Interactions Between Imprinting Effects in the Mouse (original) (raw)
Abstract
Mice with uniparental partial or complete disomies for any one of 11 identified chromosomes show abnormal phenotypes. The abnormalities, or imprinting effects, can be attributable to an incorrect dosage of maternal or paternal copies of imprinted gene(s) located within the regions involved. Here we show that combinations of partial disomies may result in interactions between imprinting effects that seemingly independently affect fetal and/or placental growth in different ways or modify neonatal and postnatal imprinting effects. Candidate genes within the regions have been identified. The findings are generally in accord with the “conflict hypothesis” for the evolution of genomic imprinting but do not clearly demonstrate common growth axes within which imprinted genes may interact. Instead, it would seem that any gene that represses or limits embryonic/fetal growth to the advantage of the mother—by any developmental means—will have been subject to evolutionary selection for paternal allele repression. Likewise, any gene that favors embryonic/fetal development at consequent cost to the mother—by any developmental means—will have faced selection for maternal allele repression. The classical Igf2-Igf2r axis may therefore be unique. The findings involve reinterpretation of older imprinting data and consequently revision of the mouse imprinting map.
THE discovery that certain chromosomal regions can lead to developmental abnormalities when both copies are exclusively maternally or paternally derived (Cattanach and Kirk 1985) has contributed significantly to the recognition and understanding of the phenomenon of genomic imprinting. The tools for this approach have been the uniparental whole chromosome disomies and uniparental partial chromosome disomies (conventionally called maternal and paternal duplications) that can readily be generated using Robertsonian and reciprocal chromosome translocations, respectively. Application, step by step, to the whole genome has identified 11 regions (imprinting regions) distributed over seven different chromosomes in which developmental abnormalities occur when two copies from one parent and none from the other are present. Both parental copies are therefore clearly required for normal development (Cattanach and Kirk 1985; Cattanach and Beechey 1997). Over the years the size of the imprinting regions has been progressively reduced through the use of new translocations and, with few exceptions, all of the uniparentally expressed imprinted genes have located within these reduced regions (Beechey et al. 2004).
The developmental abnormalities identified have been diverse. Described as imprinting effects, these have ranged from early embryonic to late fetal lethalities, neonatal abnormalities and inviabilities, prenatal fetal and placental growth effects, and postnatal growth retardations sometimes with other subtle defects (Cattanach and Beechey 1997). A general feature of all these effects has been their lack of variation, irrespective of genetic background or translocation used. However, there have been clear exceptions. Here, we reevaluate these exceptions and, together with new data, show that the cause is the co-occurrence of imprinting effects attributable to both chromosomes involved in the translocations used. This poses questions of interest in relation to Moore and Haig's (1991) “conflict hypothesis” for the role of imprinting. According to the hypothesis, maternally expressed genes favor the well-being of the mother over the fetus, while paternally expressed genes favor the development of the fetus at the expense of the mother, providing a reason why both paternal and maternal alleles are required. In the situations to be described, two maternally expressed alleles at one imprinted gene locus are set against two paternally expressed alleles at another. The question is then whether this will cause further problems in development or reestablish a balance. The combination of maternal duplication for one region with paternal duplication for another therefore offers the possibility of ascertaining whether the genes involved operate on the same or different growth axes, as illustrated for the paternally expressed _Igf2_-maternally expressed Igf2r interaction (Filson et al. 1993). Interpretation is difficult because it is often uncertain which genes are involved and how they affect development. The findings suggest that genomic imprinting is a highly complex process involving numbers of different growth axes that operate differently in different tissues and at different times in development. The “conflict hypothesis” provides a basis for many of these imprinting effects, the multiplicity suggesting a continuity in the evolutionary adaptation of imprinting as a means of favoring fetal growth by paternal genes and its negative control by maternal genes. The reinterpretations of the imprinting regions and effects require changes to the mouse imprinting map (Beechey et al. 2004).
This article describes interactions involving proximal chromosome 2 (Chr 2) with proximal Chr 11, proximal Chr 7 with central Chr 7, proximal Chr 7 with proximal Chr 11, and distal Chr 2 with distal Chr 9.
MATERIALS AND METHODS
Mouse breeding:
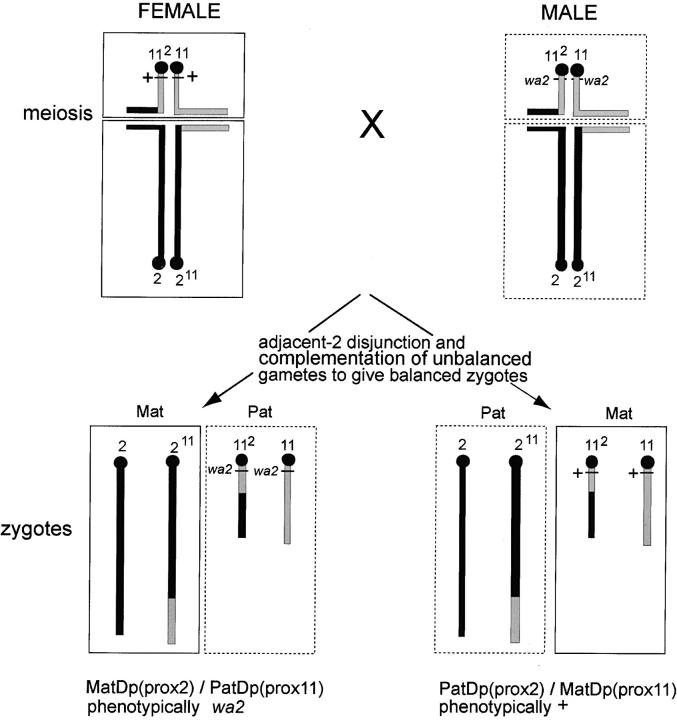
The imprinting effects studied were investigated in mice with maternal and paternal duplication (MatDp and PatDp; see standard nomenclature rules, Mouse Genome Database 2004) for selected regions of chromosomes and in the case of chromosome 11 (Chr 11) also with uniparental whole chromosome disomies (MatDi and PatDi). The duplication classes were generated by the standard genetic method of intercrossing genetically marked and unmarked translocation heterozygotes and identifying the MatDp and PatDp classes with the aid of the markers (Cattanach and Beechey 1997). A vital consideration in the present studies is that with reciprocal translocations two chromosomes are of necessity always involved and therefore, in creating animals with, for example, MatDp for a region of one chromosome, these animals also have PatDp for the corresponding region for the other (Figure 1).
Figure 1.—
System for generating uniparental partial disomies (MatDp and PatDp) using reciprocal translocations. In the example shown the translocation is T(2;11)30H, involving Chrs 2 and 11, and the screen is for MatDp and PatDp for the regions proximal to the breakpoints in the two chromosomes. The region of Chr 11 proximal to the breakpoint in the male parent is marked with wa2. Complementary adjacent-2 disjunction at meiosis (homologous centromeres going to the same pole) in both parents can generate chromosomally balanced offspring showing the recessive wa2 phenotype. These will be genetically PatDp for the proximal region of Chr 11 and, simultaneously, MatDp for the proximal region of Chr 2, MatDp(prox2)/PatDp(prox11). Unmarked PatDp(prox2)/MatDp(prox11) young will also be produced. In the reciprocal cross (not shown), with wa2 on the maternal Chr 11, the wa2 marker will identify the PatDp(prox2)/MatDp(prox11) class. Any marker gene located proximally on either chromosome can be used. With adjacent-1 disjunction (homologous centromeres going to opposite poles) in both parents, offspring with MatDp(dist2)/PatDp(dist11) and PatDp(dist2)/MatDp(dist11) can be similarly generated. These can be recognized in reciprocal crosses with the aid of markers that are located distal to the breakpoints on one or the other chromosome.
Translocation and marker gene stocks, with one exception, are maintained on a mixed C3H/HeH-101/H genetic background. The exception comprised the marker gene stock for distal Chr 2, which was maintained on a mixed C57BL/6J-JU/Ct-+c +a genetic background. The translocations used are identified according to standard nomenclature rules such that the chromosomes involved are identified in brackets and the laboratory of origin specified in the symbol following. Thus, T(2;11)30H indicates that this translocation involves Chrs 2 and 11 and that this was the thirtieth translocation named by Harwell. Throughout the text, these symbols are reduced wherever possible to T30H, etc. The Robertsonian translocation used to generate uniparental Chr 11 disomies was Rb(11.13)4Bnr, or Rb4Bnr. The marker genes used in the various crosses are specified in the figure legends. The Oed-Sml mutation (Cattanach et al. 2000) used in the distal Chr 2 studies was also maintained on the mixed C3H/HeH-101/H background.
All animal studies were carried out under the guidance of the Medical Research Council in “Responsibility in the Use of Animals for Medical Research” (July 1993) and Home Office Project license nos. 30/1518 and 30/2065.
FISH mapping of Gatm on Chr 2:
Mitotic chromosome spreads from the bone marrow of a T(2;9)11H heterozygote were prepared by standard methods. These were used for FISH with bacterial artificial chromosomes RP23-101F14 and RP-23-99H13 (UK MRC Mouse Genome Sequencing Program), each of which contains the Gatm gene, by a modified method of Buckle and Rack (1993). Hybridization signals were detected using avidin-FITC biotinylated antiavidin (Vector, Burlingame, CA). Images were captured using a fluorescent microscope with SmartCapture 2 software (Digital Scientific, UK) and merged with captured images of the same cells in which chromosomes were DAPI stained. In all cells the DAPI staining was reversed and enhanced by the software to produce reversed DAPI banding (or pseudo-G-bands) that allowed precise location of the signal on the mouse ideogram.
