Interdependent Recruitment of SAGA and Srb Mediator by Transcriptional Activator Gcn4p (original) (raw)
Abstract
Transcriptional activation by Gcn4p is enhanced by the coactivators SWI/SNF, SAGA, and Srb mediator, which stimulate recruitment of TATA binding protein (TBP) and polymerase II to target promoters. We show that wild-type recruitment of SAGA by Gcn4p is dependent on mediator but independent of SWI/SNF function at three different promoters. Recruitment of mediator is also independent of SWI/SNF but is enhanced by SAGA at a subset of Gcn4p target genes. Recruitment of all three coactivators to ARG1 is independent of the TATA element and preinitiation complex formation, whereas efficient recruitment of the general transcription factors requires the TATA box. We propose an activation pathway involving interdependent recruitment of SAGA and Srb mediator to the upstream activation sequence, enabling SWI/SNF recruitment and the binding of TBP and other general factors to the promoter. We also found that high-level recruitment of Tra1p and other SAGA subunits is independent of the Ada2p/Ada3p/Gcn5p histone acetyltransferase module but requires Spt3p in addition to subunits required for SAGA integrity. Thus, while Tra1p can bind directly to Gcn4p in vitro, it requires other SAGA subunits for efficient recruitment in vivo.
Transcription initiation by RNA polymerase II (Pol II) is dependent on a set of general transcription factors (GTFs), including the TATA binding protein (TBP), which recognize the core promoter and facilitate initiation from the correct start site (13). Eukaryotic transcriptional activators bind to upstream activation sequences (UASs) and stimulate preinitiation complex (PIC) assembly by disrupting repressive nucleosome structures and recruiting TBP, other GTFs, and Pol II to the promoter. Typically, activators execute these functions indirectly by recruiting cofactors or coactivators to the UAS region (20, 41). The SWI/SNF complex of Saccharomyces cerevisiae is a coactivator that uses ATP hydrolysis to displace or destabilize nucleosomes (42, 62). The coactivator SAGA contains a histone acetyltransferase (HAT) subunit, Gcn5p, that acetylates the amino-terminal tail of histone H3 (24, 33). Histone acetylation destabilizes higher-order chromatin structure (60) and may stimulate binding of coactivators harboring a bromodomain (15, 27, 29, 47). SAGA also binds to TBP in vitro (4, 58, 61) and enhances TBP recruitment by activators in vivo (6, 18, 36, 53), most likely functioning as an adaptor between the activators and TBP. The Srb mediator is a coactivator that can interact directly with Pol II to form a holoenzyme complex. In vitro, mediator stimulates basal and activated transcription and enhances phosphorylation of the C-terminal domain of the largest Pol II subunit by TFIIH (40). The mediator is absent from the C-terminal-domain-phosphorylated, elongating form of Pol II (51, 63) and interacts exclusively with nonphosphorylated Pol II at the promoter. Mediator can also interact with various GTFs (9, 40, 55, 56), possibly including TBP (30, 66), and it promotes the recruitment of TBP as well as Pol II to promoters in vivo (35, 38, 39, 53).
Gcn4p is a transcriptional activator of amino acid biosynthetic genes in yeast (45) that is induced at the translational level by starvation for any amino acid (26). Gcn4p activation function is dependent on clusters of hydrophobic residues in its activation domain (16, 28) that contribute to its binding to SAGA, SWI/SNF, and mediator in vitro (17, 23, 43, 68) and its ability to recruit SWI/SNF (72) and Gcn5p HAT activity (33) to target promoters in vivo. Mutations have been identified in multiple subunits of SAGA, SWI/SNF, and Srb mediator that diminish transcriptional activation by Gcn4p (5, 21, 44, 48, 50, 53, 64) and decrease the recruitment of TBP and Pol II by Gcn4p to target promoters in vivo (53).
The molecular mechanisms of coactivator recruitment by Gcn4p are not well understood. Three subunits of SWI/SNF, Swi2p, Snf5p, and Swi1p, can bind directly to Gcn4p in vitro (46). However, we found that Gcn4p cannot recruit Snf2p and Snf5p to target promoters in vivo when the SWI/SNF complex is disrupted, suggesting that SWI/SNF recruitment depends on multiple contacts between Gcn4p and SWI/SNF subunits (72). Indeed, it was shown that particular segments of Snf5p and Swi1p make additive contributions to the binding of SWI/SNF by Gcn4p in vitro (52). Similarly, we found that optimal recruitment of mediator by Gcn4p requires subunits from the head and tail domains of mediator, although the Gal11p/Med2p/Pgd1p triad from the tail domain is efficiently recruited by Gcn4p when separated from the rest of mediator by deleting the Sin4p subunit (73). It has been proposed that Gcn4p recruits SAGA through a direct interaction with the Ada2p (3) or Tra1p (10) subunit, but these hypotheses have not been tested directly in vivo. Here, we present evidence that optimal Gcn4p recruitment of Tra1p and other SAGA subunits is dependent on both the integrity of SAGA and the Spt3p subunit but occurs independently of the Ada2p/Ada3p/Gcn5p HAT module.
We also investigated whether recruitment of one coactivator enhances the ability of Gcn4p to recruit other coactivators. Genetic evidence suggests that H3 acetylation by Gcn5p (SAGA) enhances recruitment of SWI/SNF via the bromodomain in Swi2p (25). However, we and others found that substantial recruitment of SWI/SNF by Gcn4p occurs independently of both Swi2p (72) and the Gcn5p subunit of SAGA (65, 72). On the other hand, mutations that disrupt SAGA greatly reduced SWI/SNF recruitment, indicating that a non-HAT function of SAGA is important for SWI/SNF recruitment by Gcn4p (72). Here we show that a non-HAT SAGA function also stimulates recruitment of Srb mediator at a subset of Gcn4p target genes. In contrast to recent findings on Gal4p (7, 11, 37), we find that mediator is required for recruitment of SAGA and that SWI/SNF recruitment is independent of PIC formation. These and other new findings allow us to propose an activation pathway for Gcn4p involving interdependent recruitment of SAGA, mediator, and SWI/SNF to the UAS, which enables subsequent recruitment of TBP, other GTFs, and Pol II to the downstream promoter for PIC assembly.
MATERIALS AND METHODS
Yeast strains and plasmids.
All strains and plasmids are listed in Tables 1 and 2, respectively. The wild-type (WT) parent strain BY4741 and deletion derivatives thereof were described previously (69) and purchased from Research Genetics. The presence of all reported deletion alleles was confirmed by PCR amplification or complementation of mutant phenotypes by plasmid-borne wild-type genes (64). Strains carrying gcn4Δ::hisG were created by transformation with plasmid pHQ1240 (72). myc-tagged strains were constructed as previously described (64). To construct tra1Δ::HIS3 strains harboring episomal TRA1-FL, the parent strains were first transformed with URA3 plasmid p4122 carrying TRA1-FL. The resulting strains were transformed with plasmid pHQ1376 containing tra1Δ::HIS3 digested with SspI and selected on SC-His. Deletion of chromosomal TRA1 in the resulting transformants was indicated by their sensitivity to 5-fluoroorotic acid and confirmed by PCR analysis. Strains carrying the arg1-ΔTATA allele in the chromosome were constructed as described previously (53).
TABLE 1.
Yeast strains used in this study
| Name | Parent | Relevant genotypec | Reference |
|---|---|---|---|
| SPT7-myc | |||
| HQY453 | BY4741a | SPT7-myc13::HIS3* | This work |
| HQY457 | 249a | SPT7-myc13::HIS3*_gcn4_Δ::kanMX4 | 64 |
| HQY508 | 1799a | SPT7-myc13::HIS3*_ahc1_Δ::kanMX4 | This work |
| HQY579 | 4282a | SPT7-myc13::HIS3*_ada2_Δ::kanMX4 | This work |
| HQY580 | 3534a | SPT7-myc13::HIS3*_ada3_Δ::kanMX4 | This work |
| HQY484 | 7285a | SPT7-myc13::HIS3*_gcn5_Δ::kanMX4 | This work |
| HQY497 | 1038a | SPT7-myc13::HIS3*_ada1_Δ::kanMX4 | This work |
| HQY496 | 7309a | SPT7-myc13::HIS3*_ada5_Δ::kanMX4 | This work |
