Neural Stem Cells as Engraftable Packaging Lines Can Mediate Gene Delivery to Microglia: Evidence from Studying Retroviral env-Related Neurodegeneration (original) (raw)
Abstract
The induction of spongiform myeloencephalopathy by murine leukemia viruses is mediated primarily by infection of central nervous system (CNS) microglia. In this regard, we have previously shown that CasBrE-induced disease requires late, rather than early, virus replication events in microglial cells (W. P. Lynch et al., J. Virol. 70:8896–8907, 1996). Furthermore, neurodegeneration requires the presence of unique sequences within the viral env gene. Thus, the neurodegeneration-inducing events could result from microglial expression of retroviral envelope protein alone or from the interaction of envelope protein with other viral structural proteins in the virus assembly and maturation process. To distinguish between these possible mechanisms of disease induction, we engineered the engraftable neural stem cell line C17-2 into packaging/producer cells in order to deliver the neurovirulent CasBrE env gene to endogenous CNS cells. This strategy resulted in significant CasBrE env expression within CNS microglia without the appearance of replication competent virus. CasBrE envelope expression within microglia was accompanied by increased expression of activation markers F4/80 and Mac-1 (CD11b) but failed to induce spongiform neurodegenerative changes. These results suggest that envelope expression alone within microglia is not sufficient to induce neurodegeneration. Rather, microglia-mediated disease appears to require neurovirulent Env protein interaction with other viral proteins during assembly or maturation. More broadly, the results presented here prove the efficacy of a novel method by which neural stem cell biology may be harnessed for genetically manipulating the CNS, not only for studying neurodegeneration but also as a paradigm for the disseminated distribution of retroviral vector-transduced genes.
The simplest and best-defined model for analyzing the details of retroviral neuropathogenesis resides in a group of murine C-type leukemia viruses (MuLVs) that cause spongiform neurodegeneration of motor system neurons from the neocortex through the spinal cord (reviewed recently in reference 43). The prototypic virus of this class is the ecotropic host range virus referred to as CasBrE (1, 15). Genetic recombination analyses indicate that the principal determinants of MuLV neurovirulence map to the env gene (11, 41, 42, 63), which encodes the surface glycoprotein responsible for binding and entry of retrovirus into cells. It has been widely proposed that the gene product of env (the envelope protein) of neurovirulent retroviruses may be directly toxic to the central nervous system (CNS) (21, 22, 43, 62). However, indiscriminate overexpression of env alone in the brains of susceptible mice is not sufficient to precipitate acute clinical or histopathologic disease (32). Induction of neurodegeneration requires late retroviral replication events within host microglia, in particular those events associated with envelope synthesis (32).
Hence, the study of retroviral pathogenesis has focused on the infection of microglia. In vivo, microglial infection by CasBrE appears to result in the generation of unique Env proteins (10, 28, 30, 32). It remains unresolved, however, whether the microglial Env proteins themselves are directly neurotoxic, or whether the synthesis and assembly of Env protein corrupts microglial function and compromises neuronal survival from a loss of microglial support. To investigate how retroviral interactions within microglia lead to disrupted CNS function, we sought ways to genetically manipulate the microglial compartment. Prior transgenic approaches to achieve either global or cell-type-specific CNS CasBrE env expression have been unsuccessful in approximating the expression associated with CNS viral infection (22, 31a, 62). Furthermore, attempts to genetically manipulate the microglial compartment by using bone marrow chimeras have been hampered because of a very slow turnover of parenchymal microglial cells (20, 24, 26). Virus-based vectors offer a potential alternative for manipulating the microglial compartment since they have been demonstrated to be effective vehicles for the in vivo transfer of exogenous genes directly to endogenous cells in the CNS (58). However, delivering genes of interest to microglia throughout the brain is a challenge to viral vectorology (49, 58). In fact, the relatively anatomically restricted effectiveness of retrovirus- and, indeed, many virus-based vector systems has been one of the obstacles to their broader use for therapeutic gene transfer to the CNS. We recognized that surmounting this limitation to answer our particular research question might provide a method for improving the efficacy of viral vector-mediated gene transfer for much broader applications.
Neural stem cells (NSCs) are immature, uncommitted cells that exist in the developing and adult nervous system and are responsible for giving rise to the vast array of more specialized neural cells of the CNS (reviewed in references 33, 36, 39, 49, 57, 59, and 60). They are operationally defined by their ability to self-renew and to differentiate into cells of most (if not all) neuronal and neuroglial lineages and to populate developing or degenerating CNS regions. We previously demonstrated that migratory NSCs are well suited for gene therapy of broad regions of CNS because they are easily expanded and genetically manipulated in culture and, following transplantation into germinal zones, are integrated in a cytoarchitecturally appropriate manner throughout the brain, where they express the foreign genes. We have shown them to be capable of delivering therapeutic gene products in a widely disseminated manner, cross-correcting host neurons and glia by creating virtually chimeric regions of the brain (25, 51, 54). Their facility to distribute themselves extensively and disseminate foreign gene products prompted us to use these NSCs in a somewhat unconventional manner. We explored the possibility of engineering engraftable NSC clones into a packaging/producer line for retroviral vectors in order to deliver the env gene from a neurovirulent virus directly to host microglial cells within the brain. In other words, we examined whether NSCs, as packaging/producer cells, could serve as “platforms” for the widespread dissemination of replication-incompetent foreign gene-expressing viral vectors, just as they had for other diffusible and nondiffusible factors.
The notion of transplanting packaging/producer cells (i.e., the cells that provide the structural proteins for vector assembly) is not new; however, their usefulness in vivo has been limited by the strong propensity of the engineered fibroblast cell lines to become tumors in transplant recipients (14, 52). That NSCs can participate in the normal development of many regions at multiple stages along the murine neuroaxis may provide the key to making an engraftable CNS gene delivery system feasible.
Thus, the goals of our experimental approach were twofold. First, we were interested in whether NSCs engineered into a retroviral packaging/producer line would make an efficient in vivo gene delivery system. Second, we wanted to test whether microglial expression of env alone was sufficient to precipitate spongiform neurodegeneration. We recognized that approaching this particular problem as a proof of principle for a NSC-based strategy for retrovirus-mediated transgene delivery was actually ideal. Because microglia are not of neuroectodermal origin, nor can NSCs give rise to microglia, expression of this index transgene (env) in these cells would help rigorously prove host cell transduction via the retrovirus and rule out misidentification of engineered donor-derived cells as host cells.
We report that NSC-derived packaging cells can, indeed, act as in vivo gene delivery systems. This technique helped demonstrate that expression of env within microglia is insufficient to induce acute spongiform neurodegeneration, suggesting that defective retroviral assembly, rather than direct Env neurotoxicity, is the basis for pathogenesis. In a broader context, we show that a clone of migratory NSCs can be engineered ex vivo to package and release replication-defective retroviral particles and that these engineered NSCs, when transplanted into the brain, can serve as platforms for the wider dissemination of these vectors in order to direct the transfer of a desired gene to endogenous CNS host cells.
MATERIALS AND METHODS
Cells, plasmids, and viruses.
The C17-2 NSC line was derived as previously described (47). In brief, neonatal mouse cerebellar external germinal zone cells were infected with a defective retrovirus vector encoding v-myc (transcribed from the long terminal repeat [LTR]) and neo (transcribed from an internal simian virus 40 [SV40] promoter) and selected in G418. The resulting cells were then infected with a second retroviral vector, BAG, encoding lacZ transcribed from the LTR and neo transcribed from an internal SV40 promoter (44). The first vector enabled these cells to grow in culture indefinitely, while the second vector endowed the cells with β-galactosidase (β-Gal) expression as a genetic cellular marker (50). These stable _lacZ_-expressing NSCs are free of helper virus (47, 50).
Mus dunni fibroblasts were used for virus focus assays as outlined previously (8), and replication-competent virus stocks were generated in Fisher rat embryo cells.
The replication-defective, CasBrE _env_-encoding CasE virus was made in PA317 cells (37) as previously outlined (32), with viral titers of 105 to 106 per ml of culture supernatant. No helper virus is present within these stocks.
All cells were grown in Dulbecco’s modified Eagle medium (high glucose) supplemented with 10% fetal bovine serum, glutamine, and antibiotics (penicillin, streptomycin, and amphotericin B [Fungizone]).