Mapping and PCR expression studies with Rasgrf1:
Total RNA was isolated from the cerebellum from two sets of adult MatDp(dist9) mice generated with T(2;9)11H and T(4;9)45H and also from wild-type siblings. RNA samples were prepared using RNAzol B (Biotechnology Europe) and 1 μg of total RNA per sample was reverse transcribed with M-MLV reverse transcriptase (Life Technologies) using oligo (dT)15 (Promega, Madison, WI). Rasgrf1 cDNA was amplified using forward primer CDCFO1 and reverse primer CDCRO2, as described by Plass et al. (1996). As Rasgrf1 is expressed predominantly from the paternal allele in cerebellum, little or no expression would be expected in the MatDp(dist2) samples if the gene lies distal to the breakpoints. If the gene lies proximal to the breakpoints, expression would be detected.
RESULTS
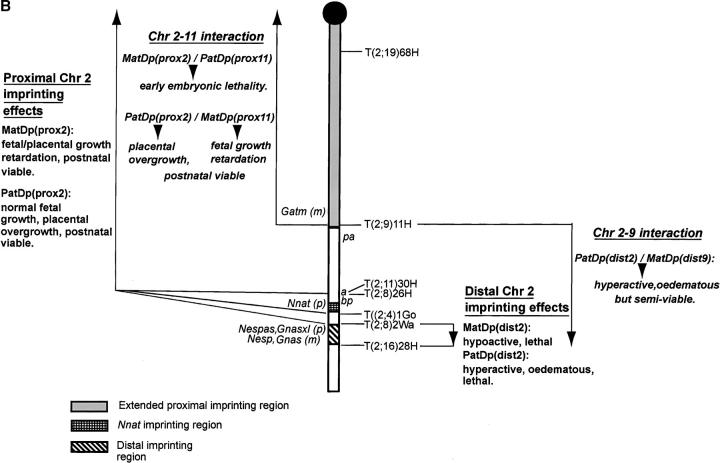
Proximal Chr 2-Proximal Chr 11 interaction
Background:
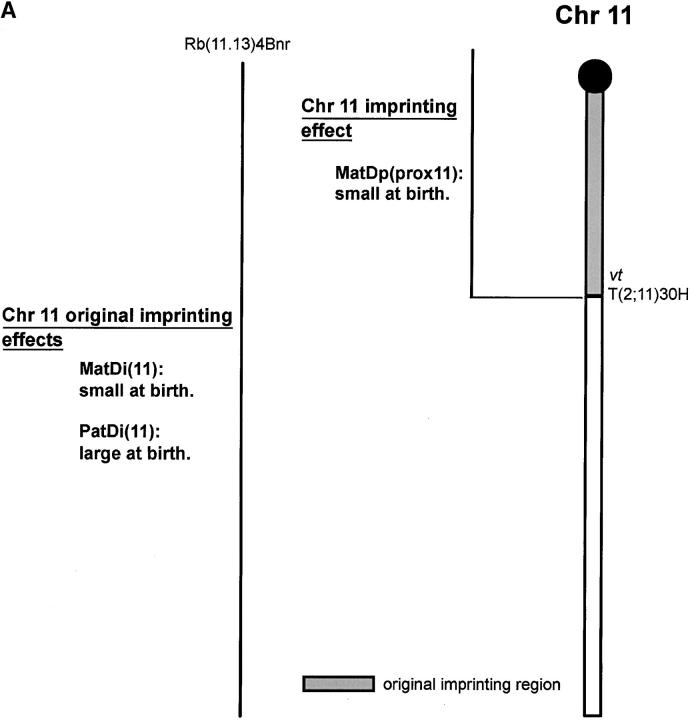
Mice with two maternal copies of Chr 11, MatDi(11), and no paternal copies show prenatal growth retardation and have small placentas. Although small at birth, they are generally fully viable, and postnatal growth is normal such that they remain small through to adulthood. The reciprocal genotype, PatDi(11), shows enhanced fetal and placental growth. They are large at birth and fully viable through to adulthood when they are still seen as oversized (Figure 2A; Cattanach and Kirk 1985; Cattanach et al. 1996).
Figure 2.—
Imprinting maps of Chr 11. (A) Original map identifying the proximal location of the imprinting region and showing the imprinting effects detected. The Chr 11 gene vt identified the disomies and the MatDp class generated with Rb(11.13)4Bnr and T30H, respectively. (B) New map in which the imprinting region has been reduced, first with T57H using the Chr 11 gene, wa2, and then with T41Ad, screening distally using the Chr 11 gene, vt. The imprinted Grb10 and U2af1-rs1 loci locate within the smaller region. The imprinting effect seen with T30H is now shown as a Chr 2–Chr 11 interaction (indicated by italics). The Chr 7–Chr 11 interaction detected with T40Ad and using wa2 as the marker is also indicated by italics. m, maternally expressed imprinted gene; p, paternally expressed imprinted gene.
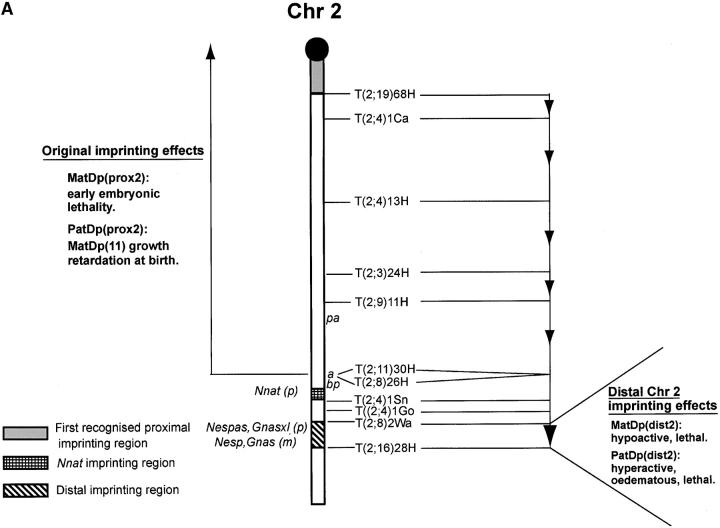
The MatDi(11) growth retardation is also found with two maternal copies of the region proximal to the T(2;11)30H breakpoint in Chr 11, MatDp(prox11)/PatDp(prox2), thereby identifying the region of Chr 11 where the gene(s) responsible for the growth effects must lie. However, no progeny representing the PatDp(prox11)/MatDp(prox2) class, which was expected to show the Chr 11-enhanced prenatal growth effect, were found in the reciprocal T30H cross. Further studies indeed indicated that the class could not even be detected prenatally, suggesting that maternal Chr 2 proximal to the T30H breakpoint is also subject to imprinting. On this basis MatDp(prox2) appeared to cause an early embryonic lethality (Cattanach and Kirk 1985). With the use of a series of Chr 2 translocations with yet more proximal breakpoints (Figure 3A) and screening for known distal Chr 2 imprinting effects (to be described later) at birth, the size of the proximal region was progressively reduced (Cattanach 1986). The region responsible for the MatDp(prox2) imprinting lethality was finally defined as that lying above the most proximal translocation (T68H) breakpoint and thus very close to the centromere (Figure 3A). No imprinted genes have been found in this region.
Figure 3.—
Imprinting maps of Chr 2. (A) Original map showing the three imprinting regions: the proximal one lying close to the centromere; the distal one lying between T2Wa and T28H; and the Nnat region, without visible phenotype, above it. The proximal imprinting effect was initially observed with T30H and the region reduced step by step using the translocations shown and with recovery of the MatDp(dist2) effect indicating absence of the lethal proximal imprinting effect. The distal imprinting effects are recognized with all translocations other than T28H. The PatDp(dist2) effect is lethal with all translocations other than T11H. The marker genes used are as shown or the distal imprinting effect itself was used as a marker. (B) New map in which the proximal region now extends distally to T11H and encompasses the imprinted Gatm locus. Both the Chr 2–Chr 11 and Chr 2–Chr 9 interactions are indicated by italics. The marker genes used are as shown. m, maternally expressed imprinted genes; p, paternally expressed imprinted genes.
New data:
Chr 11:
New work on Chr 11 first with T(5;11)57H and then with T(11;13)41Ad, in neither of which is the second component chromosome (Chrs 5 or 13) imprinted, has reduced the imprinting region to a segment close to the centromere (Cattanach et al. 1998; Figure 2B). Significantly, this smaller region encompasses the loci of the two known Chr 11 imprinted genes, Gbr10 and U2af1-rsl (Monk et al. 2003). The latter has not been found to affect growth (Sunahara et al. 2000) but a Gbr10 knockout has been found to cause a fetal and placental overgrowth when maternally transmitted (Charalambous et al. 2003). As a maternally expressed growth suppressor, Gbr10 is the likely candidate gene for the imprinting effects, two expressed maternal copies suppressing growth with MatDp(prox11) and an absence of an expressed copy resulting in overgrowth with PatDp(prox11).
Chr 2:
With Chr 2, new work with three different translocations, T(2;8)26H, T(2;4)1Go, and T(2;8)2Wa, the breakpoints of which all lie close to that of T30H (Figure 3B), have provided reason to reinterpret the conclusions on the proximal imprinting effect and the location of the region. In none of these new translocations is the second chromosome involved (Chrs 4 or 8) imprinted, and none generated the MatDp(prox2) early embryonic imprinting lethality detected with T(2;11)30H (Table 1). Instead, a different MatDp(prox2) imprinting effect was found, comprising a fetal/placental growth retardation (Table 2; Figure 3B). The class was also detectable at birth by growth retardation and was found to be fully viable through to adulthood.
TABLE 1.
