Convergence and divergence in the mechanism of SNARE binding by Sec1/Munc18-like proteins (original) (raw)
Abstract
Sec1/Munc18-like (SM) proteins functionally interact with soluble _N_-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) in membrane fusion, but the mechanisms of these interactions differ. In vertebrates, SM proteins that mediate exocytosis (Munc18-1, 18-2, and 18c) bind to the closed conformation of syntaxins 1–4, which requires the N-terminal Habc domains and SNARE motifs of these syntaxins. In contrast, SM proteins that mediate Golgi and endoplasmic reticulum fusion (Sly1 and Vps45) bind only to short N-terminal sequences of syntaxins 5, 16, or 18, independently of their Habc domains and SNARE motifs. We now show that Munc18-1, Sly1, and Vps45 interact with cognate syntaxins via similar, autonomously folded N-terminal domains, but the syntaxin 5-binding surface of the Sly1 N-terminal domain is opposite to the syntaxin 1-binding surface of the Munc18-1 N-terminal domain. In transfected cells, the N-terminal domain of Sly1 specifically disrupts the structure of the Golgi complex, supporting the notion that the interaction of Sly1 with syntaxin 5 is essential for fusion. These data, together with previous results, suggest that a relatively small N-terminal domain of SM proteins is dedicated to mechanistically distinct interactions with SNAREs, leaving the remaining large parts of SM proteins free to execute their as yet unknown function as effector domains.
Keywords: SM proteins‖syntaxins‖Sly1‖Vps45p
Intracellular membrane fusion involves several families of conserved proteins, including soluble _N-_ethylmaleimide-sensitive factor attachment protein receptor (SNARE) and Sec1/Munc18-like (SM) proteins (1). SNAREs are localized on opposing membranes before fusion and form complexes with each other to force the membranes into close apposition during fusion (2, 3). SM proteins are cytosolic proteins of ≈600–700 residues that directly or indirectly bind to SNAREs. The entire sequences of SM proteins are homologous to each other, whereas SNAREs only share a single 70-residue homologous sequence, the so-called SNARE motif, that is instrumental in the formation of SNARE complexes (reviewed in refs. 1 and 4). In contrast to the many SNARE isoforms in vertebrates and yeast, only seven SM protein isoforms are expressed in vertebrates (Munc18-1, 18-2, 18c, Sly1, Vps45, Vps33a, and Vps33b), and only four SM protein isoforms in yeast (Sec1p, Sly1p, Vps45p, and Vps33p). SNAREs and SM proteins often function in multiple fusion reactions, suggesting that they are not responsible for the specificity of fusion (reviewed in ref. 5). At least in synaptic vesicle exocytosis and yeast vacuole fusion, deletion of the respective SM proteins (Munc18-1 and Vps33p) has a more severe effect on fusion (6, 7) than deletion of corresponding SNARE proteins [e.g., deletion of synaptobrevin 2 or SNAP-25 at the synapse, or deletion of Vam3p and Vam7p at the vacuole (8–10)]. Thus, the function of SM proteins in fusion likely extends beyond SNAREs, but their precise role and the significance of their binding to SNAREs remain unclear.
The most common mode by which SM proteins interact with SNAREs is through direct binding to syntaxins, a subclass of Q-SNAREs (e.g., binding of Munc18s to syntaxins 1–4, Sly1 to syntaxins 5 and 18, and Vps45 to syntaxin 16). In addition, some SM proteins (e.g., Vps33) interact with SNAREs indirectly (reviewed in ref. 5). All syntaxins seem to contain an N-terminal domain composed of three α-helices, the so-called Habc domain (11–17). In syntaxins involved in exocytosis (syntaxin 1–4), the Habc domain folds back onto the central SNARE motif to form a closed conformation (18), whereas in other syntaxins (such as Vam3p and Tlg2p/syntaxin 16), a constitutively open conformation is observed (14, 15). In syntaxin 1, most of the cytoplasmic sequence is required for Munc18 binding (19, 20), because Munc18-1 selectively binds to the closed syntaxin conformation (18, 21). In contrast, in syntaxins 5, 16, and 18, only a short N-terminal peptide sequence is necessary to capture Sly1 and Vps45 fully (15, 16). Structurally, Munc18-1 is composed of three domains, a small domain 1 at the N terminus (residues 1–134), a complex middle domain 2 composed of central (residues 135–247) and C-terminal sequences (residues 480–594), and a large domain 3 (residues 248–479; refs. 21 and 22). The three domains form a central cavity that “rides” on the closed conformation of syntaxin 1, with most of the contacts provided by the short N-terminal domain 1 (21). The fact that Sly1 and Vps45 are homologous to Munc18-1 over their entire length indicates that they may have similar overall structures. Thus, it is puzzling that a short N-terminal sequence in syntaxins 5, 16, and 18 is sufficient for full binding of Sly1 or Vps45, because this sequence would be too small to fill a cavity in Sly1 and Vps45 that corresponds to the syntaxin-binding cavity in Munc18-1.
The observation that Munc18-1, Sly1, and Vps45 are essential for the same fusion reactions as the syntaxins to which they bind indicates that these SM proteins and syntaxins function in fusion by binding to each other. At the same time, the differences between SM proteins in syntaxin binding suggest that their mechanism of action may be different. To address this paradox, we have now studied the structural basis for the interaction of Sly1, Vps45, and Munc18-1 with their respective syntaxins. Our results suggest that, although Sly1 and Vps45, on the one hand, and Munc18-1, on the other hand, interact with syntaxins via similar domains, the exact interaction mechanisms differ dramatically. Based on these and previous data, we propose a model whereby SM proteins execute similar functions that are linked to specific SNARE proteins by distinct mechanisms.
