Importin α-regulated nucleation of microtubules by TPX2 (original) (raw)
Abstract
The importin α-regulated microtubule-associated protein TPX2 is known to be critical for meiotic and mitotic spindle formation in vertebrates, but its detailed mechanism of action and regulation is not understood. Here, the site of interaction on TPX2 for importin α is mapped. A TPX2 mutant that cannot bind importin α is constitutively active in the induction of microtubule-containing aster-like structures in Xenopus egg extract, demonstrating that no other importin α or RanGTPase target is required to mediate microtubule assembly in this system. Further, recombinant TPX2 is shown to induce the formation and bundling of microtubules in dilute solutions of pure tubulin. In this purified system, importin α prevents TPX2-induced microtubule formation, but not TPX2–tubulin interaction or microtubule bundling. This demonstrates that TPX2 has more than one mode of interaction with tubulin and that only one of these types of interaction is abolished by importin α. The data suggest that the critical early function in spindle formation regulated by importin α is TPX2-mediated microtubule nucleation.
Keywords: importin α/nuclear localization sequence/Ran/spindle assembly/TPX2
Introduction
The microtubule cytoskeleton is completely reorganized at the onset of mitosis in order to build the mitotic spindle apparatus. The interphase microtubule array disappears and mitotic microtubules assemble from the two centrosomes and around chromosomes. This rearrangement is under the global control of cdc2 kinase, which coordinates changes in the activity of many cell cycle-regulated factors including microtubule-associated proteins (MAPs) and motor proteins (Cassimeris and Spittle, 2001). Ultimately, these changes modify the parameters determining microtubule dynamics in such a way that microtubules are organized into the bipolar spindle structure that segregates the two sets of chromosomes in mitosis (Compton, 2000; Karsenti and Vernos, 2001; Wittmann et al., 2001). The molecular bases of the changes in microtubule behaviour are not understood in detail, but it is now recognized that the small GTPase Ran plays an essential role in spindle assembly (Carazo-Salas et al., 1999; Kalab et al., 1999; Ohba et al., 1999; Wilde and Zheng, 1999; Zhang et al., 1999). It has been suggested that Ran acts as a marker for chromosome positioning in spindle formation and nuclear envelope assembly and that it regulates the activity of factors which mediate essential functions in mitotic spindle formation (Clarke and Zhang, 2001; Dasso, 2002; Hetzer et al., 2002).
One target of RanGTP in spindle formation is the MAP TPX2 (Gruss et al., 2001), a protein that was studied initially because of its preferential expression in proliferating cells (Heidebrecht et al., 1997) and its ability to target the motor protein Xklp2 to spindle microtubules (Wittmann et al., 1998, 2000). A role for TPX2 in spindle pole organization was proposed initially on the basis of experiments performed in Xenopus egg extract (Wittmann et al., 1998, 2000). More recently, it was shown that TPX2 also functions early in spindle formation in the assembly of microtubules around chromatin (Gruss et al., 2001). Evidence for this early role of TPX2 in spindle formation and organization has come from experiments both in Xenopus egg extracts and in human somatic cells, i.e. from both meiotic and mitotic cell types (Gruss et al., 2001, 2002).
Ran modulates TPX2 activity during mitosis in a way that is mechanistically identical to its action in nucleocytoplasmic transport during interphase: it releases TPX2 from the import receptors importin α and β (Gruss et al., 2001). It was proposed that TPX2, when released from the inhibitory effect of importin binding, would be sufficient to induce microtubule assembly (Gruss et al., 2001). Consistent with this hypothesis, addition to Xenopus M-phase extracts of concentrations of recombinant TPX2 sufficient to saturate endogenous importin α caused polymerization of microtubules. Conversely, RanGTP- or TPX2-mediated microtubule polymerization could be inhibited by addition of an excess of importin α (Gruss et al., 2001).
However, there is good evidence that the release of TPX2 from its import receptors is not the only Ran-regulated step in spindle assembly. Evidence for a role for several other RanGTP-regulated factors in spindle assembly has been presented (Nachury et al., 2001; Wiese et al., 2001; Wilde et al., 2001). However, it is uncertain whether any of these factors has a direct role in microtubule nucleation events as opposed to later steps in the assembly process. It is therefore important to determine their precise function in microtubule assembly and spindle formation. For TPX2, it is still an open question exactly which step of spindle assembly is blocked when importin α binds to TPX2. In other words, the precise mechanism by which TPX2 induces microtubule assembly in Xenopus egg extracts is not known.
To understand better the function of TPX2 and its regulation, we have now identified the major interaction site of TPX2 with importin α and generated a mutant form of TPX2 with greatly decreased affinity for importin α. Our data demonstrate that the lack of association of TPX2 with importin α is sufficient to promote microtubule assembly in meiotic egg extracts. We also show that TPX2 is sufficient to induce microtubule assembly in a solution of purified tubulin. Regulation of TPX2-mediated microtubule formation by importin α is recapitulated in a mixture of highly purified components. Although tubulin–TPX2 interaction is not prevented by importin α in vitro, the ability of TPX2 to induce microtubule formation is abrogated by importin α. These results support the hypothesis that TPX2 is an importin α-regulated and therefore chromatin-induced nucleator of spindle microtubules.
Results
TPX2 has one critical binding site for importin α
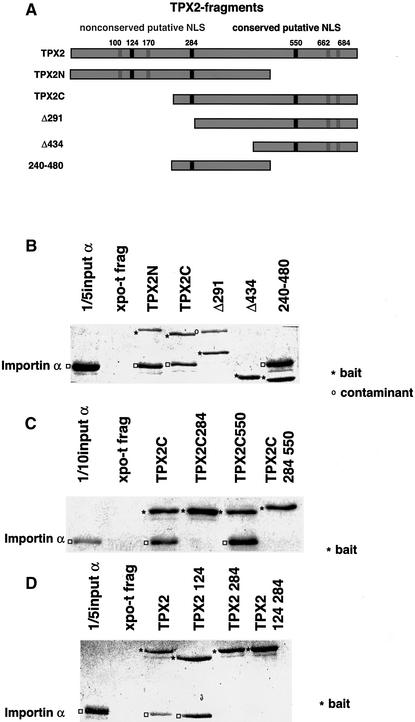
To determine whether TPX2 binding to importin α is sufficient to regulate TPX2-mediated microtubule nucleation activity and to investigate further Ran- and TPX2-mediated spindle formation, we mapped the importin α-binding site in TPX2. The computer program NUCDISC (Nakai and Horton, 1999) predicted seven possible nuclear localization sequences (NLSs) in the Xenopus TPX2 protein (Figure 1A), which we considered as potential interaction sites for importin α. Of these, only three were conserved between Xenopus, mouse, chicken and man (Figure 1A, black squares). To test whether the conserved predicted NLSs were indeed importin α-binding sites, different protein A-tagged fragments of the TPX2 protein were expressed in Escherichia coli and purified (Figure 1A), and their interaction with importin α was tested in a pull-down assay. TPX2 derivatives were immobilized on IgG–Sepharose via the protein A tag, and importin α binding was assayed.
