Latency in Human Immunodeficiency Virus Type 1 Infection: No Easy Answers (original) (raw)
For some viruses, long-term persistence in the host depends on the establishment of latency, a reversibly nonproductive state of infection. The alphaherpesviruses provide a dramatic illustration. Herpes simplex virus and varicella-zoster virus establish latency in sensory neurons, persisting in these long-lived cells by using a limited program of gene expression (10). Herpes simplex virus and varicella-zoster virus can be reactivated from latency, but during intervals between reactivation, there is little ongoing virus replication. Latency thus represents the principal form of persistence.
This is in sharp contrast to human immunodeficiency virus type 1 (HIV-1) infection. HIV-1 replicates continuously, even during the prolonged asymptomatic period between primary infection and the development of AIDS (41). During the asymptomatic phase, there are typically 104 to 105 copies of viral RNA/ml of plasma. This continuous replication propels the relentless evolution of viral variants (49), allowing viral escape from immune responses (40; reviewed in reference 25) and antiretroviral drugs (31; reviewed in reference 37). Viral replication also drives the loss of CD4+ T cells (33), although the mechanism remains controversial (for a review, see reference 19). The immune system exerts some control over viral replication, as demonstrated by increases in viremia with acute experimental depletion of CD8+ T cells in the simian immunodeficiency virus model (24, 48). However, this immune control fails to completely arrest the replication of the virus. The persistence of HIV-1 is therefore not dependent on latency. Nevertheless, HIV-1 can establish latent infection in resting memory CD4+ T cells (8, 9). Although not essential for persistence, latency has clinical importance as a mechanism of HIV-1 persistence in individuals treated with antiretroviral drugs. Here it is argued that HIV-1 latency renders the infection intrinsically incurable by antiretroviral therapy alone.
LATENCY AND IMMUNOLOGIC MEMORY
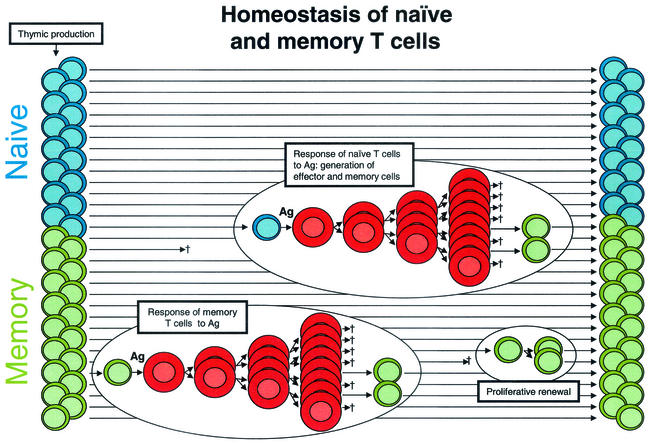
HIV-1 latency is a consequence of the normal physiology of CD4+ T lymphocytes (Fig. 1). At any given time, most CD4+ T lymphocytes are in a resting G0 state. Resting lymphocytes are profoundly quiescent cells with a low metabolic rate and a unique morphology characterized by a small cytoplasmic volume. In adults, about half of the resting cells are naïve, having yet to encounter an appropriate antigen (Ag). The remaining cells are memory cells that have previously responded to an Ag (Fig. 1). Ag-driven responses involve a burst of cellular proliferation and differentiation, giving rise to effector cells. Most effector cells die quickly, but a subset survives and reverts to the resting G0 state. They persist as memory cells, with an altered pattern of gene expression enabling long-term survival and rapid responses to the Ag in the future (for a review of immunologic memory, see reference 27).
FIG. 1.
Normal T-cell homeostasis. Most of the CD4+ T cells in the body are small resting cells that circulate throughout the lymphoid tissues, poised to respond to a specific Ag. Approximately half are naïve cells (blue) that have not encountered an Ag since emerging from the thymus. The remainder are memory cells (green) that have previously responded to Ag. Following their encounter with Ag, resting cells undergo blast transformation and begin to proliferate. These lymphoblasts (red) undergo several rounds of cell division, giving rising to effector cells. Most effector cells eventually die, but a fraction revert to a resting memory state. The memory pool is maintained by the long life span of the cells and a gradual process of proliferative renewal.
Several processes contribute to the stability of the naïve and memory pools. Most importantly, resting CD4+ T cells have a long life span. Early measurements suggested that in uninfected humans, memory cells die or divide with a half-life (_t_1/2) of 6 months while naïve cells have an even longer intermitotic half-life (32, 34). The naïve population is replenished with newly generated T cells from the thymus even in adults (13), while the memory pool is maintained by a process of Ag-independent proliferative renewal in which memory cells occasionally go through the cell cycle in response to cytokines (Fig. 1). For murine CD8+ T cells, this process is driven by interleukin-15 (2). CD4+ T cells also undergo proliferative renewal in response to as-yet-unknown stimuli. The combined effects of a long life span and proliferative renewal provide lifelong immunologic memory of many infectious agents. For example, immunity to measles virus is lifelong, and hepatitis C virus-specific CD4+ T cells persist for decades after clearance of the virus (51).