Methods
Reagents.
All chemicals used were of reagent grade; plasmids are described in Supporting Text, which is published as supporting information on the PNAS web site, www.pnas.org.
Yeast two-hybrid assays were performed in the yeast strain L40 cotransfected with bait and prey vectors in the indicated combinations, and quantitative β-galactosidase measurements were performed essentially as described (16). β-galactosidase activities are expressed as units calculated as 1,000 × OD420/min/ml yeast/OD600.
GST-Pulldown Assays.
Recombinant GST-fusion proteins were expressed in Escherichia coli BL21 cells (Novagen) and affinity-purified on glutathione-agarose (23). T7-tagged N-terminal fragments of rat Sly1, mouse Vps45, and Sly1/Vps45-chimera protein were produced in E. coli BL21_trxB_(DE3) (Novagen) by using pET-21 (16). Beads with attached recombinant proteins were incubated with bacterial extract expressing T7-tagged proteins, washed and analyzed on SDS/polyacrylamide gel, followed by Coomassie blue staining or Western blotting with anti T7⋅Tag (Novagen) monoclonal antibodies (18). Binding of rat syntaxin 1A to rat GST-Munc18-1 fusion proteins was assessed by using total rat brain homogenate as described (19).
Transfection Experiments.
Vero cells were transfected with pCMV5-based expression vectors encoding the indicated SM protein fragments and analyzed by immunocytochemistry as described (16) by using mouse monoclonal antibody against GM130 and EEA (Transduction Laboratories, Lexington, KY), calnexin (gift from H. Kramer, University of Texas Southwestern Medical Center, Dallas), and rabbit polyclonal antibody against myc epitope (Santa Cruz Biotechnology).
Protein Expression for NMR Studies.
Uniformly 15N- or 15N,13C-labeled recombinant GST-fusion proteins were produced in bacteria grown in minimal media supplemented with 15NH4Cl and with or without [13C6]glucose (CIL, Andover, MA) as the sole nitrogen and carbon sources (11). GST-fusion proteins were affinity-purified on glutathione-Sepharose (Amersham Pharmacia), cleaved from GST with thrombin (Sigma) and purified by FPLC (18). To produce the 15N-labeled syntaxin 51–33 peptide bound to the Sly1 N-terminal domain, affinity purification of the labeled fragment was performed in the presence of excess partially purified, unlabeled Sly12–147. Synthetic peptides of the N-terminal 33 residues of rat syntaxin 5 or the 27 residues of rat syntaxin 1A were purchased from the Center for Biomedical Inventions (University of Texas Southwestern Medical Center) or Abgent (San Diego), respectively.
NMR Spectroscopy.
NMR data were acquired at 27°C on Varian INOVA500 or INOVA600 spectrometers by using H2O/D2O 95:5 (vol/vol) as the solvent. 1H,15N heteronuclear single quantum correlation (HSQC) spectra for the Sly1, Vps45, and Munc18-1 N-terminal domains, free 1–33 syntaxin 5 fragment and 15N-labeled syntaxin 51–33 complexed with unlabeled Sly12–147 were obtained at 65–100 μM protein concentrations at pH 7.5. Backbone assignments for the free Sly12–147 fragment or Sly12–147 bound to 33-mer syntaxin 5 synthetic peptide were obtained by using three-dimensional (3D) 1H,15N nuclear Overhauser effect (NOE) spectroscopy (NOESY)- and total correlation spectroscopy (TOCSY)-HSQC, HNCO, HNCACB, and CBCACONH spectra acquired on 1.2 mM, 15N- and 15N,13C-labeled samples at pH 5.6, with or without 2 mM peptide. 1H-15N HSQC peaks of 0.5 mM of the 15N-labeled syntaxin 51–33 fragment bound to unlabeled Sly12–147 were assigned by using 3D 1H,15N NOESY- and TOCSY-HSQC spectra acquired at pH 5.6.
Results
Definition of N-Terminal Ligand-Binding Domains in SM Proteins.
To explore whether SM proteins generally contain small, autonomously folded N-terminal domains, we produced domain 1 of rat Munc18-1 (residues 1–136; refs. 21 and 22) or the corresponding sequences of rat Sly1 (residues 2–147) and mouse Vps45 (residues 1–128) as 15N-labeled recombinant proteins and examined the proteins by NMR spectroscopy. We recorded 1H,15N HSQC spectra that constitute protein fingerprints with one crosspeak for each residue. The HSQC spectra of all three N-terminal SM protein fragments exhibited a high dispersion of crosspeaks in the proton dimension (see Fig. 7, which is published as supporting information on the PNAS web site), demonstrating that these protein fragments form independently folded domains.