The amphotropic packaging plasmid pPAM3 (Fig. 1A; a gift from A. D. Miller, Fred Hutchinson Cancer Research Center) has been previously described (37). Plasmid pPGKPuro (a gift from P. W. Laird, University of Southern California) contains the puromycin resistance gene encoding puromycin _N_-acetyltransferase under the control of the phosphoglycerate kinase (PGK) promoter, followed by PGK polyadenylation sequences cloned into pBluescript SK+ (Stratagene) (Fig. 1B). The structure of the pCasE vector is shown in Fig. 1C. The CasE vector was constructed by using the defective Friend spleen focus-forming virus (SFFV)-based retroviral vector pSFF (6) owing to the exceptional capacity of pSFF to be packaged into viral particles. The CasE vector is also efficiently packaged in retroviral particles and readily spreads in amphotropic packaging cell culture by ping-pong transfer owing to the presence of both an amphotropic env (encoded by pPAM3) and an ecotropic env (encoded by pCasE) (32). Thus, CasE viral vector spread occurs by using whichever cellular receptors are not interfered with by endogenous cellular env expression (23, 28).
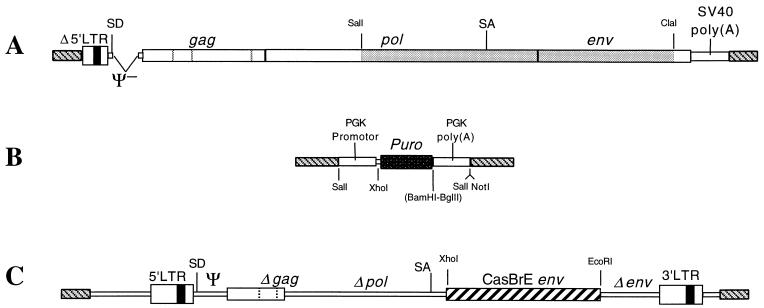
FIG. 1.
Vectors used for converting C17-2 NSCs to packaging cells capable of delivering CasBrE env to target cells. C17-2 cells were converted to a packaging cell line by transfection with the packaging plasmid pPAM3 (A) developed by Miller and Buttimore (37). This plasmid was constructed using a Moloney MuLV genome from which the Ψ sequence had been deleted and the pol-env region was exchanged for that of the 4070A amphotropic virus (shaded) (38). In addition, the 3′ LTR was replaced with the SV40 polyadenylation site and viral sequences 5′ of the enhancers were deleted in the 5′ LTR. The latter two modifications were designed specifically reduce the likelihood that helper virus would arise since it would require a minimum of two recombination events. C17-2 cells containing pPAM3 were identified by selection for puromycin resistance conferred by cotransfections with the selection plasmid pPGKPuro (B), which encodes puromycin N_-acetyltransferase (dark rectangle) under control of the PGK promoter followed by the PGK polyadenylation sequences. The CasBrE env retroviral expression vector pCasE (C) was constructed by introducing env into the pSFF vector (6) at the multiple cloning site in order to maximize mRNA packaging and protein expression. This vector, which is based on the defective Friend SFFV, contains significant deletions (both engineered and naturally occurring) in the gag, pol, and env genes (6). These modifications result in a failure to express normal viral proteins but allow both efficient packaging of the vector RNA into particles and expression of exogenous genes cloned into the polylinker site. (The possibility that the Δ_gag region gave rise to truncated Gag-like polyprotein, as reported for the defective SFFV parent [46], was not investigated in this study). Plasmid vector backgrounds (gray diagonal stripes) for the expression constructs A, B, and C, were pBR322, pBluescript (Stratagene), and pSP64, respectively. SD, splice donor; SA, splice acceptor.
The marginally neurovirulent CasBrE virus, clone 15-1 (41), was made in FRE cells. This virus was used as a positive control in the virus titration and cell infection assays. The infection of C17-2 NSCs with the replication-competent CasBrE virus (clone 15-1) was performed as previously outlined (32). In some virus titration experiments, the 4070A amphotropic virus (a gift from J. Cunningham, Brigham and Women’s Hospital and Harvard Medical School) was used as a positive control for detecting amphotropic virus and morphology of foci.
Generation and characterization of NSC packaging/producer cells.
The isolation, cloning, propagation, maintenance, and transplantation of engraftable multipotent murine NSC clone C17-2 has been previously detailed (32, 45, 48–50, 53). To convert them to a packaging/producer line, subclones of C17-2 NSCs were cotransfected by electroporation using 20 μg of the amphotropic packaging plasmid pPAM3 (Fig. 1) along with 0.5 μg of the puromycin selection plasmid pPGKPuro at a molar ratio of 20:1, respectively. After 48 h, transfected cells were selected in puromycin (2 μg/ml), and isolated colonies were analyzed for expression of plasmid pPAM3 by examining the cell clones for cell surface amphotropic envelope glycoprotein coat (clone 4070A) expression by immunocytochemistry (29) and fluorescence-activated cell sorting (32). The 4070A envelope was detected by using the Env protein-specific monoclonal antibody 83A25 (13), which recognizes an epitope common to many murine retroviral Env proteins. 83A25-positive colonies were then screened for packaging ability by assessing which colonies had packaged lacZ into infectious virus particles. (As noted above, a provirus containing lacZ was already integrated into the C17-2 genome [50].) This screen was performed by a virus focus assay evaluating β-Gal expression in target cells as previously reported [44]; however, in this instance, M. dunni fibroblasts were used as naive targets. C17-2 clones indicative of successful _lacZ_-viral vector packaging were then infected with the CasBrE env gene encoding replication-defective virus CasE at a multiplicity of infection of 1. CasBrE env expression was assessed by immunohistochemistry using monoclonal antibodies 667 and 697 (34).
env gene transfer by C17-2 packaging cells was assessed by both an infectious center assay and a viral focus assay (see below). For the infectious center assay, packaging cells were irradiated with 2,000 rads and seeded at various dilutions along with M. dunni target cells at 105 M. dunni cells per well of a TC-6 plate in the absence of Polybrene. Cells were grown for 4 days, and cultures were scored for foci after fixation and immunostaining for CasBrE env expression. Isolated single env+ cells, likely representing irradiated C17-2 packaging cells, were not scored as foci. The number of foci detected was compared to the number of packaging cells originally seeded and expressed as a percentage of cells with gene transfer capability. This number does not account for packaging cell losses that may have resulted from the radiation treatment. Infectious center focus morphology was also examined as a means for evaluating whether recombinant helper virus appeared (see below).
The C17-2 NSC packaging subclones, particularly BA6 and BA6-CasE, were examined prior to transplantation for the presence of several neural cell type markers to assess grossly their extent of differentiation in vitro due to the introduction of the packaging vector. For immunocytochemical analyses performed as previously described (29, 32, 48, 50), antibodies directed against the following markers were used: nestin, for immature neural progenitors and stem cells; 2′,3′-cyclic nucleotide 3′-phosphohydrolase (CNPase; Sternberger (Moncolonals, Inc.) and galactocerebroside (Chemicon), for oligodendrocytes; neurofilament (Chemicon) and neuron-specific enolase (Polysciences), for neurons and neuroblasts, respectively; and glial fibrillary acidic protein (GFAP; Dako), for astrocytes.
Tests for replication-defective and replication-competent virus production.
For detecting and quantitating cell-free virus production from packaging cells, both in culture supernatant and in transplanted brains, a focus assay (8) was used. Briefly, 105 M. dunni cells were seeded into each well of a TC-6 plate (Linbro) and exposed overnight to either filtered (0.2-μm-pore-size filter) undiluted or serially diluted 48-h supernatants taken from freshly confluent C17-2 packaging lines or serial diluted brain extracts (see below), in the presence of 8 μg of Polybrene. The medium was changed, and M. dunni cells were grown to confluence and then examined immunohistochemically for viral foci, using antibodies reactive to either the 4070A amphotropic viral coat (monoclonal antibody 83A25 [13]), the CasBrE Env protein, encoded by CasE (monoclonal antibodies 667 and 697 [34]), or β-Gal (rabbit polyclonal antibody from Cappel). Alternatively, BAG virus production was detected in a focus assay using 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) histochemistry (44). As performed, the sensitivity of this assay is 0.5 focus-forming unit (FFU)/ml of cell supernatant/brain extract.
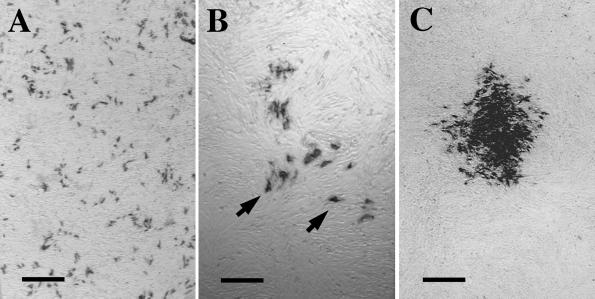
To detect the generation of recombinant helper virus, two methods were used. First, we performed the focus assay described above in duplicate, with either monoclonal antibody 667/697 or monoclonal antibody 83A25, and examined the morphology of the primary foci in detail. When replication-competent virus is present, Env protein staining in the foci is contiguous because secondary infection of surrounding uninfected M. dunni cells occurs efficiently via cell-to-cell contact as infected cells migrate. If replication-defective virus is present, the initial infectious event is terminal and the focus morphology is determined by the division and cellular migration of the originally infected target M. dunni fibroblasts. Typically, M. dunni cells divide and progeny cells migrate away from one another. At the same time, other uninfected cells divide and move between them. Under these circumstances, the foci are noncontiguous (see Fig. 3A and B) because the initial infectious event has no means to spread to M. dunni cells with which they make contact in culture.