Fig. 1. TPX2 has one high affinity binding site for importin α. (A) The computer program NUCDISC (Nakai and Horton, 1999) was used to identify predicted nuclear localization signals (NLSs) in the Xenopus TPX2 cDNA. Conserved putative NLSs are shown as black boxes, non-conserved as light grey boxes. The panel shows different fragments of the Xenopus TPX2 cDNA which were expressed in E.coli and tested for their ability to interact with purified importin α (see B). Numbers represent amino acid positions in the full-length protein. (B–D) TPX2 derivatives were immobilized on IgG–Sepharose and importin α binding was tested. Shown are Coomassie Blue-stained SDS–polyacrylamide gels of column-bound fractions that had been eluted with 2 M MgCl2. The bait proteins are marked with an asterisk, bound importin α as an open square; the Δ291 lane contains a contaminant marked with an open circle. Note that due to its tight binding to IgG–Sepharose, the Xpo-t fragment is not eluted with 2 M MgCl2, although similar amounts of all bait proteins had been coupled to IgG–Sepharose as seen after elution with SDS and Coomassie Blue staining (data not shown). (B) TPX2 fragments, (C) point mutants of the C-terminal fragment of TPX2 and (D) point mutants of the full-length TPX2 protein were tested for importin α binding.
As shown in Figure 1B, importin α did not bind to a control protein, a fragment of Xpo-t, but did bind both to an N-terminal (amino acids 1–480, Figure 1A) and a C-terminal (amino acids 240–715, Figure 1A) fragment of TPX2 as well as to an internal fragment (amino acids 240–480, Figure 1A). All these fragments include the putative NLS centred at amino acid 284. No binding was observed to two shorter C-terminal fragments of TPX2 (Δ291 and Δ434, Figure 1A), neither of which contains the 284 site.
To investigate the importin α-binding site further, we mutated the three conserved putative NLS sequences centred at amino acids 124, 284 and 550 by replacing basic residues with alanines in the context of either the full-length protein or the C-terminal fragment (amino acids 240–715). Mutation at position 550 (TPXC550) did not affect importin α binding to the C-terminus of TPX2, whereas mutation at residue 284 (TPXC284) disrupted importin α binding (Figure 1C). In the context of full-length TPX2, mutation of two basic residues (amino acids 284 and 285) to alanines was sufficient to prevent importin α binding to TPX2, indicating that the region encompassing these two amino acids is critical for the interaction, while mutation at position 124 did not change the binding properties of TPX2 (Figure 1D, note that mutation at the 124 site caused TPX2 to migrate aberrantly in the denaturing gel; sequencing of this construct failed to reveal any secondary mutations).
TPX2 function in microtubule assembly is regulated directly by importin α
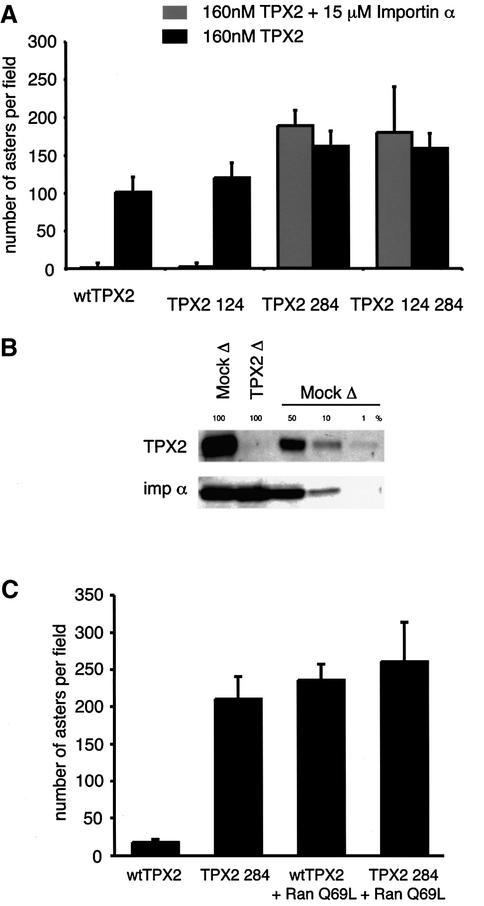
In order to test whether importin α regulates TPX2 directly, the TPX2 mutants were tested for their ability to nucleate microtubules in egg extracts. All tested mutants induced aster formation at least as well as wild-type TPX2 in these conditions (Figure 2A and data not shown). The addition of an excess of importin α inhibited nucleation of microtubules induced by either wild-type TPX2 protein or the 124 mutant protein that bind importin α in vitro (Figure 2A). TPX2 variants mutated at position 284 were, however, able to induce aster formation even in the presence of excess exogenous importin α (Figure 2A). This indicated that the sequence around position 284 is essential for the inhibitory interaction of importin α with TPX2 in Xenopus extracts.
Fig. 2. TPX2 function in microtubule assembly is regulated directly by importin α. (A) TPX2 mutants were tested for their ability to nucleate microtubules on addition to Xenopus M-phase extract to a concentration of 160 nM in the presence of rhodamine-labelled tubulin. Results were quantified by counting structures from three fixed and squashed samples (Sawin and Mitchison, 1991). Error bars represent standard deviations. All tested mutants were able to induce assembly of aster-like structures (black histograms). Addition of importin α (light grey histograms) inhibited assembly of microtubules induced by either the wild-type TPX2 (wt) protein or the 124 mutant protein, but not by the 284 mutant protein or the double mutant TPX2 124 284. (B) The amounts of TPX2 (upper panel) and importin α (lower panel) were compared in mock-treated Xenopus M-phase extract or TPX2-depleted extracts. TPX2 was not detectable in depleted extracts but was still visible in 1% of mock-treated extracts, indicating that the efficiency of depletion was >99%. (C) TPX2-depleted extract was reconstituted with either wild-type TPX2 protein or TPX2 284 in recombinant form using a final concentration of 20 nM. The wild-type TPX2 did not induce microtubule assembly. TPX2 284, on the other hand, was able to form asters at the same concentration. A GTP hydrolysis-deficient mutant form of Ran (Q69L) in its GTP-bound form enabled wild-type TPX2 to nucleate a similar number of asters but did not increase the number of asters nucleated by the mutant TPX2 (TPX2 284).