| HQY581 | 4228a | SPT7-myc13::HIS3*_spt3_Δ::kanMX4 | This work |
| HQY582 | 2666a | SPT7-myc13::HIS3*_spt8_Δ::kanMX4 | This work |
| HQY498 | LSO2b | SPT7-myc13::HIS3*_med2_Δ::kanMX4 | This work |
| HQY664 | 6611a | SPT7-myc13::HIS3*_srb2_Δ::kanMX4 | This work |
| HQY499 | 4734a | SPT7-myc13::HIS3*_srb5_Δ::kanMX4 | This work |
| HQY536 | 3119a | SPT7-myc13::HIS3*_rox3_Δ::kanMX4 | This work |
| HQY472 | 1586a | SPT7-myc13::HIS3*_swi2_Δ::kanMX4 | This work |
| HQY706 | HQY457b | SPT7-myc13::HIS3*_gcn4_Δ::kanMX4 arg1_-Δ_TATA | This work |
| ADA2-myc | |||
| HQY392 | BY4741a | ADA2-myc13::HIS3* | This work |
| HQY503 | HQY392b | ADA2-myc13::HIS3*_gcn4_Δ::hisG | This work |
| HQY546 | 1799a | ADA2-myc13::HIS3*_ahc1_Δ::kanMX4 | This work |
| HQY668 | 3534a | ADA2-myc13::HIS3*_ada3_Δ::kanMX4 | This work |
| HQY420 | 7285a | ADA2-myc13::HIS3*_gcn5_Δ::kanMX4 | This work |
| HQY418 | 1038a | ADA2-myc13::HIS3*_ada1_Δ::kanMX4 | This work |
| HQY520 | 7309a | ADA2-myc13::HIS3*_ada5_Δ::kanMX4 | This work |
| HQY551 | 3218a | ADA2-myc13::HIS3*_spt7_Δ::kanMX4 | This work |
| HQY419 | 4228a | ADA2-myc13::HIS3*_spt3_Δ::kanMX4 | This work |
| HQY669 | 2666a | ADA2-myc13::HIS3*_spt8_Δ::kanMX4 | This work |
| HQY500 | LSO2b | ADA2-myc13::HIS3*_med2_Δ::kanMX4 | This work |
| HQY667 | 6611a | ADA2-myc13::HIS3*_srb2_Δ::kanMX4 | This work |
| HQY477 | 4734a | ADA2-myc13::HIS3*_srb5_Δ::kanMX4 | This work |
| HQY573 | 3119a | ADA2-myc13::HIS3*_rox3_Δ::kanMX4 | This work |
| HQY666 | 1586a | ADA2-myc13::HIS3*_swi2_Δ::kanMX4 | This work |
| SRB6-myc | |||
| HQY464 | BY4741a | SRB6-myc13::HIS3* | This work |
| HQY470 | HQY464b | SRB6-myc13::HIS3*_gcn4_Δ::hisG | 64 |
| HQY563 | 7285a | SRB6-myc13::HIS3*_gcn5_Δ::kanMX4 | This work |
| HQY567 | 1038a | SRB6-myc13::HIS3*_ada1_Δ::kanMX4 | This work |
| HQY568 | 7309a | SRB6-myc13::HIS3*_ada5_Δ::kanMX4 | This work |
| HQY564 | 3218a | SRB6-myc13::HIS3*_spt7_Δ::kanMX4 | This work |
| HQY562 | 1586a | SRB6-myc13::HIS3*_swi2_Δ::kanMX4 | This work |
| HQY705 | HQY470b | SRB6-myc13::HIS3*_gcn4_Δ::hisG arg1_-Δ_TATA | This work |
| GAL11-myc | |||
| HQY438 | BY4741a | GAL11-myc13::HIS3* | This work |
| HQY439 | 249a | GAL11-myc13::HIS3*_gcn4_Δ::kanMX4 | 64 |
| HQY552 | 7285a | GAL11-myc13::HIS3*_gcn5_Δ::kanMX4 | This work |
| HQY549 | 1038a | GAL11-myc13::HIS3*_ada1_Δ::kanMX4 | This work |
| HQY550 | 7309a | GAL11-myc13::HIS3*_ada5_Δ::kanMX4 | This work |
| HQY544 | 3218a | GAL11-myc13::HIS3*_spt7_Δ::kanMX4 | This work |
| HQY662 | 1586a | GAL11-myc13::HIS3*_swi2_Δ::kanMX4 | This work |
| TRA1-FL | |||
| HQY825 | 249a | _tra1_Δ::_HIS3_[_TRA1-FL_]_gcn4_Δ::kanMX4 | This work |
| HQY830 | BY4741a | _tra1_Δ::_HIS3_[_TRA1-FL_] | This work |
| HQY836 | 4282a | _tra1_Δ::_HIS3_[_TRA1-FL_] _ada2_Δ::kanMX4 | This work |
| HQY837 | 3534a | _tra1_Δ::_HIS3_[_TRA1-FL_]_ada3_Δ::kanMX4 | This work |
| HQY835 | 7285a | _tra1_Δ::_HIS3_[_TRA1-FL_]_gcn5_Δ::kanMX4 | This work |
| HQY826 | 1038a | _tra1_Δ::_HIS3_[_TRA1-FL_]_ada1_Δ::kanMX4 | This work |
| HQY827 | 7309a | _tra1_Δ::_HIS3_[_TRA1-FL_]_ada5_Δ::kanMX4 | This work |
| HQY838 | 3218a | _tra1_Δ::_HIS3_[_TRA1-FL_]_spt7_Δ::kanMX4 | This work |
| HQY833 | 4228a | _tra1_Δ::_HIS3_[_TRA1-FL_]_spt3_Δ::kanMX4 | This work |
| HQY834 | 2666a | _tra1_Δ::_HIS3_[_TRA1-FL_]_spt8_Δ::kanMX4 | This work |
| SW12-myc | |||
| HQY383 | HQY470b | SWI2-myc13::HIS3*_gcn4_Δ::hisG | This work |
| HQY707 | HQY383b | SWI2-myc13::HIS3*_gcn4_Δ::hisG arg1_-Δ_TATA | This work |
| Strains with myc-tagged GTFs | |||
| HQY382 | HQY366b | TBP1-myc13::HIS3*_gcn4_Δ::hisG | 53 |
| HQY366 | BY4741a | TBP1-myc13::HIS3* | 53 |
| HQY692 | HQY366b | TBP1-myc13::HIS3*arg1_-Δ_TATA | 53 |
| HQY422 | HQY403b | RPB3-myc13::HIS3*_gcn4_Δ::hisG | 53 |
| HQY403 | BY4741a | RPB3-myc13::HIS3* | 53 |
| HQY693 | HQY403b | RPB3-myc13::HIS3*arg1_-Δ_TATA | 53 |
| HQY727 | 249a | TOA1-myc13::HIS3*_gcn4_Δ::kanMX4 | This work |
| HQY728 | BY4741a | TOA1-myc13::HIS3* | This work |
| HQY704 | HQY728b | TOA1-myc13::HIS3*arg1_-Δ_TATA | This work |
| HQY690 | 249a | SUA7-myc13::HIS3*_gcn4_Δ::kanMX4 | This work |
| HQY691 | BY4741a | SUA7-myc13::HIS3* | This work |
| HQY698 | HQY691b | SUA7-myc13::HIS3*arg1_-Δ_TATA | This work |
| HQY777 | 249a | TFA1-myc13::HIS3*_gcn4_Δ::kanMX4 | This work |
| HQY787 | BY4741a | TFA1-myc13::HIS3* | This work |
| HQY788 | HQY787b | TFA1-myc13::HIS3*arg1_-Δ_TATA | This work |
| HQY778 | 249a | TFG2-myc13::HIS3*_gcn4_Δ::kanMX4 | This work |
| HQY779 | BY4741a | TFG2-myc13::HIS3* | This work |
| HQY780 | HQY779b | TFG2-myc13::HIS3*arg1_-Δ_TATA | This work |
| HQY785 | 249a | KIN28-myc13::HIS3*_gcn4_Δ::kanMX4 | This work |
| HQY786 | BY4741a | KIN28-myc13::HIS3* | This work |
| HQY776 | HQY786b | KIN28-myc13::HIS3*arg1_-Δ_TATA | This work |
TABLE 2.
Plasmids used in this study
| Plasmid | Description | Reference |
|---|---|---|
| YEplac195 | Vector | 22 |
| pHQ1239 | GCN4-HA3 in YEplac195 | 64 |
| pHQ1303 | GCN4 in YEplac195 | 72 |
| pHQ1304 | gcn4-14Ala in YEplac195 | 72 |
| pHQ1344 | arg1_-Δ_TATA in YIplac211 | 53 |
| pHQ1376 | tra1::HIS3::tra1 in pUC18 | This work |
| p4122 | TRA1-FL3 in YCplac 33 | This work |
Plasmids pHQ1239, pHQ1303, and pHQ1304 were described previously (64, 72), as was pHQ1344 (53). To construct tra1Δ::HIS3, 5′ noncoding and 3′ noncoding regions of TRA1 were amplified by PCR and inserted into pUC18. For the resulting construct, the Eco47III-SspI fragment of HIS3 from pRS403 (22) was inserted at the EcoRV site (at position −35 upstream of TRA1) to produce plasmid pHQ1376. Plasmid p4122 containing the TRA1-FL allele was created in several steps. A fragment encoding the 3× Flag epitope (FLAG3) and containing a 5′ XbaI site and 3′ blunt end was created by PCR with Turbo PFU Taq polymerase using plasmid p3xFLAG-CMV-7.1 (Sigma) as template, 5′ primer GGGGTCTAGAATGGACTACAAAGACCATGACGGTGAT, and 3′ primer CTTGTCATCGTCATCCTTGTAGTC. (Underlined sequences in PCR primers are the relevant restriction sites used for cloning [in this case, XbaI].) In parallel, a TRA1 fragment containing nucleotides +1 to +1018 of the open reading frame (ORF) and harboring a 5′ blunt end and 3′ PstI site was PCR amplified from genomic DNA using 5′ primer ATGTCACTCACTGAGCAGATCGAG and 3′ primer GGGGCTGCAGGTTCAGAAGGGCAGTCTTGTAAAA. These two PCR products were ligated together and cloned into pBluescript SK(−) digested with XbaI and PstI. The resulting plasmid was used as template to PCR amplify the TRA1 ORF from +1 to +1018 fused in-frame to the coding sequences for FLAG3 using 5′ primer ATGGACTACAAAGACCATGACGGTGAT and 3′ primer GGGGCTGCAGGTTCAGAAGGGCAGTCTTGTAAAA, generating a fragment with a blunt 5′ end and a PstI site at the 3′ end. Independently, a fragment spanning nucleotides −875 to −1 upstream of the TRA1 ORF, containing a SacI site at the 5′ end and a blunt 3′ end, was PCR amplified from genomic DNA using 5′ primer GGGGGAGCTCCAAGAGAGAGCGCTGAAACACTAand 3′ primer CGGCAAAATGCGGTATTCTTTGTAA. These last two fragments were ligated together and cloned between the SacI and PstI sites of pBluescript SK(−). The sequence of the resulting plasmid was verified by DNA sequencing and then digested with SacI and SnaBI (a unique site in the TRA1 coding region). The resulting SacI-SnaBI fragment was isolated and used to replace the corresponding segment in the full-length TRA1 gene cloned into YCplac33, producing p4122.
Biochemical methods.
The chromatin immunoprecipitation (ChIP) experiments were conducted as described previously using the same primers described there (53, 64, 72). For Western analysis, whole-cell extracts (WCEs) were prepared as described previously (54) and analyzed using monoclonal anti-myc (Roche) and anti-Flag M2 antibodies (Sigma) and polyclonal anti-Gcd6p antibodies (12). Coimmunoprecipitation assays were conducted essentially as described previously (73), except that EZview Red Anti-FLAG M2 Affinity Gel (Sigma) was used to immunoprecipitate FL-Tra1p.
RESULTS
Optimal recruitment of SAGA subunits by Gcn4p is independent of Gcn5p but requires SAGA integrity and Spt3p.