FIG. 3.
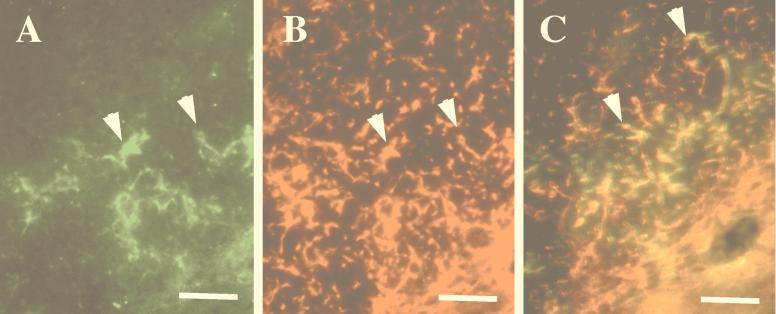
Localization of env expression within microglia demonstrates that NSCs are capable of retrovirus-mediated transgene delivery to host CNS cells. (A) Indirect immunofluorescent staining for Env protein within the deep layers of the cerebral cortex viewed through the fluorescein filter set (using biotinylated antibody 667). Note the highly arborized nature of these cells, characteristic of a microglial phenotype. (B) The same section as in panel A stained with the microglia-specific antibody rabbit anti-F4/80 as outlined elsewhere (18) and viewed through the rhodamine filter set. Note the colocalization of env and F4/80 in panels A and B, respectively (arrowheads). (C) Region of the septum in which Env protein (green) colocalizes with the microglial marker F4/80 (red) to yield yellow cells (arrowheads), further suggesting that the transplanted NSC packaging cells delivered the env gene to endogenous host cells. Magnification bars: 50 μm.
Serial supernatant passage was used as a second measure for the release of helper virus from the C17-2 packaging cell clones. Specifically, this assay used the supernatants taken from primary M. dunni cell targets (i.e., cells that were exposed to undiluted BA6 and BA6-CasE cell supernatants) once the target cells reach confluence and before they are stained for foci. These secondary supernatants were then incubated overnight with 105 naive M. dunni targets in the presence of Polybrene (8 μg/ml). After a change of medium, the secondary M. dunni target cells were cultured to confluence in TC-6 plates and then examined for cellular expression of either the amphotropic coat, CasBrE Env protein, or β-Gal or the generation of neomycin-resistant colonies. M. dunni cells examined for neomycin-resistant colonies were grown for 3 weeks in the presence of 2 μg of G418 (approximately 50% active) per ml. The sensitivity of the assay is 0.5 FFU/ml of secondary supernatant.
NSC transplantation into and analysis of engrafted mouse brains.
Transplantation of the above-mentioned NSC subclones (including BA6 and BA6-CasE packaging NSCs and 15-1-infected C17-2 NSCs) was done as previously described (32, 50, 53). All mice used were inbred Rocky Mountain Laboratories White strain (IRW) mice, which are highly susceptible to the neurodegenerative effects of CasBrE virus infection. Briefly, NSCs, maintained in culture as detailed elsewhere (29, 32, 48, 50, 53), were trypsinized from a 90% confluent dish and resuspended in phosphate-buffered saline at 5 × 104 cells/μl. On the day of birth, the lateral ventricles of cryoanesthetized pups were visualized by transillumination of the head; 2 μl of the cellular suspension (to which 0.08% trypan blue was added to assess cell viability as well as to localize the inoculum) was gently expelled via a glass micropipette inserted transcutaneously into each ventricle (gaining access to the subventricular zone). Pups were returned to maternal care until weaning.
Donor-derived cells were tentatively identified by their expression of β-Gal either by X-Gal histochemistry (50) or by an antibody directed against β-Gal (32). In addition, as previously described (29, 32), engrafted brains were analyzed immunocytochemically with antibody (83A25) reactive against the 4070A amphotropic coat of the packaging vector (unique to transplant-derived cells) and with antibodies (667B or 697) against the protein encoded by CasBrE env (potentially detectable in both donor-derived and host-derived cells).
The number and phenotype of host cells (lacZ negative) expressing env was assessed immunocytochemically by double-label immunofluorescence using antibodies directed against the neural cell type-specific markers listed above. Microglia were identified by using rat monoclonal antibodies directed to either Mac-1 (murine CD11b) (55) or F4/80 (2) or a rabbit polyclonal antibody specifically directed to the F4/80 protein (gift from S. Gordon, Oxford University) as described elsewhere (18).
Engrafted brains were tested for the production of both defective virus and replication-competent helper virus as described above. Brain extracts were prepared by homogenizing freshly isolated transplant brains in 10 volumes of 150 mM NaCl–50 mM Tris-HCl (pH 7.4)–0.1 mM EDTA (pH 7.2) (TBS-EDTA) at 4°C (using 10 strokes of a Wheaton glass homogenizer). Extracts were centrifuged a 10,000 × g for 10 min at 4°C to remove nuclei and large cellular debris. The resulting supernatants were used in virus detection assays as outlined above. Alternatively, in some cases, serial 20-μm frozen sections were taken and stained for detection of cellular engraftment, while parallel sections were extracted in 50 μl of TBS-EDTA and tested for the presence of virus. The sensitivity of these assay is 1 FFU/ml of brain extract (approximately 4 FFU/brain), or 1 FFU/tissue section.
Brains were examined for histopathological changes by hematoxylin-and-eosin staining of paraffin sections. In addition, Env protein localization was carried out in paraffin sections as outlined in reference 32 after antigen retrieval by steam heating deparaffinized slides immersed in 10 mM citrate buffer (pH 6.0) for 20 min.
Clinical neurological signs were assessed as outlined previously (8–10, 31, 32, 41).
All animal experiments were carried out in accordance with federal guidelines (39a) and in accordance with the Animal Care and Use Committees at Harvard Medical School and Northeastern Ohio Universities College of Medicine.
RESULTS AND DISCUSSION
Engineering NSCs into packaging lines.
One of the best-studied models of NSC behavior is a clone designated C17-2. It is one of a number of stable, self-renewing cellular clones originally derived from neonatal mouse cerebellum but capable of participating in the normal development of most CNS structures from fetus to adult upon implantation into mouse CNS germinal zones. The cells differentiate into neurons when transplanted into regions undergoing neurogenesis or into glia where gliogenesis predominates (25, 32, 45, 48–50, 53). For detection, C17-2 cells constitutively and stably express the lacZ reporter gene encoding β-Gal introduced by a retroviral vector. Having been used successfully in a number of animal models of neurologic disease, C17-2 cells have been useful for illustrating the range of developmental potential and therapeutic possibilities of NSCs for gene therapy and repair (25, 33, 45, 48, 52–54).
It had been previously established that clone C17-2 NSCs could be infected in vitro with replication-defective retroviral vectors (e.g., for transducing genes for β-Gal, enzymes, and neurotrophins [25, 33, 49, 53]) as well as with replication-competent retroviruses (32). These infections did not diminish the ability of these NSCs to engraft and integrate into the CNS parenchyma. Therefore, it seemed feasible to convert these cells into engraftable in situ packaging/producer lines.
In the discussions that follow, to avoid confusion, we will be careful to distinguish between procedures relevant to characterizing a retroviral vector producer line and those that coincidentally happen to look at env (the CasBrE retroviral surface glycoprotein) as the foreign gene of interest in these studies.
An NSC-based retroviral packaging line was produced as outlined in Materials and Methods by introducing the amphotropic packaging plasmid pPAM3 (used previously to generate the PA317 fibroblast packaging cell line [37]) (Fig. 1A) along with the selection plasmid pPGKPuro (Fig. 1B) into C17-2 NSCs. Colonies were selected in puromycin, and 48 clones that were immunopositive for the amphotropic coat by immunostaining with monoclonal antibody 83A25 (13) were isolated. No significant difference in the level of coat expression among clones was detected by fluorescence-activated cell sorting (not shown).