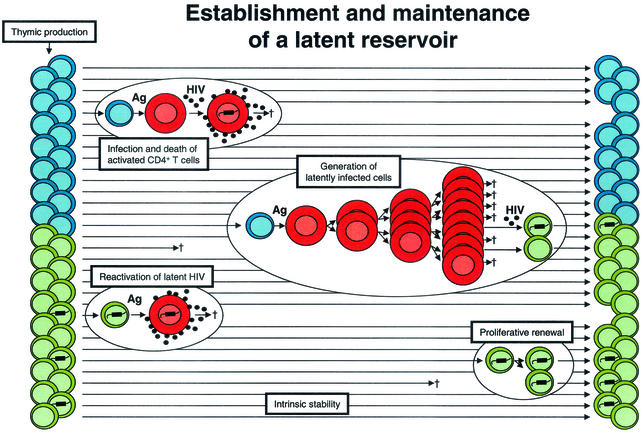
HIV-1 infection perturbs this physiology (Fig. 2). Infected individuals show decreases in thymic production of naive T cells (13) and increased T-cell activation (19). The virus replicates preferentially in activated CD4+ T cells and kills them, typically within days after infection (23, 55). Because it takes a few weeks for effector cells to revert to a resting state, most infected CD4+ lymphoblasts die before becoming memory cells. However, it has been hypothesized that some activated cells become infected when they are in the process of reverting to a resting state (Fig. 2). One could further hypothesize that some of these cells are still permissive for early steps in the virus life cycle (up to integration), but not for virus gene expression (50). HIV-1 gene expression has been clearly shown to be dependent on inducible host transcription factors that are transiently activated following exposure to Ag (14, 35, 53), and thus viral gene expression is automatically extinguished as cells return to a resting state. The result would be a stably integrated but transcriptionally silent form of the virus in a cell whose function is to survive for long periods of time. According to this hypothesis, HIV-1 latency exploits the most fundamental characteristic of the immune system, the immunologic memory that resides in long-lived resting lymphocytes.
FIG. 2.
Establishment of a latent reservoir in resting memory CD4+ T cells. CD4+ lymphoblasts (red cells) are highly susceptible to productive infection and usually die within a few days after infection. Latently infected cells with integrated HIV-1 DNA may be generated when lymphoblasts that are in the process of reverting to a resting state become infected. When latently infected cells subsequently encounter the relevant Ag, they become permissive for virus gene expression and virus production. Latently infected cells may be maintained by intrinsic stability, as well as by the process of proliferative renewal if they do not become susceptible to HIV-1-induced cytopathic effects or host cytolytic mechanisms during this process.
Interestingly, viral persistence in resting memory lymphocytes is not unique to HIV-1. Epstein-Barr virus (EBV) establishes latent infection in memory B cells (52). This allows EBV to persist despite strong immune responses. Several EBV genes function solely to facilitate the establishment and maintenance of latency. It appears that HIV-1 latency is achieved in a more passive and genetically economical fashion; it is an automatic consequence of viral tropism for activated CD4+ T cells, some of which normally revert to a resting state that is no longer permissive for virus production.
THE CLINICAL IMPORTANCE OF LATENCY
The demonstration of a stable state of latent infection in resting memory CD4+ T cells in vivo required methods for the isolation of extremely pure populations of resting CD4+ T cells, free from the activated cells in which virus replication occurs. Also required were molecular methods for demonstrating integration of the viral genome into that of host cells and culture methods for rescuing replication-competent virus from latently infected cells by cellular activation. With these methods, latently infected cells were found at a low frequency (1 in 106) in all infected individuals (8, 9). They have been detected in the blood, lymph nodes, and spleen (8; A. Shen, M. C. Zink, J. L. Mankowski, K. Chadwick, J. B. Margolick, L. M. Carruth, M. Li, J. E. Clements, and R. F. Siliciano, unpublished data). Based on recent studies of the distribution of memory T cells in mouse tissues (46), it is possible that latently infected cells are broadly distributed in nonlymphoid organs as well. Latent HIV-1 is found predominantly in resting CD4+ T cells with a memory phenotype, consistent with the model presented above (8, 42).
