RNA editing and alternative splicing: the importance of co-transcriptional coordination (original) (raw)
Abstract
The carboxy-terminal domain (CTD) of the large subunit of RNA polymerase II (pol II) is essential for several co-transcriptional pre-messenger RNA processing events, including capping, 3′-end processing and splicing. We investigated the role of the CTD of RNA pol II in the coordination of A to I editing and splicing of the ADAR2 (ADAR: adenosine deaminases that act on RNA) pre-mRNA. The auto-editing of Adar2 intron 4 by the ADAR2 adenosine deaminase is tightly coupled to splicing, as the modification of the dinucleotide AA to AI creates a new 3′ splice site. Unlike other introns, the CTD is not required for efficient splicing of intron 4 at either the normal 3′ splice site or the alternative site created by editing. However, the CTD is required for efficient co-transcriptional auto-editing of ADAR2 intron 4. Our results implicate the CTD in site-selective RNA editing by ADAR2 and in coordination of editing with alternative splicing.
Keywords: ADAR editing, RNA polymerase II, RNA processing, alternative splicing, CTD
Introduction
RNA editing by adenosine deamination is an RNA processing event that alters the nucleotide sequence of a substrate. Enzymes that catalyse adenosine (A) to inosine (I) base modification are called ADARs (adenosine deaminases that act on RNA; Bass et al, 1997). Inosine is read as guanosine by the cellular machineries. A to I editing is therefore functionally equivalent to an A to G change. ADAR editing occurs with low selectivity on completely double-stranded RNA or selectively on structured RNA, interrupted by bulges or loops (reviewed by Bass, 2002). In mammals, most selectively edited sites have been found in pre-messenger RNAs expressed in the central nervous system. RNAs coding for several subunits of a glutamate receptor, the serotonin receptor 5-HT2c and the potassium channel Kv1.1 in humans have all been shown to be edited (Sommer et al, 1991; Lomeli et al, 1994; Burns et al, 1997; Hoopengardner et al, 2003). Selective ADAR editing has been shown to result in both codon changes and formation of new splice sites (reviewed by Keegan et al, 2001; Bass, 2002; Maas et al, 2003).
Two active mammalian ADAR editing enzymes have been characterized, ADAR1 and ADAR2, with different substrate specificity (Kim et al, 1994; Maas et al, 1996; Melcher et al, 1996). The rat ADAR2 enzyme can edit its own pre-mRNA in intron 4 (Rueter et al, 1999; Fig 1). Intron 4 has been predicted to form a stem–loop structure, in which the base-pairing sequences are 1.3 kb apart from each other. This structure has been verified by evolutionary sequence conservation and mutational analysis (Dawson et al, 2004). The double-stranded region spans the AI dinucleotide created after editing, which mimics the AG sequence required for an alternative acceptor splice site. The 47 nucleotides included in the alternative exon change the open reading frame, producing premature stop codons within the alternative exon 5. Thus, it has been suggested that A to I editing of Adar2 pre-mRNA functions as an autoregulatory feedback loop to control ADAR2 levels. Up to 80% of all Adar2 pre-mRNAs extracted from whole rat brain are edited and subsequently alternatively spliced at this site (Rueter et al, 1999). For this feedback control to occur, it is crucial that splicing is restrained until editing takes place and the new 3′ splice site is formed. There are other examples where selective editing requires intron sequences and the edited site is often in close proximity to splice sites (reviewed by Keegan et al, 2001). Editing and splicing must therefore be sequential events in these transcripts.
Figure 1.
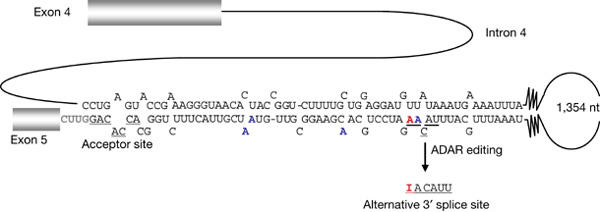
Rat Adar2 Ex 4–5 minigene. Predicted secondary structure of intron 4 of Adar2. For technical reasons, the stem has been shortened with approximately 40 base pairs interrupted by bulges and loops. A to I editing that leads to alternative splicing is in red. Other sites of editing are in blue. Normal and alternative acceptor splice sites are underlined.