In utero and birth recovery of MapDp(prox2) and Patdp(prox2) classes generated with different Chr 2 translocations
| MatDp(prox2) | PatDp(prox2) | |||
|---|---|---|---|---|
| Translocation | In utero | At birth | In utero | At birth |
| T(2;11)30H | 0/473 (0%) | 0/714 (0%) | 1/63 (1.6%) | 8/714 (1.1%) |
| T(2;8)2Wa | 13/742 (1.7%) | 1/178 (0.6%) | 3/210 (1.4%) | 1/157 (0.6%) |
| T(2;8)26H | 3/476 (0.6%) | 13/2012 (0.6%) | 33/365 (0.8%) | 17/1542 (1.1%) |
| T(2;6)1Go | 1/70 (1.4%) | 3/716 (0.4%) | 4/292 (1.4%) | 8/340 (2.3%) |
TABLE 2.
Effects of MatDp(prox2) and PatDp(prox2) generated with different translocations on fetal and placental weights
| Genotype | Translocation | Ratios of weights ofembryos to thoseof normal sibs | Ratios of weights ofplacentas to thoseof normal sibs |
|---|---|---|---|
| MatDp | T2Wa | 0.762 ± 0.026 | 0.626 ± 0.059 |
| T26H | 0.882 ± 0.057 | 0.702 ± 0.122 | |
| T1Go | 0.711 ± 0.079 | 0.704 ± 0.217 | |
| All | 0.782 ± 0.023 | 0.647 ± 0.048 | |
| PatDp | T2Wa | 0.997 ± 0.124 | 1.449 ± 0.501 |
| T26H | 0.992 ± 0.143 | 1.665 ± 0.664 | |
| T1Go | 1.076 ± 0.076 | 1.614 ± 0.297 | |
| All | 1.046 ± 0.059 | 1.586 ± 0.234 |
Reinvestigations with T30H:
To establish more firmly that MatDp(prox2) results in an early lethal imprinting effect when generated with the T30H translocation, a repeat prenatal screen was initiated. Among 269 embryos checked, none represented the MatDp(prox2) class. The zero incidence from the total T30H data differs significantly from the combined MatDp(prox2) frequency obtained with the T26H, T1Go, and T2Wa translocations both prenatally and at birth (Table 1), verifying that two distinct MatDp(prox2) imprinting phenotypes appear to be produced.
All the translocation crosses were carried out on the same genetic background. Genetic background effects cannot therefore be responsible for the differing imprinting effects. None of the new translocations involve Chr 11, however. Therefore, a more feasible explanation of the findings is that the fetal/placental growth retardation with normal postnatal viability characterizes the MatDp(prox2) imprinting effect and that the early embryonic lethality generated only with T30H derives from an interaction between the MatDp(prox2) and PatDp(prox11) imprinting effects.
PatDp(prox2) imprinting phenotype:
In addition to the MatDp(prox2) findings, the work with the three new translocations also identified for the first time an imprinting effect associated with the reciprocal PatDp(prox2) class. Fetal growth was not affected but there was a marked overgrowth of the placenta (Table 2; Figure 3B), thereby breaking the normal relationship between fetal and placental size. The placental effect has since also been seen in limited studies with T30H. Thus, among 63 fetuses screened, 2 PatDp(prox2)/MatDp(prox11) showed the expected growth retardation attributable to MatDp(prox11) imprinting (mean 1.23 g cf. 1.72 g for their normal sibs; 71%) and the PatDp(prox2) overgrowth of their placentas relative to those of their normal sibs (0.158 g cf. 0.094 g; 162%). Thus, the combination of Chr 2 and 11 imprinting effects enhances the fetal-placental growth discordance, with the PatDp(prox2) overgrowth in the placenta overriding the MatDp(prox11) placental growth reduction. Fetal size was clearly not influenced by the larger placental size.
Changes in the proximal Chr 2 imprinting region and candidate gene:
The new interpretation of the data invalidates the early refinements of the MatDp(prox2) imprinting region. The small size at birth would not readily be noted in the presence of the abnormal phenotype attributable to a MatDp(dist2) imprinting effect (to be described). As a consequence, the proximal region could be enlarged to comprise the majority of the chromosome, extending from the centromere down to the distally located T26H breakpoint (Figure 3B). However, newer prenatal studies using T11H have shown that MatDp and PatDp for the region distal to the breakpoint express only the distal Chr 2 imprinting phenotypes and not the small embryo/placenta and large placenta effects. The region, although still large, is therefore likely to lie proximal to T11H (Figure 3B). It is therefore of significance that the recently discovered imprinted gene, Gatm, which is maternally expressed in mouse placenta and postulated to be a growth suppressor (Sandell et al. 2003), maps close to the T11H breakpoint and therefore potentially within this newly defined proximal Chr 2 imprinting region. This location has been confirmed by FISH analysis with BACs in which all mitoses showed hybridization with Gatm on both the normal Chr 2 and the large rearranged Chr 29 (data not shown). Gatm is therefore the candidate gene for the placental growth effects, two expressed copies suppressing placental growth with MatDp (prox2) and an absence of an expressed copy resulting in the placental overgrowth with PatDp(prox2). An interaction between Gatm (Chr 2) and Grb10 (Chr 11) effects may account for the Chr 2-Chr 11 interaction seen with T30H.
Conclusions:
- The imprinting effect found with MatDp for the proximal region of Chr 2 no longer comprises an early embryonic lethality but rather a fetal and placental growth retardation.
- A new imprinting effect, comprising a placental overgrowth, has been found associated with PatDp for the region.
- The region now comprises a large part of Chr 2 extending down to the T11H breakpoint.
- The lethality observed with MatDp(prox2)/PatDp(prox11) using the T30H translocation can be deduced to result from an interaction between the proximal Chr 2 and proximal Chr 11 imprinting effects.
- The imprinted gene, Gatm, which is maternally expressed in the placenta, lies within this extended region and becomes the candidate gene for the placental imprinting growth effects.
- Gbr10 is the candidate locus for the proximal Chr 11 imprinting effects.
- The interaction between the Chr 2 and Chr 11 imprinting effects may therefore involve the Gatm and Gbr10 imprinted gene loci.
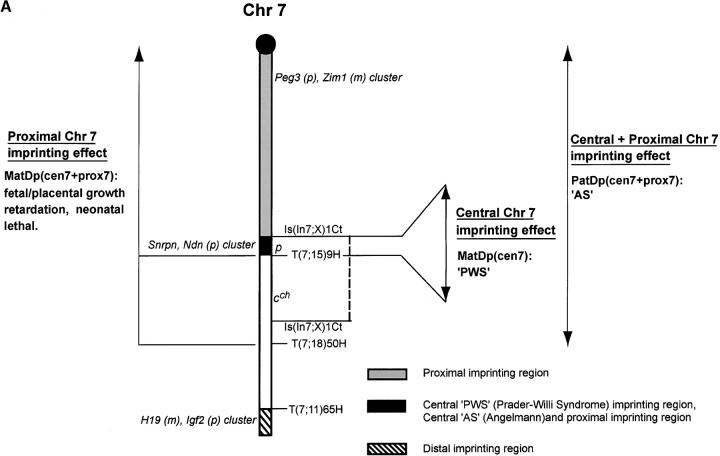
Proximal Chr 7-Central Chr 7 interaction
Background:
The imprinting of Chr 7 (Figure 4A) is complex insofar as there are at least three regions that are subject to imprinting: (a) a proximal region (Searle and Beechey 1990), (b) a central region (Cattanach et al. 1992), and (c) the well-studied distal region encompassing the H19-Igf2 imprinted gene cluster (Searle and Beechey 1990; Beechey et al. 2004).
Figure 4.—
Imprinting maps of Chr 7. (A) Earlier map showing the three imprinting regions: the proximal region encompassing the Peg3, Zim1, and Usp29 imprinted gene cluster, the adjacent central PWS/AS region, and the distal region encompassing the H19-Igf2 imprinted gene cluster. (B) The reduced proximal region centromeric to T40Ad, still encompassing the imprinted gene cluster, with its viable MatDp growth retardation imprinting effect. The central-proximal interaction effect seen with MatDp(cen7+prox7) is specified by italics. The PatDp(cen7) AS region is reduced in size. The Chr 7 marker genes are as shown but the new proximal region was identified using the wa2 Chr 11 marker. m, maternally expressed imprinted genes; p, paternally expressed imprinted genes.
Mice with two maternal copies and no paternal copies of the regions proximal to the T(7;18)50H and T(7;15)9H breakpoints on Chr 7, MatDp(prox7), show a fetal and placental growth retardation with neonatal lethality (Searle and Beechey 1990; Figure 4A). With the Is(7;X)Ct insertional translocation, which allows a central region of the chromosome to be studied separately (Cattanach et al. 1992), a distinctly different postnatal lethality occurs with MatDp for the small region that lies between the T9H breakpoint and the more proximal IsCt breakpoint (Figure 4A). As this region is homologous to the Prader-Willi/Angelman syndrome (PWS/AS) imprinting region of human Chr 15, the postnatal effect has been taken as a mouse model of PWS (Cattanach et al. 1992). Pertinent to the present study, however, is the expectation that the growth retardation/lethality seen with the T9H and T50H translocations (Searle and Beechey 1990) could be a combined phenotype derived from proximal and central Chr 7 imprinting effects.
In addition to the MatDp findings, PatDp for the region proximal to the T9H breakpoint generated a mouse model of Angelman syndrome (Cattanach et al. 1997; Figure 4A), the phenotype including abnormal brain wave patterns similar to those of AS patients. For technical reasons (B. M. Cattanach, unpublished results), it has proven impossible to investigate PatDp for the central region using the IsCt insertional translocation.
New data:
Refinement of the proximal imprinting region:
Studies using two further translocations, T(7;13)43Ad and T(5;7)30Ad, which have breakpoints proximal to the PWS/AS homologous region, have shown that MatDp for the regions proximal to both breakpoints (Figure 4B) does indeed exhibit a different imprinting effect from that seen with T(7;15)9H and T(7;18)50H. In none of these translocations is the second chromosome involved subject to imprinting. Only Chr 7 therefore needs to be considered. The new imprinting phenotype comprised a prenatal growth retardation effect, as seen with T9H and T50H, but without the neonatal lethal component. Thus, of 37 MatDp(prox7) mice generated with T40Ad and detected at birth, all but 2 survived to adulthood. This seeming discord supports the contention that the T9H and T50H neonatal lethality derives from the cumulative effects of the MatDp(prox7) Chr 7 fetal growth retardation, seen with T43Ad and T30Ad, and the MatDp(cen7) PWS early postnatal lethality.