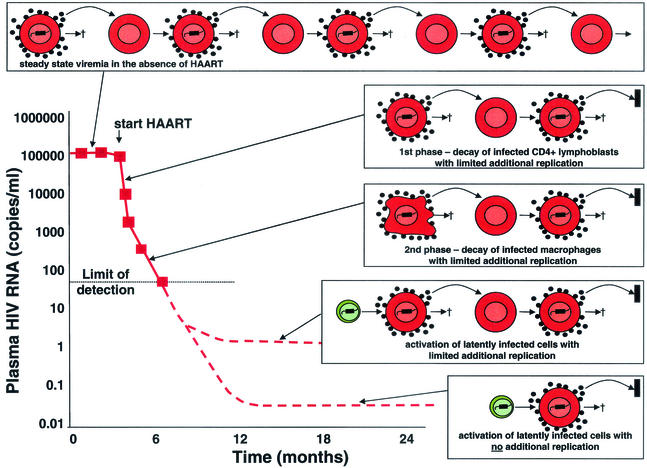
A crucial prediction of the latency model is that latently infected cells should persist even when viral replication is fully suppressed by antiretroviral drugs. With the advent of highly active antiretroviral therapy (HAART) in 1996, it became possible to test this hypothesis. Following the initiation of HAART, virus levels in plasma drop by 100-fold in the first 2 weeks (Fig. 3). Because HAART largely stops the new infection of susceptible cells, the decay of virus in plasma provides information about the relative sizes and decay rates of various compartments of productively infected cells. Initial decay is rapid because most of the virus in plasma is produced by infected CD4+ T lymphoblasts, which live only a short time after infection (_t_1/2 = 1 day) (5, 23, 38, 55). When most of these cells have been cleared, the decay of a second population of infected cells becomes apparent. These cells may be macrophages, and they decay with a _t_1/2 of about 2 weeks. Mathematical models using these estimates of cell half-life led to the prediction that eradication could be achieved within 2 to 3 years of treatment initiation if there were no additional populations of infected cells (38). The second phase brings the level of virus in plasma down to the limit of detection of current assays (50 copies of viral RNA per ml of plasma), and in compliant patients, suppression of viral replication to <50 copies/ml can be maintained for years, allowing the hypothesis that the virus can persist in latently infected resting memory CD4+ T cells to be tested.
FIG. 3.
Sources of virus in plasma during HAART. In the absence of treatment, there is an equilibrium between the production and clearance of infected cells. With HAART, there is a rapid decay of plasma virus (_t_1/2 of hours) and of the infected CD4+ T lymphoblasts that produce most of the plasma virus (_t_1/2 = 1 day) (23, 55). The second phase of decay results from the slower turnover (_t_1/2 = 2 weeks) of another population of infected cells, perhaps macrophages (38). The second phase brings the level of viremia down to a new steady state somewhat below the limit of detection of current assays for plasma HIV-1 RNA (50 copies/ml). It was initially assumed that HAART immediately stopped all new infection of susceptible cells (23, 55). More recently, it has been argued that HAART may not completely arrest viral replication but may simply reduce the number of newly infected cells generated from each productively infected cell to substantially below one (18). In patients who are responding well to HAART, there is release of virus from stable reservoirs and some degree of additional replication, giving rise to a new steady-state level of viremia that is <50 copies/ml. However, the additional replication is sufficiently limited that new drug resistance mutations rarely arise in such patients (22). If HAART stopped all new infection of susceptible cells, then virus levels in plasma would fall to a basal level, the magnitude of which would depend on the size and average rate of activation of the latent compartment (with possible contributions from other stable reservoirs).
In 1997, three groups demonstrated the presence of resting CD4+ T cells harboring replication-competent virus in patients on HAART. All three groups used methods that induce T-cell activation in order to rescue the virus from latently infected cells (7, 17, 56). The decay rate of the reservoir was then measured longitudinally in infected adults and children for whom long-term control of viremia had been maintained on HAART (16, 39). The reservoir showed striking stability, with a _t_1/2 of 44 months. At this rate of decay, eradication of a reservoir consisting of only 106 cells would take 73 years (16). More recent studies suggest that the reservoir does not decay significantly even in patients who have exhibited suppression of viremia to below 50 copies/ml for 6 to 7 years on HAART (J. D. Siliciano, J. A. Kajdas, C. Kovacs, and R. F. Siliciano, unpublished data). These results have dimmed initial hopes for eradication of the virus and have contributed to a change in the approach to treatment. Given that eradication will likely be impossible, the concept of early initiation of aggressive HAART with the goal of eradication has been replaced by a more conservative approach in which treatment (and the attendant risk of side effects and drug resistance) is delayed until later stages of the infection (36).
The notion that this reservoir persists without decay is consistent with the universal clinical observation that virus levels in plasma rebound in all patients who stop treatment, typically in 2 weeks. Sequence analysis of the rebound virus is consistent with the idea that activation of latently infected resting memory CD4+ T cells is a possible source (6, 57).
The model presented above views the generation of latently infected cells as a result of infection of activated CD4+ T cells in the periphery. There is also evidence for generation of latently infected cells in the thymus in a SCID/hu mouse model (3), but in infected individuals, most latently infected cells have a memory rather than a naïve phenotype (8, 42). Direct infection of mature resting CD4+ T cells is also a possibility, but resting CD4+ T cells from the peripheral blood do not readily support viral replication. Naïve cells lack CCR5, the coreceptor utilized by most viruses in the reservoir (42). CCR5 is expressed on a very small subset of resting memory cells, but even if entry occurs, reverse transcription proceeds only very slowly (43), and in resting cells there may be a block at the subsequent step of nuclear import of the large preintegration complex carrying the reverse-transcribed HIV-1 DNA (4). Certain cytokines can overcome these blocks (54), and in the cytokine-rich microenvironment of the peripheral lymphoid tissues (59), it is possible that direct infection of cytokine-stimulated memory cells also leads to latency.