The carboxy-terminal domain (CTD) of the largest subunit of RNA polymerase II (pol II) has been proposed to be the main coordinator of RNA processing events (reviewed by Maniatis & Reed, 2002; Kornblihtt et al, 2004; Zorio & Bentley, 2004). The CTD has been shown to promote RNA processing by capping, splicing and cleavage/polyadenylation in mammalian cells (McCracken et al, 1997a, 1997b; Hirose & Manley, 1998; Hirose et al, 1999). The mammalian CTD consists of 52 tandem repeats with the consensus sequence Tyr-Ser-Pro-Thr-Ser-Pro-Ser (YSPTSPS; supplementary Fig 3 online). A reversible phosphorylation of the CTD occurs during the transcriptional cycle (for a review, see Dahmus, 1996; Palancade & Bensaude, 2003). At transcription initiation, pol II contains a hypophosphorylated CTD (pol IIa). Phosphorylation of the CTD at Ser 2 and Ser 5 converts pol II to a processive, elongated form (pol IIo). The phosphorylated, but not the unphosphorylated, CTD can bind to and colocalize with splicing factors (Mortillaro et al, 1996; Neugebauer & Roth, 1997). In a previous report, we proposed a model for synchronized editing and splicing, in which the CTD coordinates editing of the glutamate receptor pre-mRNA with splicing (Bratt & Öhman, 2003).
In the present study, we have investigated the role of the CTD of pol II in the coordination of editing and splicing of the ADAR2 pre-mRNA. Surprisingly, we see that the CTD is not required for efficient splicing of this pre-mRNA, and that normal and alternative splicing occurs independently of the CTD. However, the CTD is required for efficient co-transcriptional editing of this pre-mRNA. Further, the 25 first repeats of the CTD or repeats 27–52 together with the C-terminal last ten residues are sufficient to support near wild-type levels of editing.
Results and Discussion
The alternative splice site created after editing
To investigate a possible coordination of A to I editing and splicing, a rat ADAR2 minigene (A2 Ex4–5) was used in transient transfections of human embryonic kidney (HEK)293 cells. The A2 Ex4–5 reporter construct codes for a pre-mRNA spanning the region exon 4, intron 4 and exon 5 of rAdar2 (Fig 1). Normal splicing and alternative splicing, which is dependent on editing, can be detected by reverse transcription–PCR (RT–PCR) on total RNA from cells transfected with this reporter gene in the presence of a co-transfected ADAR2 expression vector. No endogenous editing was observed in HEK293 cells (Fig 2A, lane 3; supplementary Fig 1A online). Further, transiently expressed ADAR2 protein in HEK293 cells is shown by western blot (Fig 2B, lane 2). To mimic an edited substrate, the A2 Ex4–5 reporter was mutated to contain an AG instead of an AA at the alternative 3′ splice site (A2 Ex4–5 G). This was undertaken to investigate how efficiently the alternative 3′ splice site competes with the normal splice site. As more than 70% of the substrates were alternatively spliced in A2 Ex4–5 G (Fig 2A, lanes 4,5), we conclude that the splice site created by editing is sufficiently well used to explain the observed level of alternative splicing in rat brain.
Figure 2.
