with a central core of eosinophilic debris surrounded by a peripheral palisade of epithelioid histiocytes and eosinophils from a patient with Churg-Strauss syndrome (allergic granulomatosis).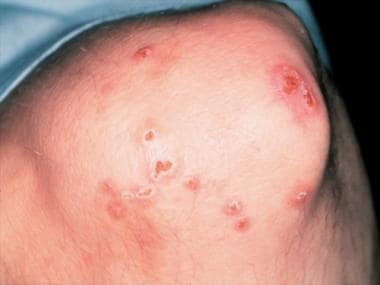
Magnified view of papules and nodules with central necrosis in a patient with Churg-Strauss syndrome (allergic granulomatosis). - Eosinophilic fasciitis (See the images below.)
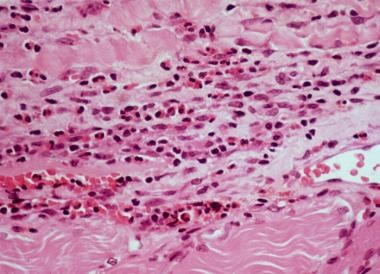
High-power photomicrograph of fascia shows heavy inflammatory infiltration with numerous eosinophils, lymphocytes, and occasional plasma cells in a patient with eosinophilic fasciitis.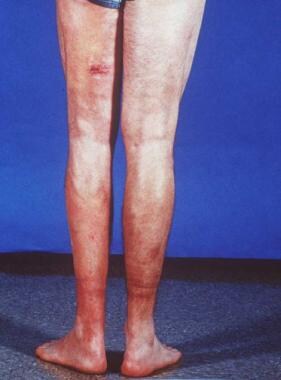
Lower back part of the legs in a patient with eosinophilic fasciitis shows hypopigmentation, induration, biopsy site, and asymmetric involvement. - Eosinophilia-myalgia syndrome (due to tryptophan in the United States in 1989)
- Toxic-oil syndrome (due to contaminated rapeseed oil in Spain in 1981)
Helminthic (ie, worm) parasitic infections include the following:
Idiopathic HES is shown in the images below:
Indurated edematous plaques of hypereosinophilic syndrome on a patient's legs.
Erythroderma in a patient with hypereosinophilic syndrome.
Neoplasias include the following:
- Lymphoma (eg, Hodgkin lymphoma, non-Hodgkin lymphoma)
- Human T-cell lymphotropic virus I (HTLV-I) infection
- Adult T-cell leukemia/lymphoma (ATLL)
- Eosinophilic leukemia (very rare)
- Gastric or lung carcinoma (ie, paraneoplastic eosinophilia)
Allergic/atopic diseases include the following:
In a retrospective review by Leverone et al of 2227 adults with peripheral blood eosinophilia, hematologic disorders comprised the most common cause (21.9%) of eosinophilia, followed by active bacterial infection (16.8%). The three most common specific causes of eosinophilia were asthma (6.4%), chronic lymphocytic leukemia (5.8%), and multiple myeloma (4.1%). [17]

Epidemiology
In the United States, compared with developing countries, eosinophilia occurs most commonly due to allergic conditions, including drug reactions and atopic asthma. Parasitic infections are rare.
Idiopathic hypereosinophilic syndrome (HES) is rare condition. The World Health Organization estimates an age‐adjusted incidence rate of approximately 0.036 per 100,000. [16]
Helminthic infections are the most common cause of eosinophilia worldwide due to the high prevalence of helminthic parasite infections, several of which are estimated to involve hundreds of millions of people.
No racial predilection exists for eosinophilia, although the occurrence of eosinophilia-associated helminthic parasitic infections is more common in certain geographic areas of the world.
No male or female predilection exists in most subtypes of eosinophilia. However, there is a marked male predominance in clonal disorders involving the PDGFRB fusion gene and a small male predominance in clonal disorders of the FGFR1 gene.
People of all ages can be affected by eosinophilia.

Prognosis
The prognosis in patients with secondary eosinophilia depends on the underlying disease. Many helminthic infections develop into chronic diseases that cause morbidity but not mortality. Similarly, allergic reactions and conditions associated with eosinophilia usually do not cause mortality.