GENETIC EVIDENCE FOR HIV-1 LATENCY
Two distinct but nonexclusive hypotheses explain the extraordinary stability of the reservoir of HIV-1 in resting memory CD4+ T cells. The first is that stability is a consequence of true latency in the long-lived population of cells that comprise the reservoir (Fig. 1). Individual memory cells may survive for months to years and may undergo proliferative renewal. If during this process HIV-1 gene expression is not upregulated to such an extent that the cells die from cytopathic effects or host effector mechanisms, then eradication can never be achieved with antiretroviral drugs, even those that completely stop all new infection of susceptible cells, because viral persistence involves host cell division, not viral replication (Fig. 2). Retroviral persistence through the proliferation of infected host cells is, of course, a well-known feature of infections by some other retroviruses, including murine mammary tumor virus, human T-cell leukemia viruses, and other classic oncoretroviruses.
The second hypothesis is that “cryptic” low-level virus replication maintains the latent pool (45). HAART can suppress viral replication by many logs, but genomic viral RNA can be detected in the plasma by more sensitive assays, even in patients whose virus levels in plasma have fallen below 50 copies/ml (12, 22). However, phylogenetic analyses of the viral quasispecies persisting in the latent reservoir have so far failed to provide definitive evidence for entry of new sequences into the stable pool of integrated virus in resting memory CD4+ T cells in patients on suppressive HAART regimens. Entry of new viruses clearly occurs when levels of virus in plasma are high, but the process is inefficient and may not occur at low levels of viremia. In addition, a reservoir that persists solely on the basis of replenishment by cryptic ongoing virus replication should harbor viral quasispecies that are contemporaneous, while a reservoir that persists because of the intrinsic stability of the host cells should retain viral quasispecies generated throughout the course of infection. To date, studies of the env and pol genes of the HIV-1 quasispecies persisting in CD4+ T cells in patients on HAART have shown an arrest in virus evolution in the majority of cases (20, 58). More convincing evidence for intrinsic stability comes from studies showing that wild-type, drug-sensitive HIV-1 is retained in a replication-competent form in resting CD4+ T cells of patients who have developed high-level drug resistance and have had continuous exposure to drugs selecting that resistance for over 10 years (47). In the presence of antiretroviral drugs, wild-type virus is profoundly unfit compared to drug-resistant virus, and thus it is difficult to explain the persistence of wild-type virus through any mechanism that involves active cycles of replication in the presence of suppressive concentrations of the drugs. Interestingly, drug-resistant viruses that arise in patients who are failing therapy can be deposited in the reservoir when virus levels in plasma are high and persist there throughout the subsequent course of infection, thereby permanently limiting treatment options (39, 47). Thus, the latent reservoir provides a stable archive reflecting the whole history of the infection. Together, these findings provide strong evidence that at least a portion of the reservoir for HIV-1 in resting CD4+ T cells is extremely stable. This notion is supported by the striking clinical observation that wild-type virus reemerges when patients with extensive drug resistance are taken off therapy (11).
What, then, is the relationship between the latent reservoir and the low level of free virus in the plasma of patients on HAART? If HAART stopped all new infection of susceptible cells, then the only viruses entering the plasma would be those derived by the activation of latently infected cells (and the viruses released from other, similarly stable reservoirs). These viruses would be unable to infect additional cells, and the result would be a low steady-state level of viremia (Fig. 3). The best that any antiretroviral regimen will ever do is to drop the levels of virus in plasma down to this theoretical basal level reflecting average release from stable preexisting reservoirs. Current HAART regimens may not achieve this basal level, and some limited degree of additional viral replication may follow the activation of individual latently infected cells. Drug resistance can develop when the level of viremia is only slightly above 50 copies/ml for sustained periods of time (21). However, when viremia is below 50 copies/ml, new cycles of replication are insufficient to allow the development of resistance over the course of several years. Direct examination of the viruses present in the plasma of patients with <50 copies of HIV RNA/ml reveals that they are archival in nature, similar to viruses found in the reservoir, and remain sensitive to the drugs in use (22). The continued release of drug-sensitive viruses over the course of several years, without the evolution of any drug resistance, is more consistent with release from a stable reservoir than with continuous low-level replication in the presence of drugs.
