Functional Inactivation of the Retinoblastoma Protein Requires Sequential Modification by at Least Two Distinct Cyclin-cdk Complexes (original) (raw)
Abstract
The retinoblastoma protein (pRb) acts to constrain the G1-S transition in mammalian cells. Phosphorylation of pRb in G1 inactivates its growth-inhibitory function, allowing for cell cycle progression. Although several cyclins and associated cyclin-dependent kinases (cdks) have been implicated in pRb phosphorylation, the precise mechanism by which pRb is phosphorylated in vivo remains unclear. By inhibiting selectively either cdk4/6 or cdk2, we show that endogenous D-type cyclins, acting with cdk4/6, are able to phosphorylate pRb only partially, a process that is likely to be completed by cyclin E-cdk2 complexes. Furthermore, cyclin E-cdk2 is unable to phosphorylate pRb in the absence of prior phosphorylation by cyclin D-cdk4/6 complexes. Complete phosphorylation of pRb, inactivation of E2F binding, and activation of E2F transcription occur only after sequential action of at least two distinct G1 cyclin kinase complexes.
The retinoblastoma protein (pRb) is a nuclear phosphoprotein that regulates growth in the G1 phase of the cell cycle. pRb exerts its growth-inhibitory effects in part by binding to and inhibiting critical regulatory proteins, including members of the E2F family of transcription factors; E2F activation is necessary for the G1-S transition (12, 61). E2F selectively associates with hypophosphorylated pRb, and phosphorylation of pRb appears to release E2F from an inhibitory complex, enabling it to promote the transcription necessary for progression into late G1 and S phase (reviewed in references 32 and 59).
pRb is phosphorylated on a still imprecisely defined number of threonine and serine residues during G1 (6, 33, 62). A temporal sequence of modifications has been defined through use of both pRb variants in which certain of these residues have been replaced and monoclonal antibodies (MAbs) specific for certain phosphorylated domains of pRb. Both serine 608 (S608) and S780 have been identified as among the sites that are initially phosphorylated (27, 63).
These phosphorylations have distinct effects on the ability of pRb to interact with its various partner proteins. Thus, pRb phosphorylated on S780 appears to lose its ability to bind to E2F (27). Phosphorylation of S807 and/or S811 is required to abolish pRb binding to c-Abl (28), while modification of threonine 821 (T821) and/or T826 is required to abolish pRb binding to LXCXE-containing proteins such as simian virus 40 large T antigen (28, 62). However, these four sites do not appear to be involved in regulating pRb binding to the E2F transcription factors.
Phosphorylation of pRb also has effects on cell physiology, ostensibly by changing its association with these and other interacting partner proteins. For example, phosphorylation of S795 is required to inactivate pRb-imposed growth suppression in a microinjection assay (6). However, the relationship between growth inhibition and E2F binding is complex: phosphorylation of pRb in vitro by cyclin D-, cyclin E-, or cyclin A-associated kinase has been reported to release E2F (6, 13), yet only action by cyclin D1–cyclin-dependent kinase 4 (cdk4) complexes, but not by cyclin E-cdk2 complexes, abrogates the growth-inhibitory property of pRb when microinjected into SaOS-2 cells (6).
Such observations raise questions concerning the identities of the cyclins and associated cdk responsible for these various phosphorylation events. D-type cyclins are induced in resting cells following growth factor stimulation (37) and are expressed throughout G1 in cycling cells. In many types of cells, cyclin E expression is induced in mid-late G1, at a time when pRb becomes extensively phosphorylated (11, 29, 35). Since cyclin A is not expressed until cells enter S phase and is degraded upon exit from mitosis (16, 29, 41, 46), it is unlikely that cyclin A functions to phosphorylate pRb in G1.
Complexes capable of phosphorylating pRb can be formed by D-type cyclins (cyclins D1, D2, and D3) with cdk4 or cdk6, by cyclin E with cdk2, or by cyclin A with either cdk2 or cdc2 (cdk1). Phosphorylation of pRb can be achieved in vitro by immunoprecipitated (IP) complexes of cyclin D-, cyclin E-, or cyclin A-associated kinases, isolated from either cell lysates or baculovirus-infected insect cells that are expressing these proteins ectopically (reviewed in references 53 and 59). Ectopic coexpression in human SaOS-2 osteosarcoma cells of pRb with either cyclin E or cyclin A will lead to pRb hyperphosphorylation, as will the coexpression of one of the D-type cyclins with cdk4 or cdk6; in all of these cases, the pRb-imposed G1 block will also be overridden (10, 14, 21, 23). The modifications of pRb effected by each of these complexes may be similar, as phosphopeptides of pRb phosphorylated in vivo by ectopically expressed cyclin D1-cdk4, cyclin E-cdk2, or cyclin A-cdk2 appear to be identical (23). Furthermore, enforced overexpression of either cyclin E or cyclin D1 in stably transfected cells will advance the cell cycle by shortening its G1 phase (25, 45, 48, 49).
These observations would seem to indicate that any of the aforementioned cyclin-cdk complexes can catalyze pRb phosphorylation. However, the identification of the kinase complexes responsible for pRb phosphorylation in vivo is complicated by other lines of evidence. While the above-specified cyclin-cdk complexes appear to drive pRb phosphorylation to similar extents when ectopically expressed in vivo (23), each may phosphorylate pRb on a different but overlapping set of residues in vitro (6, 62). In addition, when human pRb is ectopically expressed in yeast, it is fully phosphorylated only in the presence of both cyclin D1 and cyclin E (or their apparent yeast analogs) (19).
These conflicting results yield a variety of mechanistic models of pRb phosphorylation. According to one scheme, either cyclin D-cdk4/6 or cyclin E-cdk2 complexes are capable of fully phosphorylating and functionally inactivating pRb. Alternatively, as suggested by the work with yeast cells, each of these cyclin-cdk complexes may contribute partially to the phosphorylation of pRb, with full phosphorylation requiring the collaboration of both types of cyclin-cdk complexes. We provide evidence here supporting the latter mechanism. Moreover, our data suggest that the contribution of cyclin E-cdk2 complexes to pRb phosphorylation requires prior action by cyclin D-cdk4/6 complexes.
MATERIALS AND METHODS
Cell culture, plasmids, transfection, and luciferase assay.
SaOS-2 and U2-OS cells were maintained in Dulbecco’s modified Eagle medium supplemented with 15% inactivated fetal calf serum. Expression vectors for human p16_INK4A_ (38), cyclin D1, cyclin E (21), CD20, cdk2DN, cdk2, and cdk4 (58), and glutathione _S_-transferase (GST)–DP1 and E2F1-tub (13) have previously been described. U2-OS cells were transfected by calcium phosphate precipitation as described elsewhere (3). To assay for E2F-dependent transcription, U2-OS cells were transfected with the indicated plasmids, a β-galactosidase-expressing plasmid, and either a wild-type (3× E2F)-luciferase or mutant (3× mut E2F)-luciferase reporter plasmid, harvested, and analyzed as described elsewhere (15).
Immunoblotting, immunoprecipitation, IP kinase, and phosphopeptide analysis.
For immunoblot analysis of pRb, cell lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 6% acrylamide, transferred to nitrocellulose (Schleicher & Schuell) or Hybond ECL nitrocellulose (Amersham), probed with anti-human pRb MAb G3-245 (Pharmingen) or anti-human pRb antibody C15 (Santa Cruz Biotechnology), which recognizes the carboxy terminus of pRb, and developed with peroxidase-conjugated secondary antibody (Jackson Immunoresearch Lab) and enhanced chemiluminescence as recommended by the manufacturer (Amersham). pRb, cyclin E, and cdk6 were immunoprecipitated with MAbs 21C9 (60), HE111, and C15 (Santa Cruz Biotechnology), respectively, using standard techniques (39). IP kinase assays were performed as described previously (39), with an equivalent amount of protein analyzed in each sample. Control experiments confirmed that immunoprecipitation from increasing amount of lysate resulted in a proportional increase in kinase activity, indicating that the assay was in the linear range. For phosphopeptide analysis, pRb was immunoprecipitated from sorted [32P]orthophosphate-labeled U2-OS cells, resolved by gel electrophoresis, and transferred to nitrocellulose filters, which were probed by anti-pRb MAb to confirm that the radioactive band comigrated with pRb. The band was excised from the filter, digested with trypsin, and subjected to electrophoresis and chromatography as described previously (42). All images were scanned with a LaCie Silverscanner III into Adobe Photoshop 4.0.