Candidate genes:
Clusters of imprinted genes have been identified for both regions. Peg3, which maps within the proximal cluster close to the centromere (Monk et al. 2003), is the likely candidate for the growth-promoting effect as the gene is expressed in the embryo and placenta and the paternally inherited knockout shows a fetal growth retardation (Li et al. 1999). The closely associated Zim1 and Usp29 are other possible candidate genes (Kim et al. 1999; Li et al. 1999) but are not known to affect fetal growth. On the basis of work on the knockout, Ndn is the likely candidate for PWS in the mouse and elements of the disorder in humans (Ren et al. 2003).
Absence of a PatDp(prox7) imprinting effect:
The T43Ad and T30Ad studies also showed that PatDp for the regions proximal to their breakpoints was not associated with an imprinting effect and notably no component of the recognized AS-type imprinting effect (Cattanach et al. 1997). This, therefore, further refines the position of the central AS imprinting region on the paternal chromosome to the small region between the T30Ad and T9H breakpoints. The human PWS/AS homologous region lies in this segment of mouse Chr 7 (Figure 4B).
Conclusions:
- The proximal Chr 7 imprinting effect found with MatDp now consists of a fetal and placental growth retardation with full viability.
- The region is restricted to the segment proximal to the T43Ad breakpoint.
- The lethal imprinting effect found with MatDp for the larger T9H-centromere region can be attributed to the combination of the proximal and central imprinting effects.
- Peg3 is the candidate gene for the imprinting effect of proximal growth retardation. Ndn may be the candidate gene for PWS in the mouse.
- PatDp for the region proximal to the T30Ad breakpoint does not give an imprinting effect. It can be concluded that, as expected, the mouse AS effect is attributable to the more distal region between the T30Ad and T9H breakpoints, which encompasses the PWS/AS homologous region.
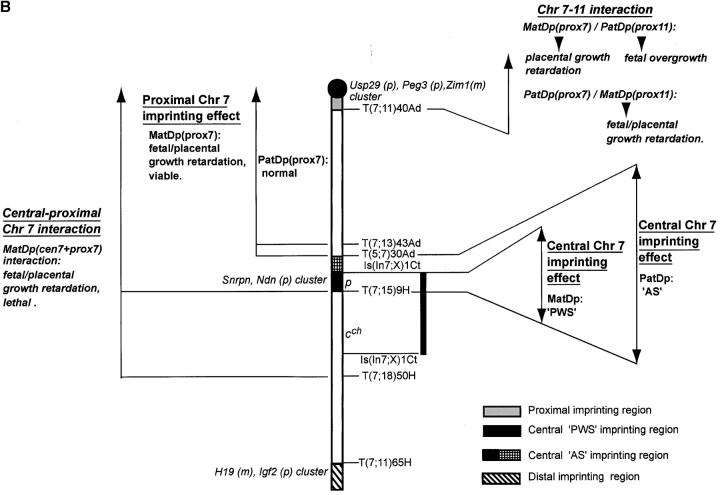
Proximal Chr 7-Proximal Chr 11 interaction
Background:
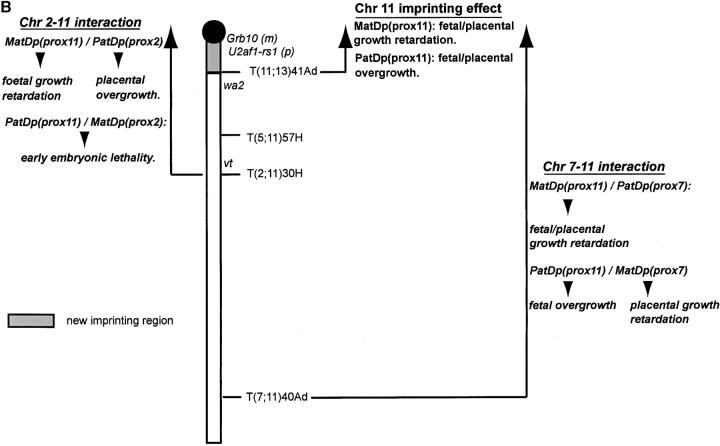
As described above, the proximal Chr 11 imprinting effects comprise a fetal and placental growth retardation with MatDp for the region proximal to the T41Ad breakpoint and a fetal and placental overgrowth with PatDp for the region (Figure 2B). A proximal Chr 7 imprinting effect is found with MatDp for the region proximal to the T43Ad breakpoint (Figure 4B), comprising a fetal and placental growth retardation (see previous section).
New data:
T(7;11)40Ad:
Evidence of an interaction between the proximal Chr 7 and 11 imprinting effects was made with a new translocation, T(7;11)40Ad. The initial objective of this study was to distinguish whether the proximal Chr 7 imprinting effect was attributable to regions proximal or distal to the translocation breakpoint, which lies very close to the centromere (Figure 4B). The T40Ad breakpoint in Chr 11 is located fairly distally (Figure 2B) such that studies upon the region proximal to the breakpoint clearly include the proximal Chr 11 imprinting region.
MatDp(prox7)-PatDp(prox11) interaction:
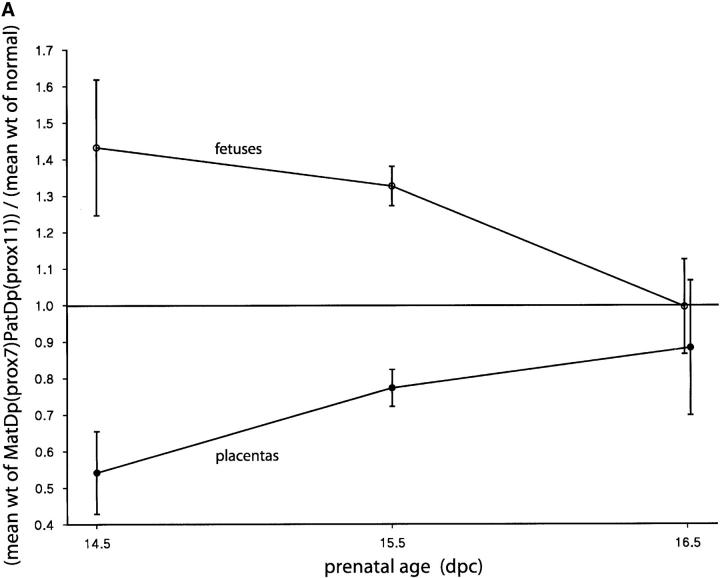
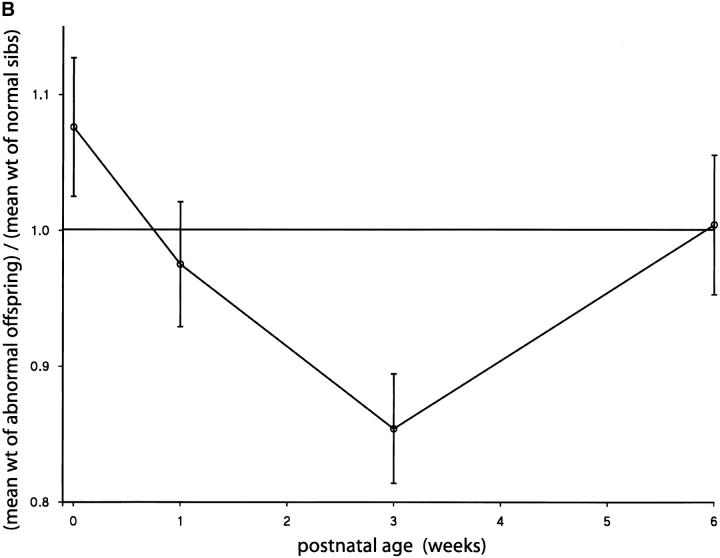
MatDp(prox7)/PatDp(prox11) fetuses generated with the T40Ad translocation were found to be significantly larger than their normal sibs (Figure 5A) at 14.5 and 15.5 days, thus exhibiting the expected PatDp(prox11) imprinting effect on fetal growth (Cattanach and Kirk 1985). By contrast, placental sizes were significantly reduced (Figure 5A), apparently showing the MatDp(prox7) imprinting effect in this tissue. Both effects were transitory as at a later age both fetal size and placental size approached normality. The postnatal growth of these mice at 3 weeks of age appeared to be reduced, but normal size was attained by adulthood (Figure 5B). As previously discussed, Peg3 may be regarded as candidate gene for the proximal Chr 7 growth retardation with Grb10 remaining the candidate gene for the Chr 11 growth effect. An interaction between the Peg3 and Grb10 imprinting effects might therefore be indicated.
Figure 5.—
Fetal and placental growth anomalies attributable to an interaction between MatDp(prox7) and PatDp(prox11) imprinting effects; ratios relative to normal sibs. (A) Fetal weights were significantly larger than those of normal sibs, averaged over all litters, at 14.5 and 15.5 days (P = 0.010 and <0.000001, respectively) but not at 16.5 days (P = 0.974). Placental weights were significantly smaller than those of normal sibs, averaged over all litters, at 14.5 and 15.5 days (P = 0.0098 and 0.0012, respectively) but not at 16.5 days (P = 0.56). (B) Postnatal weights were not significantly different from those of normal sibs at birth, 1 week, and 6 weeks (P = 0.313, 0.60, and 0.93, respectively) but were significantly different at 3 weeks (P = 0.0019). Overall, there was no significant difference between weight ratios at all ages (P = 0.012).