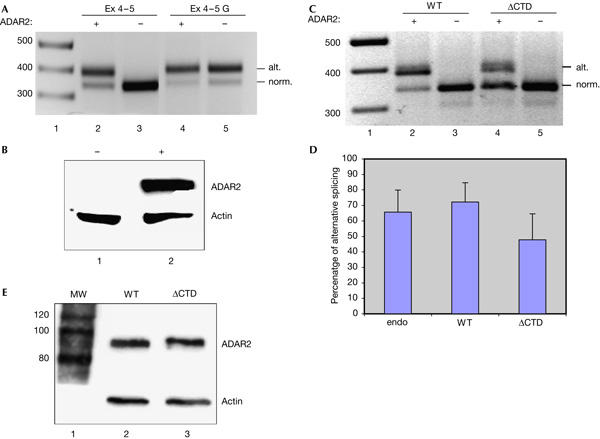
ADAR2 pre-mRNA processing and the influence of the carboxy-terminal domain. (A) A2 Ex 4–5 (lanes 2,3) and A2 Ex 4–5 G (lanes 4,5) were analysed by reverse transcription–PCR (RT–PCR). Expected band size for alternative splicing was 387 base pairs (bp) and for normal splicing 340 bp. Alternative (alt.) and normal (norm.) splicing is indicated on the right. The size of fragments in lane 1 is in base pairs. (B) Western blot analysis of exogenously expressed ADAR2 in human embryonic kidney 293 cells without (−) and with (+) a transiently transfected ADAR2 expression vector. An α-actin antibody is used as a control for equal loading. (C) Editing/splicing analysed by RT–PCR. The A2 Ex4–5 reporter was transcribed by a wild-type RNA polymerase II (pol II; WT, lanes 2,3) or a pol II with C-terminal domain deletion (ΔCTD, lanes 4,5). The presence or absence of ADAR2 expression is indicated by (+) or (−). Alternative (alt.) and normal (norm.) splicing is indicated on the right. (D) Average percentage of alternative splicing of the A2 Ex 4–5 RNA in the presence of ADAR2 for transfected wild-type (WT) and CTD-deleted pol II (ΔCTD) and three independent experiments transcribed by endogenous pol II (endo). (E) Western blot analysis of exogenous rADAR2 in cells transfected with α-amanitin-resistant polymerases. The level of ADAR2 was normalized to the expression of actin as indicated on the right. The size of proteins indicated to the left in the marker lane (MW) is in kilodaltons.
The CTD of RNA pol II is required for efficient editing
To investigate whether the CTD of pol II is required for efficient editing of Adar2 pre-mRNA during ongoing transcription, we used transiently transfected expression vectors for α-amanitin-resistant pol II large subunits (Gerber et al, 1995) with an intact wild-type CTD (WT) or a deletion of the entire CTD (ΔCTD). Transcription by pol II that has incorporated the transiently transfected α-amanitin-resistant large subunit was favoured by inhibiting the endogenous pol II with α-amanitin. The amount of editing and alternative splicing was compared to the usage of the normal 3′ splice site of intron 4 after RT–PCR on total RNA from transfected HEK293 cells (Fig 2C). Editing can be measured as the amount of alternative splicing, as the alternative acceptor site is created after editing. A twofold induction of editing and subsequent alternative splicing of A2 Ex4–5 pre-mRNA was observed when the minigene was transcribed by wild-type pol II compared with ΔCTD (Fig 2C, lanes 2,4). In five independent experiments, pol II ΔCTD produced on average only 47% of alternatively spliced transcripts, whereas wild-type CTD pol II produced 72% of alternatively spliced mRNA (Fig 2D). Moreover, the splicing ratio during transcription by an exogenously expressed wild-type RNA pol II is similar to the ratio when transcribed by the endogenous pol II (Fig 2D). The reduced ratio of alternative splicing versus normal splicing in the absence of the CTD indicated that editing and/or alternative splicing was inhibited by deletion of this domain of pol II. These results indicate that efficient editing, or possibly alternative splicing, requires the presence of the CTD. All editing/alternative splicing was dependent on expression of ADAR2 (Fig 2C, lanes 2–5). To rule out an indirect effect of CTD deletion due to altered ADAR2 expression, we assayed the ADAR2 protein level by western blot (Fig 2E). The level of ADAR2 expression was not affected by the mutant polymerase, indicating that the decrease in editing efficiency during ΔCTD pol II transcription is not due to a decrease in the concentration of ADAR2.
To confirm the results shown by RT–PCR and to compare pre-mRNA levels in relation to spliced products, an RNase protection assay (RPA) on the Ex4–5 Adar2 reporter transcript was carried out (supplementary Fig 2 online). Comparison of the protection products corresponding to pre-mRNA with the normal and alternatively spliced mRNAs showed that (i) deletion of the CTD does not seem to affect the normal splicing of intron 4 in contrast to previous observations on splicing of other introns (McCracken et al, 1997b; Fong & Bentley, 2001) and (ii) alternative splicing is significantly inhibited by the CTD deletion (supplementary Fig 2 online). The latter result therefore confirms the RT–PCR experiments in Fig 2C,D. These results show that the CTD has a minor effect on the normal splicing of ADAR2 intron 4 and a major effect on the efficiency of editing and/or alternative splicing of this intron. In addition, we have found that both the Q/R and the R/G editing site in GluR-B are dependent on the CTD for the coordination of editing and splicing (K. Ryman, N.F., Eva Bratt, D.L.B. & M.O., unpublished observations).