Flow cytometry, cell cycle analysis, and cell sorting.
Cells were cotransfected with a CD20 expression plasmid and identified by immunostaining with anti-CD20 MAb B1 (gift of J. Gribben, Dana-Farber Cancer Institute, Boston, Mass.) and fluorescein isothiocyanate (FITC)-conjugated secondary antibody (Jackson Immunoresearch Lab). Cell cycle analysis was performed as described elsewhere (58). To obtain pure populations of transfected cells, the cells were sorted first by labeling with anti-CD20 MAb followed by ferromagnetic bead-conjugated secondary antibody (Miltenyi Biotec) and subjected to magnetic separation as recommended by the manufacturer. CD20-positive cells were then stained with FITC-conjugated secondary antibody, and cells that stained at least 10 times brighter than the mean of the negative (control) population were isolated by flow cytometry. The resultant population of sorted cells was over 95% pure. Multiple independent reproducible sorting experiments were performed to obtain sufficient cells for biochemical analysis.
In situ extraction of pRb and immunofluorescence analysis.
Cells were grown on coverslips, transfected with the indicated expression plasmids, and immunostained with anti-CD20 MAb and then with FITC-conjugated anti-mouse secondary MAb to identify transfected cells. Where indicated, coverslips were subjected to low-salt detergent extraction as described elsewhere (43). The coverslips were then stained with rabbit polyclonal anti-pRb antibody (C15; Santa Cruz Biotechnology) and phycoerythrin- or Texas red-conjugated anti-rabbit secondary MAb (Jackson Immunoresearch Lab) and analyzed by fluorescence microscopy. Fifty CD20-expressing cells were counted in each experimental sample. As a control for the integrity of the nuclear membrane, coverslips from the same or parallel transfections were first stained with anti-CD20 followed by cy3-conjugated secondary antibody, subjected to low-salt detergent extraction, and then stained with anti-snRNP (ANA human reference serum 5; Centers for Disease Control and Prevention, Atlanta, Ga.) followed by FITC-conjugated anti-human secondary antibody.
GST-DP1-E2F1 pulldown and phosphatase treatment.
Sepharose 4B–GST-DP1-E2F1 complexes were produced in bacteria as described previously (13). To precipitate E2F-associated pRb, the asynchronously growing or (where indicated) transfected, but not sorted, cells were lysed in ELB with protease and phosphatase inhibitors [250 mM NaCl, 50 mM HEPES (pH 7.5)], 10 mM EDTA, 0.1% Nonidet P-40, aprotinin (10 μg/ml), leupeptin (10 μg/ml), 0.1 mM phenylmethylsulfonyl fluoride or 0.2 mM 4-(2-aminoethyl)benzene sulfonyl fluoride (AEBSF), 50 mM NaF, 10 mM β-glycerophosphate, sodium orthovanadate (10 μg/ml)] and cleared by centrifugation. One milligram of lysate was diluted in 9 volumes of HEMGN buffer (25 mM HEPES [pH 7.6], 0.1 mM EDTA, 12.5 mM MgCl2, 10% glycerol, 0.1% Nonidet P-40) containing 100 mM KCl, 1 mM dithiothreitol, 0.2 mM AEBSF, leupeptin (5 μg/ml) and aprotinin (10 μg/ml) (13) and incubated with Sepharose 4B–GST-DP1-E2F1 (or Sepharose 4B-GST alone as a control) for 1 h at 4°C. Beads were washed four times in HEMGN buffer at 4°C, resuspended in 3× Laemmli sample buffer, and subjected to SDS-PAGE and immunoblot analysis. Following GST pulldown or immunoprecipitation with anti-pRb MAb 21C9, the Sepharose 4B or protein G beads were (where indicated) washed twice in lambda phosphatase buffer and subjected to phosphatase treatment with 2,000 to 4,000 U of lambda phosphatase as recommended by the manufacturer (New England Biolabs). Where indicated, freshly prepared sodium orthovanadate (10 mM) was added prior to phosphatase treatment.
RESULTS
Selective inhibition of G1 cyclin-cdk complexes.
To dissect the steps in pRb phosphorylation in G1, we wanted to be able to inhibit selectively either cyclin D-cdk4/6 complexes or cyclin E-cdk2 complexes. The cdk inhibitor p16_INK4A_ has previously been shown to inhibit cdk4/6 activity (52), and a dominant-negative form of cdk2, termed cdk2DN, has been shown to inhibit cdk2 activity (58). However, the specificity of these inhibitors has not been well characterized. To verify the efficacy and specificity of p16_INK4A_ and cdk2DN, we introduced each separately into cells overexpressing various cyclins and cdks and monitored their effects on cyclin-cdk activity.
One measure of cyclin D- and cyclin E-associated kinase function can be derived from the observed ability of either kinase to overcome a pRb-imposed cell cycle arrest. In the pRb-deficient osteosarcoma cell line SaOS-2, ectopic expression of pRb causes cells to arrest in G1. Coexpression of either cyclin D-cdk4/6 complexes or cyclin E-cdk2 complexes with pRb overcomes this pRb-imposed arrest (21).
We first chose to examine the effects of the inhibitors p16_INK4A_ and cdk2DN on this function of cyclin D- and cyclin E-associated kinase activity. Plasmids encoding pRb and cyclin D1 plus cdk4 or cyclin E were transfected into SaOS-2 cells with or without cointroduction of either p16_INK4A_ or cdk2DN. (The levels of endogenous cdk2 in SaOS-2 cells are sufficient to form active complexes with ectopically expressed cyclin E; the lack of endogenous cdk4/6 activity necessitated the ectopic expression of cdk4 in these experiments.) Within each bulk population of cells, those that were successfully transfected were identified by staining cultures with an antibody specific for CD20, a cell surface marker expressed by a cotransfected plasmid. The cell cycle state of the transfected cells was analyzed by staining for DNA content with propidium iodide.
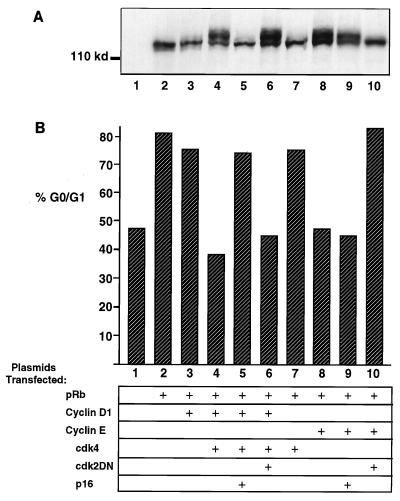
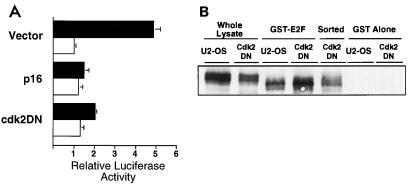
As had been previously shown, transfection of SaOS-2 cells with a pRb expression plasmid led to a G1 arrest, and transfection of either cyclin D1 plus cdk4 or cyclin E neutralized the pRb-imposed G1 arrest (Fig. 1B). Cotransfection of p16_INK4A_ reversed the effects of cyclin D1 plus cdk4 and restored the cell cycle arrest. However, p16_INK4A_ did not affect the ability of cyclin E to neutralize the pRb-imposed cell cycle arrest, suggesting a specificity of p16_INK4A_ for cyclin D1-cdk4 but not for cyclin E (Fig. 1A; compare lanes 5 and 9). Conversely, cotransfection of cdk2DN along with cyclin E abrogated the effect of cyclin E and restored the pRb-imposed cell cycle arrest. cdk2DN had no effect on the ability of cyclin D1 plus cdk4 to neutralize the pRb-imposed cell cycle arrest, suggesting a specificity of cdk2DN for inhibiting the actions of cyclin E-cdk2 but not cyclin D1-cdk4 (Fig. 1A; compare lanes 6 and 10).