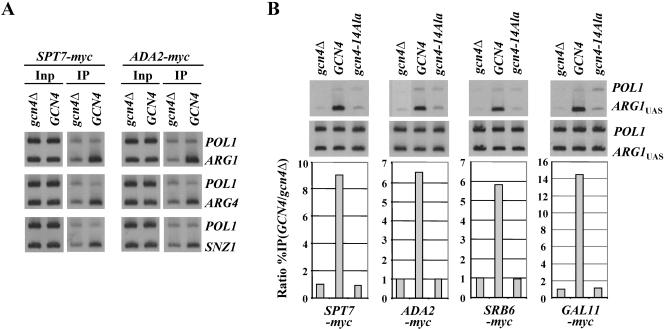
Using the ChIP assay and yeast strains containing functional myc-tagged forms of SAGA subunit Spt7p or Ada2p, we first established that Gcn4p recruits SAGA to the UASGCRE elements at ARG1, ARG4, and SNZ1. As shown in Fig. 1A, the binding of myc-Spt7p and myc-Ada2p to UAS elements at all three genes occurred at higher levels in cells containing GCN4 on a high-copy-number plasmid compared to gcn4Δ cells when synthesis of Gcn4p was induced by starvation for isoleucine and valine. (We have shown that expression of a myc-tagged form of Gcn4p from a high-copy-number plasmid increases binding of myc-Gcn4p at the ARG1 UAS by approximately twofold compared to that seen with myc-Gcn4p expressed from a single-copy plasmid [data not shown]. The elevated UAS occupancy afforded by this modest overexpression enhances our ability to quantify recruitment of SAGA and other coactivators by Gcn4p. We showed recently that overexpression of Gcn4p did not alter the subunit requirements for recruitment of Srb mediator to ARG1 compared to that seen with Gcn4p produced at native levels [73]; hence, we believe that the higher promoter occupancy obtained with Gcn4p overexpression does not qualitatively alter the requirements for coactivator recruitment.) The recruitment of both SAGA subunits to ARG1 requires the hydrophobic residues in the Gcn4p activation domain, as a strain containing 14 Ala substitutions in these residues (encoded by gcn4-14Ala) showed low levels of myc-Spt7p and myc-Ada2p binding, similar to that seen in gcn4Δ cells (Fig. 1B). The 14-Ala substitutions do not reduce binding of myc-tagged Gcn4p to the ARG1 UAS (72). High-level recruitment of two myc-tagged subunits of Srb mediator, Srb6p and Gal11p, also requires the hydrophobic residues in the activation domain (Fig. 1B).
FIG. 1.
Gcn4p recruits myc-Spt7p, myc-Ada2p, myc-Srb6p, and myc-Gal11p, dependent on hydrophobic residues in the activation domain. (A) SPT7-myc gcn4Δ and ADA2-myc gcn4Δ strains bearing empty vector (gcn4Δ) and SPT7-myc GCN4 and ADA2-myc GCN4 strains carrying high-copy-number GCN4-HA plasmid pHQ1239 were cultured in SC medium lacking Ile and Val and treated with sulfometuron for 2 h to induce Gcn4p synthesis by starvation for Ile/Val and then subjected to ChIP analysis with myc antibodies. DNA was extracted from the immunoprecipitates (IP), and 5% of the input (Inp) samples and a 1,000-fold dilution of the Inp and the undiluted IP DNA samples were PCR amplified using primers specific for the _POL1_ORF, _ARG1_UAS, _SNZ1_UAS, or ARG4 promoter (UAS and TATA sequence), in the presence of [33P]dATP. The PCR products were resolved by polyacrylamide gel electrophoresis and visualized by autoradiography. (B) The SPT7-myc gcn4Δ and ADA2-myc gcn4Δ strains described above, along with SRB6-myc gcn4Δ and GAL11-myc gcn4Δ strains, bearing empty vector (gcn4Δ), high-copy-number GCN4 plasmid pHQ1303 (GCN4), or high-copy-number gcn4-14Ala plasmid pHQ1304 (gcn4-14Ala), were subjected to ChIP analysis as above, and the results were quantified with a phosphorimager. The ratios of the _ARG1_UAS signals to the POL1 signals in the IP samples were normalized for the corresponding ratios for the Inp samples, and the resulting values measured for the GCN4 strain were normalized to the corresponding values obtained for the gcn4Δ strain to produce the “ratio %IP(GCN4/gcn4Δ)” values plotted in the histograms for each protein.
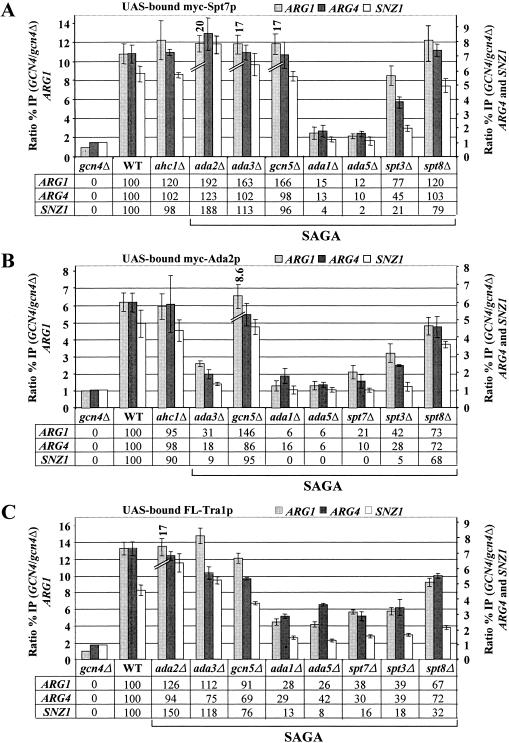
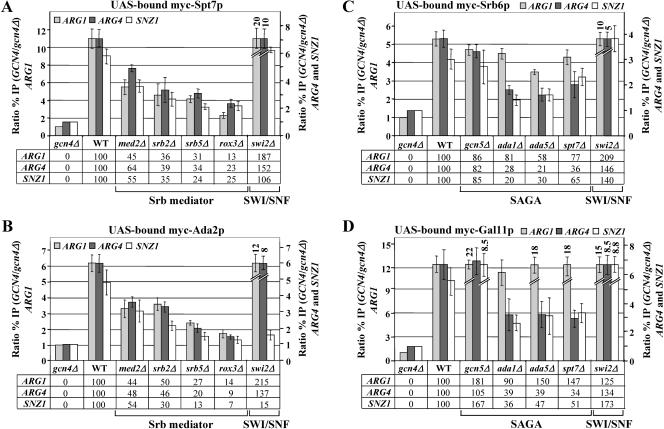
Ada2p is a subunit of the ADA complex in addition to SAGA (19, 23). To demonstrate that both Spt7p and Ada2p are recruited by Gcn4p as subunits of SAGA, we created _SPT7_-myc and _ADA2_-myc strains harboring deletions of the genes encoding SAGA subunit Ada1p, Ada5p, or Spt7p, which is required for SAGA integrity in vitro (23, 61, 71). Likewise, we analyzed an _ADA2_-myc strain lacking Ahc1p, required for integrity of the ADA complex (19). These strains were subjected to ChIP analysis to measure the effects of disrupting SAGA or ADA on Gcn4p-dependent recruitment of the myc-tagged proteins to the ARG1, ARG4, and SNZ1 UAS elements. The results of multiple, replicate ChIP assays were quantified and summarized in Fig. 2A and B. The values beneath the histograms in these figures give the Gcn4p-dependent component of myc-Spt7p or myc-Ada2p binding in the mutant strains as a percentage of that seen in the wild-type strain. (They correspond to the difference in heights of the histogram bars in the mutants and that measured in the gcn4Δ strain as a percentage of the corresponding difference calculated for the wild-type strain.)
FIG. 2.
Deletion of Spt3p or subunits required for SAGA integrity, but not Gcn5p, impairs recruitment of SAGA by Gcn4p. (A) ChIP analysis of a gcn4Δ SPT7-myc strain (gcn4Δ) and GCN4 SPT7-myc strains containing WT SAGA subunits or the indicated SAGA subunit deletions and harboring high-copy-number GCN4-HA plasmid pHQ1239 was carried out as described in Fig. 1 using anti-myc antibodies. (B) Same as panel A except that ADA2-myc strains were analyzed. (C) Same as panel A except that TRA1-FL strains were analyzed and anti-Flag M2 antibodies were used. The ratio %IP (GCN4/gcn4Δ) values as defined in Fig. 1 were calculated for the _ARG1_UAS, _SNZ1_UAS, and ARG4 probes, and the average results obtained from two or more independent cultures and two or more PCR amplifications for each culture were plotted in the histograms with standard errors shown as error bars. The numbers under the histograms, corresponding to percentages of the WT Gcn4p-dependent binding of myc-Spt7p, myc-Ada2p, or FL-Tra1p, were calculated by subtracting unity from all ratio %IP (GCN4/gcn4Δ) values for each mutant and expressing the result as a percentage of the corresponding WT value.
The results shown in Fig. 2A indicate that recruitment of myc-Spt7p by Gcn4p is strongly dependent on ADA1 and ADA5, as deletions of these genes reduced binding of myc-Spt7p to the UAS elements at all three genes to nearly the same low levels observed in gcn4Δ cells. Deletion of SPT3 also led to significant reductions in binding of myc-Spt7p at all three genes, but not as severe as those given by ada1Δ or ada5Δ. By contrast, the ada2Δ, ada3Δ, gcn5Δ, and spt8Δ deletions produced little or no reduction in myc-Spt7p recruitment by Gcn4p (Fig. 2A). These last results indicate that the function of the Gcn5p/Ada2p/Ada3p module of SAGA in histone H3 acetylation is not required for high-level binding of SAGA at the UASGCRE. In fact, it appeared that recruitment of Spt7p to ARG1 was even higher than WT in the ada2Δ, ada3Δ, and gcn5Δ mutants.