Eosinophilia associated with nonmyeloid malignancies does not affect their individual prognosis, including mortality rates. The mortality and morbidity associated with clonal and idiopathic causes depends on the degree of tissue involvement, damage, or both at diagnosis; how quickly therapy is implemented; and treatment responsiveness.
The prognosis in patients with primary eosinophilias is determined by the following:
- Degree of organ involvement at diagnosis
- The timeliness of treatment
- Responsiveness to treatment
- Underlying cytogenetic and molecular pathophysiology

- Gotlib J. World Health Organization-defined eosinophilic disorders: 2017 update on diagnosis, risk stratification, and management. Am J Hematol. 2017 Nov. 92 (11):1243-1259. [QxMD MEDLINE Link]. [Full Text].
- Spry CJF, ed. Eosinophils: A Comprehensive Review and Guide to the Scientific and Medical Literature. Oxford, UK: Oxford University Press; 1988.
- Gotlib J. Molecular classification and pathogenesis of eosinophilic disorders: 2005 update. Acta Haematol. 2005. 114(1):7-25. [QxMD MEDLINE Link].
- Prieto R, Richter JE. Eosinophilic esophagitis in adults: an update on medical management. Curr Gastroenterol Rep. 2013 Jun. 15(6):324. [QxMD MEDLINE Link].
- Feldman RE, Lam AC, Sadow PM, Bleier BS. P-glycoprotein is a marker of tissue eosinophilia and radiographic inflammation in chronic rhinosinusitis without nasal polyps. Int Forum Allergy Rhinol. 2013 May 15. [QxMD MEDLINE Link].
- Kasamatsu Y, Kida T, Shigeru M, Tagashira T, Murai N, Takai E, et al. Clinically suspected acute myopericarditis with cardiac tamponade associated with peripheral blood eosinophilia presenting in early pregnancy: a case report. J Med Case Rep. 2013 May 13. 7(1):129. [QxMD MEDLINE Link]. [Full Text].
- Tefferi A, Patnaik MM, Pardanani A. Eosinophilia: secondary, clonal and idiopathic. Br J Haematol. 2006 Jun. 133(5):468-92. [QxMD MEDLINE Link].
- Criado PR, Criado RF, Avancini Jde M, Santi CG. Drug reaction with Eosinophilia and Systemic Symptoms (DRESS) / Drug-induced Hypersensitivity Syndrome (DIHS): a review of current concepts. An Bras Dermatol. 2012 May-Jun. 87(3):435-49. [QxMD MEDLINE Link].
- Watanabe H. Recent Advances in Drug-Induced Hypersensitivity Syndrome/Drug Reaction with Eosinophilia and Systemic Symptoms. J Immunol Res. 2018. 2018:5163129. [QxMD MEDLINE Link]. [Full Text].
- Weller PF, Bubley GJ. The idiopathic hypereosinophilic syndrome. Blood. 1994 May 15. 83(10):2759-79. [QxMD MEDLINE Link]. [Full Text].
- Bain BJ. Relationship between idiopathic hypereosinophilic syndrome, eosinophilic leukemia, and systemic mastocytosis. Am J Hematol. 2004 Sep. 77(1):82-5. [QxMD MEDLINE Link]. [Full Text].
- Gotlib J, Cools J, Malone JM 3rd, et al. The FIP1L1-PDGFRalpha fusion tyrosine kinase in hypereosinophilic syndrome and chronic eosinophilic leukemia: implications for diagnosis, classification, and management. Blood. 2004 Apr 15. 103(8):2879-91. [QxMD MEDLINE Link]. [Full Text].
- Tefferi A. Modern diagnosis and treatment of primary eosinophilia. Acta Haematol. 2005. 114(1):52-60. [QxMD MEDLINE Link].
- Fletcher S, Bain B. Eosinophilic leukaemia. Br Med Bull. 2007. 81-2:115-27. [QxMD MEDLINE Link]. [Full Text].
- Arefi M, Robledo C, Peñarrubia MJ, de Coca AG, Cordero M, Hernández-Rivas JM, et al. Genomic analysis of clonal eosinophils by CGH arrays reveals new genetic regions involved in chronic eosinophilia. Eur J Haematol. 2014 Nov. 93(5):422-8. [QxMD MEDLINE Link].