FIG. 1.
Specificity of function of p16_INK4A_ and cdk2DN. (B) Cell cycle analysis of SaOS-2 cells cotransfected with the indicated plasmids and a CD20 expression plasmid. DNA content was analyzed by propidium iodide staining and fluorescence-activated cell sorting analysis of CD20-positive cells. % G0/G1, percent of CD20-positive cells with 2N DNA content. (A) Anti-pRb immunoblot of lysates prepared from the same transfected SaOS-2 cells analyzed in panel B.
A more direct way to measure the activity of cyclin D-cdk4/6 or cyclin E-cdk2 is to monitor the ability of these complexes to catalyze the phosphorylation of pRb in the cell. The level of phosphorylation of pRb affects the migration rate of the protein upon SDS-PAGE analysis. Hypophosphorylated or unphosphorylated pRb migrates more rapidly than does hyperphosphorylated pRb (1, 4, 8, 40). When pRb is ectopically expressed alone in SaOS-2 cells, it remains in a rapidly migrating, largely unphosphorylated form. When it is expressed in the presence of active kinase, it becomes hyperphosphorylated; this phosphorylation can be detected as reduced electrophoretic mobility.
Analysis of pRb electrophoretic migration rate and thus phosphorylation state was undertaken to confirm the efficacy and specificity of the cyclin-cdk inhibitors p16_INK4A_ and cdk2DN. As had been previously shown (10, 14, 21, 23), ectopic expression of pRb alone in SaOS-2 cells resulted in a rapidly migrating form of pRb, and when cells also expressed either cyclin D1 plus cdk4 or cyclin E, a hyperphosphorylated, more slowly migrating pRb appeared (Fig. 1A). Coexpression of p16_INK4A_ prevented the cyclin D1-plus-cdk4-mediated, but not cyclin E-mediated, hyperphosphorylation of pRb (Fig. 1A; compare lanes 5 and 9). Coexpression of cdk2DN, in contrast, prevented the cyclin E-mediated, but not cyclin D1-plus-cdk4-mediated, hyperphosphorylation of pRb (Fig. 1A; lanes 6 and 10). Thus, as measured by both reduction in pRb phosphorylation and restoration of a pRb-imposed cell cycle arrest, these data suggest that p16_INK4A_ is a specific inhibitor of D-type cyclin kinase complexes and that cdk2DN is a specific inhibitor of cyclin E-cdk2 complexes.
Phosphorylation of pRb by endogenous cyclin-cdk.
Having found evidence for the selectivity of inhibitors of cyclin D-cdk4/6 and cyclin E-cdk2, we next wanted to dissect the role that each cyclin kinase plays in pRb phosphorylation. As shown above, ectopic overexpression of either cyclin D1 plus cdk4 or cyclin E will result in the phosphorylation of ectopically expressed pRb in SaOS-2 cells and in the attendant loss of pRb-imposed growth suppression. However, we suspected that the ectopic overexpression of cyclins, cdk, and pRb might result in a distortion of the normal substrate specificities of the active kinase complexes, thereby obscuring their usual physiologic contributions to pRb phosphorylation. We therefore sought to examine the functioning of the cyclins, cdks, and pRb expressed endogenously by a cell.
To do so, we studied the phosphorylation of the wild-type pRb expressed by cells of the human U2-OS osteosarcoma line. Importantly, the phosphorylation and functioning of pRb in these cells appears to be very similar to what is observed in primary cells: both cyclin D-associated and cyclin E-associated kinase activities are present in U2-OS cells, pRb undergoes a cell cycle-dependent phosphorylation, and inhibition of pRb phosphorylation results in a G1 cell cycle arrest.
To assess the contributions of cyclin D-cdk4/6 complexes and cyclin E-cdk2 complexes to pRb phosphorylation, we sought to inhibit each, as before, with either p16_INK4A_ or cdk2DN. We first confirmed, as has been previously reported (22, 30), that the ectopic expression of each of these proteins also resulted in a pRb-imposed G1 arrest (data not shown). Thus, both of these inhibitory proteins are effective in U2-OS cells as they were in the previously manipulated SaOS-2 cells.
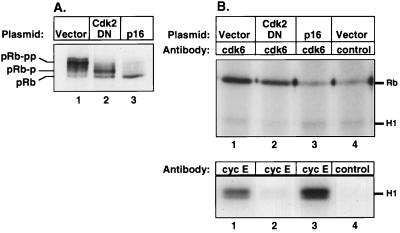
Having established the efficacy of p16_INK4A_ and cdk2DN in U2-OS cells, we next analyzed the state of phosphorylation of pRb in the presence of these inhibitors. We transfected a CD20 expression plasmid along with empty vector or expression plasmids for either p16_INK4A_ or cdk2DN into U2-OS cells and isolated a purified population of CD20-expressing U2-OS cells by a two-step sorting process of magnetic bead selection and flow cytometry. We then analyzed the phosphorylation state of pRb by SDS-PAGE and immunoblotting (Fig. 2A). Ectopic expression of p16_INK4A_ prevented significant pRb phosphorylation, as evidenced by the presence of a single rapidly migrating band of pRb by SDS-PAGE and immunoblotting. Interestingly, expression of cdk2DN permitted partial phosphorylation of pRb, as indicated by the presence of pRb forms that migrated at a rate intermediate between that of the fastest-migrating unphosphorylated pRb and that of the slowest-migrating, fully phosphorylated forms of pRb seen in mock-transfected cells.
FIG. 2.
Cyclin D-associated kinase only partially phosphorylates pRb. U2-OS cells were cotransfected with empty vector (lanes 1 and 4), cdk2DN expression plasmid (lane 2), or p16_INK4A_ expression plasmid (lane 3) as described in the legend to Fig. 1, and pure populations of transfected cells were isolated by magnetic bead selection and flow cytometry. (A) Anti-pRb immunoblot of lysates prepared from purified transfected U2-OS cells. (B) Immunoprecipitations from lysates of purified transfected U2-OS cells with anti-cdk6 antibody (upper panel, lanes 1 to 3) or anti-cdk6 antibody preincubated with peptide (lane 4), or with anti-cyclin E antibody (lower panel, lanes 1 to 3) or isotype-matched irrelevant control (lane 4), were tested for kinase activity against either GST-pRb-COOH (Rb) or histone H1 (H1) substrate.
We wished to confirm biochemically that the kinase inhibitors p16_INK4A_ and cdk2DN in the transfected sorted cells each specifically inhibited D-type cyclin or cyclin E kinase activity. To do so, we immunoprecipitated cyclin-cdk complexes from transfected, sorted cells and tested them for enzymatic activity against either histone H1 or GST-pRb substrate. Since the number of transfected sorted cells was limiting, we assayed cyclin kinase activity with the most sensitive reagents available. U2-OS cells are known to express more cdk6 than cdk4 (54), and in control experiments, IP kinase activity could be detected from fewer cells with anti-cdk6 antibody than with anti-cdk4 or anti-cyclin D1 antibody (data not shown). Therefore, immunoprecipitates obtained with anti-cdk6 antibody were analyzed as a measure of D-type cyclin kinase activity.
Cyclin D-associated kinase activity was detected in lysates of both mock-transfected and cdk2DN-transfected cells (Fig. 2B, upper panel, lanes 1 and 2) but not in lysates of cells transfected with p16_INK4A_ (lane 3). In contrast, cyclin E-associated kinase activity was detected in lysates of mock-transfected cells but not in lysates of cells transfected with cdk2DN (lower panel, lanes 1 and 2). Surprisingly, the same amounts of protein from lysates of p16_INK4A_-transfected cells also contained ample cyclin E kinase activity (lane 3).