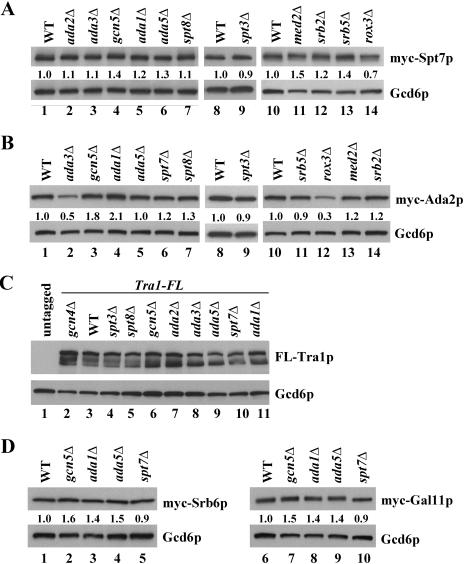
Similar to our findings on myc-Spt7p, recruitment of myc-Ada2p was greatly reduced by deletions of ADA1, ADA5, and SPT7; somewhat less impaired by deletion of SPT3; and relatively unaffected by deletions of SPT8 or GCN5 (Fig. 2B). The ada3Δ mutation reduced the recruitment of myc-Ada2p at all three promoters; however, this probably results from a reduced steady-state level of myc-Ada2p in ada3Δ cells (see below) (57). Deletion of AHC1 had no effect on recruitment of myc-Spt7p and myc-Ada2p by Gcn4p (Fig. 2A and B). We showed previously that none of the SAGA subunit deletions reduced binding of myc-tagged Gcn4p to these target genes (53). We also conducted Western analysis on whole-cell extracts of the SPT7-myc and ADA2-myc strains to determine whether the reduced levels of myc-Spt7p and myc-Ada2p recruitment in SAGA mutants might result from their reduced expression. The results in Fig. 3A (lanes 1 to 9) eliminate this possibility for myc-Spt7p, which is expressed at wild-type levels in all relevant SAGA mutants. The same was true for myc-Ada2p, except that its expression was reduced in the ada3Δ strain (Fig. 3B, lanes 1 to 9).
FIG. 3.
Western analysis of tagged SAGA or mediator subunits in coactivator mutants. (A-D) myc- or Flag-tagged strains used in Fig. 2 or 5 or the untagged parent strain BY4741 (panel C) was grown under the same conditions used for ChIP analysis, and WCEs were prepared and subjected to Western blot analysis using anti-myc, anti-Flag M2, or anti-Gcd6p antibodies, the last serving as loading control. The Western signals obtained using the ECL chemiluminescence kit (Amersham) were quantified by video densitometry using NIH image software, and the ratios of myc-Spt7p, myc-Ada2p, myc-Srb6p, or myc-Gal11p to Gcd6p signals are listed for each mutant relative to the WT strain between the two blots.
The results thus far indicate that Ada1p, Ada5p, Spt7p, and Spt3p are required, but that the Gcn5p/Ada2p/Ada3p HAT module is dispensable, for high-level recruitment of SAGA subunits by Gcn4p. Thus, if Ada2p is a direct target of the Gcn4p activation domain, as suggested previously (3), it cannot be recruited efficiently as an isolated subunit, or as a component of the ADA complex, outside of the intact SAGA complex.
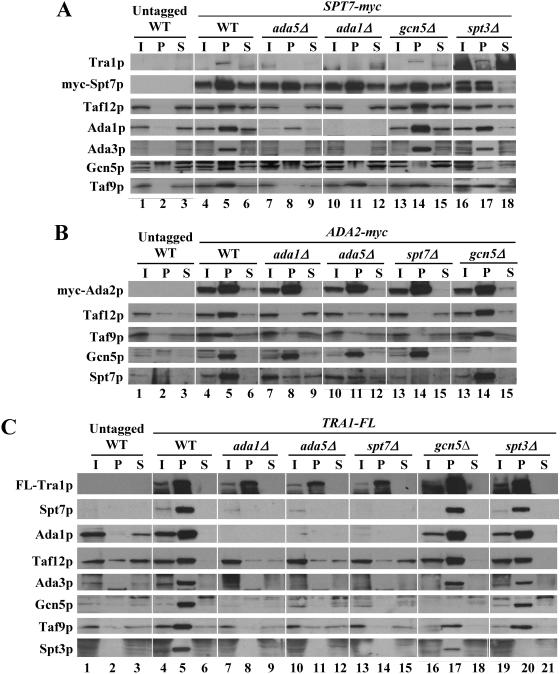
Ada1p, Ada5p, and Spt7p are required to purify an intact SAGA complex from yeast cells (23, 61, 71). To confirm that myc-Spt7p and myc-Ada2p are dissociated from other SAGA subunits in the ada1Δ, ada5Δ, and spt7Δ mutants in vivo, we immunoprecipitated these proteins with anti-myc antibodies and probed the immune complexes for other SAGA subunits. As expected, SAGA subunits Taf12p, Ada3p, Gcn5p, and Tra1p were largely or completely dissociated from myc-Spt7p in ada1Δ and ada5Δ strains, although Taf9p remained strongly associated with myc-Spt7p in the ada1Δ mutant. Ada1p was associated with myc-Spt7p at a reduced level in ada5Δ cells, although the total level of Ada1p was reduced in this mutant extract (Fig. 4A). Similarly, myc-Ada2p was dissociated from Taf12p and Taf9p in ada1Δ, ada5Δ, and spt7Δ mutants, and its interaction with Spt7p was greatly reduced in the ada1Δ and ada5Δ cells (Fig. 4B). By contrast, Gcn5p remained fully associated with myc-Ada2p in the ada1Δ, ada5Δ, and spt7Δ mutants, presumably reflecting an intact Ada2p/Ada3p/Gcn5p subcomplex in addition to the ADA complex in such mutants with disrupted SAGA (2). As expected, deletion of GCN5 or SPT3 had little effect on association of other SAGA subunits with myc-Spt7p or myc-Ada2p (Fig. 4A and B) (71). Thus, in agreement with the previous findings cited above, we conclude that Ada1p, Ada5p, and Spt7p are required for SAGA integrity in vivo.
FIG. 4.
Coimmunoprecipitation analysis of SAGA integrity in coactivator mutants. WCEs from the appropriate yeast strains were immunoprecipitated with monoclonal c-myc or Flag M2 antibodies. The immune complexes were collected, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and subjected to Western analysis to detect the proteins listed on the left of each panel or with anti-myc or anti-Flag M2 antibodies to detect the myc- or Flag-tagged proteins. I, 10% of the input WCEs; P, 50% of the pellet fraction from the immunoprecipitates; S, 10% of the supernatant fractions.
The simplest way to explain the fact that recruitment of myc-Ada2p and myc-Spt7p is reduced in all three mutants where SAGA integrity is disrupted (ada1Δ, ada5Δ, and spt7Δ) is to propose that Gcn4p interacts with only one or two SAGA subunits and that all other subunits must be connected to these targeted proteins to be efficiently recruited by Gcn4p in vivo. Previous evidence indicated that Tra1p is a direct target of the activators Hap4p (10) and Gal4p (7), and it was shown that Gcn4p can interact directly with purified Tra1p in vitro in the absence of other SAGA subunits (10). Assuming that Tra1p is a target of Gcn4p, we wished to determine whether Tra1p can be recruited by Gcn4p in mutant cells where SAGA is disrupted.
To answer this question, we deleted chromosomal TRA1 in the panel of SAGA mutants described above and replaced it with a functional Flag-tagged allele of TRA1 expressed from its own promoter on a single-copy plasmid (TRA1-FL). ChIP analysis of the resulting strains showed that recruitment of FL-Tra1p by Gcn4p was significantly reduced in the mutants lacking Ada1p, Ada5p, Spt7p, and Spt3p but occurred at essentially wild-type levels in the mutants lacking Ada2p, Ada3p, or Gcn5p (Fig. 2C). Western analysis showed that expression of FL-Tra1p was essentially unaffected by all of the deletions under consideration (Fig. 3C), and the coimmunoprecipitation experiments in Fig. 4A and C confirmed that Tra1p was dissociated from other SAGA subunits in the ada1Δ, ada5Δ, and spt7Δ strains but not in spt3Δ cells or in other SAGA mutants. Thus, we conclude that dissociation of FL-Tra1p from other SAGA subunits reduces the efficiency of FL-Tra1p recruitment by Gcn4p in vivo.
Wild-type recruitment of SAGA does not require the ATPase subunit of SWI/SNF but is dependent on multiple Srb mediator subunits.
Since Gcn4p can interact specifically with SAGA in vitro, it was possible that efficient SAGA recruitment in vivo would be independent of other coactivators. To explore this possibility, we asked whether recruitment of SAGA is dependent on SWI/SNF and Srb mediator by constructing SPT7-myc and ADA2-myc alleles in deletion mutants lacking Swi2p; the ATPase subunit of SWI/SNF; or the Med2p, Srb2p, Srb5p, or Rox3p subunit of Srb mediator. We have shown (53) that all four mediator mutants are defective in transcriptional activation by Gcn4p, with rox3Δ cells exhibiting the largest reductions in mRNA levels at all three target genes under study. The swi2Δ mutant displayed little defect in ARG1 and ARG4 mRNA induction but a marked decrease in Gcn4p-dependent induction of SNZ1 mRNA. Furthermore, we found that none of these mutations reduced binding of myc-Gcn4p to the target genes in vivo (53).
All four mediator subunit deletions impaired the recruitment of SAGA subunits in the following order of increasing severity: med2Δ, srb2Δ, srb5Δ, and rox3Δ (Fig. 5A and B). Western analysis shows that these reductions do not arise from decreased steady-state levels of myc-Spt7p or myc-Ada2p in the mediator mutants, with the possible exception of the rox3Δ strain (Fig. 3A and B, lanes 10 to 14). Even in this instance, however, the 75 to 87% reductions in myc-Spt7p recruitment (Fig. 5A) significantly exceed the ∼30% reduction in myc-Spt7p expression in rox3Δ cells (Fig. 3A).
FIG. 5.
Interdependent recruitment of SAGA and Srb mediator by Gcn4p. (A) ChIP analysis of a gcn4Δ SPT7-myc strain carrying empty vector (gcn4Δ) or GCN4 SPT7-myc strains containing no coactivator mutations (WT) or the indicated deletions of Srb mediator subunits, or swi2Δ, and harboring high-copy-number GCN4-HA plasmid pHQ1239, conducted as described in Fig. 1 and 2. (B) Same as panel A except that ADA2-myc strains were employed. (C-D) Same as panels A and B, except that SRB6-myc and GAL11-myc strains were employed, containing no coactivator mutations (WT) or the indicated deletions of SAGA subunits or swi2Δ.