- Shomali W, Gotlib J. World Health Organization-defined eosinophilic disorders: 2019 update on diagnosis, risk stratification, and management. Am J Hematol. 2019 Oct. 94 (10):1149-1167. [QxMD MEDLINE Link]. [Full Text].
- Leverone N, Tran S, Barry J, Akuthota P. Diagnoses associated with peripheral blood eosinophilia: A 5-year review. Ann Allergy Asthma Immunol. 2021 Nov. 127 (5):597-598. [QxMD MEDLINE Link]. [Full Text].
- Weller PF. Eosinophilia in travelers. Med Clin North Am. 1992 Nov. 76(6):1413-32. [QxMD MEDLINE Link].
- Abe Y, Sasaki Y, Yagi M, Mizumoto N, Onozato Y, Umehara M, et al. Endoscopic Diagnosis of Eosinophilic Esophagitis: Basics and Recent Advances. Diagnostics (Basel). 2022 Dec 16. 12 (12):[QxMD MEDLINE Link]. [Full Text].
- Di Micco P, Scudiero O, Lombardo B, Lodigiani C. Idiopathic Hypereosinophilia and Venous Thromboembolism: Is There a Pathophysiological or Clinical Link? Description of an Intriguing Clinical Case. J Blood Med. 2020. 11:73-76. [QxMD MEDLINE Link]. [Full Text].
- [Guideline] Butt NM, Lambert J, Ali S, Beer PA, Cross NC, Duncombe A, et al. Guideline for the investigation and management of eosinophilia. Br J Haematol. 2017 Feb. 176 (4):553-572. [QxMD MEDLINE Link]. [Full Text].
- Boyer DF. Blood and Bone Marrow Evaluation for Eosinophilia. Arch Pathol Lab Med. 2016 Oct. 140 (10):1060-7. [QxMD MEDLINE Link]. [Full Text].
- Soter S, Barta I, Antus B. Predicting Sputum Eosinophilia in Exacerbations of COPD Using Exhaled Nitric Oxide. Inflammation. 2013 May 17. [QxMD MEDLINE Link].
- [Guideline] Aceves SS, Alexander JA, Baron TH, Bredenoord AJ, Day L, Dellon ES, et al. Endoscopic approach to eosinophilic esophagitis: American Society for Gastrointestinal Endoscopy Consensus Conference. Gastrointest Endosc. 2022 Oct. 96 (4):576-592.e1. [QxMD MEDLINE Link]. [Full Text].
- Kirchhof MG, Wong A, Dutz JP. Cyclosporine Treatment of Drug-Induced Hypersensitivity Syndrome. JAMA Dermatol. 2016 Nov 1. 152 (11):1254-1257. [QxMD MEDLINE Link].
- Hana CK, Caldera H. Hypereosinophilic Syndrome, Multiorgan Involvement and Response to Imatinib. Cureus. 2020 Jun 7. 12 (6):e8493. [QxMD MEDLINE Link]. [Full Text].
- Jain N, Cortes J, Quintas-Cardama A, et al. Imatinib has limited therapeutic activity for hypereosinophilic syndrome patients with unknown or negative PDGFRalpha mutation status. Leuk Res. 2009 Jun. 33(6):837-9. [QxMD MEDLINE Link].
- Gotlib J. Tyrosine Kinase Inhibitors and Therapeutic Antibodies in Advanced Eosinophilic Disorders and Systemic Mastocytosis. Curr Hematol Malig Rep. 2015 Sep 24. 79(5):441-7. [QxMD MEDLINE Link].
- Fraticelli P, Kafyeke A, Mattioli M, Martino GP, Murri M, Gabrielli A. Idiopathic hypereosinophilic syndrome presenting with severe vasculitis successfully treated with imatinib. World J Clin Cases. 2016 Oct 16. 4 (10):328-332. [QxMD MEDLINE Link]. [Full Text].
- Allen JN, Davis WB. Eosinophilic lung diseases. Am J Respir Crit Care Med. 1994 Nov. 150(5 Pt 1):1423-38. [QxMD MEDLINE Link].
- Cohen AJ, Steigbigel RT. Eosinophilia in patients infected with human immunodeficiency virus. J Infect Dis. 1996 Sep. 174(3):615-8. [QxMD MEDLINE Link].