To test whether the importin α interaction is sufficient to regulate TPX2, Xenopus egg extract was immunodepleted of endogenous TPX2 protein. The efficiency of depletion was >99% as judged from a western blot experiment and comparing TPX2-depleted extract with 1% of mock-treated extract (Figure 2B, upper panel). Importin α remained unaffected (Figure 2B, lower panel). We then reconstituted with either wild-type TPX2 or the TPX2 284 mutant (Figure 2C). The amount of exogenous wild-type TPX2 that could be added to the extract without inducing microtubule assembly was determined first. Although this concentration varied for different extract preparations, wild-type TPX2 consistently failed to induce microtubule assembly when added back to 20 nM final concentration, indicating that its activity was inhibited efficiently by endogenous importin α at this concentration (Figure 2B). In contrast, 20 nM TPX2 284 efficiently induced aster formation. Furthermore, the presence of the non-hydrolysing Ran Q69L mutant (Bischoff et al., 1994) in its GTP-bound form (RanQ69LGTP) enabled wild-type TPX2 to nucleate aster formation efficiently but did not increase the number of asters nucleated by the mutant TPX2 (Figure 2B). This indicated that the wild-type protein was active only once released from importin α by RanGTP but that the TPX2 284 mutant was constitutively active in microtubule assembly. Thus, TPX2 has one critical importin α-binding site whose mutation renders it able constitutively to induce microtubule assembly in egg extract and prevents its regulation by RanGTP.
TPX2 is a microtubule nucleator in vitro
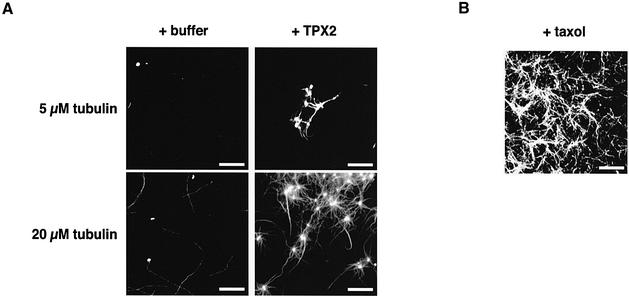
These experiments suggested a direct or indirect involvement of TPX2 in de novo microtubule formation. To test whether the effect was direct, TPX2 was added to different concentrations of purified porcine tubulin in buffer (see Materials and methods). The mixture was incubated at 37°C for 12 min and then fixed. In these assays, the human TPX2 protein was used as it is easier to produce in soluble recombinant form. The presence of 800 nM TPX2 in the reaction with 20 µM tubulin efficiently induced the assembly of apparently organized and bundled microtubule structures (Figure 3A). These structures were reminiscent of microtubule asters. At lower tubulin concentration, smaller aster-like structures were still formed (Figure 3A, top). Very few microtubules assembled spontaneously at this lower tubulin concentration in the absence of added TPX2. The structures formed at both tubulin concentrations were distinct from those induced by taxol addition, indicating that TPX2 has specific effects on microtubule assembly and organization (Figure 3B).
Fig. 3. In vitro TPX2-induced microtubule nucleation. (A) Addition of TPX2 to different concentrations of purified porcine tubulin (5 µM, upper panels, 20 µM, lower panels) induced the assembly of aster-like structures containing bundled microtubules (right panels), unlike tubulin alone (left panels). (B) Addition of the microtubule-stabilizing drug taxol induced microtubule polymerization and bundling at 20 µM tubulin. Bar: 10 µm.
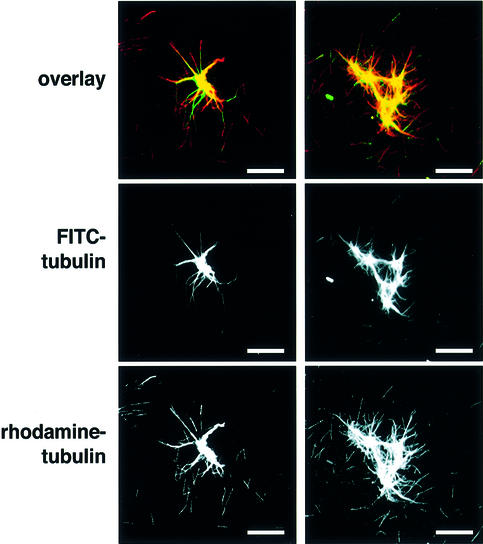
To test whether the small aster-like structures formed at low tubulin concentration could seed further microtubule assembly in the absence of soluble TPX2, a two-step experiment was performed. Aster seeds were formed in a low concentration of fluorescein (FITC)-labelled tubulin in the presence of TPX2. These asters were then pelleted and added to a higher concentration of rhodamine-labelled tubulin (Figure 4). The final structures obtained retained a FITC-labelled centre, while rhodamine-labelled microtubules were incorporated both at the growing microtubule ends and along the length of the pre-formed astral microtubules, demonstrating that the aster-like structures could act as seeds for further microtubule assembly even after removal of soluble TPX2 by centrifugation.
Fig. 4. TPX2 asters act as seeds for microtubule assembly. TPX2 asters were pre-assembled in 5 µM tubulin supplemented with FITC-labelled tubulin (middle panels, and green colour in overlay), re-purified and exposed to 20 µM tubulin supplemented with rhodamine-labelled tubulin (lower panels, and red colour in overlay). Microtubules elongated from the ends of the seed microtubules as well as along the pre-formed astral microtubules. The images are of two representative examples from the same reaction. Bar: 10 µm.
TPX2 induces aster seed formation through microtubule nucleation and bundling
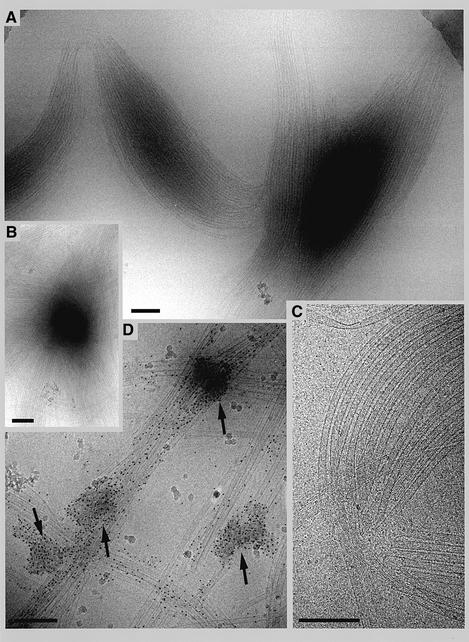
By comparing the typical fluorescence of TPX2-induced asters with that of individual in vitro polymerized microtubules (see, for example, Figure 3A, 20 µM tubulin panels), we observed that TPX2 asters consisted mostly of microtubule bundles, which were decorated by TPX2 as visualized by immunofluorescence (data not shown, but see Wittmann et al., 2000). We next examined the structure of these asters and TPX2 location in more detail by cryo-electron microscopy. At the low concentration of tubulin used, microtubules were only observed when TPX2 was present (data not shown). Two structural features were observed. The first were amorphous aggregates of varying size, which only formed in the presence of both TPX2 and tubulin (data not shown). Microtubules emanated randomly in all directions from these aggregates (Figure 5). Many structures contained an array of co-linear microtubules, which overlapped and bent at the region corresponding to the aster centre (Figure 5A–C). In order to visualize TPX2 in these structures, we used a protein A-tagged version of human TPX2 (hTPX2), which could be visualized by 5 nm colloidal gold particles coated with antibody. No gold labelling was seen when microtubules were polymerized with taxol in the absence of TPX2 (data not shown). In the presence of TPX2, gold labelling was seen both on the aggregates (Figure 5D, arrows) and along the microtubules growing outwards from these aggregates (Figure 5D). No preferential gold labelling could be detected at the ends of microtubules. The observed binding of TPX2 along microtubules is consistent with the interaction between TPX2 and pre-polymerized microtubules described previously (Wittmann et al., 2000).