The foregoing observations confirmed that the ectopically expressed inhibitors functioned selectively at the biochemical level as intended. Furthermore, the presence of D-type cyclin kinase activity, but not cyclin E kinase activity, in cdk2DN-transfected cells suggests that cyclin D kinase complexes can achieve only partial phosphorylation of pRb, as manifested by the intermediate migration rate of pRb prepared from these cells. These experiments yielded an additional conclusion as well: despite the high endogenous cyclin E kinase activity present in p16_INK4A_-transfected U2-OS cells, pRb remained in the rapidly migrating, unphosphorylated state when cyclin D kinase complexes were inhibited by p16_INK4A_ expression. This observation provided clear indication that phosphorylation of pRb by cyclin E-cdk2 required the prior modification by cyclin D-cdk4 complexes. Thus, the strong, apparently constitutive presence of cyclin E-cdk2 activity in these cells was unable on its own to drive pRb phosphorylation.
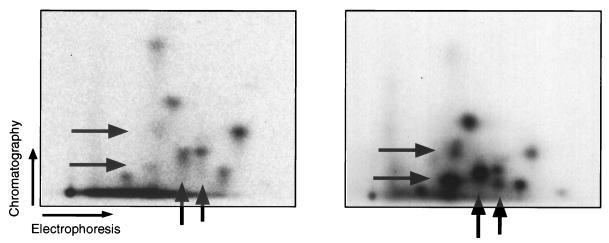
These data indicated that cyclin D-cdk4/6 complexes achieve only partial phosphorylation of pRb but provided no indication as to whether these particular cyclin-cdk complexes modified only a specific subset of the possible phosphorylation sites on pRb or whether they catalyzed nonspecific phosphorylation at all possible cdk sites, albeit incompletely. To resolve this issue, we performed tryptic phosphopeptide analysis of pRb from cdk2DN-transfected or from mock-transfected sorted cells.
Several phosphopeptides were present in significantly lower amounts in the pRb isolated from cdk2DN-transfected cells than in the pRb isolated from mock-transfected cells (Fig. 3). These represent sites in pRb that remain unphosphorylated or poorly phosphorylated in the presence of D-type cyclin kinase activity but in the absence of cyclin E kinase activity. We conclude that endogenous D-type cyclins are capable of phosphorylating in vivo only a subset of those sites that are modified in fully phosphorylated pRb.
FIG. 3.
Phosphopeptide analysis of pRb. [32P]orthophosphate-labeled pRb was immunoprecipitated from purified cells transfected with CD20 and cdk2DN expression plasmid (left) or CD20 alone (right) and subjected to tryptic phosphopeptide analysis. Arrows indicate phosphopeptides underrepresented in pRb from cdk2DN-transfected cells compared to pRb from asynchronously growing cells.
Nuclear tethering of partially phosphorylated pRb.
The results cited above suggested that the cyclin D- and cyclin E-associated kinase complexes collaborate to effect the complete phosphorylation of pRb and that this process occurs in a specific sequence, with cyclin D-cdk4/6 complexes initiating this process and cyclin E-cdk2 complexes completing it. In addition, cdk2DN-transfected cells, in which pRb is partially phosphorylated by cyclin D-cdk4/6 complexes, are arrested in G1. However, we were unable to assess the degree of functional inactivation of partially phosphorylated pRb, as the inhibition of cdk2 activity in these cells might also effect other essential steps in late G1. We therefore undertook to evaluate pRb function by criteria other than its ability to halt G1 progression.
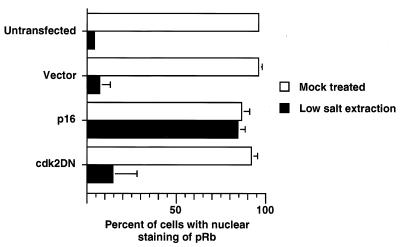
We first sought to understand the effect of partial phosphorylation on the association of pRb with certain nuclear structures. This association appears to reflect the ability of pRb to interact with other nuclear proteins, still unidentified, that may be important in growth regulation. In early G1, pRb is bound tightly to the nucleus and cannot be extracted by a detergent-containing low-salt buffer. As cells progress through G1, pRb is inactivated by phosphorylation, and this modification correlates with a decreased affinity for the nucleus such that it can be leached from the nucleus by a low-salt wash (43).
To assay pRb nuclear affinity, U2-OS cells were grown on coverslips and transfected with an empty vector or with a plasmid expressing either p16_INK4A_ or cdk2DN. First, transfected cells were identified by immunostaining for CD20 (expressed by a cotransfected plasmid). The cells were then subjected to in situ extraction with a detergent-containing low-salt buffer and fixed in methanol-acetone as described elsewhere (43). This low-salt wash disrupted the plasma membrane and lysed the cells, while the nuclei remained attached to the coverslips. However, sufficient plasma membrane remained after low-salt extraction to enable identification of the previously immunostained transfected cells. Fluorescence-activated cell sorting analysis of these samples confirmed that cells transfected with a p16_INK4A_ or cdk2DN expression plasmid were arrested in G1 (data not shown). In untransfected or mock-transfected cells, the majority of nuclei lost pRb immunoreactivity upon hypotonic wash, indicating that pRb is loosely tethered to the nuclei of these cells (Fig. 4). In p16_INK4A_-transfected cells, however, the majority of nuclei retained pRb immunoreactivity, while the nuclei of cdk2DN-transfected cells lost pRb immunoreactivity, following hypotonic wash, indicating that in these cells, which lack cyclin E-associated kinase activity, the actions of cyclin D-cdk4/6 kinase complexes alone serve to loosen the binding of pRb from nuclear structures. This effect was specific to pRb, as a second nuclear antigen (snRNP) remained detectable by immunofluorescence in each of the populations of transfected cells, both before and after hypotonic wash (data not shown). By this criterion, cyclin D-cdk4/6 complexes were able to effect a functional alteration of pRb.
FIG. 4.
Nuclear affinity of partially phosphorylated pRb. U2-OS cells grown on coverslips were transfected with the indicated plasmids and CD20 expression plasmid, subjected to indirect immunofluorescence with anti-CD20 antibody, and then mock treated or subjected to low-salt extraction prior to indirect immunofluorescence with anti-pRb antibody. The mean and standard error of the mean from three independent experiments are shown. For each experimental sample, 50 CD20-positive cells were analyzed.
Regulation of E2F by partially phosphorylated pRb.
The above-described nuclear tethering experiment assesses the interaction of pRb with as yet unidentified nuclear protein(s). The physiologic roles of most of the proteins that interact with pRb remain unclear. Among the nuclear partners of pRb, however, are the E2F transcription factors, which have a clearly defined, highly significant function in G1; these factors operate to drive expression of a number of important genes in the late G1 and S phases. The E2F complexes that are associated with pRb are unable to activate the transcription of promoters bearing E2 sites; such transcription is achieved only when pRb undergoes inactivation and liberates E2F activity. For these reasons, we attempted to assess whether the partial phosphorylation of pRb by cyclin D-cdk4/6 affected its association with E2Fs.
To do so, we transfected U2-OS cells with a luciferase reporter construct under the transcriptional control of a synthetic wild-type (3× E2F) or mutant (3× mut) E2 promoter (31) along with an empty vector or plasmid encoding p16_INK4A_ or cdk2DN. Luciferase activity was normalized to β-galactosidase activity, expressed by a cotransfected plasmid, to provide an internal control for transfection efficiency.
Cells transfected with the 3× E2F plasmid alone contained fivefold higher luciferase activity than cells transfected with the 3× mut construct (Fig. 5A). However, cells cotransfected with p16_INK4A_ or cdk2DN contained basal levels of luciferase activity, whether transfected with 3× E2F or 3× mut. Hence, as gauged by this reporter assay, the partial phosphorylation of pRb achieved by cyclin D-cdk4/6 complexes did not succeed in causing it to liberate E2F activity. These observations are in agreement with previous analyses of E2F-dependent transcription in p16_INK4A_-transfected or cdk2DN-transfected cells (20, 22, 51).