- Heimall J, Freeman A, Holland SM. Pathogenesis of hyper IgE syndrome. Clin Rev Allergy Immunol. 2009 May 19. epub ahead of print. [QxMD MEDLINE Link].
- Nair P, Pizzichini MM, Kjarsgaard M, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009 Mar 5. 360(10):985-93. [QxMD MEDLINE Link].
- Nand R, Bryke C, Kroft SH, et al. Myeloproliferative disorder with eosinophilia and ETV6-ABL gene rearrangement: efficacy of second-generation tyrosine kinase inhibitors. Leuk Res. 2009 Aug. 33(8):1144-6. [QxMD MEDLINE Link].
- Roufosse F, Goldman M, Cogan E. Hypereosinophilic syndrome: lymphoproliferative and myeloproliferative variants. Semin Respir Crit Care Med. 2006 Apr. 27(2):158-70. [QxMD MEDLINE Link].
- Lindsley AW, Schwartz JT, Rothenberg ME. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J Allergy Clin Immunol. 2020 Jul. 146 (1):1-7. [QxMD MEDLINE Link]. [Full Text].
- Abe Y, Sasaki Y, Yagi M, Mizumoto N, Onozato Y, Umehara M, et al. Endoscopic Diagnosis of Eosinophilic Esophagitis: Basics and Recent Advances. Diagnostics (Basel). 2022 Dec 16. 12 (12):[QxMD MEDLINE Link]. [Full Text].
- Indurated edematous plaques of hypereosinophilic syndrome on a patient's legs.
- Erythroderma in a patient with hypereosinophilic syndrome.
- Granuloma with a central core of eosinophilic debris surrounded by a peripheral palisade of epithelioid histiocytes and eosinophils from a patient with Churg-Strauss syndrome (allergic granulomatosis).
- Magnified view of papules and nodules with central necrosis in a patient with Churg-Strauss syndrome (allergic granulomatosis).
- High-power photomicrograph of fascia shows heavy inflammatory infiltration with numerous eosinophils, lymphocytes, and occasional plasma cells in a patient with eosinophilic fasciitis.
- Lower back part of the legs in a patient with eosinophilic fasciitis shows hypopigmentation, induration, biopsy site, and asymmetric involvement.
- Egg of Schistosoma hematobium, with its typical terminal spine.


Author
Coauthor(s)
Specialty Editor Board
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Received salary from Medscape for employment. for: Medscape.
Marcel E Conrad, MD Distinguished Professor of Medicine (Retired), University of South Alabama College of Medicine
Marcel E Conrad, MD is a member of the following medical societies: Alpha Omega Alpha, American Association for the Advancement of Science, American Association of Blood Banks, American Chemical Society, American College of Physicians, American Physiological Society, American Society for Clinical Investigation, American Society of Hematology, Association of American Physicians, The Society of Federal Health Professionals (AMSUS), International Society of Hematology, Society for Experimental Biology and Medicine, SWOG
Disclosure: Partner received none from No financial interests for none.
Chief Editor
Emmanuel C Besa, MD Professor Emeritus, Department of Medicine, Division of Hematologic Malignancies and Hematopoietic Stem Cell Transplantation, Kimmel Cancer Center, Jefferson Medical College of Thomas Jefferson University
Emmanuel C Besa, MD is a member of the following medical societies: American Association for Cancer Education, American Society of Clinical Oncology, American College of Clinical Pharmacology, American Federation for Medical Research, American Society of Hematology, New York Academy of Sciences
Disclosure: Nothing to disclose.
Additional Contributors
Pradyumna D Phatak, MBBS, MD Chair, Division of Hematology and Medical Oncology, Rochester General Hospital; Clinical Professor of Oncology, Roswell Park Cancer Institute
Pradyumna D Phatak, MBBS, MD is a member of the following medical societies: American Society of Hematology
Disclosure: Received honoraria from Novartis for speaking and teaching.
Acknowledgements
Daniel R Lucey, MD, MPH Chief, Fellowship Program Director, Department of Internal Medicine, Division of Infectious Diseases, Washington Hospital Center; Professor, Department of Internal Medicine, Uniformed Services University of the Health Sciences
Daniel R Lucey, MD, MPH is a member of the following medical societies: Alpha Omega Alpha and American College of Physicians
Disclosure: Nothing to disclose.