Fig. 5. Visualization of TPX2-mediated microtubule assemblies by cryo-electron microscopy. (A–C) Recombinant zz-tagged TPX2 (0.8 µM) was incubated for 10 min at 37°C with 5 µM purified tubulin in BRB80. Reactions were spotted directly on to copper grids, washed, shock-frozen and structures were visualized as described previously (Dubochet et al., 1985). (D) Reactions were performed in the presence of 5 nm-gold-labelled anti-rabbit IgG to visualize zz-tagged TPX2. Bar: 200 nm.
Differential regulation of the nucleation and bundling activities of TPX2 by importin
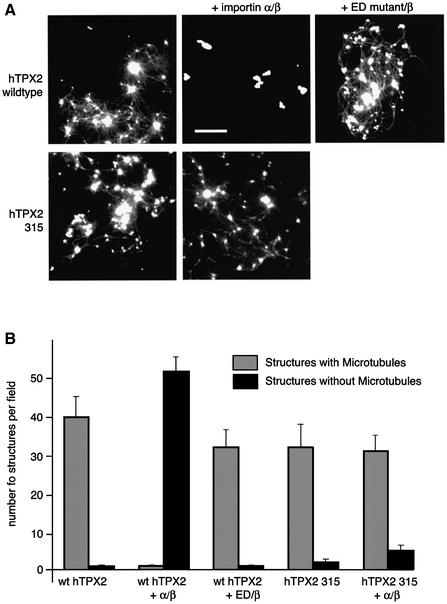
Next we examined whether importin α and β could also regulate the ability of TPX2 to nucleate microtubules in the reaction containing only human TPX2, tubulin and buffer. Either wild-type importin α or a mutant form of importin α that is defective in binding NLS proteins, the ED mutant (Gruss et al., 2001), was added to the assembly reaction together with importin β. As shown above, TPX2 induced the formation of aggregates from which microtubules emanated (Figure 6). In the presence of importin α and β, only the aggregates were observed, but no organized microtubules. Adding the same concentration of the ED mutant had little effect on TPX2 microtubule nucleation (Figure 6). A mutant form of hTPX2, in which the NLS sequence equivalent to that in Xenopus TPX2 284 was mutated, hTPX2 315, was as active as the wild-type protein in inducing microtubules but was no longer inhibited by the addition of importin α and β (Figure 6). As expected, this mutant form of hTPX2 failed to bind importin α when tested in vitro (data not shown). Thus, importin α and β regulate the ability of TPX2 to induce microtubule assembly in the purified system as they do in the complete egg extract. Importin α alone also inhibited TPX2-induced microtubule assembly, but less efficiently than together with importin β. It was shown previously that TPX2 has a higher affinity for the α/β complex than for α alone (Gruss et al., 2001).
Fig. 6. TPX2 is regulated by importin α in the purified reaction. (A) Wild-type hTPX2 (upper panels) induced both tubulin–TPX2 aggregates and outgrowing microtubules. Addition of importin α and β (upper middle panel) but not of the ED mutant protein and importin β (upper right panel) inhibited microtubule outgrowth, but aggregates were still observed. A mutant form of hTPX2 lacking the importin α-binding site (hTPX2 315, lower panels) induced microtubule asters (lower left panel), but its activity was not affected by importin α and β (lower right panel). Bar: 10 µm. (B) Aggregates with microtubules attached (light grey histograms) and aggregates with no microtubules attached (black histograms) were counted in at least 10 fields. Error bars represent standard errors.
Importin α could inhibit microtubule nucleation by TPX2 by sterically hindering TPX2 binding to tubulin. The fact that tubulin- and TPX2-containing aggregates (Figures 3, 5 and 6) were seen even in the presence of importin α (Figure 6) suggested, however, that TPX2–tubulin interaction was not abrogated by importin α interaction. To test this, hTPX2 or hTPX2 315 were incubated with taxol-polymerized microtubules in the presence or absence of importin α and β. The microtubules were then spun through a sucrose cushion and, after separation of the pelleted material by SDS–PAGE, western blot was used to detect the bound TPX2 (Figure 7A). The same amount of TPX2 was seen to co-pellet with microtubules in the presence or absence of the importins (Figure 7A, lanes 2 and 3). Thus, although the ability of TPX2 to nucleate microtubules is abolished by importin α, the binding of TPX2 to pre-polymerized microtubules is not prevented.
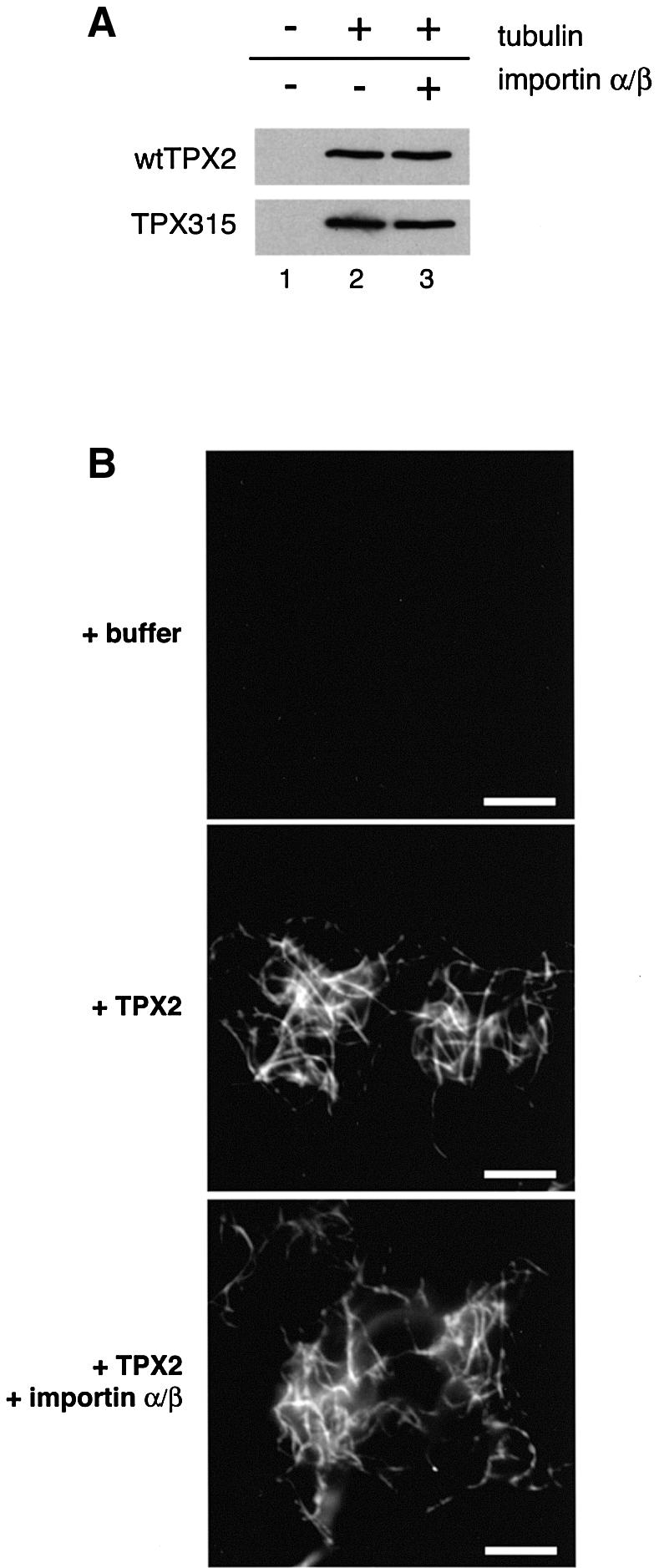
Fig. 7. TPX2 binds to and bundles microtubules independently of importin α. (A) Wild-type hTPX2 or mutant hTPX2 (hTPX2 315) was incubated with or without taxol-polymerized microtubules in the presence or absence of importin α and β as indicated, samples were pelleted and TPX2 detected in pellet fractions by SDS–PAGE and western blotting. (B) Microtubules were polymerized from a solution of 40 µM tubulin in the presence of taxol and diluted 1:40. Either buffer alone (top panel), 0.2 µM TPX2 (middle panel), or TPX2 and both 5 µM importin α and importin β (lower panel) were added. Bar: 10 µm.
The microtubules formed under the influence of TPX2 both in extracts and in the pure system are bundled (see above). We wished to test whether TPX2-induced bundling was also regulated by importin α/β. Microtubules were assembled in the presence of taxol and then diluted in taxol-containing buffer and cooled to avoid further microtubule polymerization (Figure 7B, top panel, note that images were acquired under conditions where single microtubules were not seen). Addition of TPX2 caused these pre-polymerized microtubules to form bundles (Figure 7B, middle panel). This bundling activity was unaffected by the addition of importin α and β (Figure 7B, lower panel) even when a large excess over TPX2 was added. This suggests that the ability of TPX2 to bind pre-polymerized microtubules may be a reflection of the interaction that allows it to bundle microtubules, and that this interaction is not affected by importin α.
Discussion
TPX2 interaction with importin α
In recent years, evidence has been presented that the RanGTPase regulates different target proteins at several stages in the process of assembly of the mitotic spindle (Clarke and Zhang, 2001; Dasso, 2002; Hetzer et al., 2002). One of the identified targets of Ran in spindle assembly is TPX2. In Xenopus M-phase extracts, TPX2 is released from importin α and β by RanGTP, which is produced preferentially in the vicinity of chromosomes due to the presence on chromatin of RCC1, the RanGEF (Carazo-Salas et al., 1999). Once released, TPX2 participates in the induction of microtubule assembly around chromatin at the initial stages of spindle formation (Gruss et al., 2001).
In this study, we have identified the site on TPX2 that is critical for binding to importin α in vitro and in Xenopus M-phase extracts. Mutation of two amino acids in the full-length protein abolished interaction between TPX2 and importin α in vitro. The mutant TPX2 protein was fully functional in microtubule assembly, but was insensitive to importin α’s inhibitory effect and thus to regulation by Ran, resulting in a constitutively active form of TPX2. This observation is important for two reasons. First, it represents proof of the model that Ran acts indirectly on TPX2 (and possibly other proteins involved in spindle assembly) by interacting with importin β and, as a consequence, by releasing TPX2 from importin α and β. Secondly, it shows that the interaction of TPX2 and importin α is sufficient to regulate the process of microtubule production in Xenopus M-phase extracts. No other Ran target, including NuMA, has to be activated to induce microtubule assembly. In addition, RanGTP cannot induce microtubule assembly in these extracts after depletion of TPX2 (Gruss et al., 2001), showing that TPX2 release is both necessary and sufficient for this early stage of spindle assembly. This further suggests that the TPX2- and Ran-induced aster-like structures seen in the egg extract are in fact identical, and intermediates in spindle assembly. Note that additional Ran targets, such as activities that promote microtubule stability (Carazo-Salas et al., 2001; Wilde et al., 2001) and antiparallel organization (Wilde et al., 2001), seem to be essential for spindle assembly and remain to be characterized.
Taken together, our data confirm the idea that the interaction of TPX2 with importin α and β in M-phase extracts is crucial for the inhibition of TPX2 activity. This mechanism might be particularly important in large cells, such as oocytes and embryonic cells, to prevent ectopic microtubule assembly, but TPX2-dependent microtubule assembly is also critical for spindle organization in somatic cells (Gruss et al., 2002). Furthermore, Ran plays an essential role in spindle formation in the small cells of the Caenorhabditis elegans zygote (Askjaer et al., 2002; Bamba et al., 2002). Interaction with import receptors could also be essential for the efficient inhib ition of TPX2 activity in the interphase cytoplasm. Overexpression of TPX2 in human somatic cells leads to severe defects in cytoplasmic microtubule organization in interphase (Gruss et al., 2002). The efficient binding of newly translated TPX2 to import receptors followed by its import into the nucleus would alleviate such damaging effects by preventing TPX2 from premature interaction with tubulin or microtubules during interphase. TPX2 would then only be activated following nuclear envelope breakdown at the beginning of mitosis.
The mechanism of TPX2 action in microtubule assembly
TPX2 originally was characterized functionally as a protein that binds to polymerized microtubules and targets the motor Xklp2 to them (Wittmann et al., 1998, 2000). These observations defined TPX2 as a MAP. Its binding to both microtubules and Xklp2 results in targeting of the complex to microtubules where dynein activity mediates the movement of both TPX2 and Xklp2 towards microtubule minus ends at the spindle poles (Wittmann et al., 1998, 2000). In this context, TPX2 and Xklp2 are required to help focus the spindle poles, a function that may reflect the activity of TPX2 in bundling microtubules reported here.
Many MAPs stabilize microtubules (Cassimeris and Spittle, 2001). For example, when XMAP215 was partially depleted from Xenopus extracts, microtubules nucleated from centrosomes were less stable (Tournebize et al., 2000). In contrast, in Xenopus extracts depleted of TPX2, the average length of microtubules nucleated by centrosomes was not decreased, even under conditions where NLS-containing proteins were released from importins by RanGTP (Gruss et al., 2002). Taken together with the data presented here, these observations clearly favour the model that TPX2 functions in nucleation rather than in stabilization of microtubules.
The conserved γ-tubulin ring complex is considered to be the main microtubule-nucleating activity in eukaryotic cells, particularly during centrosome-mediated microtubule nucleation (Schiebel, 2000). XMAP215 recently was reported also to play an important role in centrosomal nucleation (Popov et al., 2002). RanGTP-mediated microtubule assembly, a reflection of chromatin-induced microtubule formation, was abolished in Xenopus egg extract depleted of either γ-tubulin or XMAP215 (Wilde and Zheng, 1999). Thus, TPX2, XMAP215 and γ-tubulin are all necessary for Ran-induced nucleation of stable microtubule structures in these mitotic extracts.
It may, at first, seem strange that so many different activities are required for microtubule nucleation, but the nucleation process is a collection of structurally distinct steps that may need several activities. One step is the templating of a tube containing 13 protofilaments, the function proposed for the γ-tubulin ring complex (Moritz and Agard, 2001). A second is the formation of tubulin oligomers that are stable enough to allow further addition to the growing tube. Protofilament stabilization may occur before as well as after tube formation, and stabilization during these two processes may not utilize the identical mechanism (Schiebel, 2000).
Seen from this perspective, the participation of multiple factors in microtubule nucleation appears more reasonable and there are additional reasons why several nucleating activities may be required. TPX2 activity, as mentioned above, is restricted to M phase, and the activity of other microtubule nucleators is also likely to be under cell cycle control. XMAP215 activity has been proposed to be diminished in mitosis by cell cycle-dependent phosphorylation (Vasquez et al., 1999). Moreover, while depletion of TPX2 from mitotic cells had little effect on centrosome-nucleated microtubules, chromatin-dependent microtubules were either greatly diminished in number or absent (Gruss et al., 2002). This indicates that different classes of microtubules contribute to mitotic microtubule structures. Since the absence of TPX2 prevented bipolar spindle formation, the two types of microtubule must perform distinct functions during spindle assembly. These observations could reflect a more general phenomenon by which specific nucleators would only be required for certain periods during the cell cycle, or at certain locations in the cell to which microtubule formation needs to be targeted.
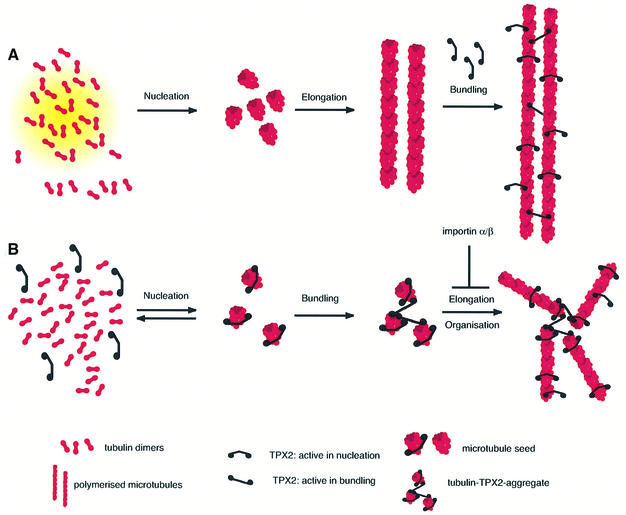
In relation to the promotion of microtubule nucleation by TPX2, it is notable that, despite numerous attempts, no stable interaction between dimeric tubulin and TPX2 has been observed (I.Vernos, personal communication; our unpublished data). However, in the presence of pure tubulin, TPX2 induces the formation of tubulin-containing aggregates, aster-like structures and bundles of microtubules. Importin α completely inhibited the ability of TPX2 to induce the formation of microtubules in the recombinant system, as it does in complete egg extract. Nevertheless, importin α did not prevent either TPX2 bundling of pre-assembled microtubules or the induction of TPX2- and tubulin-containing aggregates. These observations are important indicators of how TPX2 might function. They show that TPX2 must have at least two different modes of interaction with tubulin. First, TPX2 functions on tubulin dimers or oligomers and might promote their assembly into small, relatively stable intermediates, which are competent to elongate microtubules. It is only this function in the TPX2-mediated microtubule assembly process that is inhibited by importin α: seeds can still form, but they cannot nucleate microtubule formation (Figure 8). Secondly, TPX2 can bundle microtubules. This results in arrays of parallel microtubules and probably also contributes to the aster-like structures observed upon microtubule assembly in the presence of TPX2. If TPX2 bridges interactions of small microtubule intermediates inducing the formation of an aggregate consisting of multiple seed-like structures, microtubules can grow in different directions from this aggregate and asters would be formed (see Figure 8). Although TPX2 does not detectably induce tubulin aggregates in egg extract, probably due to the presence and influence of multiple additional tubulin-interacting activities, we speculate that the aggregates we see in the pure system reflect the initial binding between tubulin and TPX2 that is a necessary first step in the importin α-regulated process of generating well-organized microtubule structures.
Fig. 8. A model of the activities of TPX2 in microtubule nucleation and bundling. (A) Microtubules are assembled in the absence of TPX2, e.g. by the action of taxol. Small intermediates (‘seeds’) are stabilized and microtubules elongate from these seeds. Added TPX2 can interact with a polymerized microtubule at several sites and promote parallel bundling. The bundling activity is not inhibited by importin α/β. (B) Microtubules are assembled in the presence of TPX2. TPX2 initially nucleates small seeds, which eventually are bridged with one another by TPX2, reflecting TPX2 bundling activity. From these clusters of seeds, microtubules can grow in all directions, and organized, aster-like structures are formed. The function of TPX2 in generating seeds that are competent for further elongation is inhibited by importin α/β.
Materials and methods
Cloning of TPX2 expression constructs
Xenopus TPX2 fragments were PCR amplified and cloned into the _Nco_I and _Bam_HI sites of the zzpQE60 expression vector, which encodes two N-terminal IgG-binding domains of Staphylococcus aureus protein A (Gruss et al., 2001). In the TPX2 mutant constructs, the following amino acids were changed to alanines: 124, K124 and K126; 284, K284 and R285; 550, K550 and K551; h315, K315 and R316. Mutations were inserted by site-directed mutagenesis, and the mutated regions of the open reading frame were sequenced.
Expression and purification of recombinant proteins
TPX2 (full-length protein and fragments) was expressed in E.coli and purified as described previously (Gruss et al., 2001). For binding assays, Xenopus TPX2 was purified further on a Mono S column (Pharmacia). Importin α and the ED mutant of importin α were expressed and purified as described previously (Görlich et al., 1994; Gruss et al., 2001). RanQ69L was expressed, purified and loaded with GTP as described previously (Weis et al., 1996).
TPX2–importin α binding assay
Bacterially expressed zz-tagged Xenopus TPX2 (900 pmol) was pre-bound to 10 µl of IgG–Sepharose in buffer A (50 mM Tris–HCl pH 7.5, 500 mM NaCl, 5 mM MgCl2, 10 mM β-mercaptoethanol, 0.01% Triton X-100) for 1 h at 23°C. Beads were washed five times with 500 µl of buffer A, and 150 pmol of importin α were added and incubated for 1 h at 23°C. After binding, beads were washed as before, and bound protein was eluted with 100 µl of 1 M MgCl2, 50 mM Tris–HCl pH 7.5, precipitated with acetone and separated by SDS–PAGE (Laemmli, 1970) followed by Coomassie Blue staining.