In contrast to the effects of mediator mutations, the swi2Δ mutant exhibits wild-type or higher levels of myc-Spt7p recruitment at all three target genes and wild-type or higher levels of myc-Ada2p binding at the ARG1 and ARG4 promoters. The only recruitment deficit observed in swi2Δ cells was a strong reduction in myc-Ada2p binding at SNZ1 (Fig. 5B). We conclude that recruitment of SAGA by Gcn4p is critically dependent on mediator but largely independent of SWI/SNF ATPase function. The fact that swi2Δ reduces the recruitment of Ada2p but not Spt7p at SNZ1 may indicate more stringent requirements for retention of the ADA subcomplex compared to the rest of SAGA at this promoter. Indeed, we previously observed more stringent requirements for Gcn4p recruitment of SWI/SNF (72) and Srb mediator (73) at SNZ1 versus ARG1. Our finding that SAGA recruitment is significantly elevated at ARG1 and ARG4 in swi2Δ cells (Fig. 5A and B) may indicate that nucleosome remodeling by SWI/SNF somehow limits the recruitment of SAGA by Gcn4p.
Recruitment of Srb mediator requires SAGA complex but not SWI/SNF function.
We asked next whether recruitment of mediator is dependent on SAGA or SWI/SNF activity by introducing functional SRB6-myc or GAL11-myc alleles (64) into the ada1Δ, ada5Δ, spt7Δ, gcn5Δ, and swi2Δ mutants and conducting ChIP analysis. The results in Fig. 5C and D indicate that wild-type (or higher) levels of myc-Srb6p and myc-Gal11p recruitment occurred in swi2Δ cells, showing that SWI/SNF ATPase activity is dispensable for recruitment of Srb mediator by Gcn4p. Inactivating the HAT activity of SAGA by deletion of GCN5 also had little effect on Srb mediator recruitment by Gcn4p. However, recruitment of myc-Srb6p and myc-Gal11p at the ARG4 and SNZ1 promoters was substantially reduced in the ada1Δ, ada5Δ, and spt7Δ mutants that disrupt SAGA integrity and impair recruitment of SAGA itself by Gcn4p (Fig. 5C and D, ARG4 and SNZ1). The reduction in recruitment of myc-Srb6p and myc-Gal11p in these SAGA mutants does not arise from reduced expression of mediator subunits (Fig. 3D). Thus, a non-HAT function dependent on the integrity of SAGA complex is needed for optimal recruitment of Srb mediator by Gcn4p to ARG4 and SNZ1. Deletion of SPT3 also impaired recruitment of myc-Gal11p to ARG4 and SNZ1 by 60 to 70% (data not shown), in accordance with the reduction in recruitment of SAGA itself conferred by spt3Δ (Fig. 2). Because efficient recruitment of SAGA at these genes requires mediator subunits, it appears that recruitment of SAGA and mediator is highly interdependent at ARG4 and SNZ1.
Surprisingly, SAGA is much less important for recruitment of Srb mediator at ARG1 compared to ARG4 and SNZ1. The ada1Δ, ada5Δ, and spt7Δ mutations reduced the recruitment of myc-Srb6p to ARG1 by only 20 to 40% and had little effect on recruitment of myc-Gal11p by Gcn4p to this gene (Fig. 5C and D). The spt3Δ mutation likewise had a small effect on myc-Gal11p recruitment at ARG1, reducing it by only ∼25% (data not shown). The difference between the results obtained for myc-Srb6p and myc-Gal11p may be related to our recent finding that the Gal11p/Med2p/Pgd1p triad from the tail domain of mediator is an in vivo target of Gcn4p that can be recruited to ARG1 independently of the rest of mediator (73). Thus, perhaps binding of the mediator tail domain at ARG1 can be maintained independently of SAGA, whereas the mediator head domain (to which Srb6p belongs) requires SAGA function for maximal recruitment by Gcn4p. It is currently unclear why recruitment of both mediator head and tail subunits is less dependent on SAGA at ARG1 than at ARG4 and SNZ1.
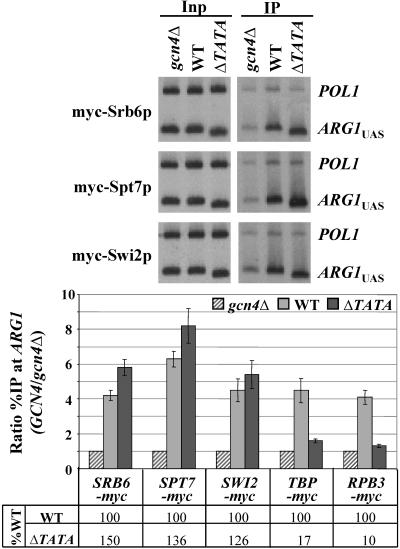
Deletion of the TATA element at ARG1 does not reduce recruitment of SAGA, Srb mediator, or SWI/SNF to the UASGCRE by Gcn4p.
We previously reported that deletion of the TATA element at ARG1 (ΔTATA mutation) greatly reduced recruitment of TBP and Pol II to the promoter by Gcn4p and impaired ARG1 expression, producing arginine auxotrophy (53). These findings indicated that TBP recruitment is a prerequisite for high-level Pol II binding at ARG1. It was reported recently that recruitment of SWI/SNF and Srb mediator at RNR3 was impaired by mutations in Rpb1p and a TFIID subunit, suggesting a requirement for PIC assembly for retention of SWI/SNF and mediator at this gene (59). Hence, to determine whether recruitment of SAGA, Srb mediator, and SWI/SNF by Gcn4p is dependent on stable TBP and Pol II binding to the promoter, we asked whether the ΔTATA mutation at ARG1 would reduce recruitment of myc-tagged subunits of SAGA, Srb mediator, and SWI/SNF to the ARG1 UAS. As shown in Fig. 6, subunits of SAGA, Srb mediator, and SWI/SNF were recruited by Gcn4p at the same level, or even higher levels, to the UAS of TATA-less ARG1 compared to wild-type ARG1. For comparison, we included in Fig. 6 the quantification of our previous results indicating that recruitment of TBP and Pol II to the promoter was greatly impaired by the ΔTATA mutation (53).
FIG. 6.
Deletion of the ARG1 TATA does not affect recruitment of SAGA, Srb mediator, and SWI/SNF by Gcn4p. Transformants of gcn4Δ strains carrying empty vector (gcn4Δ) or high-copy-number GCN4-HA plasmid pHQ1239 (WT) and of gcn4Δ arg1-ΔTATA strains carrying pHQ1239 (ΔTATA), harboring SRB6-myc, SPT7-myc, or SWI2-myc, were subjected to ChIP analysis as described in Fig. 1 and 2. (Data for TBP-myc and RPB3-myc shown in the histogram were published previously [53] and are provided here only for comparison.)
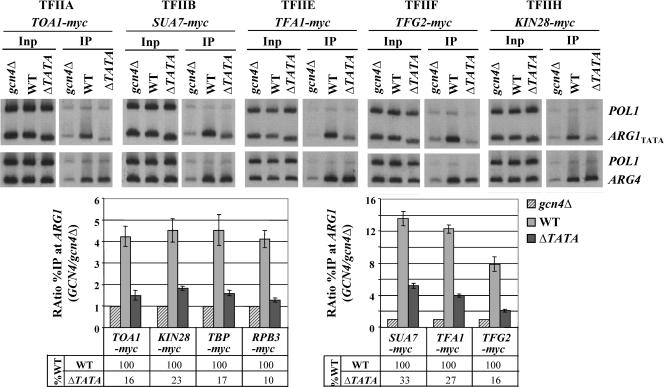
We next investigated whether recruitment of various GTFs by Gcn4p can occur independently of TBP and Pol II binding to the promoter by examining whether the ΔTATA mutation at ARG1 reduces recruitment of functional myc-tagged versions of TFIIB (myc-Sua7) or subunits of TFIIA (myc-Toa1p), TFIIE (myc-Tfa1p), TFIIF (myc-Tfg2p), or TFIIH (myc-Kin28p). These experiments were stimulated by reports of direct binding of activators to GTFs (reviewed in reference 17) and of association of GTFs with mediator (31, 40, 55, 56, 66). As shown in Fig. 7, Gcn4p recruits all of these GTFs to the ARG1 promoter in a manner dependent on the TATA element. The strong reduction in myc-Toa1p, myc-Tfg2p, and myc-Kin28p recruitment produced by the ΔTATA mutation is comparable to that observed for myc-TBP itself, suggesting that TFIIA, TFIIF, and TFIIH recruitment is wholly dependent on TBP recruitment by Gcn4p. Recruitment of TFIIB and TFIIE may be partly independent of TBP, however, as their recruitment was impaired less than that of TBP and Pol II by the ΔTATA mutation (Fig. 7).
FIG. 7.
TBP binding to TATA box is a prerequisite for recruitment of GTFs by Gcn4p. gcn4Δ, WT, and arg1-ΔTATA strains containing chromosomal TOA1-myc, SUA7-myc, TFA1-myc, TFG2-myc, or KIN28-myc alleles were subjected to ChIP analysis as described in Fig. 1 and 2. (Data for TBP-myc and RPB3-myc in the histogram were published previously [53].)
DISCUSSION
In this report, we have addressed four issues regarding the mechanism of transcriptional activation by Gcn4p in vivo. Regarding the subunit requirements for SAGA recruitment, we showed that recruitment of SAGA to the UASGCRE elements at three Gcn4p target genes is strongly dependent on Ada1p, Ada5p, and Spt7p; moderately dependent on Spt3p; and largely independent of Spt8p and the Gcn5p/Ada2p/Ada3p HAT module. Evidence was presented previously that Tra1p is a direct target of Gcn4p (10); however, we observed that recruitment of FL-Tra1p by Gcn4p was significantly reduced by deletion of ADA1, ADA5, SPT7, or SPT3. Because the SAGA complex is disrupted by the ada1Δ, ada5Δ, and spt7Δ mutations, it appears that optimal recruitment of Tra1p in vivo is dependent on its presence in the intact SAGA complex. Thus, either Gcn4p must interact with one or more SAGA subunits besides Tra1p for efficient recruitment of the entire complex or Tra1p must interact with other SAGA subunits to assume the proper conformation needed for a robust interaction with Gcn4p. Tra1p is a subunit of the NuA4 HAT complex in addition to SAGA (1), and Gcn4p was shown to interact specifically with NuA4 in vitro (68). Thus, much of the residual FL-Tra1p recruited by Gcn4p in the ada1Δ, ada5Δ, and spt7Δ strains may be in the form of NuA4.