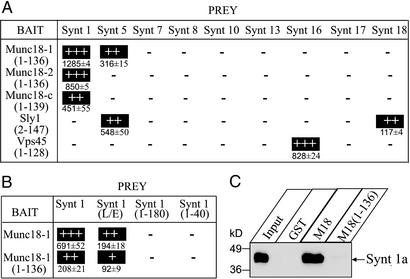
Next, we tested whether the N-terminal domains are capable of binding to syntaxins by using a yeast two-hybrid analysis (Fig. 1A). The N-terminal domains of all Munc18 isoforms bound to syntaxin 1, and the N-terminal domain of Munc18-1 (but not of Munc18-2 or 18c) additionally bound to syntaxin 5. No other syntaxins tested were bound. The syntaxin 5 interaction was not observed for full-length Munc18-1 (16) and thus may be artifactual. Similar to full-length Sly1 and Vps45 (15, 16), the N-terminal domain of Sly1 only bound to syntaxins 5 and 18, and the N-terminal domain of Vps45 only bound to syntaxin 16. Several other syntaxins used as negative controls (syntaxins 7, 8, 10, 13, and 17) did not bind to these SM proteins.
Figure 1.
Binding of different syntaxins to the N-terminal domains of SM proteins. (A and B) Yeast two-hybrid analysis of interactions between mammalian syntaxins and the N-terminal domains of mammalian SM proteins (A) or between WT or mutant syntaxin 1a with the full-length or the N-terminal domain of Munc18-1 (B) quantitated by β-galactosidase assays. Data are shown as means ± SEM from representative experiments performed in triplicate. Except where noted otherwise, syntaxins were expressed as full-length cytoplasmic sequences truncated at the transmembrane region. (C) GST pulldowns of rat brain syntaxin 1A with GST-fusion proteins of either full-length or the N-terminal domain of Munc18-1. GST alone was used as control. Input and bound proteins were analyzed by immunoblotting with the HPC-1 monoclonal antibody, and signals were visualized by enhanced chemiluminescence (ECL). Numbers on the left indicate positions of molecular mass standards.
We next examined whether binding of the N-terminal domain of Munc18-1 to syntaxin 1 mimics binding of full-length Munc18-1. For this purpose, we used the so-called LE mutant of syntaxin 1 (L165A,E166A) which impairs Munc18-1 binding because it forces syntaxin 1 into an open conformation (18). Different from GST pulldowns, which did not detect any binding of LE mutant synaxin 1 to Munc18-1 (18), the more sensitive yeast two-hybrid assays still observed such binding (Fig. 1B). This result suggests that at any given time, some of the LE mutant syntaxin 1 may still be in a partly closed conformation, resulting in weak binding to Munc18-1. The N-terminal domain of Munc18-1 bound more weakly to syntaxin 1 than full-length Munc18-1, but its binding was decreased by the LE mutation similar to full-length Munc18-1. Together, these data suggest that the N-terminal domain of Munc18-1 includes a minimal binding site for the closed conformation of syntaxin 1. Consistent with this conclusion, truncation of syntaxin 1 by deletion of the SNARE motif [Synt 1a (1–180)] or the SNARE motif and Habc domain [Synt 1a (1–40)] abolished binding of both full-length Munc18-1 and the N-terminal domain (Fig. 1B). No binding of the N-terminal Munc18-1 domain to syntaxin 1 was detected by GST pulldowns (Fig. 1C), probably because of the greater stringency of the assay.
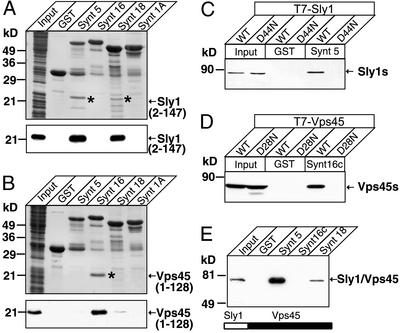
In parallel experiments, we expressed the N-terminal domains of Sly1 and Vps45 as recombinant epitope-tagged proteins in bacteria and applied the total bacterial lysates to glutathione agarose columns containing either GST alone as a control, or GST-fusion proteins of syntaxins 1a, 5, 16, and 18 as a test (Fig. 2 A and B). The N-terminal Sly1 domain only bound to syntaxins 5 and 18, and the N-terminal Vps45 domain only bound to syntaxin 16. Binding was so tight that the bound proteins were visible on Coomassie-stained gels. These experiments suggest that the N-terminal domains of Sly1 and Vps45 are fully sufficient ligand-binding domains. To test whether the N-terminal domain is essential for ligand binding also in the context of the full-length SM proteins, we made use of a mutation described in domain 1 of Drosophila Munc18-1 that replaces a conserved aspartate for an asparagine and interferes with syntaxin binding (24). When we introduced analogous mutations into Sly1 and Vps45, these SM proteins were unable to bind to their cognate syntaxins, confirming that the N-terminal domain is essential for binding (Fig. 2 C and D). Furthermore, when we replaced the N-terminal domain of Vps45 with that of Sly1 in the context of full-length Vps45, the binding specificity of Vps45 was switched from syntaxin 16 (its physiological partner) to syntaxins 5 and 18 (the normal partners for Sly1; Fig. 2E).
Figure 2.
The N-terminal domains of Sly1 and Vps 45 specifically bind to cognate syntaxins. (A and B) Pulldowns of the recombinant T7-tagged N-terminal domains of Sly1 (A) or Vps45 (B) with GST-fusion proteins containing full-length cytoplasmic sequences of the indicated syntaxins. Input and bound proteins were analyzed by Coomassie blue staining (Upper) and immunoblotting with a T7 antibody visualized by ECL (Lower). (C_–_E) Pulldowns of T7-tagged WT and mutant Sly1 and Vps45 with GST-fusion proteins containing the full-length cytoplasmic domains of various syntaxins. The experiments compared binding of WT Sly1 and Vps45 with that of point-mutants in corresponding residues of Sly1 (C: D44N) or Vps45 (D: D28N) and of a chimeric protein composed of the N-terminal domain of Sly1 fused to the rest of Vps45 (E). Input and bound proteins were analyzed by immunoblotting with a T7 antibody. Numbers on the left indicate positions of molecular mass standards.