MatDp(prox11)/PatDp(prox7):
Without a PatDp(prox7) imprinting effect for the region proximal to the T43Ad breakpoint (Figure 4B), the MatDp(prox11)/PatDp(prox7) combination presented only the expected small MatDp(prox11) fetal/placental imprinting effect (Cattanach and Kirk 1985; Figure 3B). The growth retardation of this class was evident at birth, as usual, and maintained through to adulthood (data not shown).
Conclusions:
- On the basis of the placental effect, the proximal Chr 7 imprinting region can now be reduced to a small region very close to the centromere.
- The PatDp(prox11) imprinting effect potentially attributable to Grb10 appears to operate primarily upon early fetal development, as previously suggested (Cattanach et al. 1992).
- The MatDp(prox7) imprinting effect appears to operate primarily upon the placenta, as might be expected if Peg3 is the responsible gene.
- Both the fetal and placental effects appeared transitory.
- The interactions between the proximal Chr 7 and proximal Chr 11 imprinting effects may be attributable to the Grb10 and Peg3 imprinted gene loci.
Distal Chr 2-Distal Chr 9 interaction
Background:
The distal Chr 2 imprinting region first detected in the T30H studies (Cattanach and Kirk 1985) yields two distinct imprinting effects. MatDp(dist2) newborns are seen as flat-sided, hump-backed, and hypoactive. They fail to suckle and die within a few hours of birth. The reciprocal PatDp class typically exhibit short, square bodies, edema, paddle feet, kinked tails, hyperactive behavior, and, as demonstrated by all but one of a series of Chr 2 translocations (Cattanach 1986), invariably dies within a few days of birth. Through the use of these Chr 2 translocations the imprinting region was reduced to the region lying between the T2Wa and T28H breakpoints (Figure 3A), limiting its size to ∼7 Mb (E. P. Evans, personal communication). This encompasses the Gnas cluster (Williamson et al. 1996), and a new nonfunctional mutation detected in Gnas (Cattanach et al. 2000; Skinner et al. 2002; Williamson et al. 2004) has been found to produce an edematous phenotype, that, when maternally inherited, closely resembles that of the PatDp class, although without the behavioral effect. As such, it may be concluded that the absence of maternally expressed Gnas is largely responsible for the PatDp phenotype.
The one exceptional PatDp class lacks the behavioral effect and is semiviable rather than lethal (Cattanach 1986). It is found when generated with the T(2;9)11H translocation when >50% survive to adulthood. No evidence has been found that would suggest that genetic background differences are responsible (Cattanach 1991a,b) and as the size of the duplicated region is larger rather than smaller than that generated with most of the other Chr 2 translocations (Figure 3B), it is unlikely that some other gene on this chromosome is responsible. The second chromosome involved in the T11H translocation, Chr 9, was initially not recognized as being imprinted and potentially involved.
New data:
Evidence that distal Chr 9 is imprinted:
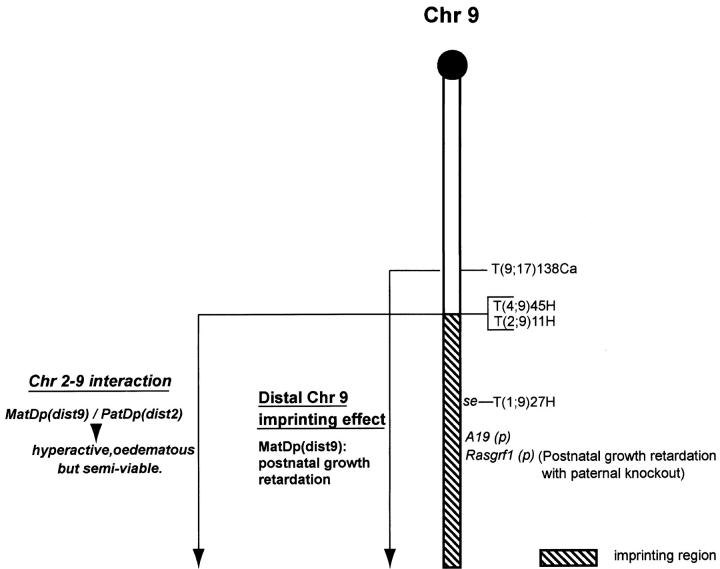
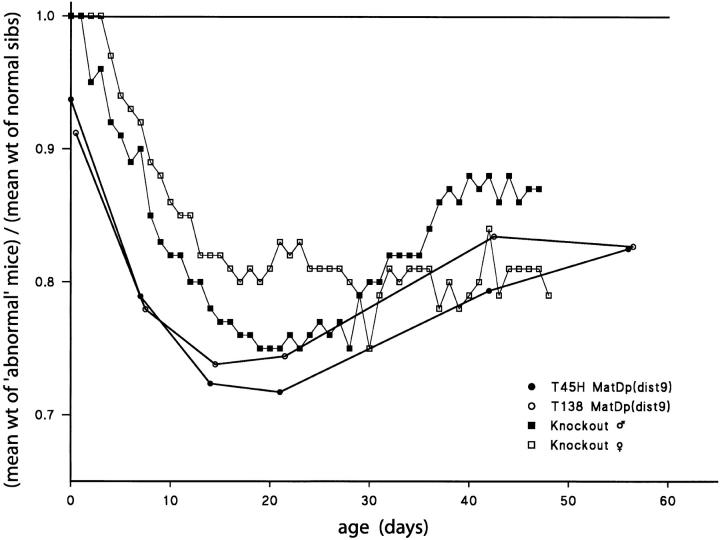
The translocation evidence on Chr 9 imprinting (Figure 6) has been ambiguous. A postnatal growth retardation was initially found in MatDp(dist9) mice generated with T(9;17)138Ca but this was not evident with another Chr 9 translocation, T(1;9)27H (Cattanach and Beechey 1994), suggesting that Chr 17 [PatDp(dist17)] was responsible for the growth effect. This conclusion proved erroneous, however, because the growth effect was subsequently found with a third Chr 9 translocation, T(4;9)45H (Figure 7), suggesting that the Chr 9 genotype, MatDp(dist9), was in fact responsible. Consistent with this conclusion is the finding that the growth curves of the T138Ca and T45H MatDp(dist9) classes accord well (Figure 7) with that of a paternally inherited knockout of the imprinted paternally expressed Chr 9 gene, Rasgrf1 (Itier et al. 1998), and also with that of a paternally inherited mutation at the locus (Clapcott et al. 2003). Rasgrf1 is paternally expressed in the neonatal and adult brain (Plass et al. 1996). Therefore, to confirm that the gene lies distally on the chromosome (Pearsall et al. 1998) and could be responsible for the MatDp(dist9) growth retardations, PCR expression studies were conducted on cerebellum samples from MatDp(dist9) mice generated with T11H and T45H (Figure 6). No evidence of paternal allele expression was found, whereas expression was found in the controls (data not shown). The lack of Rasgrf1 expression in MatDp(dist9) mice is therefore likely to be the cause of the T138Ca and T45H MatDp growth retardations (Figure 7). This raises the possibility that the loss of the behavioral effect and increased viability of the PatDp(dist2) class generated with the T(2;9)11H translocation is attributable to an interaction between the distal Chr 2 (Gnas complex) and distal Chr 9 (Rasgrf1) imprinting effects. There is another Chr 9 imprinted gene, A19, which maps close to Rasgrf (de la Puente et al. 2002). However, as it is a noncoding gene, it is unlikely to be involved in any interaction with the Chr 2 imprinting effect.
Figure 6.—
Imprinting map of Chr 9. New map showing the location of the imprinting region distal to the T45H breakpoint and the Chr 2–Chr 9 interaction (in italics) obtained using the T11H translocation. The Chr 2 marker gene, bp, was used in the T11H studies; the Chr 9 genes, d and ln, in the T138Ca studies; the Chr 4 gene, b, in the T45H studies; and the Chr 1 gene, ln, in the T27H studies. The latter, for a reason as yet unknown, did not distinguish the distal Chr 9 imprinting effect. The T11H breakpoint has been reassigned to a position close to that of T45H. Previously, it had been located more proximally on the chromosome.
Figure 7.—
Postnatal growth curves of MatDp(dist9) mice generated with T45H and T138Ca and those with male and female paternally inherited Rasgrf1 knockouts; ratios relative to normal sibs. Knockout data are from Itier et al. (1998).
Evidence of aRasgrf1 -PatDp(dist2) interaction:
The above T11H PCR expression studies confirmed that Rasgrf1 is not expressed in T11H MatDp(dist9)/PatDp(dist2) mice. However, to ascertain if it is the absence of Rasgrf1 that is responsible for the modified behavior and viability, MatDp(dist4)/PatDp(dist2) mice generated with T26H were investigated in crosses in which a null mutation of Rasgrf1 (Clapcott et al. 2003) was segregating. Those that did not inherit the mutation from the father showed evidence of the typical PatDp(dist2) hyperactive behavior from birth and none survived for more than 4 days (n = 14). However, 14 of 27 that carried the paternal Rasgrf1 mutation, and which therefore did not express the gene, showed little sign of the behavioral effect and survived for at least 10 days. The difference is statistically significant (P = 0.00057). Furthermore, uniquely for T26H-generated PatDp(dist2) animals, some survived to adulthood. An additional finding was that, at birth, the incidence of the PatDp(dist2) class carrying the Rasgrf1 mutation was twice that of those that did not, indicating that PatDp(dist2) prenatal viability is also enhanced in the absence of Rasgrf1. The segregation of the Rasgrf1 mutation was normal among 10 normal sibs studied and Rasgrf1 segregates normally in other crosses (Clapcott et al. 2003). Finally, to determine if an absence of Rasgrf1 also modified the phenotype of the maternally inherited Oed-Sml mutation at the Gnas locus (Cattanach et al. 2000), the same test system was applied. Mice showing the maternally inherited Oed phenotype do not show a behavioral effect like PatDp(dist2), but nevertheless few survive more than a few days. The absence of Rasgrf1 expression was not found to modify the viability or any other component of the phenotype (data not shown).