The alternative splicing is not CTD independent
To further investigate how editing and alternative splicing are influenced by the CTD, we used the A2 Ex4–5 G mutant minigene. Pre-mRNA from this mutant mimics the edited state, and alternative splicing can therefore be analysed independently of the editing event. About 70% of the A2 Ex4–5 G transcripts were alternatively spliced regardless of whether or not the CTD was deleted (Fig 3A, lanes 2,3; Fig 3B). This result implies that alternative splice site choice is not affected by the CTD. Taken together, our results indicate that the CTD is required for efficient editing, but not for efficient alternative splicing. Splicing independent of the CTD in vivo has not been characterized previously. Further analyses are required to rule out whether the CTD independence in Adar2 is due to insertion of the minigene construct into a vector or whether this is an intron that is immune to the CTD also in its natural context.
Figure 3.
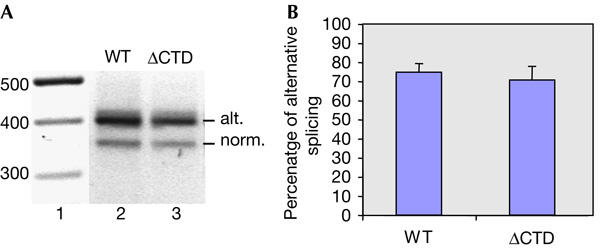
Splicing and alternative splicing of a ‘pre-edited' ADAR2 substrate. (A) Spliced A2 Ex4–5 G RNA transcribed by a wild-type RNA polymerase II (pol II; lane 2) or pol II ΔCTD (lane 3). Alternative (alt.) and normal (norm.) splicing is indicated on the right. The size of fragments in lane 1 is in base pairs. (B) Average percentage of alternative splicing of the A2 Ex 4–5 G RNA was from four independent experiments.
Partial CTD is sufficient to support efficient editing
To further investigate the importance of the CTD of pol II for editing efficiency, we used partial deletions of the heptad repeats (supplementary Fig 3 online). The amino-terminal half of the mammalian CTD mostly contains consensus heptapeptide repeats (YSPTSPS). In the C-terminal half, most of the repeats diverge from the consensus, although they are conserved among mammals. We investigated whether CTD deletion mutants containing heptads 1–25 or 27–52 could support editing of the ADAR2 alternative splice site. For these experiments, expression vectors for α-amanitin-resistant pol II with heptads 1–25 or 27–52 were co-transfected with the A2 Ex4–5 reporter and the ADAR2 expression vector. As shown by RT–PCR analysis of total RNA (Fig 4A), editing at wild-type levels can be observed with heptad repeats 1–25 after an addition of the 10-amino-acid C-terminal motif proven to be required for transcription and RNA processing (Fong et al, 2003). This motif contains an interaction site for the CTD tyrosine kinases Abl1/c-Abl, Abl2/Arg and casein kinase II (Baskaran et al, 1999; Chapman et al, 2004). The CTD is proposed to be subjected to proteolytic degradation in the absence of these last ten amino acids (Chapman et al, 2005). The pol II with heptads 27–52 including the C-terminal motif also supports efficient editing and alternative splicing (Fig 4A, lane 5). Our results indicate that either heptads 1–25 or 27–52 are sufficient to support efficient editing of the Adar2 pre-mRNA (Fig 4B). This conclusion is in agreement with two recent reports showing that 22 repeats of either the C- or N-terminal half of the CTD were sufficient for splicing and 3′-end formation of some pre-mRNAs (Ryan et al, 2002; Rosonina & Blencowe, 2004). Moreover, efficient RNA processing has previously been shown to be achieved only with heptads 1–25 or 27–52, together with the C-terminal motif of the CTD (Fong et al, 2003).
Figure 4.