Physiological Importance of Syntaxin 5 Binding by the N-Terminal Domain of Sly1.
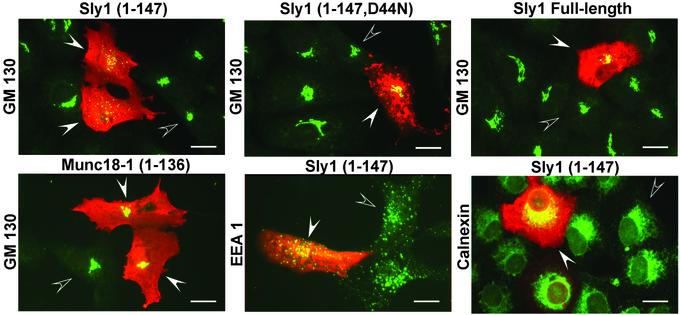
Is the binding of SM proteins to syntaxins intrinsic to their function in fusion, or is it primarily regulatory and only of incidental importance? To address this question, we exploited the selective binding of the N-terminal domain of Sly1 to only the N-terminal peptides of syntaxin 5 and 18, reasoning that if syntaxin 5 binding to Sly1 is essential for fusion, expression of its N-terminal domain should disrupt fusion and perturb the structure of the Golgi apparatus. We transfected the N-terminal Sly1 domain into Vero cells and examined the morphology of different cellular compartments after transfection (Fig. 3). As controls, we used full-length Sly1, a mutant N-terminal Sly1 domain with the D44N substitution that impairs syntaxin 5 binding, and the Munc18-1 N-terminal domain. Expression of the N-terminal domain of Sly1 specifically disrupted the Golgi complex similarly to truncated syntaxin 5 (16). All controls, including full-length Sly1, had no effect (Fig. 3). Furthermore, the morphology of the endoplasmic reticulum (ER) or of endosomes was not affected. The ER may appear to be morphologically unaltered even though Sly1 binds to the ER syntaxin 18/ufe1p (16), because the ER is a dispersed organelle, making it difficult to detect changes at the light level.
Figure 3.
Selective disruption of the Golgi complex by transfection of the Sly1 N-terminal domain. The WT or mutant (D44N) N-terminal domain of Sly1 (residues 1–147), full-length Sly1, and the N-terminal domain of Munc18-1 were expressed in transfected Vero cells as myc-tagged proteins. Transfected cells were examined by double immunofluorescence for the myc epitope (red) and the Golgi marker GM130, the ER marker calnexin, and the endosomal marker EEA1 (green); areas of overlap in the staining are indicated in yellow. Note the selective dissolution of the Golgi apparatus in cells transfected with the WT N-terminal domain of Sly1 but not with the mutant N-terminal domain, full-length Sly1, or the N-terminal domain of Munc18-1. Filled arrows point to transfected cells; open arrows point to nearby nontransfected cells. (Bars = 20 μm.)
Structural Characterization of Syntaxin Binding to SM Proteins.
The fact that the N-terminal domains of the SM proteins Munc18-1, Sly1, and Vps45 recognize syntaxins differently raises the question of how a similar, small domain can mediate such specific but distinct interactions. Is it possible that we missed a peptide-based interaction of Munc18-1 with syntaxin 1 that may be too weak to be detectable by yeast two-hybrid methods? Conversely, could the different N-terminal domains of SM proteins adopt distinct conformations, maybe as a consequence of syntaxin binding?
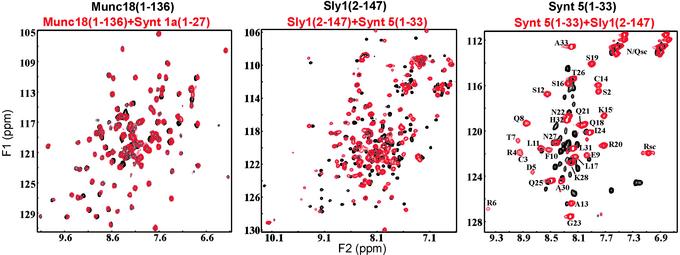
To address these questions, we used NMR spectroscopy and analyzed the interactions of peptides containing residues 1–27 of syntaxin 1A and 1–33 of syntaxin 5 with the N-terminal domains of Munc18-1 and Sly1, respectively. Addition of the unlabeled syntaxin 1 peptide to the 15N-labeled Munc18-1 N-terminal domain did not alter its HSQC spectrum (Fig. 4A), even when a tenfold excess of syntaxin 1 peptide was added (data not shown). These data confirm the yeast two-hybrid result (Fig. 1B), that the syntaxin 1 N-terminal sequence does not interact with the Munc18-1 N-terminal domain. In contrast, addition of unlabeled syntaxin 5 peptide to 15N-labeled Sly1 N-terminal domain dramatically changed its HSQC spectrum [Fig. 4B, compare black contours (free) with red contours (bound)]. In complementary experiments, addition of the unlabeled N-terminal domain of Sly1 to the 15N-labeled syntaxin 5 N-terminal peptide also induced large changes (Fig. 4C). The HSQC spectrum of the labeled free peptide exhibited a poor crosspeak dispersion characteristic of an unstructured state, whereas a much higher crosspeak dispersion caused by the formation of a defined structure was observed for the bound peptide.