Conclusions:
- MatDp for the regions distal to the T138Ca and T45H breakpoints on Chr 9 results in postnatal growth retardations similar to those found with paternal inheritance of a knockout and mutation of the paternally expressed imprinted gene, Rasgrf1.
- The Rasgrf1 locus lies distal to the translocation breakpoints on Chr 9 and on the basis of the knockout and mutation phenotypes is likely to be the gene responsible for the MatDp(dist9) growth retardation.
- The similarities between the PatDp(dist2) phenotype and that of the maternally inherited Gnas mutation (Oed-Sml) imply that the Gnas locus is responsible for the edematous component of the former phenotype.
- The absence of a behavioral effect and the enhanced postnatal viability of T11H PatDp(dist2)/MatDp(dist9) animals appears to derive from an interaction between Chr 2 (Gnas) and Chr 9 (Rasgrf1) imprinting effects.
- Specifically, the modification can be attributed to the absence of Rasgrf1 expression in these animals.
DISCUSSION
Changes in the mouse imprinting map:
The data presented modify the imprinting map of the mouse as last formally published (Cattanach and Beechey 1994) and as currently reported on the Harwell website (Beechey et al. 2004). Thus, (1) the proximal Chr 2 region is extended down to the T11H breakpoint (Figure 3B), (2) the proximal Chr 7 region is reduced to the centromeric region (Figure 4B), (3) the proximal boundary of the central region on the paternal Chr 7 has been brought closer (Figure 4B) to the recognized PWS/AS homologous region, and (4) a new imprinting region has been identified in Chr 9 distal to the T45H breakpoint (Figure 7).
Changes in the imprinting effects:
The imprinting effects have also changed such that now (1) both the proximal Chr 2 and proximal Chr 7 MatDp imprinting effects comprise fetal and placental growth retardations with effectively normal postnatal viabilities (Figures 3B and 4B), (2) PatDp for proximal Chr 2 has now been found to have an imprinting effect comprising a placental overgrowth (Figure 3B), and (3) MatDp for distal Chr 9 has now been seen to have an imprinting effect comprising a postnatal growth retardation (Figure 6).
The information obtained from the proximal-central Chr 7 study clearly separates the two imprinting regions, both observed with MatDp, for the first time (Figure 4B). Their interaction to provide a compound third imprinting effect could most simply be explained by the additive effects of the proximal prenatal growth retardation and the central postnatal lethality, giving a combined fetal/placental growth retardation with neonatal lethality. However, the other interactions, which combine MatDp for one chromosome with PatDp for another, potentially provide new insight into the regulation of fetal and placental growth and also the growth axes upon which imprinting operates.
Implications of the interactions:
Each of the individual imprinting effects, seen separately with MatDp and PatDp for the various regions, generally accord well with the Moore and Haig's (1991) “conflict hypothesis” by which maternally expressed imprinted genes favor the well-being of the mother over the growth of the fetus and paternally expressed genes favor fetal growth. Thus, MatDp genotypes with two copies of a maternally expressed gene might present extra disadvantage to fetal growth, whereas PatDp genotypes might potentially enhance fetal growth. Each of the interactions described combine MatDp for one region with PatDp for another. Therefore, if the genes involved in any imprinting effect combination operate upon the same growth axis, then some balance, such as seen with the combination of Igf2 and Igf2r knockouts to rescue the null Igf2r phenotype (Filson et al. 1993), might be expected. However, if there is a combination of MatDp and PatDp imprinting genes working in different axes, either an additive effect such as that found with the combination of Grb10 and Igf2 knockouts (Charalambous et al. 2003) or some exaggeration of the “conflict” might be generated. Interpretation of most of the interactions described here, however, is complicated by a number of uncertainties. These include correct identification of the genes involved and whether it is the presence of two expressed copies or the absence of an expressed copy of an imprinted gene that causes the observed imprinting effects. Further difficulties concern the sites of effective gene action (for example, fetus vs. placenta), the relationship between fetal and placental growth, the relationship between placental size and function, and, in one case, even transient effects.
Chr 2–Chr 11 interactions:
Such complications are found with the proximal Chr 2–proximal Chr 11 interaction (Table 3). Separately, PatDp(prox2) does not affect fetal growth but clearly operates in the placenta to cause a placental overgrowth (Table 2), while MatDp(prox11) causes a fetal and placental growth retardation that appears to be initiated first in the fetus (Cattanach et al. 1996) and perhaps therefore primarily operates here. Together, in the MatDp(prox11)/PatDp(prox2) combination, the fetus showed the Chr 11 growth retardation and the placenta showed the Chr 2 overgrowth (Table 3).
TABLE 3.
Summary of imprinting effect interactions
| Genotypes | Effects |
|---|---|
| MatDp(prox2) alone | Small placenta with fetal IUGR |
| PatDp(prox11) alone | Fetal overgrowth with large placenta |
| MatDp(prox2)/PatDp(prox11) combined | Early embryonic lethal (interaction) |
| PatDp(prox2) alone | Normal fetus with placental overgrowth |
| MatDp(prox11) alone | Fetal IUGR with small placenta |
| PatDp(prox2)/MatDp(prox11) combined | Fetal IUGR with placental overgrowth (interaction) |
| MatDp(prox7) alone | Fetal IUGR with small placenta |
| PatDp(prox11) alone | Fetal overgrowth with large placenta |
| MatDp(prox7/PatDp(prox11) combined | Transient fetal overgrowth, transient reduced placental growth (interaction) |
| PatDp(prox7) alone | Normal |
| MatDp(prox11) alone | Fetal IUGR with small placenta |
| MatDp(prox7)/PatDp(prox11) combined | Fetal IUGR with small placenta |
| PatDp(dist2) alone | Edema, hyperactive, lethal by 5 days |
| MatDp(dist9) alone | PNGR |
| PatDp(dist2)/MatDp(dist9) combined | Edema, PNGR partial viability,a normal activity (interaction) |
| MatDp(dist2) alone | Thin, flat-sided, hypoactive, lethal at birth |
| PatDp(dist9) alone | Normal |
| MatDp(dist2)/PatDp(dist9) combined | Thin, flat sided, hypoactive, lethal at birth |
In the reciprocal situation, MatDp(prox2) alone gives a placental growth retardation, with perhaps a secondary fetal growth retardation (Table 2), while PatDp(prox11) brings about the fetal overgrowth followed by a like effect in the placenta (Cattanach et al. 1996). Together, the MatDp(prox2)/PatDp(prox11) genotype might therefore have been expected to give a large fetus and a small placenta. Instead, an as-yet-undetected early embryonic lethality was indicated (Table 3). This might be interpreted in terms of a failure of the small, possibly less functional placenta to sustain a large fetus, although this was not seen with a Chr 7-Chr 11 interaction (Figure 4B, to be discussed). Whatever the exact mechanism, it might appear that the Chr 2 and Chr 11 effects operate independently and primarily in different sites (fetus and placenta, respectively). Therefore, while one cannot rule out a common growth axis, this cannot be the basis for the interaction observed.
The candidate Chr 2 and Chr 11 genes can be accommodated within the same framework. Gatm lies within the revised Chr 2 imprinting region (Figure 3B) and is expressed only in the placenta and only from the maternal allele (Sandell et al. 2003). It encodes a metabolic enzyme involved in creatine synthesis and has been postulated to be a growth suppressor. Grb10 is the candidate gene of choice for the Chr 11 effect as maternal transmission of a knockout enhances fetal and placental growth in the same way, as seen with PatDp(prox11) (Miyoshi et al. 1998; Charalambous et al. 2003). Considered to be another maternally expressed growth repressor, Grb10 encodes a signaling protein that interacts with tyrosine kinase receptors that include growth factor receptors. With PatDp(prox2)/MatDp(prox11), the large placenta could be attributed to the absence of the maternally expressed Gatm suppressor, while the small fetus could be attributed to the presence of two maternal copies of the Grb10 suppressor. In the reciprocal MatDp(prox2)/PatDp(prox11) genotype, two maternal copies of the Gatm suppressor operating in the placenta with the absence of Gbr10 expression to suppress growth in the fetus might explain the lethality. There is no evidence that the two genes may operate in the same growth axis.
Chr 7–Chr 11 interaction:
With the Chr 7–Chr 11 combination, evidence of yet another fetal and placental growth interaction is suggested (Table 3). Separately, both MatDp(prox7) and PatDp(prox11) affect fetal and placental growth, the former reducing growth and the latter increasing it. Postnatally, both genotypic classes retain their growth differences through to adulthood. When together, the Chr 11 effect initially (14.5 days) overrode the Chr 2 effect in the fetus, and the reverse occurred in the placenta to give a large fetus with small placenta (Figure 5A). At later prenatal ages, a different interaction was observed: the fetal growth rate appeared to decline while the placental growth rate increased, and newborn mice of this genotype were normal sized. There was no significant difference in the growth rates thereafter (Figure 5B). This may present a different situation from the Chr 2–Chr 11 interaction in which the limited prenatal and more extensive historical postnatal data (Cattanach and Kirk 1985; B. M. Cattanach, unpublished results) showed no evidence of a temporal effect. The overall picture for the Chr 7–Chr 11 interaction therefore suggests that the two imprinting effects operate in both the fetus and the placenta but perhaps at different but overlapping stages of development, and this does not immediately suggest a common growth axis. Without any detectable PatDp(prox7) imprinting effect, the PatDp(prox7)/MatDp(prox11) combination produced only the MatDp(prox11) fetal and placental growth retardation.