These clones were then screened for the ability to package the endogenous lacZ gene, which was originally introduced into NSCs by a retroviral vector at the time of their derivation from the cerebellum (50). Eighteen pPAM3-transfected clones with packaging ability were identified. These clones expressed very low β-Gal virus titers of 100 to 102 FFU/ml. To identify whether any of these clones could more efficiently package a pSFF expression vector, the 18 clones were infected with replication-defective CasE virus, which encodes the CasBrE Env protein (Fig. 1C). The CasE vector was observed to spread throughout the 18 C17-2 packaging cultures, most likely because of incorporation of pCasE RNA into virions expressing both amphotropic (4070A) and ecotropic (CasBrE) coats. Assessment of the C17-2 NSC packaging cell clones ability to package and transfer the gene of interest (env) to naive targets indicated that all 18 subclones packaged env into infectious viral particles with titers of 102 to 104 FFU/ml. In particular, one subclone, BA6, consistently and reproducibly transduced CasBrE env with high efficiency, producing titers of 104 to 105 FFU/ml, even after extensive passaging. Therefore, we concentrated our efforts on this subclone, designated BA6-CasE.
To evaluate the ability of BA6-CasE to deliver env by cell-to-cell contact (a more sensitive means for identifying a cell’s infectious potential), irradiated BA6-CasE cells were evaluated by an infectious center assay (see Materials and Methods). Of the BA6-CasE cells seeded, 31% ± 11% (n = 4) generated distinguishable foci. This result was indicative of successful delivery of virus to surrounding susceptible fibroblasts and suggested the potential of the line to deliver genes in vivo. Attempts to subclone cells from the BA6-CasE population did not yield lines with a higher-efficiency env transduction. Both BA6 and BA6-CasE cells were negative for the production of replication-competent amphotrophic or recombinant CasBrE helper virus when assayed by serial supernatant passage.
Interestingly, the BA6 and BA6-CasE cells packaged lacZ at 100 to 102 FFU/ml even though 80 to 100% of these cells constitutively express β-Gal from an integrated provirus. This situation is akin to that noted previously where C17-2 cells, infected with replication-competent virus, had lacZ viral titers which were 2 to 3 logs lower than the infecting virus titers (32). This phenomenon may reflects the relative levels of message for each vector. However, it may also relate to (i) the ability of the CasE vector to more efficiently ping-pong through the cultures as outlined previously (28, 32) and/or (ii) the presence of gag sequences present in the CasE vector, which could increase the efficiency of packaging (5). It is also possible that over the extended passing of the C17-2 cells, the packaging sequences in the integrated _lacZ_-encoding provirus mutated, rendering the lacZ mRNA less suitable for viral packaging. Fortuitously, this ability of the BA6 packaging line to efficiently express β-Gal but inefficiently package _lacZ_-containing viral vectors allowed us to continue using this genetic marker for tentatively identifying transplanted C17-2 cells, since the likelihood that host target cells will be infected by _lacZ_-expressing virus, versus the _env_-containing virus CasE, is comparatively low (i.e., 2 to 3 orders of magnitude minimally).
To assess whether the cellular and molecular derivation of BA6 and BA6-CasE cells from C17-2 NSCs resulted in an alteration of their progenitor-like phenotype, cells were examined immunohistochemically for nestin expression. Both BA6 and BA6-CasE cells expressed nestin at the same levels as the parent C17-2 NSCs. In addition, proliferating cells were not immunoreactive to antibodies against GFAP, galactocerebroside, neurofilament, neuron-specific enolase, and CNPase, suggesting that they maintained an immature, undifferentiated NSC/progenitor-like phenotype.
It should also be noted that the packaging NSC subclone BA6, into which the _env_-encoding vector was transduced to make BA6-CasE cells, was phenotypically indistinguishable from BA6-CasE except for lacking the _env_-encoded protein. In other words, BA6 packaging NSCs could in the future be transfected with vectors encoding other cDNAs for different research purposes. BA6 and BA6-CasE, as the best packaging and transducing NSC subclones, were expanded and used for transplantation into neonatal IRW mouse brains as outlined below.
Transplantation of packaging NSCs into the brains of IRW mice.
To control for the possibility that the BA6 NSC packaging cell line (NSCs without the CasBrE env) could cause neuropathologic changes when engrafted into the brain, BA6 cells were introduced into the lateral ventricles of newborn IRW mouse brains. The brains of 16 animals were examined for engraftment and for the induction of spongiform neurodegeneration. Areas showing the most reproducible levels of engraftment in all mice examined were the forebrains and olfactory bulbs (OBs) (Fig. 2A and B). (Therefore, as in prior studies [32], for uniformity across experiments, analyses focused on these regions.) To evaluate whether there were any untoward effects in response to engrafted BA6 cells only, we examined transplanted mice daily for clinical neurologic signs and examined brains for histopathology at 15, 18, 25, and 28 days postimplantation (dpi) (n = 4 at each time point). No clinical signs were noted through the course of these experiments, and histopathologic analysis revealed no spongiform neurodegenerative changes (not shown). Furthermore, no evidence of inflammation was observed, nor was overt microglial or astrocytic activation detectable by immunohistochemical staining for F4/80 or GFAP, respectively (not shown). These results were in agreement with previous studies in our laboratory which demonstrated that CNS expression of replication-restricted retrovirus from C17-2 NSCs does not induce acute neuropathologic changes in the brain (32).
FIG. 2.
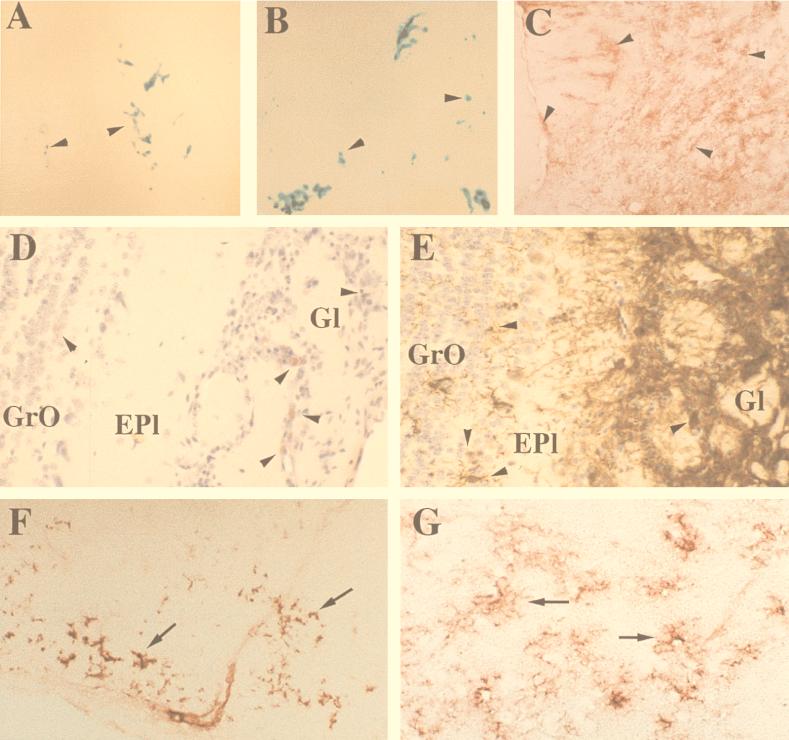
Engraftment of BA6 and BA6-CasE retroviral vector packaging NSCs and associated expression in vivo of the foreign gene of interest (env). (A and B) Low-magnification micrographs of representative sections through the forebrain and OBs of mice transplanted with either BA6 packaging NSCs (A, at 25 dpi) or BA6-CasE packaging NSCs (B, at 31 dpi), the latter a subclone of BA6 specifically engineered to package into replication-incompetent retroviral vectors the index gene of interest, env. The sections were processed with X-Gal histochemistry to show donor-derived _lacZ_-expressing cells as blue (arrowheads). (C and E to G) Immunostaining for the gene product encoded by CasE (env) in the brains of mice transplanted with BA6-CasE cells. (C) A frozen section from the OB immunostained for env, using aminoethyl carbazole as the chromogen (red, arrowheads), is representative of the intense and widespread env expression observed in the OBs and forebrains of BA6-CasE-transplanted animals. To assess the likelihood that transplanted NSCs are delivering env to endogenous CNS cells, panels D and E directly compare the distribution of β-Gal+ cells (32) with _env_-expressing cells in adjacent paraffin sections from the OB (subjected to antigen retrieval prior to immunohistochemistry using antibody 697). Panel D demonstrates a rather restricted number of β-Gal+ cells (aminoethyl carbazole; red, arrowheads) across the multiple layers of the OB. In comparison, env expression (diaminobenzidine; brown-stained cells, arrowheads) in panel E appears as intense immunoreactivity in practically all cells of the glomerular layer (Gl), as well as a large number of cells in the external plexiform (Epl) and granule layers (GrO), implying that the engrafted NSC-derived packaging cells may successfully deliver the index foreign gene, env, to host cells. Representative env expression is also detected in cells of deep cortical layers and corpus callosum in panel F and at higher magnification in the septum in panel G (red, arrows), two CNS regions documented to be susceptible to the neurodegenerative effects of the CasBrE virus (10, 32) (see also Fig. 3). As seen particularly well in panel G, the _env_-expressing cells in these regions are highly ramified, a morphology characteristic of microglia, a cell type of nonneuroectodermal origin. Immunostaining of sections corresponding to those in panels F and G for β-Gal expression failed to detect the presence of β-Gal+ donor derived NSCs (not shown). Furthermore, most env+ tissue sections examined in the septum and deep cortex actually had few if any detectable β-Gal+ cells (donor derived), further suggesting that the env+ cells were of host rather than donor origin. Magnifications: A and B, ×25; C, ×125; D to G, ×250.