Figure 4.
NMR analysis of the interactions of the N-terminal domains of Sly 1 and Munc18 with the N-terminal sequences of their cognate syntaxins. Panels display superpositions of 1H,15N HSQC spectra. (A) Spectra of the 15N-labeled Munc18-1 N-terminal domain (residues 1–136; 65 μM) in the absence (black) or presence (red) of unlabeled syntaxin 1 N-terminal peptide (residues 1–27; 200 μM). (B) Spectra of the 15N-labeled Sly1 N-terminal domain (residues 2–147; 65 μM) in the absence (black) and presence (red) of unlabeled syntaxin 5 N-terminal peptide (residues 1–33; 200 μM). (C) Spectra of the 15N-labeled syntaxin 5 N-terminal sequence (residues 1–33; 100 μM) in the absence (black) and presence (red) of unlabeled Sly1 N-terminal domain (residues 2–147; 100 μM). Assignments of the crosspeaks for the bound syntaxin 5 sequence are shown in C. Asn, Gln, and Arg side-chain NH groups are indicated by “sc.”
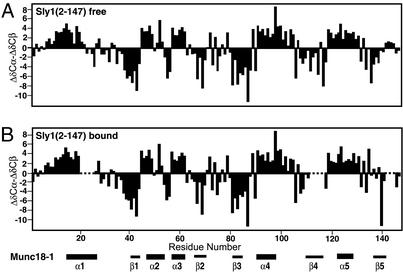
The complex between the N-terminal domain of Sly1 and the syntaxin 5 peptide exhibited severe broadening of resonances (Fig. 4) because of slow exchange between the bound and free proteins. Although this broadening prevented determination of a high-resolution structure of the complex, we could assign most of the backbone resonances of the free and bound 15N,13C-labeled N-terminal domain of Sly 1 by using triple resonance experiments. In addition, we assigned most of the backbone 1H and 15N-resonances of the 15N-labeled syntaxin 5 peptide bound to the N-terminal Sly1 domain by using 15N-edited experiments (see Tables 1–3, which are published as supporting information on the PNAS web site). Comparison of the Cα and Cβ chemical shifts of the Sly1 N-terminal domain derived from these assignments with the chemical shifts characteristic of a random coil allowed elucidation of the secondary structure elements of the domain (25). This comparison revealed that five α-helices and five β-strands were present in the free and the syntaxin 5 peptide-bound Sly1 N-terminal domain (Fig. 5). The positions of the α-helices and β-strands correspond to those observed in the crystal structure of Munc18-1 (21, 22), confirming that the N-terminal domains of Sly1 and Munc18-1 have a similar architecture (Fig. 5). Furthermore, although the syntaxin 5 peptide induced widespread shifts in the HSQC spectrum of the Sly1 N-terminal domain, it did not induce large changes in secondary structure (Fig. 5).
Figure 5.
Secondary structure of the free and peptide-bound N-terminal domain of Sly 1. Graphs display the differences between the observed Cα and Cβ chemical shifts in the N-terminal domain of Sly1 from the chemical shifts expected for a random coil conformation (ΔδCα-ΔδCβ; ref. 25). These differences are plotted as a function of residue number for the free Sly1 N-terminal domain (A) and the domain bound to the syntaxin 5 N-terminal peptide (B). Five regions corresponding to α-helices (positive chemical shift differences) and five regions corresponding to β-strands (negative chemical shift differences) are observed. Residues 21–26, 112–114, and 143–146 of the bound form could not be assigned. The distribution of the corresponding structural elements in neuronal Munc18-1 is shown at the bottom.
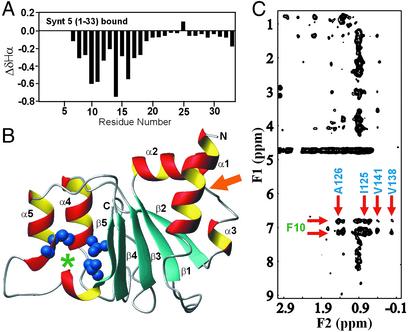
In the syntaxin 5 peptide bound to the Sly1 N-terminal domain, residues 21–33 were characterized by sharp resonances (Fig. 4C), and their Hα chemical shifts exhibited only small deviations from those expected for a random coil (Fig. 6A). In contrast, residues 7–20 displayed upfield-shifted Hα chemical shifts that are characteristic of α-helical structures, which was further supported by the observation of sequential NH/NH NOEs throughout this region (data not shown). The N-terminal residues of the syntaxin 5 peptide (residues 3–6) displayed broad resonances and downfield-shifted amide protons (Fig. 4C), consistent with a β-strand, but their Hα protons could not be assigned unambiguously. Viewed together, these results show that the 20 N-terminal residues of syntaxin 5 bind to Sly1 in a primarily α-helical conformation, whereas residues 21–33 remain unbound.
Figure 6.