The Chr 7 and Chr 11 candidate genes can again be accommodated within the interpretation. The best candidate for proximal Chr 7 is Peg3, a gene of unknown function although expressed in a variety of embryonic tissues and placenta (Kuroiwa et al. 1996; Relaix et al. 1998). A Peg3 mutation has been shown to cause a similar fetal and placental growth retardation (Li et al. 1999) to that seen with MatDp(prox7). There is no reported interaction with the Chr 11 candidate gene, Grb10.
Chr 2–Chr 9 interactions:
Finally, with the Chr 2–Chr 9 combination achieved using the T11H translocation some basis for the observed interaction is indicated. PatDp(dist2) alone causes the anomalous phenotype at birth of short square body, edema, “paddle” feet, kinked tail, and notable hyperactivity, with death invariably following within a few days when generated with all translocations other than T11H (Figure 2B). MatDp(dist9) alone causes a postnatal growth retardation starting soon after birth (Figure 7). When combined in PatDp(dist2)/MatDp(dist9) animals, achieved with T11H, the key observation is that the hyperactivity of the edematous PatDp(dist2) class is lost and the viability substantially improved (Table 3). The connection between the two effects is not immediately clear but consideration of the genes involved provides one basis for hypothesis. For Chr 2, the complex Gnas locus has a clear relationship with the PatDp(dist2) phenotype because (1) it lies within the 7-Mb region of Chr 2 involved (Williamson et al. 1996), and (2) the Oed-Sml mutation, which gives an almost identical edematous phenotype when maternally inherited, has been shown to result from a base-pair substitution within exon 6 of Gnas itself (Skinner et al. 2002). For Chr 9, Rasgrf1 is surely responsible for the postnatal growth retardation because (1) the gene lies within in the appropriate region of the chromosome, (2) both a knockout (Itier et al. 1998) and a null mutation (Clapcott et al. 2003) of the gene, on paternal transmission, cause postnatal growth retardations similar to that of PatDp(dist2)/MatDp(dist9) mice (Figure 7), (3) Rasgrf1 expression is absent in brain of PatDp(dist2)/MatDp(dist9), and (4) the extreme behavior and inviability of PatDp(dist2) animals generated with another translocation (T26H) that does not involve Chr 9 is reduced when combined with a null Rasgrf1 mutation.
The basis for the interaction cannot be precisely specified but a likely possibility is that the PatDp(dist2) behavioral and inviability effects stem from a neuronal abnormality in the brain. Consistent with this hypothesis is the fact that the maternally expressed Gnas and the paternally expressed Gnasxl each produce, in addition to their respective full-length proteins, shortened forms of their proteins in neural tissue (Crawford et al. 1993; Pasolli et al. 2000; Pasolli and Huttner 2001). The absence of maternal neuronal Gnas or the presence of two paternal copies of neuronal Gnasxl might therefore account for the PatDp(dist2) behavioral and inviability phenotype. Several observations suggest that it is the absence of the Gnas neural protein, however, that might be involved, but the evidence is not strong. Thus:
- Partially trisomic mice with one maternal copy in addition to two paternal copies of Chr 2 have not been found to show the behavioral effect (Beechey and Peters 1994). This observation should exclude a role for the Gnasxl neural protein, but few such animals have been produced and other abnormalities attributable to the chromosomal unbalance may have disguised the PatDp(dist2) behavioral effect.
- Mice with a maternally inherited Gnas knockout (Yu et al. 1998), which should lack both full-length Gnas protein and Gnas neural protein, exhibit a behavioral effect. This might implicate Gnas, but it is not clear if the behavioral abnormality equates to that seen with PatDp(dist2).
- When maternally derived, the Oed-Sml mutation results in a nonfunctional full-length Gnas protein (Williamson et al. 2004) but, because the mutation lies downstream of the genome sequence for the neural protein, the latter should not be affected. Indeed, such mice do not show the PatDp(dist2) behavioral effect.
On balance it would seem most likely that the absence of the Gnas neural protein is responsible for the abnormal behavior in PatDp(dist2) mice, just as the absence of a functional maternal copy of full-length Gnas protein accounts for the edema component of the phenotype (Williamson et al. 2004).
In the case of Rasgrf1, there are two potential links with Gnas/Gnasxl neuronal proteins. First, brain is a tissue in which Rasgrf1 is imprinted (Plass et al. 1996). Second, mice with paternally inherited Oed-Sml or null Rasgrf1 mutations both show diminished IGF1 levels (Itier et al. 1998; J. Peters, unpublished results), and the growth factor has an important role in the development and repair of the central nervous system, stimulating neurone proliferation and myelination (Zumkeller 1997; Müller et al. 1999). It is of further note that the Rasgrf1 interaction with PatDp(dist2) enhances prenatal as well as postnatal viability. This suggests that IGF1 is again involved and has a role in prenatal as well as postnatal development. Overall, while it is clear that there is an interaction between Gnas and Rasgrf1, it is still unclear if this operates through a common growth axis.
Conclusions:
The findings illustrate considerable complexity in the interactions of imprinted genes in development. It seems likely that some of these genes operate largely or exclusively in the placenta (proximal Chr 2), while others may operate primarily in the fetus (subproximal Chr 6; Beechey et al. 2004), and in other cases (proximal Chrs 7 and 11) they may operate in both. Temporal factors may also be involved. Placental and fetal growths do not appear to be interdependent, but rather are independently regulated, and placental size in itself may (Chr 2–Chr 11 interaction) or may not (Chr 7–Chr 11 interaction) be important in sustaining fetal development. The interactions described raise a multitude of questions concerning development and the role of imprinting that may be investigated by the use of combinations of candidate gene knockouts or combinations of uniparental duplications and such knockouts.
Much of the data presented are consistent with the conflict hypothesis (Moore and Haig 1991). Growth enhancements, whether in the fetus or placenta, are seen with PatDps, and growth suppression is evident with MatDps. This applies irrespectively of whether the candidate genes are growth repressors, for MatDps, or promoters, for PatDps. It may therefore be that the imprinted genes do serve a maternal-fetal conflict. The anomaly that the main growth-promoting gene, Igf1, is not imprinted is also possibly resolved by the Chr 2–Chr 9 findings as these suggest that Igf1 may be regulated by imprinted genes to behave as if it were imprinted. However, Hurst and McVean (1998) have reviewed a number of observations that do not fit with the conflict hypothesis of which the preweaning growth-retarded PatDp(cen7) (Cattanach et al. 1992) and maternal Ube3a knockout (Jiang et al. 1998) “Angelman” mice provide significant examples. Nevertheless, imprinting clearly plays a vital role in the regulation of early mammalian development, and its observed diversity in terms of numerous growth axes, tissues implicated, and timing of action suggests a continuity of the evolutionary maternal-fetal conflict. The original role of imprinting may have been to aid placentation (Hall 1990), initially promoting the growth of the trophoblast through the actions of paternal genes (Barton et al. 1984; Surani et al. 1984) with maternal genes operating to control the growth of this foreign tissue. However, to account for the observed multiplicity in the control of fetal and early postnatal growth by imprinting, it may be concluded that subsequently, throughout evolution, any mechanism that might favor fetal growth has been exploited by paternal genes, again counterbalanced by growth control by maternal genes. This concept is not inconsistent with the conclusion of Reik et al. (2003) that imprinted genes have central roles in controlling fetal demand for, and placental supply of, maternal nutrients.
Acknowledgments
We thank David Papworth for statistical analyses of the data and Anne-Marie Woodword, Lynn Jones, Jackie McNaughton, Mark Harrison, and Debra Brooker for their dedicated and conscientious work with the detailed mouse breeding.
References
- Barton, S. C., M. A. H. Surani and M. L. Norris, 1984. Role of paternal and maternal genomes in mouse development. Nature 311**:** 374–376. [DOI] [PubMed] [Google Scholar]
- Beechey, C. V., and J. Peters, 1994. Dosage effects of the distal chromosome 2 imprinting region. Mouse Genome 92**:** 353–354. [Google Scholar]
- Beechey, C. V., B. M. Cattanach, A. Blake and J. Peters, 2004 Mouse imprinting map. MRC Mammalian Genetics Unit, Oxfordshire, UK (http://www.mgu.har.mrc.ac.uk/imprinting/imprinting.html).
- Buckle, V. J., and K. A. Rack, 1993 Fluorescent in situ hybrisation, pp. 59–80 in Human Genetic Disease Analysis: A Practical Approach, Ed. 2, edited by K. E. Davies. IRL Press, Oxford.
- Cattanach, B. M., 1986. Parental origin effects in mice. J. Embryol. Exp. Morphol. 97**(Suppl.):** 137–150. [PubMed] [Google Scholar]
- Cattanach, B. M., 1991a Chromosome imprinting and its significance for mammalian development, pp. 41–71 in Genome Analysis, edited by K. E. Davies and S. M. Tilgham. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Cattanach, B. M., 1991. b Lack of genetic background modification upon distal Chr 2 imprinting. Mouse Genome 89**:** 273. [Google Scholar]
- Cattanach, B. M., and C. V. Beechey, 1994. Further evidence that differential recoveries of maternal and paternal disomies are not attributable to imprinting. Mouse Genome 92**:** 504. [Google Scholar]
- Cattanach, B. M., and C. V. Beechey, 1997 Genomic imprinting in the mouse: possible final analysis, pp. 118–145 in Genomic Imprinting, edited by W. Reik and A. Surani. IRL Press/Oxford University Press, Oxford/London/New York.