FIG. 5.
Repression of E2F by partially phosphorylated pRb. (A) Repression of E2F transcriptional activity by p16_INK4A_ and cdk2DN. Cells transfected with the indicated plasmid and either a wild-type (3× E2F; ▪) or a mutant (3× mut; □) E2 promoter-luciferase reporter construct were analyzed for luciferase activity. The mean and standard error of the mean from three independent experiments are shown. (B) Identification of E2F-associated pRb by immunoblot analysis of pRb from lysates prepared from asynchronously growing (lanes 1 and 3) or cdk2DN-transfected but not sorted (lanes 2 and 4) U2-OS cells before (lanes 1 and 2) or after precipitation with Sepharose 4B–GST-DP1-E2F1 (lanes 3 and 4) or Sepharose 4B-GST alone (lanes 6 and 7) or prepared from cdk2DN-transfected sorted U2-OS cells (lane 5).
Since both p16_INK4A_-transfected and cdk2DN-transfected U2-OS cells are arrested in G1, a cell cycle phase-specific regulation unrelated to the phosphorylation state of pRb could be invoked to explain the absence of E2F-mediated transcriptional activity observed in these cells. We therefore wished to confirm and extend the above-mentioned results by an independent test of the ability of partially phosphorylated pRb to interact with E2F.
To identify specifically the forms of phosphorylated pRb that retain the ability to bind E2Fs, we probed lysates of asynchronously growing, unmanipulated U2-OS cells with bacterially produced GST-DP1-E2F1 conjugated to Sepharose 4B (GST-E2F). Indeed, a form of pRb having intermediate electrophoretic mobility was found to associate with GST-E2F (Fig. 5B, lane 3). This form of pRb also comigrated with pRb from cdk2DN-transfected, sorted cells (compare lane 3 with lane 5 and Fig. 2A), suggesting that the two pRb forms are phosphorylated to similar extents. Identical results were obtained from lysates of the HaCat human keratinocyte cell line (data not shown).
To provide yet more evidence that pRb in cdk2DN-transfected cells continues to bind E2F, lysates of cdk2DN-transfected U2-OS cells were also probed with GST-E2F. In this experiment, transfected cells were not sorted prior to analysis. Upon precipitation with GST-E2F, the form of pRb isolated from these cells showed identical electrophoretic mobility intermediate to that of E2F-associated pRb from asyncrhonously growing U2-OS cells and that of pRb from cdk2DN transfected, sorted cells (Fig. 5B; compare lane 3 with lanes 4 and 5). Furthermore, this form of pRb was precipitated in greater amounts from cdk2DN-transfected, but unsorted, cells than from untransfected cells, providing additional evidence that this represents pRb that which has been partially phosphorylated by cyclin D-cdk4/6 complexes alone. Equivalent amounts of protein from each lysate were analyzed in each GST-E2F precipitation (lanes 3 and 4), as confirmed by immunoblot analysis of an equivalent amount of protein from cell lysates obtained prior to precipitation with GST-E2F (lanes 1 and 2), and the specificity of the interaction was confirmed by the lack of pRb association with GST alone in either sample (lanes 6 and 7).
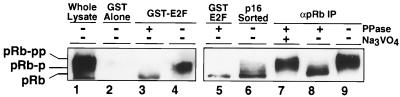
The reduction in mobility of pRb on SDS-PAGE and Western blot analysis is a widely accepted indicator of pRb phosphorylation. Nonetheless, it was possible that the isoforms of pRb with different electrophoretic mobilities observed in association with E2F were the result of pRb degradation rather than phosphorylation. Indeed, proteolysis of pRb with ICE-like proteases has recently been shown to result in a species of pRb lacking a carboxy-terminal 5-kDa peptide. This proteolytic fragment of pRb migrates slightly faster than the unphosphorylated full-length protein (5, 24, 55). To exclude the possibility that the isoforms of pRb observed in association with E2F were not simply degradation fragments, Western blot analysis was performed with an antibody recognizing an epitope on the carboxy-terminal 5-kDa cleavage product (C15; Santa Cruz Biotechnology). Indeed, multiple fast-migrating species of GST-E2F-associated pRb were also detected by this antibody (Fig. 6, lane 4), indicating that these isoforms of pRb are not proteolytic fragments lacking their carboxy termini. Upon phosphatase treatment, the different species of pRb all migrated at a single rate (lanes 3 and 5), similar to that of the major species of pRb in cells transfected with p16_INK4A_ (lane 6), to that of immunoprecipitated pRb after phosphatase treatment (lane 8 and data not shown), and to that of human pRb ectopically expressed in SaOS-2 cells (data not shown). These data confirm that at least some of the species of GST-E2F-associated pRb are phosphorylated, to variable extents.
FIG. 6.
Phosphatase treatment of E2F-associated pRb. Shown are immunoblots of untreated and phosphatase-treated pRb with an antibody specific for the carboxy terminus of pRb (αpRb). (Left) Immunoblot of pRb from lysates prepared from asynchronously growing U2-OS cells (lane 1) or after precipitation with Sepharose 4B–GST-DP1-E2F1 before (lane 4) or after (lane 3) treatment with phosphatase (PPase). (Right) Immunoblot of pRb after precipitation with Sepharose 4B–GST-DP1-E2F1 and subsequent phosphatase treatment (lane 5), pRb isolated from p16-transfected, sorted cells (lane 6), and pRb immunoprecipitated from lysates of asynchronously growing U2-OS cells before (lane 9) or after treatment with phosphatase in the absence (lane 8) or presence (lane 7) of sodium orthovanadate (Na3VO4).
Taken together, these experiments provide a clear demonstration that partially phosphorylated species of pRb are still active in binding to and repressing E2F. Along with the previous observations, they provide strong evidence that functional inactivation of pRb occurs only after completion of a sequential, cooperative phosphorylation process that is initiated by cyclin D-cdk4/6 complexes and then continued by cyclin E-cdk2 complexes.
DISCUSSION
Attempts to identify the cyclin-cdk complexes responsible for the phosphorylation and functional inactivation of pRb have been frustrated by conflicting evidence generated by different types of experiments. Resolution of this issue is essential for understanding the control of cell cycle progression, as pRb phosphorylation appears to be critical for the advance of most cell types through the G1 phase into S phase. Some experiments have gauged the activities of G1 cyclin-cdk complexes through their incubation in vitro with pRb substrate (6, 26, 27, 33, 36, 56, 62). Yet others have studied the phosphorylation of pRb in cells in which high, superphysiologic levels of various cyclins and cdk has been achieved through introduction of different expression vectors (10, 14, 21, 23, 26) Each of these approaches would appear to be vulnerable to artifact. The specificity of G1 cyclin kinases for different substrates is relatively weak (27), and a recent study has shown that by increasing the amount of a cyclin-cdk above a certain threshold level, one can cause the phosphorylation of additional sites beyond those modified at lower levels of these complexes (6). With these observations in mind, we avoided the ectopic expression of active cyclins and cdks and instead attempted to inhibit the functions of endogenous cyclin-cdk complexes expressed at physiologic levels by the cell.
Our analysis of phosphopeptides of pRb from cells arrested by cdk2DN, in which cdk2 activity has been effectively blocked, indicates that D-type cyclin-directed phosphorylation in vivo is restricted to a subset of sites on pRb. Tryptic phosphopeptides obtained from pRb that has been phosphorylated both in vitro and in vivo have been extensively characterized (6, 7, 19, 23, 26, 33, 36, 42, 62). However, in the present experiments, the lack of precision in phosphopeptide analysis of manipulated, sorted cells makes it difficult to draw further conclusions from our data. For example, the population of cdk2DN-transfected cells may contain a small amount of contaminating untransfected cells, so that we cannot conclusively determine that all the pRb phosphopeptides from these cells represent phosphorylation by D-type cyclin kinases alone. More significantly, however, the relative absence of specific pRb phosphopeptides from cdk2DN-transfected cells indicates that these sites are less favored targets for phosphorylation by D-type cyclin-associated kinases in vivo and indeed unlikely to be modified at all by these cyclin-cdk complexes. We suspect but cannot prove directly that these particular sites are targeted specifically by cyclin E-cdk2 complexes following initial phosphorylation of pRb on other sites by cyclin D-cdk4/6 complexes.