Delineation of the syntaxin 5/Sly1-binding mode. (A) Differences (ΔδHα) between the observed Hα chemical shifts of the syntaxin 5 N-terminal peptide bound to the Sly1 N-terminal domain and the chemical shifts expected for a random coil conformation. Negative ΔδHα values are indicative of α-helix formation. The Hα protons of residues 1–6 could not be assigned. (B) 2D 13C-filtered, 13C-edited NOESY spectrum of 15N,13C-labeled Sly1 N-terminal domain bound to unlabeled syntaxin 5 peptide. This spectrum reveals intermolecular NOEs between protons from the syntaxin 5 peptide (F1 dimension) and protons from the Sly1 N-terminal domain (F2 dimension). Arrows point to NOEs between F10 of syntaxin 5 and methyl-containing residues of Sly1 (I125, A126, V138, and V141). (C) Homology model of the structure of the Sly1 N-terminal domain based on the crystal structure of Munc18-1. Helices and β-strands are labeled as α1–α5 and β1–β5, respectively. The side chains of I125, A126, V138, and V141 are shown as solid blue spheres. The binding site for F10 of syntaxin 5 is indicated with a green asterisk. The orange arrow points at the site where syntaxin 1 binds to the N-terminal domain of Munc18-1 (helices α2, α3, and strand β2).
The Syntaxin 5-Binding Site of Sly1.
To determine where in the N-terminal domain of Sly1 syntaxin 5 binds, we searched for intermolecular NOEs between the N-terminal Sly1 domain and the syntaxin 5 peptide by using two-dimensional (2D) and 3D 13C-filtered,13C-edited NOESY-HSQC experiments. We observed numerous NOEs between methyl groups from Sly1 and Phe-10 from syntaxin 5 (Fig. 6B). In the N-terminal sequence of syntaxin 5, Phe-10 is a conserved residue that is essential for Sly1 binding (15, 16). Sly1 methyl groups involved in the NOEs with Phe-10 were unambiguously assigned to Ile-125, Ala-126, Val-138, and Val-141. A model of the Sly1 N-terminal domain built by homology with the crystal structure of Munc18-1 (Fig. 6C; refs. 21 and 22) shows that these side chains form a hydrophobic pocket between α-helix 5 and β-strand 5, demonstrating that Phe-10 of syntaxin 5 and the region between α-helix 5 and β-strand 5 of Sly1 form an integral part of the interacting surface. This surface of the Sly1 N-terminal domain is opposite to the syntaxin 1-binding surface of the Munc18-1 N-terminal domain (see Fig. 6C). Note that D44 is located at the C terminus of strand 1 in the N-terminal Sly 1 domain, and that the disruption of syntaxin 5 binding caused by the D44N mutation (Fig. 2) is most likely caused by overall destabilization of the whole N-terminal domain.
Discussion
This study identifies a small, evolutionarily conserved domain at the N terminus of SM proteins. In Munc18-1, this domain forms a minimal binding site for syntaxin 1 that still recognizes the closed conformation of syntaxin 1 but does not completely account for syntaxin 1 binding (Fig. 1). In Sly1 and Vps45, however, this domain largely accounts for syntaxin binding (Figs. 2 and 4), which is consistent with previous data in yeast showing that the N-terminal half of Sly1p binds to Sed5p (26). Binding of the N-terminal syntaxin 5 peptide did not cause large conformational changes in the Sly1 N-terminal domain (Fig. 5). A comparison of the syntaxin-bound Munc18-1 structure from rat (21) with the syntaxin-free Munc18 structure from squid (22) suggests that syntaxin 1 binding to Munc18-1 also does not cause a conformational change; thus, syntaxin binding generally does not induce an allosteric signal in SM proteins. The similarity between the N-terminal domains of Munc18-1 and Sly1 raised the possibility that Munc18-1 may interact with syntaxin 1 by means of two independent mechanisms, a high-affinity interaction with the closed conformation of syntaxin 1 (18), and a second interaction with the N-terminal syntaxin 1 peptide analogous to the binding of Sly1 to the N-terminal syntaxin 5 peptide (15). However, we observed no significant changes in the HSQC spectrum of the N-terminal Munc18-1 domain upon addition of the N-terminal syntaxin 1 peptide (Fig. 4). Because we would have detected even low-affinity interactions in these experiments, this result effectively rules out a peptide-based interaction for Munc18-1, analogous to that of Sly1 and Vps45. Thus, despite the similar structures of the small N-terminal domains of Munc18-1, Sly1, and Vps45, these recognize their syntaxin ligands by different mechanisms. Based on sequence homologies, it is possible that analogous independently folded N-terminal domains also constitute ligand-binding domains in other SM proteins, even if their ligand is not a syntaxin.
The question arises whether the binding of SM proteins to syntaxins is intrinsic to their essential function in fusion, or is primarily regulatory and only of incidental importance. In the case of Munc18, the evidence regarding this question is inconclusive. The LE mutant of syntaxin 1 that induces an open syntaxin conformation with a decreased affinity for Munc18-1 (18) can substitute for syntaxin 1 in Caenorhabditis elegans (27) and also rescues deletions of the active zone proteins RIM and unc13 (27, 28). One interpretation of this result is that Munc18 performs an essential function that constitutes the conserved function of SM proteins in membrane fusion but is independent of syntaxin 1 binding. In this interpretation, Munc18 binding to syntaxin 1 regulates fusion, possibly by inhibiting the participation of syntaxin 1 in SNARE complexes in a manner that is controlled by RIM and (M)unc13. However, the findings that syntaxin 1 and Munc18-1 are essential in the same fusion reaction (7, 29), that deletion of Munc18-1 depresses syntaxin 1 levels (7), and that overexpression of Munc18-1 promotes fusion instead of inhibiting it (30), argue against this interpretation. Because the binding of Munc18-1 to the closed conformation of syntaxin 1 seems to be their only direct interaction (Fig. 4), the most parsimonious interpretation would be that their direct binding is responsible for the essential function of Munc18 in fusion. According to this interpretation, the syntaxin LE mutation in C. elegans is a functional syntaxin and is simultaneously capable of rescuing unc13 and RIM mutations because it still binds to (M)unc18, but with a large decrease in affinity (see Fig. 1). The decreased affinity may still be sufficient to support Munc18-1 binding during exocytosis, but at the same time may lead to a partial uncoupling of the control of exocytosis which is normally executed by unc13 and RIM. At present, it is not possible to definitively distinguish between these two interpretations.