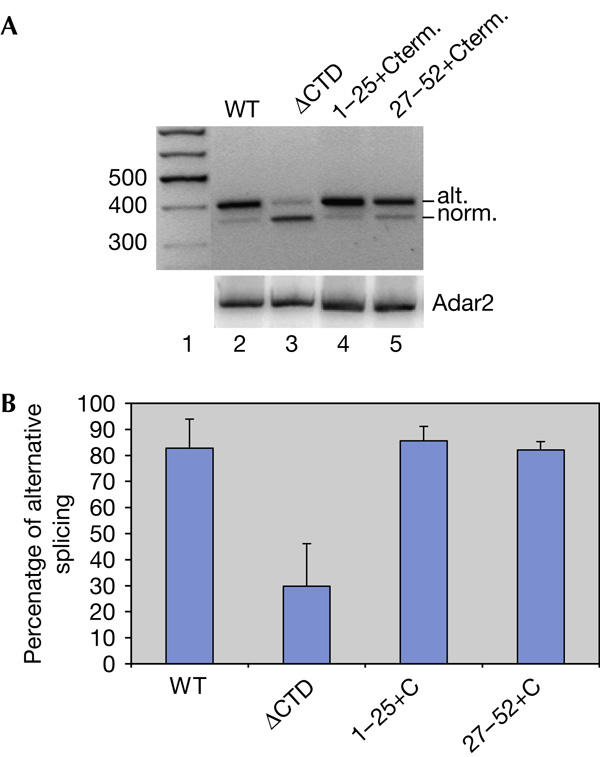
Editing and alternative splicing of transcripts using different carboxy-terminal domain deletion mutants. (A) Alternative (alt.) and normal (norm.) splicing was analysed by reverse transcription–PCR (RT–PCR). The A2 Ex4–5 reporter was transcribed by an RNA polymerase II (pol II) with a wild-type C-terminal domain (CTD; WT), CTD deleted (ΔCTD), heptad repeats 1–25 with the additional ten-residue C-terminal motif (1–25+Cterm.) and repeats 27–52 including the ten-residue C-terminal motif (27–52+Cterm.). The size of fragments in lane 1 is in base pairs. Bottom: semiquantitative RT–PCR with 32P-labelled primers shows expression of ADAR2 in each lane. (B) Quantification of RT–PCR results using the different CTD deletion mutants. The A2 Ex4–5 reporter was transcribed by an RNA pol II with a wild-type CTD (WT), a deleted CTD (ΔCTD), heptad repeats 1–25 with the additional ten-residue C-terminal motif (1–25+C) or heptad repeats 27–52 including the ten-residue C-terminal motif (27–52+C). The average percentage of alternatively spliced PCR products from two independent experiments is shown.
To our knowledge, this is the first time the efficiency of RNA editing has been shown to be dependent on the CTD of pol II. These results indicate that the CTD works as a landing pad for different factors depending on the nature of the transcript. Further, the CTD seems to work as a coordinator of the RNA processing events rather than a simple enhancer of processing. The CTD might coordinate editing and alternative splicing through direct or indirect interactions with the ADAR2 protein. Understanding the connection between RNA editing and the transcription machinery is important to unravel the mechanism behind the recognition of transcript-selective editing in the mammalian cell.
Methods
Constructs. Plasmids for expression of α-amanitin-resistant pol II, pATRpb1 WT (Nguyen et al, 1996), pATRpb1 Δ0–1 (Fong & Bentley, 2001), pATRpbl 1–25+C-term and pATRpb1 27–52 including the C-terminal motif, have been described. For expression of ADAR2, the plasmids pcDNA3 FLAG/rADAR2 and pcDNA3 FLAG EMPTY were used (Bratt & Öhman, 2003). The editing/splicing reporter pRC-CMV-A2 Exon 4–5 plasmid is as described by Rueter et al (1999). The pRC-CMV-A2 Exon 4–5 was mutated to pRC-CMV-A2 Exon 4–5-G using QuikChange Site directed mutagenesis (Stratagene, La Jolla, CA, USA). Primers used for the mutagenesis are available from the author on request.
Transfections. HEK293 cells were transfected with LIPOFECTAMINE™ 2000 (Invitrogen, Carlsbad, CA, USA) and treated with α-amanitin as described (Fong & Bentley, 2001).
RNA analysis. RNA was extracted with TRIzol reagent (Invitrogen), treated with DNase I and RT–PCR amplified with primers A2-rev and A2–5′. Primers DAM 3′ and DAM 5′ were used to evaluate expression of ADAR2. RPA was carried out with an antisense probe of 380 nucleotides transcribed from a PCR product spanning the intron 4 to exon 5 boundary. Oligonucleotide sequences are available on request.