MOLECULAR MECHANISMS AND THERAPEUTIC APPROACHES
Among the proposed mechanisms for HIV-1 latency are the lack of activation-dependent host transcription factors in resting cells (14, 35, 53), silencing based on chromatin structure changes or epigenetic modifications at the site of integration (26), premature termination of transcription due to the absence of sufficient levels of Tat and associated host factors (1, 28), and inefficient export of RNAs for structural proteins (44). Unfortunately, none of these mechanisms has been confirmed in purified resting CD4+ T cells from infected individuals because of the low frequency of latently infected cells and the absence of any method for separating latently infected cells from uninfected resting CD4+ T cells. Understanding the mechanism of latency is critical for determining whether this reservoir can ever be eliminated. If latency operates at the level of transcription, then no therapeutic approach with an RNA or protein target can directly affect the reservoir. Thus, there is interest in strategies that “flush” cells out of latency. Interleukin-2 and antibodies to CD3 induce T-cell activation in vivo. However, initial studies in patients have shown significant toxicities and no meaningful decay of the latent reservoir (15). An ideal agent would induce expression of latent HIV-1 without inducing global T-cell activation. However, this is a tall order given that HIV-1 gene expression is so intimately linked to T-cell activation. Recently, two groups have demonstrated that a naturally occurring, non-tumor-promoting phorbol ester, prostratin, induces the expression of latent HIV-1 in resting CD4+ T cells without inducing cellular proliferation or enhancing de novo HIV-1 infection (29, 30). Even if this strategy proves successful, there remains the possibility that other stable reservoirs exist, each of which would have to be dealt with in a cell type-specific fashion.
SUMMARY
In summary, HIV-1 latency represents a formidable therapeutic challenge for which there are no easy answers. As a result of viral tropism for activated CD4+ T cells and the reversion of some of these cells to a profoundly quiescent and long-lived memory state, the virus can persist through the same mechanisms responsible for the most fundamental characteristic of the immune system, immunologic memory. The dependence of viral gene expression on host factors that are inactive in resting T cells means that viral gene expression can be largely or completely extinguished in latently infected cells. In the absence of virus gene expression, latently infected cells will differ from their uninfected counterparts by only the presence of 10 kb of viral DNA integrated into a host cell chromosome. In this case, the virus is persisting as genetic information in a stably integrated form in rare memory cells that cannot be distinguished from their uninfected counterparts. It is difficult to envision any targeting mechanism that will allow specific elimination of this reservoir. Optimism stems from the fact that antiretroviral drugs come very close to stopping the active replication of the virus, with its attendant damage to the immune system. In some patients on HAART, the evolution of drug-resistant viruses, which is the principal cause of treatment failure, is largely halted. The development of nontoxic, convenient, and affordable combinations of antiretroviral drugs may allow infected individuals to live a normal life despite the indefinite persistence of latent HIV-1 in resting memory CD4+ T cells.
Acknowledgments
This work was supported by grants from the Elizabeth Glaser Pediatric AIDS Foundation (D.P) and the Doris Duke Charitable Foundation (D.P. and R.F.S.) and by NIH grants AI43222 and AI51178 (R.F.S.).
REFERENCES
- 1.Adams, M., L. Sharmeen, J. Kimpton, J. M. Romeo, J. V. Garcia, B. M. Peterlin, M. Groudine, and M. Emerman. 1994. Cellular latency in human immunodeficiency virus-infected individuals with high CD4 levels can be detected by the presence of promoter-proximal transcripts. Proc. Natl. Acad. Sci. USA 91**:**3862-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker, T. C., E. J. Wherry, D. Boone, K. Murali-Krishna, R. Antia, A. Ma, and R. Ahmed. 2002. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 195**:**1541-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks, D. G., S. G. Kitchen, C. M. Kitchen, D. D. Scripture-Adams, and J. A. Zack. 2001. Generation of HIV latency during thymopoiesis. Nat. Med. 7**:**459-464. [DOI] [PubMed] [Google Scholar]