In contrast to the uncertainty surrounding Munc18 binding to syntaxin 1, the importance of Sly1 binding to syntaxin 5 for fusion itself is supported by clear evidence. In yeast, Sly1p functions on the acceptor membrane together with sed5p (the syntaxin 5 homolog) after docking, but before SNARE complex assembly (31). We previously showed that the N-terminal sequence of syntaxin 5, when overexpressed in tissue culture cells, disrupts the Golgi complex, suggesting that it acts as a dominant negative inhibitor of Golgi membrane traffic (16). This action was most likely executed by binding of the syntaxin 5 fragment to Sly1 because the dominant negative effect was abolished by a mutation that inhibits Sly1 binding in vitro. The current data now show that the peptide binds to Sly1 at a discrete binding site in an autonomously folded small N-terminal domain without inducing a conformational change (Fig. 5); thus, the overexpressed N-terminal syntaxin 5 sequence is unlikely to abolish Sly1 function by a global structural effect on Sly1. Furthermore, we found that overexpression of the isolated Sly1 N-terminal domain also disrupts the Golgi complex (Fig. 3). This result demonstrates that interfering with the Sly1/syntaxin 5 complex by overexpressing the N-terminal sequences of either syntaxin 5 or Sly1 has the same effect on the Golgi, probably because the transfected fragments uncouple the interaction between the endogenous proteins, but cannot functionally substitute for them. Thus, at least for Sly1, binding to syntaxin 5 is an intrinsic part of fusion and not a regulatory event.
Based on the current identification of the N-terminal ligand-binding domain in SM proteins and previous results (see references above), we suggest that the function of SM proteins is more uniform than indicated by the diversity of their interactions with SNAREs. Our proposal is that SM proteins share a similar overall structure in which a small, autonomously folded N-terminal domain mediates the interaction with SNAREs, whereas the larger C-terminal domains constitute effector domains that execute similar but unknown functions of SM proteins in fusion. According to this proposal, Munc18s developed a radically different interaction mechanism with syntaxins (i.e., binding of the closed conformation of syntaxins as opposed to a small N-terminal peptide) during the evolution of multicellular organisms to allow for a better temporal control of SNARE complex assembly in exocytosis. The effector surface of these domains may involve the loop between domains 2 and 3, as suggested by the mutation that led to the discovery of Sly1 and that renders Golgi fusion independent of the rab protein ypt1p (32). In this case, the effector surface would be located on the opposite side of the syntaxin-binding region. Alternatively, the central cavity that forms the syntaxin 1-binding site in Munc18-1 (21) may represent an effector region that is already occupied by syntaxin 1 as a substrate, or even inhibited by syntaxin 1. Although the actual activity performed by the effector domains is unclear, indirect data indicate that fusion cannot proceed to SNARE complex assembly in the absence of SM proteins (e.g., 33, 34), suggesting that the most likely function of the SM protein effector domains is to gate fusion via SNARE proteins, possibly by connecting the membrane tethering apparatus to the SNARE machinery.
Supplementary Material
Supporting Information
Acknowledgments
We thank I. Kornblum and E. Borowicz for technical assistance. This work was supported by National Institutes of Health Grant NS37200 (to J.R.).
Abbreviations
SNARE
soluble _N-_ethylmaleimide-sensitive factor attachment protein receptor
SM
Sec1/Munc18-like
HSQC
heteronuclear single quantum correlation
ER
endoplasmic reticulum
NOE
nuclear Overhauser effect
Note Added in Proof.
After submission of this paper, the crystal structure of a yeast Sed5p N-terminal peptide bound to Sly1p was described and revealed the complete binding mode between the two proteins (35).