The present experiments suggest that cyclin D-cdk4/6 complexes alone were unable to inactivate pRb in vivo. We were not able to directly measure the growth-suppressive properties of pRb which has been modified by cyclin D-cdk4/6 complexes, since the concurrent inhibition of cdk2 activity might also affect other essential steps in G1. Instead, we analyzed other characteristics associated with active pRb. pRb mediates growth suppression in part by binding to and apparently sequestering a number of other cellular growth-promoting proteins. Prominent among these are members of the E2F family of transcription factors. Only hypophosphorylated pRb binds E2F, and pRb inactivated by deletion, mutation, or phosphorylation is incapable of binding to or repressing E2F (2, 13, 17, 18, 47, 57). As shown here, E2F-mediated transcription is repressed in cdk2DN-transfected cells, despite phosphorylation of pRb by D-type cyclin kinase. These results provided an indication that the partial modification of pRb effected by cyclin D-cdk4/6 complexes does not succeed in causing release of E2Fs from the inhibitory effects of pRb.
While we suppose here that E2F activity is controlled by the state of phosphorylation of pRb, an alternative scenario might be suggested by recent work demonstrating that E2F activity can in certain circumstances be modulated directly by cyclin E-cdk2, independent of any involvement of pRb (9, 64). This mechanism, if operative, would force us to reinterpret the experiments here in which we analyzed E2F activity in cells expressing the cdk2DN plasmid which interferes directly with cdk2 activity. However, using the same cells that we have studied here, others have shown that the effects of the cdk2DN allele can be reversed by wild-type simian virus 40 large T antigen but not by a mutant thereof that is incapable of binding pRb (22). Such results strongly support the notion that the effects on E2F activity observed by us here derive from the functioning of pRb, which in the present case are regulated by its state of phosphorylation.
Further evidence supporting the notion that partial phosphorylation of pRb, obtained by blocking cdk2 activity, does not abrogate the binding of pRb to E2F comes from our analysis of both an osteosarcoma and a keratinocyte cell line. Specifically, the form of pRb that migrates at a rate intermediate between those of fully phosphorylated and unphosphorylated pRb retains its ability to bind E2Fs and is enriched in a population of cdk2DN-transfected cells.
In contrast to our observations, others have recently reported that pRb that is phosphorylated in vitro by cyclin D1-cdk4 complexes, cyclin E-cdk2 complexes, or cyclin A-cdk2 complexes can no longer bind E2F (6, 13). However, we would argue that the substrate specificities of protein kinases are often abrogated in vitro and, as mentioned above, that the excessive amounts of cyclin-cdk complexes can phosphorylate additional sites that are not modified by lower amounts of these complexes (6). This notion is borne out by our own earlier results showing that ectopic expression of cyclin E or cyclin D in the living cell can drive pRb phosphorylation to completion, which would not seem to reflect the normal activities of the endogenous proteins in cells.
As demonstrated some years ago, phosphorylated forms of pRb lose their binding affinity to nuclear structures, as manifested by the ability to leach them from the nuclei of detergent-permeabilized cells with low-salt buffers (43). As shown here, in cdk2DN-transfected cells, pRb undergoes partial phosphorylation by cyclin D-cdk4/6, retains its ability to bind and repress E2Fs, but loses its tight tethering to the nucleus. These data indicate that pRb undergoes a succession of functional alterations as the cell traverses G1; in this case, the loss of nuclear tethering of pRb precedes the loss of ability to bind E2F. Since E2F binding appears to be tightly connected with the growth-controlling functions of pRb, we believe that loss of nuclear binding is not equivalent, as previously assumed, to loss of the ability to inhibit cell proliferation (43).
Previous experiments have suggested that pRb phosphorylation occurs in a sequential manner in other types of cells. In particular, full phosphorylation of human pRb ectopically expressed in yeast requires both D-type and E cyclin kinase activity or the activity of analogous yeast cyclins. In this yeast expression system, the presence of cyclin D1 alone also leads to partial pRb phosphorylation (19). Further correlative evidence for sequential phosphorylation of pRb comes from analysis of phytohemagglutinin-stimulated T cells. In these cells, the initial forms of phosphorylated pRb appear at a point in time when only cyclin D-associated (and not cyclin E-associated) kinase can be detected, while the slowest-migrating fully phosphorylated form or pRb does not appear until after cyclin E is active (7, 39, 44). Together, these observations provide correlative evidence supporting the mechanistic model that has been tested here directly by the specific inhibition of the responsible cyclin-cdk complexes.
Although our observations further elucidate pRb phosphorylation in G1, several critical issues remain. While we can conclude that cyclin D-cdk4/6 complexes are able to phosphorylate pRb only partially in vivo, we lack direct evidence demonstrating that cyclin E-cdk2 completes this process. Cell physiologic evidence suggests that pRb undergoes functional inactivation at a time in the cell cycle when cyclin E-cdk2 becomes active in mid/late G1, a period when cyclin A-cdk2 complexes are not yet active (29). Nonetheless, other, still undefined kinases in addition to cyclin E-cdk2 may contribute to the complete phosphorylation of pRb that occurs in mid/late G1. Furthermore, during S phase, the activities of cyclin A-associated complexes may extend the work of the cyclin D- and cyclin E-associated complexes that is initiated in G1.
A second issue is provoked by the observation that cyclin E-cdk2 appears unable to phosphorylate pRb that has not been previously modified by the actions of cyclin D-cdk4/6. The molecular mechanism by which this occurs is unclear. We do not believe that this is merely an artifact of p16_INK4A_ expression, as others have also recently observed that cyclin E kinase is unable to phosphorylate pRb in the absence of cyclin D kinase activity (34). Cyclin E kinase complexes may not be able to recognize or gain access to native unphosphorylated pRb that is bound to certain tethering sites in the nucleus; our work indicates that cyclin D-cdk4/6-mediated phosphorylation releases pRb from these tethers. Alternatively, cyclin D-cdk4/6-mediated phosphorylation may evoke a conformational change in pRb that makes it into a better substrate for cyclin E-cdk2. Such a model of progressive phosphorylation of a protein by distinct kinases is not without precedent. For example, the phosphorylation of glycogen synthase kinase on certain sites by glycogen synthase kinase 3 requires its prior phosphorylation on other sites by casein kinase II (50).
A third issue is suggested by the observation that the entire pool of cellular pRb appears to be held in a functionally inactive state after the cell has passed the restriction point in late G1. This would seem to require that pRb molecules that are synthesized de novo in S and G2 phases undergo phosphorylation shortly after their synthesis at a time when cyclin E is no longer active. More importantly, this phosphorylation would appear to occur during periods in S, G2, and M phase when D-type cyclins may no longer be active. For example, cells in S phase that are then deprived of serum mitogens will continue their cell cycle advance and apparently continue to successfully phosphorylate pRb in the presence of only cyclin A-cdc2 complexes. We speculate therefore that B-like cyclin–cdk complexes (i.e., involving cyclin A or B) may be able to completely phosphorylate pRb even in the absence of preparatory phosphorylation by D-type cyclin-associated cdk and may thus, with respect to pRb, be more wide-ranging in their substrate specificities than are the cyclin D- and cyclin E-associated kinase complexes.
Finally, the purpose of sequential, cooperative phosphorylation is unclear. It may facilitate an additional dimension of control on pRb inactivation. Alternatively, it may create a gradation of gene activations in which certain transcription factors are liberated by the actions of cyclin D-cdk4/6 on pRb while yet others, such as E2Fs, are released only following the actions of cyclin E-cdk2. It may also provide the cell cycle apparatus with additional specificity in regulating alternative cell fates such as proliferation and differentiation.
ACKNOWLEDGMENTS
We thank members of the Weinberg lab, MIT Center for Cancer Research, B. Dynlacht, S. Mittnacht, G. Paradis, and P. Utz for advice and assistance, J. Gribben, B. Dynlacht, S. van den Heuvel, W. Krek, and H. Huber for reagents, and M. Meyerson and R. Beijersbergen for critical reading of the manuscript.
R.A.W. is an American Cancer Society Professor of Biology. A.S.L. is a Margaret and Herman Sokol postdoctoral fellow and was a Howard Hughes Medical Institute Physician postdoctoral fellow. This work was supported by NIH grant K11-CA69242.
REFERENCES
- 1.Buchkovich K, Duffy L A, Harlow E. The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell. 1989;58:1097–1105. doi: 10.1016/0092-8674(89)90508-4. [DOI] [PubMed] [Google Scholar]
- 2.Chellappan S P, Hiebert S, Mudryj M, Horowitz J M, Nevins J R. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65:1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- 3.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen P L, Scully P, Shew J Y, Wang J Y, Lee W H. Phosphorylation of the retinoblastoma gene product is modulated during the cell cycle and cellular differentiation. Cell. 1989;58:1193–1198. doi: 10.1016/0092-8674(89)90517-5. [DOI] [PubMed] [Google Scholar]
- 5.Chen W D, Otterson G A, Lipkowitz S, Khleif S N, Coxon A B, Kaye F J. Apoptosis is associated with cleavage of a 5 kDa fragment from RB which mimics dephosphorylation and modulates E2F binding. Oncogene. 1997;14:1243–1248. doi: 10.1038/sj.onc.1201096. [DOI] [PubMed] [Google Scholar]
- 6.Connell-Crowley L, Harper J W, Goodrich D W. Cyclin D1/cdk4 regulates retinoblastoma protein-mediated cell cycle arrest by site-specific phosphorylation. Mol Biol Cell. 1997;8:287–301. doi: 10.1091/mbc.8.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Caprio J A, Furukawa Y, Ajchenbaum F, Griffin J D, Livingston D M. The retinoblastoma-susceptibility gene product becomes phosphorylated in multiple stages during cell cycle entry and progression. Proc Natl Acad Sci USA. 1992;89:1795–1798. doi: 10.1073/pnas.89.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Caprio J A, Ludlow J W, Lynch D, Furukawa Y, Griffin J, Piwnica-Worms H, Huang C M, Livingston D M. The product of the retinoblastoma susceptibility gene has properties of a cell cycle regulatory element. Cell. 1989;58:1085–1095. doi: 10.1016/0092-8674(89)90507-2. [DOI] [PubMed] [Google Scholar]
- 9.Dimri G P, Nakanishi M, Desprez P Y, Smith J R, Campisi J. Inhibition of E2F activity by the cyclin-dependent protein kinase inhibitor p21 in cells expressing or lacking a functional retinoblastoma protein. Mol Cell Biol. 1996;16:2987–2997. doi: 10.1128/mcb.16.6.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowdy S F, Hinds P W, Louie K, Reed S I, Arnold A, Weinberg R A. Physical interaction of the retinoblastoma protein with human D cyclins. Cell. 1993;73:499–511. doi: 10.1016/0092-8674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- 11.Dulic V, Lees E, Reed S I. Association of human cyclin E with a periodic G1-S phase protein kinase. Science. 1992;257:1958–1961. doi: 10.1126/science.1329201. [DOI] [PubMed] [Google Scholar]
- 12.Duronio R J, O’Farrell P H, Xie J E, Brook A, Dyson N. The transcription factor E2F is required for S phase during Drosophila embryogenesis. Genes Dev. 1995;9:1445–1455. doi: 10.1101/gad.9.12.1445. [DOI] [PubMed] [Google Scholar]
- 13.Dynlacht B D, Flores O, Lees J A, Harlow E. Differential regulation of E2F transactivation by cyclin/cdk2 complexes. Genes Dev. 1994;8:1772–1786. doi: 10.1101/gad.8.15.1772. [DOI] [PubMed] [Google Scholar]
- 14.Ewen M E, Sluss H K, Sherr C J, Matsushime H, Kato J, Livingston D M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993;73:487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- 15.Geng Y, Eaton E N, Picon M, Roberts J M, Lundberg A S, Gifford A, Sardet C, Weinberg R A. Regulation of cyclin E transcription by E2Fs and retinoblastoma protein. Oncogene. 1996;12:1173–1180. [PubMed] [Google Scholar]
- 16.Girard F, Strausfeld U, Fernandez A, Lamb N J. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 1991;67:1169–1179. doi: 10.1016/0092-8674(91)90293-8. [DOI] [PubMed] [Google Scholar]
- 17.Hamel P A, Cohen B L, Sorce L M, Gallie B L, Phillips R A. Hyperphosphorylation of the retinoblastoma gene product is determined by domains outside the simian virus 40 large-T-antigen-binding regions. Mol Cell Biol. 1990;10:6586–6595. doi: 10.1128/mcb.10.12.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamel P A, Gill R M, Phillips R A, Gallie B L. Regions controlling hyperphosphorylation and conformation of the retinoblastoma gene product are independent of domains required for transcriptional repression. Oncogene. 1992;7:693–701. [PubMed] [Google Scholar]
- 19.Hatakeyama M, Brill J A, Fink G R, Weinberg R A. Collaboration of G1 cyclins in the functional inactivation of the retinoblastoma protein. Genes Dev. 1994;8:1759–1771. doi: 10.1101/gad.8.15.1759. [DOI] [PubMed] [Google Scholar]
- 20.Hengstschlager M, Hengstschlager-Ottnad E, Pusch O, Wawra E. The role of p16 in the E2F-dependent thymidine kinase regulation. Oncogene. 1996;12:1635–1643. [PubMed] [Google Scholar]
- 21.Hinds P W, Mittnacht S, Dulic V, Arnold A, Reed S I, Weinberg R A. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992;70:993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann F, Livingston D M. Differential effects of cdk2 and cdk3 on the control of pRb and E2F function during G1 exit. Genes Dev. 1996;10:851–861. doi: 10.1101/gad.10.7.851. [DOI] [PubMed] [Google Scholar]
- 23.Horton L E, Qian Y, Templeton D J. G1 cyclins control the retinoblastoma gene product growth regulation activity via upstream mechanisms. Cell Growth Differ. 1995;6:395–407. [PubMed] [Google Scholar]
- 24.Janicke R U, Walker P A, Lin X Y, Porter A G. Specific cleavage of the retinoblastoma protein by an ICE-like protease in apoptosis. EMBO J. 1996;15:6969–6978. [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang W, Kahn S M, Zhou P, Zhang Y J, Cacace A M, Infante A S, Doi S, Santella R M, Weinstein I B. Overexpression of cyclin D1 in rat fibroblasts causes abnormalities in growth control, cell cycle progression and gene expression. Oncogene. 1993;8:3447–3457. [PubMed] [Google Scholar]
- 26.Kato J, Matsushime H, Hiebert S W, Ewen M E, Sherr C J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993;7:331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- 27.Kitagawa M, Higashi H, Jung H K, Suzuki-Takahashi I, Ikeda M, Tamai K, Kato J, Segawa K, Yoshida E, Nishimura S, Taya Y. The consensus motif for phosphorylation by cyclin D1-Cdk4 is different from that for phosphorylation by cyclin A/E-Cdk2. EMBO J. 1996;15:7060–7069. [PMC free article] [PubMed] [Google Scholar]
- 28.Knudsen E S, Wang J Y. Differential regulation of retinoblastoma protein function by specific Cdk phosphorylation sites. J Biol Chem. 1996;271:8313–8320. doi: 10.1074/jbc.271.14.8313. [DOI] [PubMed] [Google Scholar]
- 29.Koff A, Giordano A, Desai D, Yamashita K, Harper J W, Elledge S, Nishimoto T, Morgan D O, Franza B R, Roberts J M. Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science. 1992;257:1689–1694. doi: 10.1126/science.1388288. [DOI] [PubMed] [Google Scholar]
- 30.Koh J, Enders G H, Dynlacht B D, Harlow E. Tumour-derived p16 alleles encoding proteins defective in cell-cycle inhibition. Nature. 1995;375:506–510. doi: 10.1038/375506a0. [DOI] [PubMed] [Google Scholar]
- 31.Krek W, Livingston D M, Shirodkar S. Binding to DNA and the retinoblastoma gene product promoted by complex formation of different E2F family members. Science. 1993;262:1557–1560. doi: 10.1126/science.8248803. [DOI] [PubMed] [Google Scholar]
- 32.La Thangue N B. DRTF1/E2F: an expanding family of heterodimeric transcription factors implicated in cell-cycle control. Trends Biochem Sci. 1994;19:108–114. doi: 10.1016/0968-0004(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 33.Lees J A, Buchkovich K J, Marshak D R, Anderson C W, Harlow E. The retinoblastoma protein is phosphorylated on multiple sites by human cdc2. EMBO J. 1991;10:4279–4290. doi: 10.1002/j.1460-2075.1991.tb05006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leone G, De Gregori J, Sears R, Jakoi L, Nevins J R. Myc and Ras collaborate in inducing accumulation of active cyclin E/Cdk2 and E2F. Nature. 1997;387:422–426. doi: 10.1038/387422a0. [DOI] [PubMed] [Google Scholar]
- 35.Lew D J, Dulic V, Reed S I. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991;66:1197–1206. doi: 10.1016/0092-8674(91)90042-w. [DOI] [PubMed] [Google Scholar]
- 36.Lin B T, Gruenwald S, Morla A O, Lee W H, Wang J Y. Retinoblastoma cancer suppressor gene product is a substrate of the cell cycle regulator cdc2 kinase. EMBO J. 1991;10:857–864. doi: 10.1002/j.1460-2075.1991.tb08018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsushime H, Roussel M F, Ashmun R A, Sherr C J. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991;65:701–713. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- 38.Medema R H, Herrera R E, Lam F, Weinberg R A. Growth suppression by p16ink4 requires functional retinoblastoma protein. Proc Natl Acad Sci USA. 1995;92:6289–293. doi: 10.1073/pnas.92.14.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyerson M, Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol. 1994;14:2077–2086. doi: 10.1128/mcb.14.3.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mihara K, Cao X R, Yen A, Chandler S, Driscoll B, Murphree A L, T’Ang A, Fung Y K. Cell cycle-dependent regulation of phosphorylation of the human retinoblastoma gene product. Science. 1989;246:1300–1303. doi: 10.1126/science.2588006. [DOI] [PubMed] [Google Scholar]
- 41.Minshull J, Golsteyn R, Hill C S, Hunt T. The A- and B-type cyclin associated cdc2 kinases in Xenopus turn on and off at different times in the cell cycle. EMBO J. 1990;9:2865–2875. doi: 10.1002/j.1460-2075.1990.tb07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mittnacht S, Lees J A, Desai D, Harlow E, Morgan D O, Weinberg R A. Distinct sub-populations of the retinoblastoma protein show a distinct pattern of phosphorylation. EMBO J. 1994;13:118–127. doi: 10.1002/j.1460-2075.1994.tb06241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mittnacht S, Weinberg R A. G1/S phosphorylation of the retinoblastoma protein is associated with an altered affinity for the nuclear compartment. Cell. 1991;65:381–393. doi: 10.1016/0092-8674(91)90456-9. [DOI] [PubMed] [Google Scholar]
- 44.Moberg K, Starz M A, Lees J A. E2F-4 switches from p130 to p107 and pRB in response to cell cycle reentry. Mol Cell Biol. 1996;16:1436–1449. doi: 10.1128/mcb.16.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohtsubo M, Theodoras A M, Schumacher J, Roberts J M, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pines J, Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature. 1990;346:760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- 47.Qian Y, Luckey C, Horton L, Esser M, Templeton D J. Biological function of the retinoblastoma protein requires distinct domains for hyperphosphorylation and transcription factor binding. Mol Cell Biol. 1992;12:5363–5372. doi: 10.1128/mcb.12.12.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quelle D E, Ashmun R A, Shurtleff S A, Kato J Y, Bar-Sagi D, Roussel M F, Sherr C J. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- 49.Resnitzky D, Gossen M, Bujard H, Reed S I. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roach P J. Control of glycogen synthase by hierarchal protein phosphorylation. FASEB J. 1990;4:2961–2968. [PubMed] [Google Scholar]
- 51.Schulze A, Zerfass K, Spitkovsky D, Henglein B, Jansen-Durr P. Activation of the E2F transcription factor by cyclin D1 is blocked by p16INK4, the product of the putative tumor suppressor gene MTS1. Oncogene. 1994;9:3475–3482. [PubMed] [Google Scholar]
- 52.Serrano M, Hannon G J, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 53.Sherr C J. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 54.Tam S W, Theodoras A M, Shay J W, Draetta G F, Pagano M. Differential expression and regulation of Cyclin D1 protein in normal and tumor human cells: association with Cdk4 is required for Cyclin D1 function in G1 progression. Oncogene. 1994;9:2663–2674. [PubMed] [Google Scholar]
- 55.Tan X, Martin S J, Green D R, Wang J Y J. Degradation of retinoblastoma protein in tumor necrosis factor- and CD95-induced cell death. J Biol Chem. 1997;272:9613–9616. doi: 10.1074/jbc.272.15.9613. [DOI] [PubMed] [Google Scholar]
- 56.Taya Y, Yasuda H, Kamijo M, Nakaya K, Nakamura Y, Ohba Y, Nishimura S. In vitro phosphorylation of the tumor suppressor gene RB protein by mitosis-specific histone H1 kinase. Biochem Biophys Res Commun. 1989;164:580–586. doi: 10.1016/0006-291x(89)91759-2. [DOI] [PubMed] [Google Scholar]
- 57.Templeton D J, Park S H, Lanier L, Weinberg R A. Nonfunctional mutants of the retinoblastoma protein are characterized by defects in phosphorylation, viral oncoprotein association, and nuclear tethering. Proc Natl Acad Sci USA. 1991;88:3033–3037. doi: 10.1073/pnas.88.8.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- 59.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 60.Whyte P, Buchkovich K J, Horowitz J M, Friend S H, Raybuck M, Weinberg R A, Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988;334:124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- 61.Wu C L, Classon M, Dyson N, Harlow E. Expression of dominant-negative mutant DP-1 blocks cell cycle progression in G1. Mol Cell Biol. 1996;16:3698–3706. doi: 10.1128/mcb.16.7.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zarkowska T, Mittnacht S. Differential Phosphorylation of the retinoblastoma protein by G1/S cyclin-dependent kinases. J Biol Chem. 1997;19:112738–12746. doi: 10.1074/jbc.272.19.12738. [DOI] [PubMed] [Google Scholar]
- 63.Zarkowska T, Harlow S U E, Mittnacht S. Monoclonal antibodies specific for underphosphorylated retinoblastoma protein identify a cell cycle regulated phosphorylation site targeted by CDKs. Oncogene. 1997;14:249–254. doi: 10.1038/sj.onc.1200824. [DOI] [PubMed] [Google Scholar]
- 64.Zerfass-Thome K, Schulze A, Zwerschke W, Vogt B, Helin K, Bartek J, Henglein B, Jansen-Durr P. p27KIP1 blocks cyclin E-dependent transactivation of cyclin A gene expression. Mol Cell Biol. 1997;17:407–415. doi: 10.1128/mcb.17.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]