- 4.Bukrinsky, M. I., N. Sharova, M. P. Dempsey, T. L. Stanwick, A. G. Bukrinskaya, S. Haggerty, and M. Stevenson. 1992. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc. Natl. Acad. Sci. USA 89**:**6580-6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavert, W., D. W. Notermans, K. Staskus, S. W. Wietgrefe, M. Zupancic, K. Gebhard, K. Henry, S.-C. Zhang, R. Mills, H. McDade, J. Goudsmit, S. A. Danner, and A. T. Haase. 1997. Kinetics of response in lymphoid tissue to antiretroviral therapy of HIV-1 infection. Science 276**:**960-964. [DOI] [PubMed] [Google Scholar]
- 6.Chun, T. W., R. T. Davey, Jr., M. Ostrowski, J. J. Shawn, D. Engel, J. I. Mullins, and A. S. Fauci. 2000. Relationship between pre-existing viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active anti-retroviral therapy. Nat. Med. 6**:**757-761. [DOI] [PubMed] [Google Scholar]
- 7.Chun, T. W., L. Stuyver, S. B. Mizell, L. A. Ehler, J. M. Mican, M. Baseler, A. L. Lloyd, M. A. Nowak, and A. S. Fauci. 1997. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 94**:**13193-13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chun, T.-W., L. Carruth, D. Finzi, X. Shen, J. A. Digiuseppe, H. Taylor, M. Hermankova, K. Chadwick, J. Margolick, T. C. Quinn, Y.-H. Kuo, R. Brookmeyer, M. A. Zeiger, P. Barditch-Crovo, and R. F. Siliciano. 1997. Quantitation of latent tissue reservoirs and total body load in HIV-1 infection. Nature 387**:**183-188. [DOI] [PubMed] [Google Scholar]
- 9.Chun, T.-W., D. Finzi, J. Margolick, K. Chadwich, D. Schwartz, and R. F. Siliciano. 1995. Fate of HIV-1-infected T cells in vivo: rates of transition to stable latency. Nat. Med. 1**:**1284-1290. [DOI] [PubMed] [Google Scholar]
- 10.Daheshia, M., L. T. Feldman, and B. T. Rouse. 1998. Herpes simplex virus latency and the immune response. Curr. Opin. Microbiol. 1**:**430-435. [DOI] [PubMed] [Google Scholar]
- 11.Deeks, S. G., T. Wrin, T. Liegler, R. Hoh, M. Hayden, J. D. Barbour, N. S. Hellmann, C. J. Petropoulos, J. M. McCune, M. K. Hellerstein, and R. M. Grant. 2001. Virologic and immunologic consequences of discontinuing combination antiretroviral-drug therapy in HIV-infected patients with detectable viremia. N. Engl. J. Med. 344**:**472-480. [DOI] [PubMed] [Google Scholar]
- 12.Dornadula, G., H. Zhang, B. VanUitert, J. Stern, L. Livornese, Jr., M. J. Ingerman, J. Witek, R. J. Kedanis, J. Natkin, J. DeSimone, and R. J. Pomerantz. 1999. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA 282**:**1627-1632. [DOI] [PubMed] [Google Scholar]
- 13.Douek, D. C., R. D. McFarland, P. H. Keiser, E. A. Gage, J. M. Massey, B. F. Haynes, M. A. Polis, A. T. Haase, M. A. Feinberg, J. L. Sullivan, B. D. Jamieson, J. A. Zack, L. J. Picker, and R. A. Koup. 1998. Changes in thymic function with age and during the treatment of HIV infection. Nature 396**:**690-695. [DOI] [PubMed] [Google Scholar]
- 14.Duh, E. J., W. J. Maury, T. M. Folks, A. S. Fauci, and A. B. Rabson. 1989. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc. Natl. Acad. Sci. USA 86**:**5974-5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dybul, M., B. Hidalgo, T. W. Chun, M. Belson, S. A. Migueles, J. S. Justement, B. Herpin, C. Perry, C. W. Hallahan, R. T. Davey, J. A. Metcalf, M. Connors, and A. S. Fauci. 2002. Pilot study of the effects of intermittent interleukin-2 on human immunodeficiency virus (HIV)-specific immune responses in patients treated during recently acquired HIV infection. J. Infect. Dis. 185**:**61-68. [DOI] [PubMed] [Google Scholar]
- 16.Finzi, D., J. Blankson, J. D. Siliciano, J. B. Margolick, K. Chadwick, T. Pierson, K. Smith, J. Lisziewicz, F. Lori, C. Flexner, T. C. Quinn, R. E. Chaisson, E. Rosenberg, B. Walker, S. Gange, J. Gallant, and R. F. Siliciano. 1999. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 5**:**512-517. [DOI] [PubMed] [Google Scholar]
- 17.Finzi, D., M. Hermankova, T. Pierson, L. M. Carruth, C. Buck, R. E. Chaisson, T. C. Quinn, K. Chadwick, J. Margolick, R. Brookmeyer, J. Gallant, M. Markowitz, D. D. Ho, D. Richman, and R. F. Siliciano. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278**:**1295-1300. [DOI] [PubMed] [Google Scholar]
- 18.Grossman, Z., M. Feinberg, V. Kuznetsov, D. Dimitrov, and W. Paul. 1998. HIV infection: how effective is drug combination treatment? Immunol. Today 19**:**528-532. [DOI] [PubMed] [Google Scholar]
- 19.Grossman, Z., M. Meier-Schellersheim, A. E. Sousa, R. M. Victorino, and W. E. Paul. 2002. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat. Med. 8**:**319-323. [DOI] [PubMed] [Google Scholar]
- 20.Günthard, H. F., S. D. W. Frost, A. J. Leigh Brown, C. C. Ignacio, K. Kee, A. S. Perelson, C. A. Spina, D. V. Havlir, M. Hezareh, D. J. Looney, D. D. Richman, and J. K. Wong. 1999. Evolution of envelope sequences of human immunodeficiency virus type 1 in cellular reservoirs in the setting of potent antiviral therapy. J. Virol. 73**:**9404-9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Günthard, H. F., J. K. Wong, C. C. Ignacio, J. C. Guatelli, N. L. Riggs, D. V. Havlir, and D. D. Richman. 1998. Human immunodeficiency virus replication and genotypic resistance in blood and lymph nodes after a year of potent antiretroviral therapy. J. Virol. 72**:**2422-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hermankova, M., S. C. Ray, C. Ruff, M. Powell-Davis, R. Ingersoll, R. T. D'Aquila, T. C. Quinn, J. D. Siliciano, R. F. Siliciano, and D. Persaud. 2001. HIV-1 drug resistance profiles in children and adults with viral load <50 copies/mL receiving combination therapy. JAMA 286**:**196-207. [DOI] [PubMed] [Google Scholar]
- 23.Ho, D. D., A. U. Neumann, A. S. Perelson, W. Chen, J. M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373**:**123-126. [DOI] [PubMed] [Google Scholar]
- 24.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189**:**991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson, W. E., and R. C. Desrosiers. 2002. Viral persistence: HIV's strategies of immune system evasion. Annu. Rev. Med. 53**:**499-518. [DOI] [PubMed] [Google Scholar]
- 26.Jordan, A., P. Defechereux, and E. Verdin. 2001. The site of HIV-1 integration in the human genome determines basal transcriptional activity and response to Tat transactivation. EMBO J. 20**:**1726-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaech, S. M., E. J. Wherry, and R. Ahmed. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2**:**251-262. [DOI] [PubMed] [Google Scholar]
- 28.Kao, S. Y., A. F. Calman, P. A. Luciw, and B. M. Peterlin. 1987. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature 330**:**489-493. [DOI] [PubMed] [Google Scholar]
- 29.Korin, Y. D., D. G. Brooks, S. Brown, A. Korotzer, and J. A. Zack. 2002. Effects of prostratin on T-cell activation and human immunodeficiency virus latency. J. Virol. 76**:**8118-8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulkosky, J., D. M. Culnan, J. Roman, G. Dornadula, M. Schnell, M. R. Boyd, and R. J. Pomerantz. 2001. Prostratin: activation of latent HIV-1 expression suggests a potential inductive adjuvant therapy for HAART. Blood 98**:**3006-3015. [DOI] [PubMed] [Google Scholar]
- 31.Larder, B. A., G. Darby, and D. D. Richman. 1989. HIV with reduced sensitivity to zidovudine (AZT) isolated during prolonged therapy. Science 243**:**1731-1734. [DOI] [PubMed] [Google Scholar]
- 32.McLean, A. R., and C. A. Michie. 1995. In vivo estimates of division and death rates of human T lymphocytes. Proc. Natl. Acad. Sci. USA 92**:**3707-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mellors, J. W., C. W. Rinaldo, Jr., P. Gupta, R. M. White, J. A. Todd, and L. A. Kingsley. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272**:**1167-1170. [DOI] [PubMed] [Google Scholar]
- 34.Michie, C. A., A. McLean, C. Alcock, and P. C. Beverley. 1992. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature 360**:**264-265. [DOI] [PubMed] [Google Scholar]
- 35.Nabel, G., and D. Baltimore. 1987. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature 326**:**711-713. [DOI] [PubMed] [Google Scholar]
- 36.Panel on Clinical Practices for Treatment of HIV Infection. 4February2002, posting date. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. [Online.] HIV/AIDS Treatment Information Service, U.S. Department of Health and Human Services, Washington, D.C., and the Henry J. Kaiser Family Foundation. http://www.hivatis.org.
- 37.Parikh, U., J. Hammond, C. Calef, B. Larder, R. Schinazi, and J. W. Mellors. 2001. Mutations in retroviral genes associated with drug resistance, p. 191-277. In C. L. Kuiken, B. Foley, B. Hahn, P. Marx, F. McCutchan, J. W. Mellors, S. Wolinsky, and B. Korber (ed.), HIV Sequence Compendium 2001. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, N.Mex.
- 38.Perelson, A. S., P. Essunger, Y. Cao, M. Vesanen, A. Hurley, K. Saksela, M. Markowitz, and D. D. Ho. 1997. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 387**:**188-191. [DOI] [PubMed] [Google Scholar]
- 39.Persaud, D., T. Pierson, C. Ruff, D. Finzi, K. R. Chadwick, J. B. Margolick, A. Ruff, N. Hutton, S. Ray, and R. F. Siliciano. 2000. A stable latent reservoir for HIV-1 in resting CD4+ T lymphocytes in infected children. J. Clin. Investig. 105**:**995-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips, R. E., S. Rowland-Jones, D. F. Nixon, F. M. Gotch, J. P. Edwards, A. O. Oguniesi, J. G. Elvin, J. A. Rothbard, C. R. M. Bangham, C. R. Rizza, and A. J. McMichael. 1991. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature 354**:**453-459. [DOI] [PubMed] [Google Scholar]
- 41.Piatak, M., Jr., M. S. Saag, L. C. Yang, S. J. Clark, J. C. Kappes, K. C. Luk, B. H. Hahn, G. M. Shaw, and J. D. Lifson. 1993. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science 259**:**1749-1754. [DOI] [PubMed] [Google Scholar]