Our finding that recruitment of SAGA subunits was reduced by spt3Δ, even though the SAGA complex is fully intact in this mutant (71), might indicate that Spt3p provides a second contact for Gcn4p in SAGA besides Tra1p. An alternative possibility, that deletion of Spt3p alters the conformation of Tra1p in a manner that reduces its interaction with Gcn4p, may be unlikely considering a recent structural model of SAGA in which Spt3p resides in a flexible domain distantly located from the bulk of Tra1p in the extended complex (70). We recently investigated whether Spt3p can interact directly with recombinant glutathione _S_-transferase (GST)-Gcn4p in a “GST pull-down” assay employed previously to measure binding of SAGA to Gcn4p in WCEs (17, 43). Whereas Spt3p bound specifically to GST-Gcn4p in a WT extract, this did not occur in an ada1Δ extract. By contrast, FL-Tra1p bound to GST-Gcn4p in both WT and ada1Δ extracts (data not shown), in accordance with previous results (10). While these data might indicate that Gcn4p does not contact Spt3p directly, it is also possible that Spt3p is not folded properly outside of SAGA or that the dissociation rate of a Gcn4p-Spt3p complex is too high to be detected with this binding assay. Because high-level SAGA recruitment by Gcn4p is dependent on Srb mediator, another intriguing possibility is that Spt3p is required for the stimulatory effect of mediator on SAGA recruitment.
It was shown recently that Spt20p/Ada5p is required for Gal4p-Tra1p interaction and for the recruitment of Tra1p to the GAL1 UAS by Gal4p in yeast cells (7). Thus, it seems that Gal4p cannot efficiently recruit Tra1p to the GAL1 promoter outside of the context of SAGA, just as we observed for Gcn4p. Deletion of SPT3 had a smaller effect on recruitment of Spt20p by Gal4p than was generally observed here for Gcn4p, and it was concluded that Spt3p is not required for SAGA recruitment by Gal4p (36).
We found that ada2Δ, ada3Δ, and gcn5Δ led to higher-than-WT levels of myc-Spt7p recruitment (Fig. 2A), that gcn5Δ led to elevated recruitment of myc-Ada2p (Fig. 2B), and that ada2Δ increased the recruitment of FL-Tra1p (Fig. 2C) to the ARG1 promoter. These findings suggest that histone acetylation by Gcn5p may antagonize SAGA recruitment to ARG1. The fact that ada3Δ did not elevate recruitment of myc-Ada2p at ARG1 can be explained by the reduced expression of myc-Ada2p in ada3Δ cells (Fig. 3B); however, it is more difficult to explain why ada3Δ and gcn5Δ did not elevate FL-Tra1p recruitment to ARG1. Thus, further study is required before drawing any firm conclusion about the impact of Gcn5p HAT activity on SAGA recruitment to ARG1. It is also intriguing that spt8Δ significantly reduced SAGA recruitment only at SNZ1. As noted above, there are more stringent requirements for coactivator recruitment by Gcn4p at SNZ1 versus ARG1 (72, 73), although the molecular basis for this difference is unknown. Presumably, the elimination of Spt8p reduces the binding of SAGA to Gcn4p to a small extent that can be compensated for by other interactions at ARG1 and ARG4 but not at SNZ1.
The second major question addressed in this report is whether the recruitment of one coactivator by Gcn4p enhances the recruitment of others. We showed previously (72) that recruitment of SWI/SNF by Gcn4p is impaired by deletions of Srb mediator subunits, including Gal11p, Med2p, and Rox3p, and that Gal11p and Med2p are required for efficient recruitment of mediator itself to ARG1 (73). Thus, high-level recruitment of SWI/SNF is dependent on recruitment of Srb mediator by Gcn4p. We also reported previously that recruitment of SWI/SNF was dependent on SAGA integrity but independent of the SAGA HAT Gcn5p (72). In contrast to the requirement for mediator and SAGA in SWI/SNF recruitment, we showed here that recruitment of SAGA and Srb mediator was not reduced by inactivating the nucleosome-remodeling function of SWI/SNF by deleting SWI2. In fact, recruitment of SAGA and mediator appeared to be increased considerably at ARG1, and also slightly at ARG4, in the swi2Δ mutant (Fig. 5). The latter findings may indicate that remodeling of the nucleosomal array at these promoters by SWI/SNF decreases retention of SAGA and mediator.
We further demonstrated here that efficient recruitment of SAGA is dependent on Srb mediator subunits Rox3p, Srb5p, Srb2p and Med2p and, likewise, that high-level recruitment of Srb mediator at ARG4 and SNZ1 is dependent on SAGA integrity but not on Gcn5p (Fig. 5). Thus, even though Gcn4p can interact directly with SAGA, mediator, and SWI/SNF in vitro, these interactions do not suffice for high-level recruitment of these coactivators by Gcn4p to target promoters in vivo. Additional work will be required to understand how recruitment of Srb mediator escapes the dependence on SAGA for wild-type recruitment to the ARG1 UASGCRE. In fact, recruitment of the tail domain of mediator (containing Gal11p) seems to be significantly elevated at ARG1 in SAGA mutants (Fig. 5D). Of even greater importance will be to determine how SAGA and Srb mediator can stimulate SWI/SNF recruitment and also mutually enhance their own recruitment by Gcn4p.
Our findings on Swi2p-independent recruitment of SAGA by Gcn4p are in agreement with a previous analysis of Gcn5p recruitment by Gcn4p to a synthetic PHO5 promoter harboring a UASGCRE (65). However, our results contrast with those of Topalidou and Thireos, who observed high-level recruitment of SAGA independent of mediator to various UASGCRE elements that are separated from core promoter sequences, such as in open reading frames (67). It is unclear at present why mediator is required for efficient SAGA recruitment by Gcn4p to intact bona fide promoters, such as ARG1 or ARG4 (Fig. 5A and B), but not to UASGCRE elements unconnected to core promoter sequences.
While Gal4p and Gcn4p are often regarded as acidic activators of a similar nature, they differ substantially with respect to their mechanisms of coactivator recruitment. Neither Bhaumik et al. nor Bryant and Ptashne observed any reduction in SAGA recruitment by Gal4p in response to mutations in mediator subunits, including srb4-ts (7) and gal11Δ (11), even though srb4-ts abolishes PIC formation at GAL1 (39). Hence, the marked dependency on Srb mediator for SAGA recruitment observed here for Gcn4p is not shared by Gal4p (Table 3). In addition, it appears that mediator, but not SAGA, is required for high-level recruitment of SWI/SNF by Gal4p (37), whereas both SAGA and mediator contribute substantially to recruitment of SWI/SNF by Gcn4p (72). Furthermore, SWI/SNF recruitment by Gal4p requires Pol II binding to the promoter (37), whereas we showed here that Gcn4p can recruit SWI/SNF independently of PIC formation. There is conflicting evidence concerning the requirement for SAGA in mediator recruitment by Gal4p (7, 11, 37), making it difficult to determine whether the situation is more similar to our findings for Gcn4p at ARG1, where mediator recruitment is largely independent of SAGA, or to our findings at ARG4 and SNZ1, where SAGA makes an important contribution to mediator recruitment by Gcn4p (Table 3).
TABLE 3.
Comparison of requirements for recruitment of mediator, SAGA, and SWI/SNF by three different yeast activatorsa
| Requirement | Coactivator recruited and activator | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mediator | SAGA | SWI/SNF | |||||||
| Gal4p | Gcn4p | Swi5p | Gal4p | Gcn4p | Swi5p | Gal4p | Gcn4p | Swi5p | |
| Mediator | − | + | ND | + | + | − | |||
| SAGA | ? | +(−) | − | − | + | − | |||
| SWI/SNF | ND | − | + | ND | − | + | |||
| PIC assembly | − | − | − | − | − | − | + | − | − |
A completely different pattern of coactivator interdependency has been described for the HO gene, at which SWI/SNF recruitment by the activator Swi5p is a prerequisite for recruitment of both SAGA (14) and Srb mediator (8), and mediator is not required for SWI/SNF recruitment (8). Note, however, that the requirement for SWI/SNF in SAGA recruitment at HO appears to be restricted to late mitosis and applies even to Gal4p- and Gcn4p-regulated promoters in this phase of the cell cycle, most likely involving a highly condensed state of promoter chromatin (32). Thus, the degree of coactivator interdependency can vary for the same activator depending on the chromatin structure of the UAS.
The third question regarding the Gcn4p recruitment program addressed here is whether PIC formation is required for high-level recruitment or retention of coactivators at the UASGCRE by Gcn4p. Previous studies of Gal4p showed that SAGA and Srb mediator can be recruited by Gal4p to the GAL1 UAS in the absence of a downstream promoter element and that Ts− mutations in TBP, TFIIB, or Pol II do not reduce recruitment of these coactivators to the GAL1 UAS even though they destroy PIC formation (6, 7, 34). Consistent with this, recruitment of SAGA and mediator precedes that of TBP, GTFs, and Pol II at GAL1 following induction by galactose (11). Thus, efficient recruitment of SAGA and mediator by Gal4p is independent of PIC formation at the GAL1 promoter. By contrast, as noted above, recruitment of SWI/SNF by Gal4p seems to require Pol II recruitment to the GAL1 promoter (37). Similarly, TBP and Pol II binding at the RNR3 promoter were shown to be required for optimal recruitment of SWI/SNF and mediator at this gene (59). By contrast, we found that recruitment of SWI/SNF, as well as SAGA and mediator, by Gcn4p was unaffected by deletion of the TATA box at ARG1, a mutation that impairs recruitment of TBP, GTFs, and Pol II. Thus, Gcn4p recruits all three coactivators to the ARG1 UAS independently of PIC assembly at the promoter. A similar conclusion was reached for mediator and SAGA using engineered PHO5 promoters with a UASGCRE either containing or lacking a TATA box (67). The fact that recruitment of Pol II, but not Srb mediator, is impaired by the ΔTATA mutation also indicates that mediator can be recruited by Gcn4p independent of its association with Pol II in the holoenzyme, as concluded previously for other activators (8, 11, 34, 49).
Finally, we found that recruitment of TFIIA, TFIIF, and TFIIH to ARG1 is completely dependent on TBP binding to the promoter, as deletion of the TATA element impaired recruitment of these GTFs to the same degree that it reduced TBP binding at ARG1. Although the TATA deletion also produced a marked reduction in TFIIB and TFIIE recruitment, there appeared to be significant residual binding of these factors to the TATA-less ARG1 promoter. Thus, TBP-independent binding of TFIIB and TFIIE to the promoter may be enhanced by their interactions with mediator or another coactivator recruited by Gcn4p to the UAS element.
Based on our findings, we can now propose a pathway for the stimulation of PIC formation by Gcn4p. Because Gcn4p can directly interact with SAGA, mediator, and SWI/SNF in vitro, and it recruits all three coactivators to the ARG1 UAS in the absence of the TATA element, we propose that Gcn4p directly recruits SAGA, Srb mediator (free of Pol II), and SWI/SNF to the UASGCRE. SAGA and mediator facilitate the recruitment of one another and also enhance SWI/SNF recruitment or retention by Gcn4p. All three coactivators function directly or indirectly to stimulate TBP binding to the TATA element, which, in turn, permits recruitment of the remaining GTFs and Pol II to the promoter to complete the assembly of a preinitiation complex.
Acknowledgments
We thank Fred Winston and Jerry Workman for generous gifts of antibodies and Chhabi Govind for critical reading of the manuscript and many helpful suggestions.
REFERENCES
- 1.Allard, S., R. T. Utley, J. Savard, A. Clarke, P. Grant, C. J. Brandl, L. Pillus, J. L. Workman, and J. Cote. 1999. NuA4, an essential transcription adaptor/histone H4 acetyltransferase complex containing Esa1p and the ATM-related cofactor Tra1p. EMBO J. 18**:**5108-5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balasubramanian, R., M. G. Pray-Grant, W. Selleck, P. A. Grant, and S. Tan. 2002. Role of the Ada2 and Ada3 transcriptional coactivators in histone acetylation. J. Biol. Chem. 277**:**7989-7995. [DOI] [PubMed] [Google Scholar]
- 3.Barlev, N. A., R. Candau, L. Wang, P. Darpino, N. Silverman, and S. L. Berger. 1995. Characterization of physical interactions of the putative transcriptional adaptor, ADA2, with acidic activation domains and TATA-binding protein. J. Biol. Chem. 270**:**19337-19344. [DOI] [PubMed] [Google Scholar]
- 4.Belotserkovskaya, R., D. E. Sterner, M. Deng, M. H. Sayre, P. M. Lieberman, and S. L. Berger. 2000. Inhibition of TATA-binding protein function by SAGA subunits Spt3 and Spt8 at Gcn4-activated promoters. Mol. Cell. Biol. 20**:**634-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger, S. L., B. Pina, N. Silverman, G. A. Marcus, J. Agapite, J. L. Regier, S. J. Triezenberg, and L. Guarente. 1992. Genetic isolation of ADA2: a potential transcription adaptor required for function of certain acidic activation domains. Cell 70**:**251-265. [DOI] [PubMed] [Google Scholar]
- 6.Bhaumik, S. R., and M. R. Green. 2001. SAGA is an essential in vivo target of the yeast acidic activator Gal4p. Genes Dev. 15**:**1935-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhaumik, S. R., T. Raha, D. P. Aiello, and M. R. Green. 2004. In vivo target of a transcriptional activator revealed by fluorescence resonance energy transfer. Genes Dev. 18**:**333-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhoite, L. T., Y. Yu, and D. J. Stillman. 2001. The Swi5 activator recruits the mediator complex to the HO promoter without RNA polymerase II. Genes Dev. 15**:**2457-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjorklund, S., O. Buzaite, and M. Hallberg. 2001. The yeast mediator. Mol. Cells 11**:**129-136. [PubMed] [Google Scholar]
- 10.Brown, C., L. Howe, K. Sousa, S. C. Alley, M. J. Carrozza, S. Tan, and J. L. Workman. 2001. Recruitment of HAT complexes by direct activator interactions with the AATM-related Tra1 subunit. Science 292**:**2333. [DOI] [PubMed] [Google Scholar]
- 11.Bryant, G. O., and M. Ptashne. 2003. Independent recruitment in vivo by Gal4 of two complexes required for transcription. Mol. Cell 11**:**1301-1309. [DOI] [PubMed] [Google Scholar]
- 12.Cigan, A. M., M. Foiani, E. M. Hannig, and A. G. Hinnebusch. 1991. Complex formation by positive and negative translational regulators of GCN4. Mol. Cell. Biol. 11**:**3217-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conaway, R. C., and J. W. Conaway. 1997. General transcription factors for RNA polymerase II. Prog. Nucleic Acid Res. Mol. Biol. 56**:**327-346. [DOI] [PubMed] [Google Scholar]
- 14.Cosma, M. P., T. Tanaka, and K. Nasmyth. 1999. Ordered recruitment of transcription and chromatin remodeling factors to a cell cycle- and developmentally regulated promoter. Cell 97**:**299-311. [DOI] [PubMed] [Google Scholar]
- 15.Dhalluin, C., J. E. Carlson, L. Zeng, C. He, A. K. Aggarwal, and M. M. Zhou. 1999. Structure and ligand of a histone acetyltransferase bromodomain. Nature 399**:**491-496. [DOI] [PubMed] [Google Scholar]
- 16.Drysdale, C. M., E. Dueñas, B. M. Jackson, U. Reusser, G. H. Braus, and A. G. Hinnebusch. 1995. The transcriptional activator GCN4 contains multiple activation domains that are critically dependent on hydrophobic amino acids. Mol. Cell. Biol. 15**:**1220-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drysdale, C. M., B. M. Jackson, R. McVeigh, E. R. Klebanow, Y. Bai, T. Kokubo, M. Swanson, Y. Nakatani, P. A. Weil, and A. G. Hinnebusch. 1998. The Gcn4p activation domain interacts specifically in vitro with RNA polymerase II holoenzyme, TFIID, and the Adap-Gcn5p coactivator complex. Mol. Cell. Biol. 18**:**1711-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudley, A. M., C. Rougeulle, and F. Winston. 1999. The Spt components of SAGA facilitate TBP binding to a promoter at a post-activator-binding step in vivo. Genes Dev. 13**:**2940-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eberharter, A., D. E. Sterner, D. Schieltz, A. Hassan, J. R. Yates, S. L. Berger, and J. L. Workman. 1999. The ADA complex is a distinct histone acetyltransferase complex in Saccharomyces cerevisiae. Mol. Cell. Biol. 19**:**6621-6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Featherstone, M. 2002. Coactivators in transcription initiation: here are your orders. Curr. Opin. Genet. Dev. 12**:**149-155. [DOI] [PubMed] [Google Scholar]
- 21.Georgakopoulos, T., and G. Thireos. 1992. Two distinct yeast transcriptional activators require the function of the GCN5 protein to promote normal levels of transcription. EMBO J. 11**:**4145-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gietz, R. D., and A. Sugino. 1988. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74**:**527-534. [DOI] [PubMed] [Google Scholar]
- 23.Grant, P. A., L. Duggan, J. Cote, S. M. Roberts, J. E. Brownell, R. Candau, R. Ohba, T. Owen-Hughes, C. D. Allis, F. Winston, S. L. Berger, and J. L. Workman. 1997. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev. 11**:**1640-1650. [DOI] [PubMed] [Google Scholar]
- 24.Grant, P. A., A. Eberharter, S. John, R. G. Cook, B. M. Turner, and J. L. Workman. 1999. Expanded lysine acetylation specificity of Gcn5 in native complexes. J. Biol. Chem. 274**:**5895-5900. [DOI] [PubMed] [Google Scholar]
- 25.Hassan, A. H., P. Prochasson, K. E. Neely, S. C. Galasinski, M. Chandy, M. J. Carrozza, and J. L. Workman. 2002. Function and selectivity of bromodomains in anchoring chromatin-modifying complexes to promoter nucleosomes. Cell 111**:**369-379. [DOI] [PubMed] [Google Scholar]
- 26.Hinnebusch, A. G. 1996. Translational control of GCN4: gene-specific regulation by phosphorylation of eIF2, p. 199-244. In J. W. B. Hershey, M. B. Mathews, and N. Sonenberg (ed.), Translational control. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 27.Hudson, B. P., M. A. Martinez-Yamout, H. J. Dyson, and P. E. Wright. 2000. Solution structure and acetyl-lysine binding activity of the GCN5 bromodomain. J. Mol. Biol. 304**:**355-370. [DOI] [PubMed] [Google Scholar]
- 28.Jackson, B. M., C. M. Drysdale, K. Natarajan, and A. G. Hinnebusch. 1996. Identification of seven hydrophobic clusters in GCN4 making redundant contributions to transcriptional activation. Mol. Cell. Biol. 16**:**5557-5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobson, R. H., A. G. Ladurner, D. S. King, and R. Tjian. 2000. Structure and function of a human TAFII250 double bromodomain module. Science 288**:**1422-1425. [DOI] [PubMed] [Google Scholar]
- 30.Kang, J. S., S. H. Kim, M. S. Hwang, S. J. Han, Y. C. Lee, and Y. J. Kim. 2001. The structural and functional organization of the yeast mediator complex. J. Biol. Chem. 276**:**42003-42010. [DOI] [PubMed] [Google Scholar]
- 31.Kim, Y. J., S. Bjorklund, Y. Li, M. H. Sayre, and R. D. Kornberg. 1994. A multiprotein mediator of transcriptional activation and its interaction with the C-terminal repeat domain of RNA polymerase II. Cell 77**:**599-608. [DOI] [PubMed] [Google Scholar]
- 32.Krebs, J. E., C. J. Fry, M. L. Samuels, and C. L. Peterson. 2000. Global role for chromatin remodeling enzymes in mitotic gene expression. Cell 102**:**587-598. [DOI] [PubMed] [Google Scholar]
- 33.Kuo, M. H., E. vom Bauer, K. Struhl, and C. D. Allis. 2000. Gcn4 activator targets Gcn5 histone acetyltransferase to specific promoters independently of transcription. Mol. Cell 6**:**1309-1320. [DOI] [PubMed] [Google Scholar]
- 34.Kuras, L., T. Borggrefe, and R. D. Kornberg. 2003. Association of the mediator complex with enhancers of active genes. Proc. Natl. Acad. Sci. USA 100**:**13887-13891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuras, L., and K. Struhl. 1999. Binding of TBP to promoters in vivo is stimulated by activators and requires Pol II holoenzyme. Nature 399**:**609-613. [DOI] [PubMed] [Google Scholar]
- 36.Larschan, E., and F. Winston. 2001. The S. cerevisiae SAGA complex functions in vivo as a coactivator for transcriptional activation by Gal4. Genes Dev. 15**:**1946-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemieux, K., and L. Gaudreau. 2004. Targeting of Swi/Snf to the yeast GAL1 UAS(G) requires the mediator, TAF(II)s, and RNA polymerase II. EMBO J. 23**:**4040-4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, X., A. Virbasius, X. Zhu, and M. R. Green. 1999. Enhancement of TBP binding by activators and general transcription factors. Nature 399**:**605-609. [DOI] [PubMed] [Google Scholar]
- 39.Li, X. Y., S. R. Bhaumik, and M. R. Green. 2000. Distinct classes of yeast promoters revealed by differential TAF recruitment. Science 288**:**1242-1244. [DOI] [PubMed] [Google Scholar]
- 40.Myers, L. C., and R. D. Kornberg. 2000. Mediator of transcriptional regulation. Annu. Rev. Biochem. 69**:**729-749. [DOI] [PubMed] [Google Scholar]
- 41.Naar, A. M., B. D. Lemon, and R. Tjian. 2001. Transcriptional coactivator complexes. Annu. Rev. Biochem. 70**:**475-501. [DOI] [PubMed] [Google Scholar]
- 42.Narlikar, G. J., H. Y. Fan, and R. E. Kingston. 2002. Cooperation between complexes that regulate chromatin structure and transcription. Cell 108**:**475-487. [DOI] [PubMed] [Google Scholar]
- 43.Natarajan, K., B. M. Jackson, E. Rhee, and A. G. Hinnebusch. 1998. yTAFII61 has a general role in RNA polymerase II transcription and is required by Gcn4p to recruit the SAGA coactivator complex. Mol. Cell 2**:**683-692. [DOI] [PubMed] [Google Scholar]
- 44.Natarajan, K., B. M. Jackson, H. Zhou, F. Winston, and A. G. Hinnebusch. 1999. Transcriptional activation by Gcn4p involves independent interactions with the SWI/SNF complex and SRB/mediator. Mol. Cell 4**:**657-664. [DOI] [PubMed] [Google Scholar]
- 45.Natarajan, K., M. R. Meyer, B. M. Jackson, D. Slade, C. Roberts, A. G. Hinnebusch, and M. J. Marton. 2001. Transcriptional profiling shows that Gcn4p is a master regulator of gene expression during amino acid starvation in yeast. Mol. Cell. Biol. 21**:**4347-4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neely, K. E., A. H. Hassan, C. E. Brown, L. Howe, and J. L. Workman. 2002. Transcription activator interactions with multiple SWI/SNF subunits. Mol. Cell. Biol. 22**:**1615-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Owen, D. J., P. Ornaghi, J. C. Yang, N. Lowe, P. R. Evans, P. Ballario, D. Neuhaus, P. Filetici, and A. A. Travers. 2000. The structural basis for the recognition of acetylated histone H4 by the bromodomain of histone acetyltransferase gcn5p. EMBO J. 19**:**6141-6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park, J. M., H. S. Kim, S. J. Han, M. S. Hwang, Y. C. Lee, and Y. J. Kim. 2000. In vivo requirement of activator-specific binding targets of mediator. Mol. Cell. Biol. 20**:**8709-8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park, J. M., J. Werner, J. M. Kim, J. T. Lis, and Y. J. Kim. 2001. Mediator, not holoenzyme, is directly recruited to the heat shock promoter by HSF upon heat shock. Mol. Cell 8**:**9-19. [DOI] [PubMed] [Google Scholar]
- 50.Pina, B., S. Berger, G. A. Marcus, N. Silverman, J. Agapite, and L. Guarente. 1993. ADA3: a gene, identified by resistance to GAL4-VP16, with properties similar to and different from those of ADA2. Mol. Cell. Biol. 13**:**5981-5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pokholok, D. K., N. M. Hannett, and R. A. Young. 2002. Exchange of RNA polymerase II initiation and elongation factors during gene expression in vivo. Mol. Cell 9**:**799-809. [DOI] [PubMed] [Google Scholar]
- 52.Prochasson, P., K. E. Neely, A. H. Hassan, B. Li, and J. L. Workman. 2003. Targeting activity is required for SWI/SNF function in vivo and is accomplished through two partially redundant activator-interaction domains. Mol. Cell 12**:**983-990. [DOI] [PubMed] [Google Scholar]
- 53.Qiu, H., C. Hu, S. Yoon, K. Natarajan, M. Swanson, and A. G. Hinnebusch. 2004. An array of coactivators is required for optimal recruitment of TBP and RNA polymerase II by promoter-bound Gcn4p. Mol. Cell. Biol. 24**:**4104-4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reid, G. A., and G. Schatz. 1982. Import of proteins into mitochondria. J. Biol. Chem. 257**:**13062-13067. [PubMed] [Google Scholar]
- 55.Sakurai, H., and T. Fukasawa. 2000. Functional connections between mediator components and general transcription factors of Saccharomyces cerevisiae. J. Biol. Chem. 275**:**37251-37256. [DOI] [PubMed] [Google Scholar]
- 56.Sakurai, H., and T. Fukasawa. 1997. Yeast Gal11 and transcription factor IIE function through a common pathway in transcriptional regulation. J. Biol. Chem. 272**:**32663-32669. [DOI] [PubMed] [Google Scholar]
- 57.Saleh, A., V. Lang, R. Cook, and J. Brandl. 1997. Identification of native complexes containing the yeast coactivator/repressor proteins NGG1/ADA3 and ADA2. J. Biol. Chem. 272**:**5571-5578. [DOI] [PubMed] [Google Scholar]
- 58.Sanders, S. L., J. Jennings, A. Canutescu, A. J. Link, and P. A. Weil. 2002. Proteomics of the eukaryotic transcription machinery: identification of proteins associated with components of yeast TFIID by multidimensional mass spectrometry. Mol. Cell. Biol. 22**:**4723-4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma, V. M., B. Li, and J. C. Reese. 2003. SWI/SNF-dependent chromatin remodeling of RNR3 requires TAF(II)s and the general transcription machinery. Genes Dev. 17**:**502-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spencer, V. A., and J. R. Davie. 1999. Role of covalent modifications of histones in regulating gene expression. Gene 240**:**1-12. [DOI] [PubMed] [Google Scholar]
- 61.Sterner, D. E., P. A. Grant, S. M. Roberts, L. J. Duggan, R. Belotserkovskaya, L. A. Pacella, F. Winston, J. L. Workman, and S. L. Berger. 1999. Functional organization of the yeast SAGA complex: distinct components involved in structural integrity, nucleosome acetylation, and TATA-binding protein interaction. Mol. Cell. Biol. 19**:**86-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sudarsanam, P., and F. Winston. 2000. The Swi/Snf family nucleosome-remodeling complexes and transcriptional control. Trends Genet. 16**:**345-351. [DOI] [PubMed] [Google Scholar]
- 63.Svejstrup, J. Q., Y. Li, J. Fellows, A. Gnatt, S. Bjorklund, and R. D. Kornberg. 1997. Evidence for a mediator cycle at the initiation of transcription. Proc. Natl. Acad. Sci. USA 94**:**6075-6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Swanson, M. J., H. Qiu, L. Sumibcay, A. Krueger, S.-J. Kim, K. Natarajan, S. Yoon, and A. G. Hinnebusch. 2003. A multiplicity of coactivators is required by Gcn4p at individual promoters in vivo. Mol. Cell. Biol. 23**:**2800-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Syntichaki, P., I. Topalidou, and G. Thireos. 2000. The Gcn5 bromodomain co-ordinates nucleosome remodeling. Nature 404**:**414-417. [DOI] [PubMed] [Google Scholar]
- 66.Thompson, C. M., A. J. Koleske, D. M. Chao, and R. A. Young. 1993. A multisubunit complex associated with the RNA polymerase II CTD and TATA-binding protein in yeast. Cell 73**:**1361-1375. [DOI] [PubMed] [Google Scholar]
- 67.Topalidou, I., and G. Thireos. 2003. Gcn4 occupancy of open reading frame regions results in the recruitment of chromatin-modifying complexes but not the mediator complex. EMBO Rep. 4**:**872-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Utley, R. T., K. Ikeda, P. A. Grant, J. Cote, D. J. Steger, A. Eberharter, S. John, and J. L. Workman. 1998. Transcriptional activators direct histone acetyltransferase complexes to nucleosomes. Nature 394**:**498-502. [DOI] [PubMed] [Google Scholar]
- 69.Winzeler, E. A., D. D. Shoemaker, A. Astromoff, H. Liang, K. Anderson, B. Andre, R. Bangham, R. Benito, J. D. Boeke, H. Bussey, A. M. Chu, C. Connelly, K. Davis, F. Dietrich, S. W. Dow, M. E. Bakkoury, F. Foury, S. H. Friend, E. Gentalen, G. Giaever, J. H. J. Hegemann, T. M. Laub, H. Liao, N. Liebundguth, D. J. Lockhart, A. Lucau-Danila, M. Lussier, N. M'Rabet, P. Menard, M. Mittmann, C. Pai, C. Rebischung, J. L. Revuelta, L. Riles, C. J. Roberts, P. Ross-McDonald, B. Scherens, M. Snyder, S. Sookhai-Mahadeo, R. K. Storms, S. Veronneau, M. Voet, G. Volckaert, T. R. Ward, R. Wysocki, G. S. Yen, K. Yu, K. Zimmermann, P. Philippsen, M. Johnston, and R. W. Davis. 1999. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285**:**901-906. [DOI] [PubMed] [Google Scholar]
- 70.Wu, P. Y., C. Ruhlmann, F. Winston, and P. Schultz. 2004. Molecular architecture of the S. cerevisiae SAGA complex. Mol. Cell 15**:**199-208. [DOI] [PubMed] [Google Scholar]
- 71.Wu, P. Y., and F. Winston. 2002. Analysis of Spt7 function in the Saccharomyces cerevisiae SAGA coactivator complex. Mol. Cell. Biol. 22**:**5367-5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoon, S., H. Qiu, M. J. Swanson, and A. G. Hinnebusch. 2003. Recruitment of SWI/SNF by Gcn4p does not require Snf2p or Gcn5p but depends strongly on SWI/SNF integrity, SRB mediator, and SAGA. Mol. Cell. Biol. 23**:**8829-8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang, F., L. Sumibcay, A. G. Hinnebusch, and M. J. Swanson. 2004. A triad of subunits from the Gal11/tail domain of Srb mediator is an in vivo target of transcriptional activator Gcn4p. Mol. Cell. Biol. 24**:**6871-6886. [DOI] [PMC free article] [PubMed] [Google Scholar]