Western blot. Cell lysates from transfected cells were immunoblotted with anti-FLAG antibody and developed with ECL-PLUS (Amersham Biosciences, Piscataway, NJ, USA).
Supplementary information is available at EMBO reports online ((http://www.nature.com/embor/journal/vaop/ncurrent/extref/7400621-s1.pdf).
Supplementary Material
Supplementary Figures
Acknowledgments
We thank H. Wahlstedt for excellent technical assistance. We are grateful to professor R. Emeson for the pRC-CMV-A2 Exon 4–5 vector. This work was supported by grants from the Swedish Natural Science Research Council and Carl Tryggers Stiftelse. D.B. and N.F. are supported by National Institutes of Health Grant GM58613.
References
- Baskaran R, Escobar SR, Wang JY (1999) Nuclear c-Abl is a COOH-terminal repeated domain (CTD)-tyrosine (CTD)-tyrosine kinase-specific for the mammalian RNA polymerase II: possible role in transcription elongation. Cell Growth Differ 10: 387–396 [PubMed] [Google Scholar]
- Bass BL (2002) RNA editing by adenosine deaminases that act on RNA. Annu Rev Biochem 71: 817–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass BL, Nishikura K, Keller W, Seeburg PH, Emeson RB, O'Connell MA, Samuel CE, Herbert A (1997) A standardized nomenclature for adenosine deaminases that act on RNA. RNA 3: 947–949 [PMC free article] [PubMed] [Google Scholar]
- Bratt E, Öhman M (2003) Coordination of editing and splicing of glutamate receptor pre-mRNA. RNA 9: 309–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CM, Chu H, Rueter SM, Hutchinson LK, Canton H, Sanders-Bush E, Emeson RB (1997) Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature 387: 303–308 [DOI] [PubMed] [Google Scholar]
- Chapman RD, Palancade B, Lang A, Bensaude O, Eick D (2004) The last CTD repeat of the mammalian RNA polymerase II large subunit is important for its stability. Nucleic Acids Res 32: 35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RD, Conrad M, Eick D (2005) Role of the mammalian RNA polymerase II C-terminal domain (CTD) nonconsensus repeats in CTD stability and cell proliferation. Mol Cell Biol 25: 7665–7674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmus ME (1996) Reversible phosphorylation of the C-terminal domain of RNA polymerase II. J Biol Chem 271: 19009–19012 [DOI] [PubMed] [Google Scholar]
- Dawson TR, Sansam CL, Emeson RB (2004) Structure and sequence determinants required for the RNA editing of ADAR2 substrates. J Biol Chem 279: 4941–4951 [DOI] [PubMed] [Google Scholar]
- Fong N, Bentley DL (2001) Capping, splicing, and 3′ processing are independently stimulated by RNA polymerase II: different functions for different segments of the CTD. Genes Dev 15: 1783–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong N, Bird G, Vigneron M, Bentley DL (2003) A 10 residue motif at the C-terminus of the RNA pol II CTD is required for transcription, splicing and 3′ end processing. EMBO J 22: 4274–4282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber HP, Hagmann M, Seipel K, Georgiev O, West MA, Litingtung Y, Schaffner W, Corden JL (1995) RNA polymerase II C-terminal domain required for enhancer-driven transcription. Nature 374: 660–662 [DOI] [PubMed] [Google Scholar]
- Hirose Y, Manley JL (1998) RNA polymerase II is an essential mRNA polyadenylation factor. Nature 395: 93–96 [DOI] [PubMed] [Google Scholar]
- Hirose Y, Tacke R, Manley JL (1999) Phosphorylated RNA polymerase II stimulates pre-mRNA splicing. Genes Dev 13: 1234–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopengardner B, Bhalla T, Staber C, Reenan R (2003) Nervous system targets of RNA editing identified by comparative genomics. Science 301: 832–836 [DOI] [PubMed] [Google Scholar]
- Keegan LP, Gallo A, O'Connell MA (2001) The many roles of an RNA editor. Nat Rev Genet 2: 869–878 [DOI] [PubMed] [Google Scholar]