- Cattanach, B. M., and M. Kirk, 1985. Chromosome 11 parental source effect upon fetal growth in mice. Genet. Res. 45**:** 220–221. [Google Scholar]
- Cattanach, B. M., J. A. Barr, E. P. Evans, M. Burtenshaw, C. V. Beechey et al., 1992. A candidate mouse model for Prader-Willi syndrome which shows an absence of Snrpn expression. Nat. Genet. 2**:** 270–274. [DOI] [PubMed] [Google Scholar]
- Cattanach, B. M., C. V. Beechey, C. Rasberry, J. Jones and D. Papworth, 1996. Time of initiation and site of action of the mouse chromosome 11 imprinting effects. Genet. Res. 68**:** 35–44. [DOI] [PubMed] [Google Scholar]
- Cattanach, B. M., J. A. Barr, C. V. Beechey, J. Martin, J. Noebels et al., 1997. A candidate model for Angelman syndrome in the mouse. Mamm. Genome 8**:** 472–478. [DOI] [PubMed] [Google Scholar]
- Cattanach, B. M., H. Shibata, Y. Hayashizaki, K. M. S. Townsend, S. Ball et al., 1998. Association of a redefined proximal mouse chromosome 11 imprinting region and U2afbp-rs/U2af1-rs1 expression. Cytogenet. Cell Genet. 80**:** 41–47. [DOI] [PubMed] [Google Scholar]
- Cattanach, B. M., J. Peters, S. Ball and C. Rasberry, 2000. Two imprinted gene mutations: three phenotypes. Hum. Mol. Genet. 9**:** 2263–2273. [DOI] [PubMed] [Google Scholar]
- Charalambous, M., F. M. Smith, W. R. Bennett, T. E. Crew, F. Mackenzie et al., 2003. Disruption of the imprinted Grb10 gene leads to disproportionate overgrowth by an _Igf2_-independent mechanism. Proc. Natl. Acad. Sci. USA 100**:** 8292–8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapcott, S. J., J. Peters, P. C. Orban, R. Brambilla and C. F. Graham, 2003. Two ENU mutations in Rasgrf1 and early mouse growth retardation. Mamm. Genome 14**:** 495–505. [DOI] [PubMed] [Google Scholar]
- Crawford, J. A., K. J. Mutchler, B. E. Sullivan, T. M. Lanigan, M. S. Clark et al., 1993. Neural expression of a novel alternatively spliced and polyadenylated GSα transcript. J. Biol. Chem. 268**:** 9879–9885. [PubMed] [Google Scholar]
- de la Puente, A., J. Hall, Y-Z. Wu, L. Gustavo, J. Peters et al., 2002. Structural characterization of Rasgrf1 and a novel linked imprinted locus. Gene 291**:** 287–297. [DOI] [PubMed] [Google Scholar]
- Filson, A. J., A. Louvi, A. Efstratiadis and E. J. Robertson, 1993. Rescue of the _T_-associated maternal effect in mice carrying null mutations in Igf-2 and reciprocally imprinted genes. Development 118**:** 731–736. [DOI] [PubMed] [Google Scholar]
- Hall, J., 1990. Genomic imprinting: review and relevance to human diseases. Am. J. Hum. Genet. 46**:** 857–873. [PMC free article] [PubMed] [Google Scholar]
- Hurst, L. D., and G. T. McVean, 1998. Do we understand the evolution of genomic imprinting? Curr. Opin. Genet. Dev. 8**:** 701–708. [DOI] [PubMed] [Google Scholar]
- Itier, J.-M., G. L. Tremp, J.-F. Léonard, M.-C. Multon, G. Ret et al., 1998. Imprinted gene in postnatal growth role. Nature 393**:** 125–126. [DOI] [PubMed] [Google Scholar]
- Jiang, Y. H., D. Armstrong, U. Albrecht, C. M. Atkins, J. L. Noebels et al., 1998. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron 21**:** 647–649. [DOI] [PubMed] [Google Scholar]
- Kim, J., X. Lu and L. Stubs, 1999. Zim1, a maternally expressed mouse Kruppel-type zinc-finger gene located in proximal chromosome 7. Hum. Mol. Genet. 8**:** 847–854. [DOI] [PubMed] [Google Scholar]
- Kuroiwa, Y., T. Kaneko-Ishino, F. Kagitani, T. Kohda, L. L. Li et al., 1996. Peg3 imprinted gene on proximal chromosome 7 encodes for a zinc finger protein. Nat. Genet. 12**:** 186–190. [DOI] [PubMed] [Google Scholar]
- Li, L.-L., E. B. Keverne, S. A. Aparicio, F. Ishino, S. C. Barton et al., 1999. Regulation of maternal behaviour and offspring growth by paternally expressed Peg3. Science 284**:** 330–333. [DOI] [PubMed] [Google Scholar]
- Miyoshi, N., Y. Kuroiwa, T. Kohda, H. Shitara, H. Yonekawa et al., 1998. Identification of the Meg1/Grb10 imprinted gene on mouse proximal chromosome 11, a candidate for the Silver-Russell syndrome gene. Proc. Natl. Acad. Sci. USA 3**:** 1102–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk, D., R. Smith, P. Arnaud, M. A. Preece, P. Stanier et al., 2003. Imprinted methylation profiles for proximal mouse chromosomes 11 and 7 as revealed by methylation-sensitive representation difference analysis. Mamm. Genome 14**:** 805–816. [DOI] [PubMed] [Google Scholar]
- Moore, T., and D. Haig, 1991. Genomic imprinting in mammalian development: a parental tug-of-war. Trends Genet. 7**:** 45–49. [DOI] [PubMed] [Google Scholar]
- Mouse Genome Database (MGD), 2004 Mouse genome informatics. The Jackson Laboratory, Bar Harbor, ME (http://www.informatics.jax.org).
- Müller, E. E., V. Locatelli and D. Cocchi, 1999. Neuroendocrine control of growth hormone secretion. Physiol. Rev. 79**:** 511–607. [DOI] [PubMed] [Google Scholar]
- Pasolli, H. A., and W. B. Huttner, 2001. Expression of the extra-large G protein α-subunit XLαs in neuroepithelial cells and young neurons during development of the rat nervous system. Neurosci. Lett. 301**:** 119–122. [DOI] [PubMed] [Google Scholar]
- Pasolli, H. A., M. Klenke, R. H. Kehlenbach, Y. Wang and W. B. Huttner, 2000. Characterization of the extra-large G protein α-subunit XLαs. J. Biol. Chem. 275**:** 33622–33632. [DOI] [PubMed] [Google Scholar]
- Pearsall, R. S., K. Imai, H. Shibata, Y. Hayashizaki, V. M. Chapman et al., 1998. The _Rasgrf1_-repeat sequence (D9Ncvs53) maps between Mod1 and Rbp1 on mouse chromosome 9 and may define a putative imprinted region. Mamm. Genome 9**:** 261–262. [DOI] [PubMed] [Google Scholar]
- Plass, C., H. Shibata, I. Kalcheva, L. Mullins, N. Kotelevtseva et al., 1996. Identification of Grf1 on mouse chromosome 9 by RLGS-M. Nat. Genet. 14**:** 106–109. [DOI] [PubMed] [Google Scholar]
- Reik, W., M. Constância, A. Fowden, N. Anderson, W. Dean et al., 2003. Regulation of supply and demand for maternal nutrients in mammals by imprinted genes. J. Physiol. 547**:** 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix, F., X-J. Wei, X. Wu and D. A. Sassoon, 1998. Peg3/Pew1 is an imprinted gene involved in the TNF-NF_k_B signal transduction pathway. Nat. Genet. 18**:** 287–291. [DOI] [PubMed] [Google Scholar]
- Ren, J., S. Lee, S. Pagliardini, M. Gérard, C. L. Stewart et al., 2003. Absence of Ndn, encoding the Prader-Willi syndrome-deleted gene necdin, results in congenital deficiency of central respiratory drive in neonatal mice. J. Neurosci. 23**:** 1569–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell, L. L., X.-J. Guan, R. Ingram and S. M. Tilghman, 2003. Gatm, a creatine synthesis enzyme, is imprinted in mouse placenta. Proc. Natl. Acad. Sci. USA 100**:** 4622–4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle, A. G., and C. V. Beechey, 1990. Genome imprinting phenomena on mouse chromosome 7. Genet. Res. 56**:** 237–244. [DOI] [PubMed] [Google Scholar]
- Skinner, J. A., B. M. Cattanach and J. Peters, 2002. The imprinted oedematous-small mutation on mouse chromosome 2 identified new roles for Gnas and Gnasxl in development. Genomics 80**:** 373–375. [DOI] [PubMed] [Google Scholar]
- Sunahara, S., K. Nakamura, K. Nakao, Y. Gondo, Y. Nagata et al., 2000. The oocyte-specific methylated region of the U2afbp-rs/U2af1-rs1 gene is dispensable for its imprinted methylation. Biochem. Biophys. Res. Commun. 268**:** 590–595. [DOI] [PubMed] [Google Scholar]
- Surani, M. A. H., S. C. Barton and M. L. Norris, 1984. Development of reconstituted mouse eggs suggests imprinting of the genome during gametogenesis. Nature 308**:** 548–550. [DOI] [PubMed] [Google Scholar]
- Williamson, C. M., J. Schofield, E. R. Dutton, A. Seymour, C.V. Beechey et al., 1996. Glomerular-specific imprinting of the mouse GSα gene: How does this relate to hormone resistance in Albright hereditary osteodystrophy? Genomics 36**:** 280–287. [DOI] [PubMed] [Google Scholar]
- Williamson, C. M., S. Ball, W. Nottingham, J. Skinner, A. Plagge et al., 2004. A _cis_-acting control region is required exclusively for the tissue-specific imprinting of Gnas. Nat. Genet. 36**:** 894–899. [DOI] [PubMed] [Google Scholar]
- Yu, S., D. Yu, E. Lee, M. Eckhaus, R. Lee et al., 1998. Variable and tissue-specific hormone resistance in heterotrimeric Gs protein α-subunit (Gs α) knockout mice is due to tissue-specific imprinting of the Gs α gene. Proc. Natl. Acad. Sci. USA 95**:** 8715–8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumkeller, W., 1997. The effect of insulin-like growth factors on brain myelination and their potential therapeutic application in myelination disorders. Eur. J. Paediatr. Neurol. 4**:** 91–101. [DOI] [PubMed] [Google Scholar]