Assays for in vitro microtubule assembly
Porcine tubulin was purified and labelled with tetramethylrhodamine or FITC (Mitchison and Kirschner, 1984; Hyman et al., 1991). Microtubule assembly reactions consisted of 9 µl of BRB80 (80 mM PIPES, 1 mM K-EGTA, 1 mM MgCl2 pH 6.8) and 5, 10, 15 or 20 µM tubulin containing 10% rhodamine-labelled tubulin and 1 mM GTP. Samples were supplemented with either buffer or TPX2 protein at the concentration specified in the text, incubated for 12 min at 37°C and fixed, spun down and analysed as described previously (Wittmann et al., 1998). For the two-step aster assembly reaction, 5 µM tubulin containing 10% FITC-labelled tubulin, 1 mM GTP and 0.8 µM TPX2 was incubated for 10 min at 37°C to assemble aster seeds. The seeds were then spun down for 5 min at 10 000 r.p.m. in an Eppendorf desktop centrifuge at 37°C and supplemented with 20 µM rhodamine-labelled tubulin mixture. The two-colour reaction was incubated a further 2 min at 37°C, fixed and spun down (Wittmann et al., 1998).
For importin α/β inhibition of TPX2-induced microtubule nucleation, importin α or the ED mutant was added to 1 µM, together with 1 µM importin β, to the nucleation assay that was processed as described above at room temperature to prevent protein precipitation. Numbers of aggregates with or without associated microtubules in a given field were counted. Error bars are standard errors of the mean.
To test the bundling activity of TPX2, microtubules were first polymerized from a solution of 40 µM tubulin in BRB80 in the presence of 10 µM taxol (paclitaxel, Sigma) and 1 mM GTP for 10 min at 37°C. Microtubules were then diluted 1:40 in BRB80, 10 µM taxol, 1 mM GTP, and incubated further for 10 min at 37°C. To exclude effects on microtubule polymerization, the diluted microtubules were put on ice, mixed with buffer or with 0.2 µM TPX2, or 0.2 µM TPX2 and both 5 µM importin α and β, incubated for 5 min on ice and fixed and processed as described previously (Wittmann et al., 1998).
Microtubule co-pelleting assay
Purified recombinant TPX2 protein (0.8 µM) was incubated for 30 min with or without 10 µM of both importin α and β at 37°C in 50 µl of BRB80 containing 4 mM MgCl2, 4 mM ATP, 4 mM GTP, 0.4 µg/µl porcine brain tubulin, 100 mM NaCl and 20 µM taxol. After centrifugation at 37°C on a BRB80 sucrose cushion (BRB80, 10% sucrose, 10 µM taxol, 2 mM GTP) at 100 000 g for 20 min, the pellets were resuspended in SDS sample buffer (Laemmli, 1970) and separated by SDS–PAGE followed by western blotting, probing for the protein A tag with secondary antibody (Giet and Prigent, 2001).
Electron microscopy
Recombinant TPX2 (0.8 µM) was incubated with purified 5 µM porcine brain tubulin for 10 min at 37°C in BRB80 containing 5 mM MgCl2. Reactions were spotted on copper grids for electron microscopy, washed twice with BRB80 and shock-frozen as described previously (Dubochet et al., 1985). To visualize zz-tagged TPX2, the reactions were performed in the presence of 5 nm gold-labelled goat anti-mouse antibodies (BBI international; 1:10 dilution). Images were taken on a CM-120 (Biotwin) electron microscope.
Microtubule assembly assay in Xenopus M-phase extracts
A 10 µl aliquot of Xenopus M-phase extract (Murray, 1991) was supplemented with rhodamine tubulin (Hyman et al., 1991), and either 15 µM importin α or buffer was added. Thereafter, 160 nM TPX2 wild-type or mutant protein was added and incubated for 25 min at 20°C. Samples (1.5 µl) were fixed and squashed (Sawin and Mitchison, 1991). Image accquisition and analysis were on a Leica confocal microscope. Reactions were quantified by counting all structures from at least three samples. Histograms represent mean values, and error bars standard deviation. TPX2 was depleted from M-phase extracts as described previously (Gruss et al., 2001). Depleted extract was reconstituted with 20 nM wild-type TPX2 or mutant TPX2.
Western blotting and antibodies
Proteins were separated by SDS–PAGE (Laemmli, 1970), transferred to nitrocellulose and decorated with antibodies against human TPX2 or importin α (Gruss et al., 2002), which were visualized with secondary antibodies coupled to horseradish peroxidase (Amersham).
Acknowledgments
Acknowledgements
We would like to thank Margy Koffa, Tobias Walter and Isabelle Vernos for critically reading the manuscript, and Thomas Mayer for technical advice. C.A.S. and R.E.C.-S. were supported by EMBL PhD fellowships, and O.J.G. was supported by EMBL and from the Louis-Jeantet Prize for Medicine awarded to I.W.M.