References
- 1.Jahn R, Südhof T C. Annu Rev Biochem. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- 2.Hanson P I, Roth R, Morisaki H, Jahn R, Heuser J E. Cell. 1997;90:523–535. doi: 10.1016/s0092-8674(00)80512-7. [DOI] [PubMed] [Google Scholar]
- 3.Weber T, Zemelman B V, McNew J A, Westermann B, Gmachl M, Parlati F, Sollner T H, Rothman J E. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y A, Scheller R H. Nat Rev Mol Cell Biol. 2001;2:98–106. doi: 10.1038/35052017. [DOI] [PubMed] [Google Scholar]
- 5.Rizo J, Südhof T C. Nat Rev Neurosci. 2002;8:641–653. doi: 10.1038/nrn898. [DOI] [PubMed] [Google Scholar]
- 6.Banta L M, Vida T A, Herman P K, Emr S D. Mol Cell Biol. 1990;10:4638–4649. doi: 10.1128/mcb.10.9.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verhage M, Maia A S, Plomp J J, Brussaard A B, Heeroma J H, Vermeer H, Toonen R F, Hammer R E, van den Berg T K, Missler M, et al. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 8.Ungermann C, Wickner W. EMBO J. 1998;17:3269–3276. doi: 10.1093/emboj/17.12.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schoch S, Deak F, Königstorfer A, Mozhayeva M, Sara Y, Südhof T C, Kavalali E T. Science. 2001;294:1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- 10.Washbourne P, Thompson P M, Carta M, Costa E T, Mathews J R, Lopez-Bendito G, Molnar Z, Becher M W, Valenzuela C F, Partridge L D, et al. Nat Neurosci. 2002;5:19–26. [Google Scholar]
- 11.Fernandez I, Ubach J, Dulubova I, Zhang X, Südhof T C, Rizo J. Cell. 1998;94:841–849. doi: 10.1016/s0092-8674(00)81742-0. [DOI] [PubMed] [Google Scholar]
- 12.Lerman J C, Robblee J, Fairman R, Hughson F M. Biochemistry. 2000;39:8470–8479. doi: 10.1021/bi0003994. [DOI] [PubMed] [Google Scholar]
- 13.Munson M, Chen X, Cocina A E, Schultz S M, Hughson F M. Nat Struct Biol. 2000;7:894–902. doi: 10.1038/79659. [DOI] [PubMed] [Google Scholar]
- 14.Dulubova I, Yamaguchi T, Wang Y, Südhof T C, Rizo J. Nat Struct Biol. 2001;8:258–264. doi: 10.1038/85012. [DOI] [PubMed] [Google Scholar]
- 15.Dulubova I, Yamaguchi T, Gao Y, Min S W, Huryeva I, Südhof T C, Rizo J. EMBO J. 2002;21:3620–3631. doi: 10.1093/emboj/cdf381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamaguchi T, Dulubova I, Min S W, Chen X, Rizo J, Südhof T C. Dev Cell. 2002;2:295–305. doi: 10.1016/s1534-5807(02)00125-9. [DOI] [PubMed] [Google Scholar]
- 17.Antonin W, Dulubova I, Araç D, Pabst S, Plitzner J, Rizo J, Jahn R. J Biol Chem. 2002;277:36449–36456. doi: 10.1074/jbc.M204369200. [DOI] [PubMed] [Google Scholar]
- 18.Dulubova I, Sugita S, Hill S, Hosaka M, Fernandez I, Südhof T C, Rizo J. EMBO J. 1999;18:4372–4382. doi: 10.1093/emboj/18.16.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hata Y, Slaughter C A, Südhof T C. Nature. 1993;366:347–351. doi: 10.1038/366347a0. [DOI] [PubMed] [Google Scholar]
- 20.Kee Y, Lin R C, Hsu S C, Scheller R H. Neuron. 1995;14:991–998. doi: 10.1016/0896-6273(95)90337-2. [DOI] [PubMed] [Google Scholar]
- 21.Misura K M, Scheller R H, Weis W I. Nature. 2000;404:355–362. doi: 10.1038/35006120. [DOI] [PubMed] [Google Scholar]
- 22.Bracher A, Perrakis A, Dresbach T, Betz H, Weissenhorn W. Struct Fold Des. 2000;8:685–694. doi: 10.1016/s0969-2126(00)00156-8. [DOI] [PubMed] [Google Scholar]
- 23.Guan K L, Dixon J E. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- 24.Harrison S D, Broadie K, van de Goor J, Rubin G M. Neuron. 1994;13:555–566. doi: 10.1016/0896-6273(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 25.Wishart D S, Sykes B D. J Biomol NMR. 1994;4:171–180. doi: 10.1007/BF00175245. [DOI] [PubMed] [Google Scholar]
- 26.Grabowski R, Gallwitz D. FEBS Lett. 1997;411:169–172. doi: 10.1016/s0014-5793(97)00720-5. [DOI] [PubMed] [Google Scholar]
- 27.Richmond J E, Weimer R M, Jorgensen E M. Nature. 2001;412:338–341. doi: 10.1038/35085583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koushika S P, Richmond J E, Hadwiger G, Weimer R M, Jorgensen E M, Nonet M L. Nat Neurosci. 2001;4:997–1005. doi: 10.1038/nn732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blasi J, Chapman E R, Yamasaki S, Binz T, Niemann H, Jahn R. EMBO J. 1993;12:4821–4828. doi: 10.1002/j.1460-2075.1993.tb06171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voets T, Toonen R F, Brian E C, de Wit H, Moser T, Rettig J, Südhof T C, Neher E, Verhage M. Neuron. 2001;31:581–591. doi: 10.1016/s0896-6273(01)00391-9. [DOI] [PubMed] [Google Scholar]
- 31.Cao X, Barlowe C. J Cell Biol. 2000;3:55–66. doi: 10.1083/jcb.149.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dascher C, Ossig R, Gallwitz D, Schmitt H D. Mol Cell Biol. 1991;11:872–885. doi: 10.1128/mcb.11.2.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato T K, Rehling P, Peterson M R, Emr S D. Mol Cell. 2000;6:661–671. doi: 10.1016/s1097-2765(00)00064-2. [DOI] [PubMed] [Google Scholar]
- 34.Bryant N J, James D E. EMBO J. 2001;20:3380–3388. doi: 10.1093/emboj/20.13.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bracher A, Weissenhorn W. EMBO J. 2002;21:6114–6124. doi: 10.1093/emboj/cdf608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information