- 42.Pierson, T., T. L. Hoffman, J. Blankson, D. Finzi, K. Chadwich, J. B. Margolick, C. Buck, J. D. Siliciano, R. W. Doms, and R. F. Siliciano. 2000. Characterization of chemokine receptor utilization of viruses in the latent reservoir for human immunodeficiency virus type 1. J. Virol. 74**:**7824-7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pierson, T. C., Y. Zhou, T. Kieffer, C. T. Ruff, C. Buck, and R. F. Siliciano. 2002. Molecular characterization of preintegration latency in human immunodeficiency virus type 1 infection. J. Virol. 76**:**8518-8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pomerantz, R. J., T. Seshamma, and D. Trono. 1992. Efficient replication of human immunodeficiency virus type 1 requires a threshold level of Rev: potential implications for latency. J. Virol. 66**:**1809-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramratnam, B., J. E. Mittler, L. Zhang, D. Boden, A. Hurley, F. Fang, C. A. Macken, A. S. Perelson, M. Markowitz, and D. D. Ho. 2000. The decay of the latent reservoir of replication competent HIV-1 is inversely correlated with the extent of residual viral replication during prolonged antitretroviral therapy. Nat. Med. 6**:**82-85. [DOI] [PubMed] [Google Scholar]
- 46.Reinhardt, R. L., A. Khoruts, R. Merica, T. Zell, and M. K. Jenkins. 2001. Visualizing the generation of memory CD4 T cells in the whole body. Nature 410**:**101-105. [DOI] [PubMed] [Google Scholar]
- 47.Ruff, C. T., S. C. Ray, P. Kwon, R. Zinn, A. Pendleton, N. Hutton, R. Ashworth, S. Gange, T. C. Quinn, R. F. Siliciano, and D. Persaud. 2002. Persistence of wild-type virus and lack of temporal structure in the latent reservoir for human immunodeficiency virus type 1 in pediatric patients with extensive antiretroviral exposure. J. Virol. 76**:**9481-9492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283**:**857-860. [DOI] [PubMed] [Google Scholar]
- 49.Shankarappa, R., J. B. Margolick, S. J. Gange, A. G. Rodrigo, D. Upchurch, H. Farzadegan, P. Gupta, C. R. Rinaldo, G. H. Learn, X. He, X. L. Huang, and J. I. Mullins. 1999. Consistent viral evolutionary changes associated with the progression of human immunodeficiency virus type 1 infection. J. Virol. 73**:**10489-10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stevenson, M. 1997. Molecular mechanisms for the regulation of HIV replication, persistence and latency. AIDS 11(Suppl. A)**:**S25-S33. [PubMed] [Google Scholar]
- 51.Takaki, A., M. Wiese, G. Maertens, E. Depla, U. Seifert, A. Liebetrau, J. L. Miller, M. P. Manns, and B. Rehermann. 2000. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat. Med. 6**:**578-582. [DOI] [PubMed] [Google Scholar]
- 52.Thorley-Lawson, D. A. 2001. Epstein-Barr virus: exploiting the immune system. Nat. Rev. Immunol. 1**:**75-82. [DOI] [PubMed] [Google Scholar]
- 53.Tong-Starksen, S. E., P. A. Luciw, and B. M. Peterlin. 1987. Human immunodeficiency virus long terminal repeat responds to T-cell activation signals. Proc. Natl. Acad. Sci. USA 84**:**6845-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Unutmaz, D., V. N. KewalRamani, S. Marmon, and D. R. Littman. 1999. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J. Exp. Med. 189**:**1735-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wei, X., S. K. Ghosh, M. E. Taylor, V. A. Johnson, E. A. Emini, P. Deutsch, J. D. Lifson, S. Bonhoeffer, M. A. Nowak, B. H. Hahn, and G. M. Shaw. 1995. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373**:**117-122. [DOI] [PubMed] [Google Scholar]
- 56.Wong, J. K., M. Hezareh, H. F. Günthard, D. V. Havlir, C. C. Ignacio, C. A. Spina, and D. D. Richman. 1997. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278**:**1291-1295. [DOI] [PubMed] [Google Scholar]
- 57.Zhang, L., C. Chung, B.-S. Hu, T. He, Y. Guo, A. J. Kim, E. Skulsky, X. Jin, A. Hurley, B. Ramratnam, M. Markowitz, and D. D. Ho. 2000. Genetic characterization of rebounding HIV-1 after cessation of highly active antiretroviral therapy. J. Clin. Investig. 106**:**839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang, L., B. Ramratnam, K. Tenner-Racz, Y. He, M. Vesanen, S. Lewin, A. Talal, P. Racz, A. S. Perelson, B. Korber, M. Markowitz, and D. D. Ho. 1999. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N. Engl. J. Med. 340**:**1605-1613. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, Z., T. Schuler, M. Zupancic, S. Wietgrefe, K. A. Staskus, K. A. Reimann, T. A. Reinhart, M. Rogan, W. Cavert, C. J. Miller, R. S. Veazey, D. Notermans, S. Little, S. A. Danner, D. D. Richman, D. Havlir, J. Wong, H. L. Jordan, T. W. Schacker, P. Racz, K. Tenner-Racz, N. L. Letvin, S. Wolinsky, and A. T. Haase. 1999. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 286**:**1353-1357. [DOI] [PubMed] [Google Scholar]