References
- Askjaer P., Galy,V., Hannak,E. and Mattaj,I.W. (2002) The Ran GTPase cycle and importins α and β are essential for spindle formation and nuclear envelope assembly in living Caenorhabditis elegans embryos. Mol. Biol. Cell, 13, 4355–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamba C., Bobinnec,Y., Fukuda,M. and Nishida,E. (2002) The GTPase Ran regulates chromosome positioning and nuclear envelope assembly in vivo. Curr. Biol., 12, 503–507. [DOI] [PubMed] [Google Scholar]
- Bischoff F.R., Klebe,C., Kretschmer,J., Wittinghofer,A. and Ponstingl,H. (1994) RanGAP1 induces GTPase activity of nuclear Ras-related Ran. Proc. Natl Acad. Sci. USA, 91, 2587–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carazo-Salas R.E., Guarguaglini,G., Gruss,O.J., Segref,A., Karsenti,E. and Mattaj,I.W. (1999) Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature, 400, 178–181. [DOI] [PubMed] [Google Scholar]
- Carazo-Salas R.E., Gruss,O.J., Mattaj,I.W. and Karsenti,E. (2001) Ran-GTP coordinates regulation of microtubule nucleation and dynamics during mitotic-spindle assembly. Nat. Cell Biol., 3, 228–234. [DOI] [PubMed] [Google Scholar]
- Cassimeris L. and Spittle,C. (2001) Regulation of microtubule-associated proteins. Int. Rev. Cytol., 210, 163–226. [DOI] [PubMed] [Google Scholar]
- Clarke P.R. and Zhang,C. (2001) Ran GTPase: a master regulator of nuclear structure and function during the eukaryotic cell division cycle? Trends Cell Biol., 11, 366–371. [DOI] [PubMed] [Google Scholar]
- Compton D.A. (2000) Spindle assembly in animal cells. Annu. Rev. Biochem., 69, 95–114. [DOI] [PubMed] [Google Scholar]
- Dasso M. (2002) The Ran GTPase: theme and variations. Curr. Biol., 12, R502–R508. [DOI] [PubMed] [Google Scholar]
- Dubochet J.M., Lepault,A.J. and McDowall,A.W. (1985) Cryo-electron microscopy of vitrified biological specimen. Trends Biochem. Sci., 10, 143–146. [Google Scholar]
- Giet R. and Prigent,C. (2001) The non-catalytic domain of the Xenopus laevis auroraA kinase localises the protein to the centrosome. J. Cell Sci., 114, 2095–2104. [DOI] [PubMed] [Google Scholar]
- Görlich D., Prehn,S., Laskey,R.A. and Hartmann,E. (1994) Isolation of a protein that is essential for the first step of nuclear protein import. Cell, 79, 767–778. [DOI] [PubMed] [Google Scholar]
- Gruss O.J. et al. (2001) Ran induces spindle assembly by reversing the inhibitory effect of importin α on TPX2 activity. Cell, 104, 83–93. [DOI] [PubMed] [Google Scholar]
- Gruss O.J., Wittmann,M., Yokoyama,H., Pepperkok,R., Kufer,T., Silljé,H., Karsenti,E., Mattaj,I.W. and Vernos,I. (2002) Chromosome-induced microtubule assembly mediated by TPX2 is required for spindle formation in HeLa cells. Nat. Cell Biol., 4, 871–879. [DOI] [PubMed] [Google Scholar]
- Heidebrecht H.J., Buck,F., Steinmann,J., Sprenger,R., Wacker,H.H. and Parwaresch,R. (1997) p100: a novel proliferation-associated nuclear protein specifically restricted to cell cycle phases S, G2 and M. Blood, 90, 226–233. [PubMed] [Google Scholar]
- Hetzer M., Gruss,O.J. and Mattaj,I.W. (2002) The Ran GTPase as a marker for chromosome position in spindle formation and nuclear envelope assembly. Nat. Cell Biol., 4, E177–E184. [DOI] [PubMed] [Google Scholar]
- Hyman A., Drechsel,D., Kellogg,D., Salser,S., Sawin,K., Steffen,P., Wordeman,L. and Mitchison,T. (1991) Preparation of modified tubulins. Methods Enzymol., 196, 478–485. [DOI] [PubMed] [Google Scholar]
- Kalab P., Pu,R.T. and Dasso,M. (1999) The Ran GTPase regulates mitotic spindle assembly. Curr. Biol., 9, 481–484. [DOI] [PubMed] [Google Scholar]
- Karsenti E. and Vernos,I. (2001) The mitotic spindle: a self-made machine. Science, 294, 543–547. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 277, 680–685. [DOI] [PubMed] [Google Scholar]
- Mitchison T. and Kirschner,M. (1984) Microtubule assembly nucleated by isolated centrosomes. Nature, 312, 232–237. [DOI] [PubMed] [Google Scholar]
- Moritz M. and Agard,D.A. (2001) γ-tubulin complexes and microtubule nucleation. Curr. Opin. Struct. Biol., 11, 174–181. [DOI] [PubMed] [Google Scholar]
- Murray A. (1991) Cell cycle extracts. In Kay,B.K. and Peng,H.B. (eds), Xenopus laevis: Practical Uses in Cell and Molecular Biology, Vol. 36. Academic Press, San Diego, CA, pp. 581–605.
- Nachury M.V., Maresca,T.J., Salmon,W.C., Waterman-Storer,C.M., Heald,R. and Weis,K. (2001) Importin β is a mitotic target of the small GTPase Ran in spindle assembly. Cell, 104, 95–106. [DOI] [PubMed] [Google Scholar]
- Nakai K. and Horton,P. (1999) PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem. Sci., 24, 34–35. [DOI] [PubMed] [Google Scholar]
- Ohba T., Nakamura,M., Nishitani,H. and Nishimoto,T. (1999) Self-organization of microtubule asters induced in Xenopus egg extracts by GTP-bound Ran. Science, 284, 1356–1358. [DOI] [PubMed] [Google Scholar]
- Popov A., Severin,F. and Karsenti,E. (2002) XMAP215 is required for the microtubule-nucleating activity of centrosomes. Curr. Biol., 12, 1326–1330. [DOI] [PubMed] [Google Scholar]
- Sawin K.E. and Mitchison,T.J. (1991) Mitotic spindle assembly by two different pathways in vitro. J. Cell Biol., 112, 925–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiebel E. (2000) γ-tubulin complexes: binding to the centrosome, regulation and microtubule nucleation. Curr. Opin. Cell Biol., 12, 113–118. [DOI] [PubMed] [Google Scholar]
- Tournebize R. et al. (2000) Control of microtubule dynamics by the antagonistic activities of XMAP215 and XKCM1 in Xenopus egg extracts. Nat. Cell Biol., 2, 13–19. [DOI] [PubMed] [Google Scholar]
- Vasquez R.J., Gard,D.L. and Cassimeris,L. (1999) Phosphorylation by CDK1 regulates XMAP215 function in vitro. Cell Motil. Cytoskeleton, 43, 310–321. [DOI] [PubMed] [Google Scholar]
- Weis K., Dingwall,C. and Lamond,A.I. (1996) Characterization of the nuclear protein import mechanism using Ran mutants with altered nucleotide binding specificities. EMBO J., 15, 7120–7128. [PMC free article] [PubMed] [Google Scholar]
- Wiese C., Wilde,A., Moore,M.S., Adam,S.A., Merdes,A. and Zheng,Y. (2001) Role of importin β in coupling Ran to downstream targets in microtubule assembly. Science, 291, 653–656. [DOI] [PubMed] [Google Scholar]
- Wilde A. and Zheng,Y. (1999) Stimulation of microtubule aster formation and spindle assembly by the small GTPase Ran. Science, 284, 1359–1362. [DOI] [PubMed] [Google Scholar]
- Wilde A., Lizarraga,S.B., Zhang,L., Wiese,C., Gliksman,N.R., Walczak,C.E. and Zheng,Y. (2001) Ran stimulates spindle assembly by altering microtubule dynamics and the balance of motor activities. Nat. Cell Biol., 3, 221–227. [DOI] [PubMed] [Google Scholar]
- Wittmann T., Boleti,H., Antony,C., Karsenti,E. and Vernos,I. (1998) Localization of the kinesin-like protein Xklp2 to spindle poles requires a leucine zipper, a microtubule-associated protein and dynein. J. Cell Biol., 143, 673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann T., Wilm,M., Karsenti,E. and Vernos,I. (2000) TPX2, a novel Xenopus MAP involved in spindle pole organization. J. Cell Biol., 149, 1405–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann T., Hyman,A. and Desai,A. (2001) The spindle: a dynamic assembly of microtubules and motors. Nat. Cell Biol., 3, E28–E34. [DOI] [PubMed] [Google Scholar]
- Zhang C., Hughes,M. and Clarke,P.R. (1999) Ran-GTP stabilises microtubule asters and inhibits nuclear assembly in Xenopus egg extracts. J. Cell Sci., 112, 2453–2461. [DOI] [PubMed] [Google Scholar]