BA6-CasE packaging NSCs were next similarly implanted into neonatal mouse brains to determine whether they could deliver significant levels of the retroviral vector-transduced gene env to endogenous dividing cells. Therefore, the distribution and phenotype of host (β-Gal-negative) cells expressing env was assessed; alternatively stated, infected env+ cells were examined to determine whether they were β-Gal negative. In addition, we examined the animals clinically and analyzed brain sections histopathologically as described above. No clinical neurological signs were noted in any BA6-CasE NSC-transplanted animals through the course of these experiments (n = 20).
Examination of the brains at 14, 22, and 31 dpi (n = 4, 4, and 12, respectively) revealed X-Gal-positive, blue donor-derived cells in the rostral forebrain and OBs of all mice (Fig. 2B). In contrast to the somewhat restricted distribution of X-Gal reactivity, immunostaining for the protein encoded by env was much more widespread, including significant expression in the OB (Fig. 2C and E), deep layers of the cerebral cortex and the corpus callosum (Fig. 2F), striatum, and septum (Fig. 2G).
When colocalization of env expression with β-Gal was attempted via double immunostaining, very little β-Gal-positive reactivity was observed in env+ cells, suggesting that engrafted NSC-derived packaging cells may, indeed, have successfully delivered the virally transduced foreign gene env to host cells. This assessment was supported by the representative data presented in Fig. 2 which demonstrated that the extensive distribution of env expression was significantly broader than, and not restricted to, the typical distribution of engrafted β-Gal-expressing (β-Gal+) packaging NSCs; for example, in the OB, comparison can be made between the extent of env immunoreactivity in Fig. 2E with the distribution of donor-derived β-Gal immunoreactivity in Fig. 2D. In addition, certain env+ tissue sections actually had few if any detectable donor-derived cells; for example, sections corresponding to those in Fig. 2F and G were immunonegative for β-Gal+ cells.
Although we believe that the dissociation between env and β-Gal expression represents endogenous CNS cells (defined by their β-Gal negativity) that were infected by the _env_-containing vector released from transplanted NSCs, it is also possible that the appearance of such cells was due simply to down regulation of lacZ expression in engrafted BA6-CasE cells. Although most (≥80%) BA6-CasE packaging NSCs are β-gal+, it was still possible for some donor-derived cells to be incorrectly identified as host cells if they were among the few cells that failed to express β-Gal. Even looking at the particular neural cell types expressing env would not be compelling in this regard (colocalization of env with various neuronal and glial markers was, indeed, detected). For example, distinguishing between cells of host and donor origin in even an extensively _env_-expressing region like the OB (Fig. 2C and E) where postnatal neurogenesis persists is difficult because NSCs can give rise to the neural cell types comprising this region (49).
However, there was one instance where observing env expression in a particular cell type would compellingly prove the env transduction phenomenon. As seen particularly well in Fig. 2G, the _env_-expressing cells in most regions are highly ramified cells with a morphology and size characteristic of microglia. Because microglia are not of neuroectodermal origin and C17-2 NSCs cannot give rise to this bone marrow-derived cell type, expression of the transgene env in these cells would strongly suggest host cell transduction by the transplanted BA6-CasE packaging NSCs and not misidentification of engineered donor-derived cells as host cells.
To prove unambiguously that this strategy for delivering foreign genes to, and genetically manipulating, endogenous host cells in the CNS via infection by transplanted packaging NSCs, we carried out rigorous cell type analysis to determine whether the env+ cells, as seen in Fig. 2C and E to G, were microglia, a cell type which must be host and not donor derived. Therefore, brain sections were double immunostained for the protein encoded by env and the microglial marker F4/80 (18, 40). The results (Fig. 3) clearly indicate the coincidence of the two markers, indicating that env was transmitted to microglia by the transplanted BA6-CasE packaging NSCs. In fact, outside of the OB, we observed few env+ cells that did not also stain with the F4/80 microglial marker.
This result interestingly also highlights the particular susceptibility of microglia to in vivo infection by murine retroviruses. What accounts for this particular viral tropism is not known. However, in assessing the CNS response to this microglial env expression we noted that in the local regions where microglia were demonstrated to be expressing env, there was an elevated expression of the microglial antigens F4/80 and Mac-1 (CD11b) (Fig. 4) compared with control engrafted (BA6) or unengrafted animals. These results suggest that CasBrE env expression in microglia was inducing microglial activation, a phenomenon believed by some to play a contributory role in neuropathogenesis (12, 16, 35). However, we have previously noted a dissociation between glial activation and the acute induction of spongiform myeloencephalopathy by the highly neurovirulent chimeric CasBrE virus FrCasE (10, 29, 31, 32).
FIG. 4.
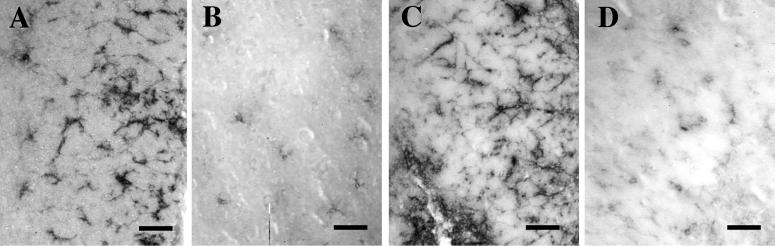
env expression in host microglia results in local microglial activation. Microglial activation was compared between BA6-CasE-transplanted (A and C) and control uninoculated (B and D) mice at 31 days of age by immunostaining brain sections from the striatum for the microglial markers F4/80 (A and B) and Mac-1 (CD11b) (C and D), using rat monoclonal antibodies (2, 56). Note that expression of both antigens is greater in this region of engraftment than in control sections from the same brain region. This elevated staining was observed only in areas where microglial env expression was noted (see also Fig. 3B), as areas in the BA6-CasE brains closely adjacent to those expressing env showed no increased staining for F4/80 and Mac-1 compared with controls (not shown). Magnification bars: 40 μm.
To rule out the possibility that microglial infection resulted from the in vivo generation of a replication-competent virus, homogenates from serial frozen engrafted brain sections or whole brain homogenates were evaluated for the presence of replication-competent virus on M. dunni fibroblasts by serial supernatant passage (see Materials and Methods). When the target cells were exposed to antibodies to detect virus infection, only colonies with patterns consistent with defective infection (noncontiguous cell staining) were evident (Fig. 5). Furthermore, passage of undiluted supernatants from cultures like that shown in Fig. 5A onto additional naive M. dunni targets failed to reveal the presence of viral foci either by staining for viral Env antigens or β-Gal or by selection for neomycin. These results support the conclusion that the microglial expression of env was due to the delivery of the _env_-encoding vector by transplanted BA6-CasE packaging NSCs and not as a result of the emergence of a recombinant virus.
FIG. 5.
Transplanted BA6-CasE packaging NSCs release defective virus encoding env without helper virus. (A) Example of the viral foci obtained after exposing naive M. dunni fibroblasts to 10% CNS homogenate extract from a mouse transplanted with the BA6-CasE packaging NSCs 7 days posttransplantation. Note that abundant env+ cells (black) are not in contiguous distinct foci, like that observed for a replication-competent 4070A amphotropic virus (C). Upon infection with a replication-defective virus, dividing, immunoreactive cells become separated due to cellular migration. Were these cells infected by a replication-competent virus, they would deliver virus to naive cells with which they make contact, as they migrate, to generate a contiguous focus (C). Dilution of a BA6-CasE brain extracts reveals the nature of a single virus focus (B). Again, note that the individual env+ cells are separated from one another (arrowheads) by uninfected cells, suggesting that the original infectious event did not result from a replication-competent virus and thus could not spread to adjacent dividing cells. Passage of supernatants from cultures such as that shown in panel A onto additional naive M. dunni fibroblasts failed to result in the appearance of detectable foci. These data support the conclusion that in vivo, NSCs continue to produce viral particles carrying the transgene of interest without producing helper virus. Magnification bars: A and C, 500 μm; B, 200 μm.
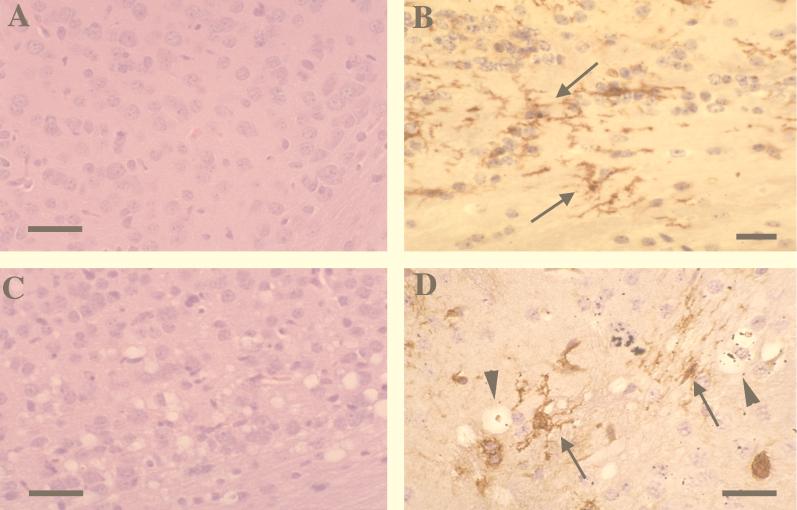
Previous reports on retrovirus-induced spongiform neurodegeneration suggested that microglial infection was directly associated with this pathologic process (3, 4, 19, 28, 29, 31, 32) and that env expression alone in the CNS might be sufficient for inducing neurodegenerative changes (21, 22, 43, 61, 62). Given that significant env expression was observed in microglia after transplantation with BA6-CasE packaging NSCs (Fig. 2F and G and 3), we evaluated engrafted mice for histopathologic changes indicative of status spongiosis. Mice examined at 22 (n = 4) and 31 (n = 12) dpi showed no evidence of spongiform neurodegeneration in regions with microglial env expression (Fig. 6A and B). Whether this was influenced by the microglial activation noted is not known. However, this picture contrasted with the spongiosis noted in positive control mice transplanted with the complete neurovirulent, replication-competent 15-1 clone of the CasBrE virus (32, 41) wherein extensive pathology was noted by 14 days after transplantation of C17-2 cells (Fig. 6C and D), and microglial activation was not detected (not shown).
FIG. 6.
env expression within host microglia as mediated by engraftment of BA6-CasE packaging NSCs is not sufficient to induce CNS spongiosis. (A) Representative hematoxylin-and-eosin-stained section from the deep cerebral cortex of a BA6-CasE packaging NSC-engrafted mouse. This section was taken from a region where significant microglial expression of env was observed. (B) Paraffin section from the cerebral cortex immunostained for _env_-encoding protein after antigen retrieval (using antibody 697) and counterstained with hematoxylin. Note that no spongiform neurodegenerative changes are observed by 31 days after engraftment of NSCs in panel A or B despite abundant env expression in microglia (B; arrows). (C and D) positive controls, demonstrating that NSC-mediated infection with the complete, replication-competent neurovirulent virus is capable of inducing neurodegeneration. A hematoxylin-and-eosin-stained section (C) and Env-immunostained (brown) and hematoxylin-counterstained section (D) show the same CNS regions presented in panels A and B but from a mouse transplanted with C17-2 NSCs containing replication-competent CasBrE virus (clone 15-1) 17 days posttransplantation. Note the abundant vacuolation (arrowheads) associated with the presence of env expression within microglia (arrows) (see also references 29, 32, and 43). Magnification bars: 50 μm.
Therefore, with respect to our novel approach for exploring the pathogenesis of spongiform myeloencephalopathy, we suggest that expression of the CasBrE env gene alone within microglia is not sufficient to mimic the neuropathogenic effects of the complete neurovirulent retrovirus. It appears that _env_-encoded protein requires interaction with additional viral structural components to cause acute vacuolar neurodegeneration. Taken together with our previous studies, which demonstrated that in vivo CasBrE virus binding and entry are also not sufficient to induce disease (32), the data suggest that retrovirus-induced CNS pathogenesis involves an interaction between the _env_-encoded protein and structural proteins from the late stages of viral replication, perhaps in the virus assembly and maturation process.
In this regard, it has been demonstrated that assembly and release are, indeed, defective in CasBrE-infected microglia in culture (28). Specifically, we have observed that infected microglia fail to process the gpr85 envelope precursor protein to gp70 and p15E. Associated with this defective processing, viral particles are observed to bud errantly into intracellular compartments rather than from the cell surface. It may be that the process of trying to assemble retroviral particles in microglia by using improperly processed or defective components alters microglial physiology to the point where microglia either (i) release a neurotoxic factor or (ii) can no longer adequately provide the support required for maintaining local neuronal homeostasis.
The results presented herein and previously (32) indicate that CasBrE env expression alone is not sufficient to induce acute spongiform neurodegeneration regardless of the cell type in which env is expressed. These results are in seeming contrast to those generated in studies examining CasBrE (22) and _ts_1 env expression in transgenic animals. In these studies it was observed that animals harboring and expressing mRNA from the respective env genes correlated with mild clinical and neuropathological changes. However, it should be noted that disease incidence was markedly reduced, and when it did appear it had a time course much longer than that noted for infection with virus. While it is possible that the mild phenotype observed in the transgenic animals was the direct result of the limited env gene expression noted in these animals, it is also possible that the lack of acute disease was due to the lack of additional retroviral proteins. In fact, given the extended time course of the transgenic experiments, it is possible that endogenous expression of gag and pol transcripts and protein could complement the transgenic env expression without resulting in recombinant virus. Such a possibility was not formally excluded in these experiments. Moreover, we have previously documented that CasBrE-induced spongiform neuropathology can arise acutely after postnatal day 10 (10, 30), and this pathology is directly associated with a small number of virus infected microglial cells, in the absence of other cellular infection (31). In the experiments described herein, CasBrE env protein expression in microglia was not significantly different from that noted in mice transplanted with the “slow” 15-1 clone of CasBrE and was consistent with that previously noted in microglial transplant experiments (31). Yet the differences in the induction of pathology are striking. The possibility that BA6-CasE C17-2 transplant recipients can develop neuropathological changes at extended time points is moot given the caveats associated with endogenous retroviral gene expression for which there are no adequate controls. To clearly address the differences noted between the transplant and transgenic studies, experiments involving microglial coexpression of neurovirulent env and additional viral structural constituents will need to be undertaken in order to understand what precipitates acute disease.
We expect that dissecting the precise CasBrE viral assembly events that occur in microglia and precipitate neural spongiform changes will provide insight into understanding the molecular pathogenesis of other CNS diseases of viral and nonviral origin. Of particular note are human immunodeficiency virus-induced cognitive-motor complex, where microglial infection appears to be critical for disease induction, and prion diseases, where microglia have been implicated in the vacuolar myeloencephalopathy associated with abnormal protease-resistant protein assemblies (7). Analyses of these pathologies at the molecular level should be facilitated by future experiments wherein transplantable packaging NSCs are used to deliver to microglia, individual or multiple retroviral or prion components, in a stepwise fashion, to selectively reconstitute the infectious process in the brain.
In a much broader sense, particular note should be made of the approach used to address these questions regarding neuropathogenesis. These data support the hypothesis that engraftable, migratory NSCs may be engineered ex vivo to serve as novel platforms for the effective dissemination in vivo of replication-defective viral vectors and their encoded genes directly to endogenous CNS host cells. The generation of a viral packaging/producer cell line that can engraft and intercalate into the CNS parenchyma provides a novel avenue for delivering genes (therapeutic or otherwise) to relatively inaccessible sites within the CNS. Certainly this strategy magnifies the often limited distribution of retroviral vector-mediated gene transfer to the CNS and therefore extends their applicability and efficacy for a much broader range of research and clinical applications. Certainly, microglia and cells of mesenchymal origin in the CNS can be targeted. This is of great significance given that microglia appear to play a significant role in a variety of genetic, sporadic, and infectious diseases (7, 12, 17, 35) and have been rather refractory to genetic manipulation due to their limited turnover in the mature CNS (24, 26, 27). Observations in the OB (a region of persistent postnatal neurogenesis where env expression extended beyond donor cell engraftment and colocalized with neuronal and glial markers) suggest that dividing cells of neuroectodermal origin may also be targeted (Fig. 2C and E). (Note in particular the intense env immunoreactivity in all OB neuronal layers in Fig. 2C.) Thus, the potential exists for manipulating multiple CNS cell types via the NSC packaging cell strategy.
Regarding the genes of interest for transfer to the CNS, while env was the index gene explored in these feasibility/proof-of-principle experiments, the approach for making packaging NSC lines transducing other types of genes (e.g., those encoding neurotrophic or cytotoxic agents) to manipulate host cells for other research or clinical demands would follow similar procedures, with anticipated similar success. Although this work was done in the postnatal mouse cerebrum, we anticipate that the same approach can be applied where mitotic cells are prevalent in a given region of the developing or adult CNS. In the fetus, such cells could be neuroblasts in most regions of CNS; in the postnatal or adult brain, such cells could include glia, microglia, endothelium, choroid, persistent centers of neurogenesis, and even neoplastic cells. Therefore, the strategy presented in this report may potentially be applicable to a wide variety of therapies for both genetic and acquired disorders of the CNS.
Given that in the present study we used a second-generation murine retrovirus packaging vector, pPAM3, as a starting point, it is likely that the application of newer packaging plasmids will only further enhance gene transfer efficacy to CNS cells. In particular, the marriage of lentivirus-based vectors to NSCs should provide a means to extensively target genes to nondividing as well as to dividing cells of the CNS. Furthermore, NSCs may be amenable to delivering other types of viral vectors that previously used producer cells of nonneural origin (adeno-associated viral vectors, herpesvirus amplicon-augmented vectors, etc.). In short, this approach may represent the interface between two gene therapy strategies, virus-mediated and cell-mediated gene delivery, maximizing the advantages of each (14, 49, 52, 58) and providing yet another strategy by which NSC biology may be harnessed for genetically manipulating the CNS.
ACKNOWLEDGMENTS
Support for this work was provided in part by a grant from the Amyotrophic Lateral Sclerosis Association and NINDS grant NS37614 to W.P.L., NINDS grant NS31065 to A.H.S., and grants from NINDS (NS34247), the American Paralysis Association, and the Paralyzed Veterans of America to E.Y.S. Mental Retardation Research Center grant NIH-P30-HD18655 to Children’s Hospital also provided support for this project.
REFERENCES
- 1.Andrews J M, Gardner M B. Lower motor neuron degeneration associated with type C RNA virus infection in mice: neuropathological features. J Neuropathol Exp Neurol. 1974;33:285–307. doi: 10.1097/00005072-197404000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Austyn J M, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;11:805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- 3.Baszler T V, Zachary J F. Murine retroviral-induced spongiform neuronal degeneration parallels resident microglial cell infection: ultrastructural findings. Lab Investig. 1990;63:612–623. [PubMed] [Google Scholar]
- 4.Baszler T V, Zachary J F. Murine retroviral neurovirulence correlates with an enhanced ability of virus to infect selectively, replicate in, and activate resident microglial cells. Am J Pathol. 1991;138:655–671. . (Erratum, 138:1058.) [PMC free article] [PubMed] [Google Scholar]
- 5.Bender M A, Palmer T D, Gelinas R E, Miller A D. Evidence that the packaging signal of Moloney murine leukemia virus extends into the gag region. J Virol. 1987;61:1639–1646. doi: 10.1128/jvi.61.5.1639-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bestwick R K, Kozak S L, Kabat D. Overcoming interference to retroviral superinfection results in amplified expression and transmission of cloned genes. Proc Natl Acad Sci USA. 1988;85:5404–5408. doi: 10.1073/pnas.85.15.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown D R, Schmidt B, Kretschmar H A. Role of microglia and host prion protein in neurotoxicity of a prion protein fragment. Nature. 1996;380:345–347. doi: 10.1038/380345a0. [DOI] [PubMed] [Google Scholar]
- 8.Czub M, Czub S, McAtee F, Portis J. Age-dependent resistance to murine retrovirus-induced spongiform neurodegeneration results from central nervous system-specific restriction of virus replication. J Virol. 1991;65:2539–2544. doi: 10.1128/jvi.65.5.2539-2544.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czub M, McAtee F J, Portis J L. Murine retrovirus-induced spongiform encephalomyelopathy: host and viral factors which determine the length of the incubation period. J Virol. 1992;66:3298–3305. doi: 10.1128/jvi.66.6.3298-3305.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czub S, Lynch W P, Czub M, Portis J L. Kinetic analysis of the spongiform neurodegenerative disease induced by a highly virulent murine retrovirus. Lab Investig. 1994;70:711–723. [PubMed] [Google Scholar]
- 11.DesGroseillers L, Barrette M, Jolicoeur P. Physical mapping of the paralysis-inducing determinant of a wild mouse ecotropic neurotropic virus. J Virol. 1984;52:356–363. doi: 10.1128/jvi.52.2.356-363.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickson D W, Lee S C, Mattiace L A, Yen S-H C, Brosnan C. Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer’s disease. Glia. 1993;7:75–83. doi: 10.1002/glia.440070113. [DOI] [PubMed] [Google Scholar]
- 13.Evans L H, Morrison R P, Malik F G, Portis J, Britt W J. A neutralizable epitope common to the envelope glycoproteins of ecotropic, polytropic, xenotropic, and amphotropic murine leukemia viruses. J Virol. 1990;64:6176–6183. doi: 10.1128/jvi.64.12.6176-6183.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gage F H. Cell therapy. Nature. 1998;392(Suppl.):18–24. [PubMed] [Google Scholar]
- 15.Gardner M B, Henderson B E, Officer J E, Rongey R W, Parker J C, Oliver C, Estes J D, Huebner R J. A spontaneous lower motor neuron disease apparently caused by indigenous type-C RNA virus in wild mice. JNCI. 1973;51:1243–1254. doi: 10.1093/jnci/51.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giulian D, Corpuz M. Microglial secretion products and their impact on the nervous system. Adv Neurol. 1993;59:315–320. [PubMed] [Google Scholar]
- 17.Glass J D, Johnson R T. Human immunodeficiency virus and the brain. Annu Rev Neurosci. 1996;19:1–26. doi: 10.1146/annurev.ne.19.030196.000245. [DOI] [PubMed] [Google Scholar]
- 18.Gordon S, Lawson L, Rabinowitz S, Crocker P R, Morris L, Perry V H. Antigen markers of macrophage differentiation in murine tissues. Curr Top Microbiol Immunol. 1992;181:1–37. doi: 10.1007/978-3-642-77377-8_1. [DOI] [PubMed] [Google Scholar]
- 19.Gravel C, Kay D G, Jolicoeur P. Identification of the infected target cell type in spongiform myeloencephalopathy induced by the neurotropic Cas-Br-E murine leukemia virus. J Virol. 1993;67:6648–6658. doi: 10.1128/jvi.67.11.6648-6658.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hickey W F, Vass K, Lassmann K. Bone marrow-derived elements in the central nervous system: an immunohistochemical and ultrastructural survey of rat chimeras. J Neuropathol Exp Neurol. 1992;51:246–256. doi: 10.1097/00005072-199205000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Jolicoeur P, Gravel C, Kay D G. Pathogenesis of murine spongiform myeloencephalopathy induced by a murine retrovirus. In: Roos R P, editor. Molecular neurovirology. Totowa, N.J: Humana Press, Inc.; 1992. pp. 199–224. [Google Scholar]
- 22.Kay D G, Gravel C, Pothier F, Laperriere A, Robitalle Y, Jolicoeur P. Neurological disease induced in transgenic mice expressing the env gene of the Cas-Br-E murine retrovirus. Proc Natl Acad Sci USA. 1993;90:4538–4542. doi: 10.1073/pnas.90.10.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozak S L, Kabat D. Ping-pong amplification of a retroviral vector achieves high level gene expression: human growth hormone production. J Virol. 1990;64:3500–3508. doi: 10.1128/jvi.64.7.3500-3508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krall W J, Challita P M, Perlmutter L S, Skelton D C, Krone D B. Cells expressing human glucocerebrosidase from a retroviral vector repopulate macrophages and central nervous system microglia after murine bone marrow transplantation. Blood. 1994;83:2737–2748. [PubMed] [Google Scholar]
- 25.Lacorraza H D, Flax J D, Snyder E Y, Jendoubi M. Expression of human β-hexosaminidase α-subunit gene (the gene defect of Tay Sachs disease) in mouse brains upon engraftment of transduced progenitor cells. Nat Med. 1996;2:424–429. doi: 10.1038/nm0496-424. [DOI] [PubMed] [Google Scholar]
- 26.Lawson L J, Perry V H, Gordon S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience. 1992;48:405–415. doi: 10.1016/0306-4522(92)90500-2. [DOI] [PubMed] [Google Scholar]
- 27.Ling E-A, Wong W-C. The origin and nature of ramified and amoeboid microglia: a historical review and current concepts. Glia. 1993;7:9–18. doi: 10.1002/glia.440070105. [DOI] [PubMed] [Google Scholar]
- 28.Lynch W P, Brown W J, Spangrude G J, Portis J L. Microglia infection by a neurovirulent murine retrovirus results in defective processing of envelope protein and intracellular budding of virus particles. J Virol. 1994;68:3401–3409. doi: 10.1128/jvi.68.5.3401-3409.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lynch W P, Czub S, McAtee F J, Hayes S F, Portis J L. Murine retrovirus-induced spongiform encephalopathy: productive infection of microglia and cerebellar neurons in accelerated CNS disease. Neuron. 1991;7:365–379. doi: 10.1016/0896-6273(91)90289-c. [DOI] [PubMed] [Google Scholar]
- 30.Lynch W P, Portis J L. Murine retrovirus-induced spongiform encephalopathy: disease expression is dependent on postnatal development of the central nervous system. J Virol. 1993;67:2601–2610. doi: 10.1128/jvi.67.5.2601-2610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lynch W P, Robertson S J, Portis J L. Induction of focal spongiform neurodegeneration in developmentally resistant mice by implantation of murine retrovirus-infected microglia. J Virol. 1995;69:1408–1419. doi: 10.1128/jvi.69.3.1408-1419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31a.Lynch, W. P., and A. H. Sharpe. Unpublished data.
- 32.Lynch W P, Snyder E Y, Qualtiere L F, Portis J L, Sharpe A H. Late virus replication events in microglia are required for murine retrovirus-induced spongiform neurodegeneration: evidence from neural progenitor-derived chimeric mouse brains. J Virol. 1996;70:8896–8907. doi: 10.1128/jvi.70.12.8896-8907.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez-Serrano A, Bjorklund A. Immortalized neural progenitor cells for CNS gene transfer and repair. Trends Neurosci. 1997;20:530–538. doi: 10.1016/s0166-2236(97)01119-3. [DOI] [PubMed] [Google Scholar]
- 34.McAtee F J, Portis J L. Monoclonal antibodies specific for wild mouse neurotropic retrovirus: detection of comparable levels of virus replication in mouse strains susceptible and resistant to paralytic disease. J Virol. 1985;56:1010–1022. doi: 10.1128/jvi.56.3.1018-1022.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGeer P L, Kawamata T, Walker D G, Akiyama H, Tooyama I, McGeer E G. Microglia in degenerative neurological disease. Glia. 1993;7:84–92. doi: 10.1002/glia.440070114. [DOI] [PubMed] [Google Scholar]
- 36.McKay R. Stem cells in the central nervous system. Science. 1997;276:66–71. doi: 10.1126/science.276.5309.66. [DOI] [PubMed] [Google Scholar]
- 37.Miller A D, Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986;6:2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller A D, Law M, Verma I M. Generation of helper-free amphotropic retroviruses that transduce a dominant-acting, methotrexate-resistant dihydrofolate reductase gene. Mol Cell Biol. 1985;5:431–437. doi: 10.1128/mcb.5.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrison S J, Shah N M, Anderson D J. Regulatory mechanisms in stem cell biology. Cell. 1997;88:287–298. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]
- 39a.National Institutes of Health. Guide for the care and use of laboratory animals, rev. ed. Department of Health and Human Services publication no. (NIH) 85-23. Bethesda, Md: National Institutes of Health; 1985. [Google Scholar]
- 40.Perry V H, Hume D A, Gordon S. Immunohistochemical localization of macrophages and microglias in the adult and developing mouse brain. Neuroscience. 1985;15:313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- 41.Portis J L, Czub S, Garon C F, McAtee F J. Neurodegenerative disease induced by the wild mouse ecotropic retrovirus is markedly accelerated by long terminal repeat and gag-pol sequences from nondefective Friend murine leukemia virus. J Virol. 1990;64:1648–1656. doi: 10.1128/jvi.64.4.1648-1656.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Portis J L, Czub S, Robertson S, McAtee F, Chesebro B. Characterization of a neurologic disease induced by a polytropic murine retrovirus: evidence for differential targeting of ecotropic and polytropic viruses in the brain. J Virol. 1995;69:8070–8075. doi: 10.1128/jvi.69.12.8070-8075.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Portis J L, Lynch W P. Dissecting the determinants of neuropathogenesis of the murine oncornaviruses. Virology. 1998;247:127–136. doi: 10.1006/viro.1998.9240. [DOI] [PubMed] [Google Scholar]
- 44.Price J, Turner D L, Cepko C L. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci USA. 1987;84:156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosario C M, Yandava B D, Kosaras B, Zurakowski D, Sidman R L, Snyder E Y. Differentiation of engrafted multipotent progenitors towards replacement of missing granule neurons in meander tail cerebellum may help determine the locus of mutant gene action. Development. 1997;124:4213–4224. doi: 10.1242/dev.124.21.4213. [DOI] [PubMed] [Google Scholar]
- 46.Ruscetti S, Troxler D, Linemeyer D, Scolnick E. Three laboratory strains of spleen focus-forming virus: comparison of their genomes and translational products. J Virol. 1980;33:140–151. doi: 10.1128/jvi.33.1.140-151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryder E F, Snyder E Y, Cepko C L. Establishment and characterization of multipotent neural cell lines using retrovirus vector-mediated oncogene transfer. J Neurobiol. 1990;21:356–375. doi: 10.1002/neu.480210209. [DOI] [PubMed] [Google Scholar]
- 48.Snyder E, Yoon C, Flax J, Macklis J. Multipotent neural precursors can differentiate toward replacement of neurons undergoing targeted apoptotic degeneration in adult mouse neocortex. Proc Natl Acad Sci USA. 1997;94:11645–11650. doi: 10.1073/pnas.94.21.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snyder E Y. Neural stem-like cells: developmental lessons with therapeutic potential. Neuroscientist. 1998;4:408–425. [Google Scholar]
- 50.Snyder E Y, Deitcher D L, Walsh C, Arnold-Aldea S, Hartwieg E, Cepko C L. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell. 1992;68:33–51. doi: 10.1016/0092-8674(92)90204-p. [DOI] [PubMed] [Google Scholar]
- 51.Snyder E Y, Flax J D. Transplantation of neural progenitors and stem like cells as a strategy for gene therapy and repair of neurodegenerative diseases. Ment Retard Dev Disabil Res Rev. 1995;1:27–38. [Google Scholar]
- 52.Snyder E Y, Senut M. Use of non-neural cells for gene delivery. Neurobiol Dis. 1997;4:69–102. doi: 10.1006/nbdi.1997.0138. [DOI] [PubMed] [Google Scholar]
- 53.Snyder E Y, Taylor R M, Wolfe J H. Neural progenitor cell engraftment corrects lysosomal storage throughout the MPS VII mouse brain. Nature. 1995;374:367–370. doi: 10.1038/374367a0. [DOI] [PubMed] [Google Scholar]
- 54.Snyder E Y, Wolfe J H. CNS cell transplantation: a novel therapy for storage diseases? Curr Opin Neurol. 1996;9:126–136. doi: 10.1097/00019052-199604000-00013. [DOI] [PubMed] [Google Scholar]
- 55.Springer T, Galfre G, Secher D S, Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979;9:301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- 56.Springer T, Galfre G, Secher D S, Milstein C. Monoclonal xenogenic antibodies to murine cell surface antigens: identification of novel leukocyte differentiation antigens. Eur J Immunol. 1978;8:539–551. doi: 10.1002/eji.1830080802. [DOI] [PubMed] [Google Scholar]
- 57.Stemple D L, Manhanthappa N K. Neural stem cells are blasting off. Neuron. 1997;18:1–4. doi: 10.1016/s0896-6273(01)80018-0. [DOI] [PubMed] [Google Scholar]
- 58.Verma I M, Somia N. Gene therapy: promises, problems, and prospects. Nature. 1997;389:239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 59.Weiss S, Reynolds B A, Vescovi A L, Morshead C, Craig C, van der Kooy D. Is there a neural stem cell in the mammalian forebrain. Trends Neurosci. 1996;19:387–393. doi: 10.1016/s0166-2236(96)10035-7. [DOI] [PubMed] [Google Scholar]
- 60.Whittemore S R, Snyder E Y. The physiologic relevance and functional potential of central nervous system-derived cell lines. Mol Neurobiol. 1996;12:13–38. doi: 10.1007/BF02740745. [DOI] [PubMed] [Google Scholar]
- 61.Wong P K Y, Yuen P H. Molecular basis of neurologic disorders induced by a mutant ts1, of Moloney murine leukemia virus. In: Roos R, editor. Molecular neurovirology: pathogenesis of viral CNS infections. Totowa, N.J: Humana Press, Inc.; 1992. pp. 161–197. [Google Scholar]
- 62.Yu Y E, Choe W, Zhang W, Stoica G, Wong P K. Development of pathological lesions in the central nervous system of transgenic mice expressing the env gene of ts1 Moloney murine leukemia virus in the absence of viral gag and pol genes and viral replication. J Neurovirol. 1997;3:274–282. doi: 10.3109/13550289709029468. [DOI] [PubMed] [Google Scholar]
- 63.Yuen P H, Malehorn D, Knupp C, Wong P K Y. A 1.6-kilobase-pair fragment in the genome of the ts1 mutant of Moloney murine leukemia virus TB that is associated with temperature sensitivity, nonprocessing of Pr80env, and paralytogenesis. J Virol. 1985;54:364–373. doi: 10.1128/jvi.54.2.364-373.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]