- Kim U, Wang Y, Sanford T, Zeng Y, Nishikura K (1994) Molecular cloning of cDNA for double-stranded RNA adenosine deaminase, a candidate enzyme for nuclear RNA editing. Proc Natl Acad Sci USA 91: 11457–11461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt AR, de la Mata M, Fededa JP, Munoz MJ, Nogues G (2004) Multiple links between transcription and splicing. RNA 10: 1489–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomeli H, Mosbacher J, Melcher T, Hoger T, Geiger JR, Kuner T, Monyer H, Higuchi M, Bach A, Seeburg PH (1994) Control of kinetic properties of AMPA receptor channels by nuclear RNA editing. Science 266: 1709–1713 [DOI] [PubMed] [Google Scholar]
- Maas S, Melcher T, Herb A, Seeburg PH, Keller W, Krause S, Higuchi M, O'Connell MA (1996) Structural requirements for RNA editing in glutamate receptor pre-mRNAs by recombinant double-stranded RNA adenosine deaminase. J Biol Chem 271: 12221–12226 [DOI] [PubMed] [Google Scholar]
- Maas S, Rich A, Nishikura K (2003) A-to-I RNA editing: recent news and residual mysteries. J Biol Chem 278: 1391–1394 [DOI] [PubMed] [Google Scholar]
- Maniatis T, Reed R (2002) An extensive network of coupling among gene expression machines. Nature 416: 499–506 [DOI] [PubMed] [Google Scholar]
- McCracken S, Fong N, Rosonina E, Yankulov K, Brothers G, Siderovski D, Hessel A, Foster S, Shuman S, Bentley DL (1997a) 5′-Capping enzymes are targeted to pre-mRNA by binding to the phosphorylated carboxy-terminal domain of RNA polymerase II. Genes Dev 11: 3306–3318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken S, Fong N, Yankulov K, Ballantyne S, Pan G, Greenblatt J, Patterson SD, Wickens M, Bentley DL (1997b) The C-terminal domain of RNA polymerase II couples mRNA processing to transcription. Nature 385: 357–361 [DOI] [PubMed] [Google Scholar]
- Melcher T, Maas S, Herb A, Sprengel R, Seeburg PH, Higuchi M (1996) A mammalian RNA editing enzyme. Nature 379: 460–464 [DOI] [PubMed] [Google Scholar]
- Mortillaro MJ, Blencowe BJ, Wei X, Nakayasu H, Du L, Warren SL, Sharp PA, Berezney R (1996) A hyperphosphorylated form of the large subunit of RNA polymerase II is associated with splicing complexes and the nuclear matrix. Proc Natl Acad Sci USA 93: 8253–8257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer KM, Roth MB (1997) Distribution of pre-mRNA splicing factors at sites of RNA polymerase II transcription. Genes Dev 11: 1148–1159 [DOI] [PubMed] [Google Scholar]
- Nguyen VT, Giannoni F, Dubois MF, Seo SJ, Vigneron M, Kedinger C, Bensaude O (1996) In vivo degradation of RNA polymerase II largest subunit triggered by α-amanitin. Nucleic Acids Res 24: 2924–2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palancade B, Bensaude O (2003) Investigating RNA polymerase II carboxyl-terminal domain (CTD) phosphorylation. Eur J Biochem 270: 3859–3870 [DOI] [PubMed] [Google Scholar]
- Rosonina E, Blencowe BJ (2004) Analysis of the requirement for RNA polymerase II CTD heptapeptide repeats in pre-mRNA splicing and 3′-end cleavage. RNA 10: 581–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueter SM, Dawson TR, Emeson RB (1999) Regulation of alternative splicing by RNA editing. Nature 399: 75–80 [DOI] [PubMed] [Google Scholar]
- Ryan K, Murthy KG, Kaneko S, Manley JL (2002) Requirements of the RNA polymerase II C-terminal domain for reconstituting pre-mRNA 3′ cleavage. Mol Cell Biol 22: 1684–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer B, Kohler M, Sprengel R, Seeburg PH (1991) RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell 67: 11–19 [DOI] [PubMed] [Google Scholar]
- Zorio DA, Bentley DL (2004) The link between mRNA processing and transcription: communication works both ways. Exp Cell Res 296: 91–97 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures