Stimulation of naïve T-cell adhesion and immunological synapse formation by chemokine-dependent and -independent mechanisms (original) (raw)
Abstract
Chemokines adsorbed to the cell surface play an important role in the initial interactions of T cells with endothelial cells, and may also have a role in T-cell interactions with dendritic cells. Therefore, we examined the effect of surface-adsorbed chemokines on the interaction of naïve murine splenic T cells with supported bilayers containing intercellular adhesion molecule (ICAM)-1, or with bone marrow-derived cultured dendritic cells in the presence and absence of relevant MHC–peptide complexes. Naïve T cells formed immunological synapses, defined as a ring of lymphocyte function associated (LFA)-1–ICAM-1 interactions surrounding a central cluster of MHC–peptide complexes, on supported planar bilayers containing ICAM-1 and relevant MHC–peptide complexes. Chemokines stimulated an increase in the percentage of naïve cells that adhered to ICAM-1, but did not increase the average number of LFA-1–ICAM-1 interactions in the contact area. In contrast, relevant MHC–peptide complexes resulted in a small increase in the proportion of interacting T cells, but stimulated an 8-fold increase in the number of LFA-1–ICAM-1 interactions in each contact formed. Naïve T cells displayed a significant basal adhesion to bone marrow dendritic cells that was further increased when relevant chemokines were adsorbed to the dendritic cell surface. However, basal and antigen-stimulated T-cell adhesion to dendritic cells was not sensitive to pertussis toxin. Thus, there are chemokine-independent mechanisms that initiate adhesion between T cells and dendritic cells.
Introduction
T cells are stimulated by the interaction of a clonotypic T-cell receptor (TCR) with MHC–peptide complexes (MHCps) on the surface of antigen-presenting cells (APCs). Since both the TCR and MHCp are integral membrane proteins, their interaction requires contact between the T cell and APC. The specialized cell–cell junction formed by T cells and APCs in response to TCR triggering is described as an immunological synapse composed of supramolecular activation clusters (SMACs). The dominant pattern that correlates with changes of transcriptional regulation is a central SMAC (cSMAC) of TCR–MHCp interactions surrounded by a peripheral SMAC (pSMAC) of lymphocyte function associated (LFA)-1–intercellular adhesion molecule (ICAM)-1 interactions.1–3 The most potent APCs for stimulation of naïve T cells are dendritic cells (DCs).4 A reversible antigen-independent binding mechanism allows naive T cells to survey DCs for cognate antigen.5,6 T cells responding to antigen form clusters with DCs, both in vitro and in vivo.5–7 This clustering creates intimate T cell–APC contacts in which the T cells receive stimulatory signals from engaged TCR and co-stimulatory signals from CD28–CD86 interactions. It is likely that other receptor ligand pairs are involved in this process, possibly including chemokine receptors and their ligands.
Circulating naïve T cells are rounded and non-polarized with a uniform radial distribution of cell surface receptors.8,9 These cells are relatively non-motile and their integrin adhesion molecules are maintained in a low activity state.10–12 Chemokines rapidly transform these undifferentiated cells into polarized, motile cells as they first trigger an increase in integrin avidity and then set the stage for extravasation.13 DCs produce chemokines and use these chemokines to attract specific subpopulations of T cells.14 Therefore, one of the receptor systems that could be involved in the initial interaction of the T cell with DCs are the chemokine receptors and their ligands.
One of the most notable characteristics of DCs is their antigen-independent interaction with naïve T cells.5,15,16 Trautmann and colleagues15,16 demonstrated that adhesion of naïve T cells to DCs induces a calcium response and the polarization of some molecules involved in immunological synapse formation. Since chemokine signalling triggers calcium mobilization and polarization in responding cells,8 we hypothesized that chemokine receptors may be involved in initiating adhesion of naïve T cells to DCs. Chemokine receptor signalling results in the rapid polarization of T lymphocytes. Exposure to chemoattractant gradients induces the formation of a cell leading edge or lamellapodium, and a cell trailing edge or uropod.10 In addition to the morphological polarization of the cell, studies have demonstrated a polarization of cell signalling molecules, cellular receptors and actin polymerization. Chemokine-induced actin polymerization at the leading edge of responding cells17 might promote the T cell to interact with and probe the APC surface by generating membrane protrusions. A prominent feature of T cell–DC interactions in vivo is the presence of T-cell protrusions that indent the DC surface.18 Concomitant polarization of cell surface receptors might facilitate T cell–APC interaction. For example, the T-cell lamellipodium is 5–10-fold more sensitive to MHCp or anti-CD3 mAb than the uropod,19,20 suggesting that components of responding cells are compartmentalized even before recognition of antigen and formation of the immunological synapse.
To study the role of chemokines in the T-cell response to antigen, we examined immunological synapse formation by naïve T cells interacting with antigen-presenting planar phospholipid bilayers in the presence or absence of chemokine adsorbed to the same surface. Naïve T cells form immunological synapses with these bilayers in the absence of any chemokine. Addition of chemokines to the system has no effect on the accumulation of receptors (IEk, CD80 and ICAM-1) within the immunological synapse. However, chemokines (CXCL12 and CCL21) increase the number of cells that interact with the substrate in the presence and absence of agonist MHCp. Therefore, the population of cells capable of response to antigen is increased. Similarly, when we examined conjugate formation by naïve T cells and DCs in the presence or absence of chemokines, we found that the initial antigen-independent adhesion is increased by the immobilization of chemokines on the DC surface. In contrast, naïve T cells interact with antigen-pulsed DCs equally well in the presence or absence of chemokines, and pertussis toxin had little effect on these interactions. Thus, DCs were able to efficiently initiate antigen presentation to naïve T cells in the absence of pertussis toxin-sensitive signalling.
Materials and Methods
Purification of naïve T cells
2B4 transgenic mice were obtained from O. Kanagawa (Washington University, St. Louis, MO). T cells from the spleen of 2B4 mice were purified by nylon wool adherence followed by incubation on tissue culture-treated plastic dishes. The cells did not proliferate in response to Con A, indicating a high T-cell purity.
Preparation of bone-marrow derived dendritic cells
Bone-marrow derived DCs were prepared as previously described.21 The bone marrow cell suspensions were depleted of T cells by treatment with Thy 1 antibody as 10% AT83A supernatant provided by E. R. Unanue (Washington University, St. Louis, MO) and 10% rabbit compliment at 37° for 45 min with occasional mixing. The remaining cells were then washed and resuspended at 106 cells/ml, plated onto tissue culture-treated plastic dishes and incubated at 37° overnight for removal of adherent monocytes. The next day, the cells were washed, resuspended at 2·5×105 cells/ml, and differentiated by culture with 1000 units/ml granulocyte monocyte colony stimulating factor (GM-CSF) and interleukin-4 (IL-4). On day 4, the cells were washed and recultured in GM-CSF and IL-4. Finally, on day 6, the cells were treated with 1000 units/ml tumour necrosis factor α (TNFα) to induce their maturation and left unpulsed or pulsed with 1 µm moth cytochrome C (MCC) 91-103 peptide.
Formation of planar phospholipid bilayers
Glycosylphosphatidylinositol (GPI)-CD80 and GPI-ICAM-1 expressed in baby hamster kidney (BHK) cells, and GPI-IEk expressed in Chinese hamster ovary (CHO) cells, were purified and labelled with Cy3 (Amersham, Pharmacia, Piscataway, NJ) for CD80, Cy5 (Amersham, Pharmacia) for ICAM-1, and Oregon green (Molecular Probes, Eugene, OR) for IEk.3 The proteins were then reconstituted into egg phosphatidylcholine liposomes as described previously.22 Planar bilayers were formed by sandwiching liposome droplets between two clean glass coverslips in a parallel plate flow chamber (Bioptechs, Butler, PA). To generate lower densities of the agonist MHCp, MHC molecules were loaded with mixtures of MCC 91-103 and the null peptide MCC 99A. The MCC 99A concentration was varied to maintain a total peptide concentration of 100 µm in all experiments. This ‘null’ MHCp has been found to synergize with the agonist peptide, allowing responses to 10–100-fold lower concentrations of the agonist MHCp.23 The mixture of slow-interacting agonist and fast-interacting null peptides may reflect a more physiological situation, in which some self peptides act as coagonists with the MHCp. Molecular densities of ICAM-1, CD80, I-Ek and MCC 91-103 loaded I-Ek were determined by radioimmunoassay using iodinated YN1/1,24 1610A1,25 14-4-4 and D426 for detection, respectively. Early on in these studies we had hypothesized that the CD28–CD80 interaction would play an important role in immunological synapse formation by naïve murine CD4 T cells. However, when these experiments were underway we determined that CD80 inclusion in the bilayer had no effect on synapse formation.27 Therefore, some later experiments were performed without CD80.
Adsorption of chemokines to planar bilayers
Chemokines were adsorbed to the planar phospholipid bilayers based on the heparin-binding activity of chemokines.28 Planar phospholipid bilayers were formed between a coverslip and the microaqueduct slide in a Bioptechs parallel plate flow chamber (Bioptechs). Heparin (2 mg/ml) was dissolved in HEPES buffered saline (HBS) with 1% human serum albumin (HSA), injected into the flow chamber, and allowed to interact with the bilayers for 20 min at 37°. The bilayers were then washed thoroughly to remove unbound heparin. The chemokine CXCL12 or CCL21 (1 µg/ml) was injected into the flow cell and allowed to adsorb to the bilayers for 10 min at 37°. Unbound chemokines were then removed by thorough washing. Radioimmunoassays were performed to ensure adsorption of chemokine to the bilayer using iodinated antibody to CXCL12 for detection (R&D Systems, Minneapolis, MN).
Planar bilayer adhesion assays
Naïve T cells were injected into the flow chamber and allowed to interact with bilayers for 20 min at 37°. Transmitted light images of input cells were acquired. Unbound cells were then removed by gentle washing at a wall shear stress of ∼1 dyne/cm2. Images were acquired of the remaining bound cells. The percentage of adherent cells was then determined by comparison of images of input and bound cells.
Determination of bound receptor densities
The density of bound molecules in the cell contact areas was determined as previously described.27 Briefly, the specific activity or number of fluorescent units per molecule was determined by imaging bilayers containing fluorescently labelled receptors at known molecular densities (determined by radioimmunometric assay as above). Regions of the synapse with fluorescence values exceeding the noise level in the neighbouring bilayer areas (usually ∼5% of the bilayer values) were marked and the fluorescence density and area of these regions were measured. The density of molecules in a neighbouring area outside the area of accumulation was used to estimate the density of free molecules, which was then subtracted from the total value in the area of accumulation to define the density of specific receptor–ligand interactions in the defined area. The threshold for detection is about 10 molecules/µm2 accumulated in an area of 1 µm2 (10 molecules).
Cell conjugate assays
Conjugates between DCs and naïve T cells were established and quantified as described.15 Briefly, 100 µl DCs in HBS at a concentration of 5×107 DCs/ml was attached to poly l-lysine coated coverslips assembled in a flow chamber. In some cases, chemokine was then adsorbed to the DCs by incubation of 1 µg/ml chemokine at 37° for 20 min. Unbound chemokine was removed by gentle washing. T cells were labelled at 2×106 cells/ml with cell tracker green (Molecular Probes) using the manufacturer's recommended conditions so that they could easily be distinguished from unlabelled DCs by both morphology and fluorescence. In some cases, T cells were pretreated with 100 ng/ml pertussis toxin for 2 hr at 37°. Then, 100 µl T cells at a concentration of 2·5×108 cells/ml was injected into the flow chamber in HBS with 1% HSA and allowed to interact with the DCs for 20 min at 37°. Since protein was present in the buffer, few T cells interacted with the poly l-lysine coated glass coverslips. Transmitted light and fluorescent images were acquired of the input cells. Then, unbound cells were removed from the flow chamber by gentle washing at a shear stress of ∼1 dyne/cm2. Images were acquired of the remaining bound cells. The percentage of T cells interacting with DCs was then determined and normalized for the number of DCs attached to the coverslip in each flow chamber.
Statistical analysis
Statistical analyses were performed using the _t_-test, assuming that data from adhesion assays were normally distributed about the mean. As the distribution of the data is not known we also performed the Mann–Whitney rank test, which does not make assumptions about the distribution of the data.
Results
Immunological synapse formation by naïve T cells
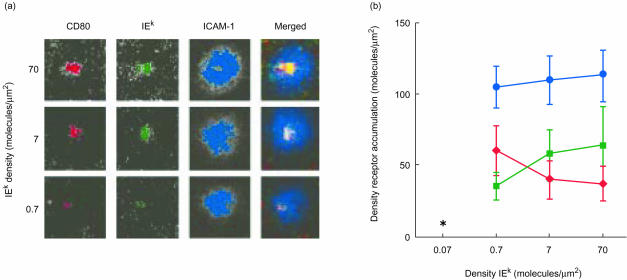
We tested the effect of chemokines on adhesion and mature immunological synapse formation by naïve T cells. We defined a mature immunological synapse as a central cluster of TCR–MHCp interactions (cSMAC) surrounded by a ring of LFA-1–ICAM-1 interactions (pSMAC).2,3 In order to determine the effect of chemokines we first needed to better characterize the spontaneous formation of immunological synapses by naïve T cells in the absence of chemokines. We previously demonstrated that naïve T cells are capable of forming mature immunological synapses on planar phospholipid bilayers containing Cy3-labelled GPI-anchored CD80, Oregon green-labelled GPI-IEk MCC 91-103, and Cy5-labelled GPI-ICAM-1.27Figure 1 shows examples of synapses formed by naïve splenic T cells over a range of densities of MHCp in the presence of fixed densities of ICAM-1 and CD80. The mature immunological synapses formed by naïve T cells were similar to the mature immunological synapses formed by previously activated T cells.3 Immunological synapse formation required only 2–5 min from the initial T-cell interaction with the bilayer and was maintained for at least 1 hr.
Figure 1.
Immunological synapse formation by naïve T cells. (a) T cells were purified from the spleen of 2B4 T-cell receptor transgenic mice and allowed to settle onto planar phospholipid bilayers containing 170 molecules/µm2 Cy3-labelled GPI-CD80 (red), 115 molecules/µm2 Cy5-labelled GPI-ICAM-1 (blue), and the indicated densities of Oregon green-labelled GPI-IEk-MCC 91-103 (green). Red/green/blue merged images are shown in the far-right column. (b) Density of accumulated GPI-CD80 (red diamonds), GPI-IEk (green squares) and GPI-ICAM-1 (blue circles) in the immunological synapse of naïve T lymphocytes interacting with bilayers as in (a). *No immunological synapse formed. Images were acquired following 30 min of T-cell interaction with the bilayers. Results shown are representative of three experiments.
The lowest density of agonist MHCp required for immunological synapse formation by naïve T cells was 0·7 molecules/µm2 I-Ek-MCC 91-103 in the presence of 70 molecules/µm2 of I-Ek-MCC 91-103 K99A. ICAM-1 accumulated in the synapse to an average density of approximately 100 molecules/µm2 (Fig. 1b). This accumulated receptor density remained relatively constant over decreasing IEk densities in the bilayer. CD80 accumulated in the central cluster of the immunological synapse to an average density of approximately 35 molecules/µm2 (Fig. 1b). The decrease in CD80 accumulation seen with increasing initial I-Ek densities in the bilayer in the experiment presented in Fig. 1 was not always observed. In two of three experiments, the density of CD80 accumulation remained constant. The level of engaged CD80 was independent of the initial I-Ek density in the bilayer down to 0·7 molecules/µm2 initial I-Ek. In contrast, the density of engaged I-Ek molecules within the immunological synapse decreased with decreasing initial I-Ek densities in the bilayer (Fig. 1b). Naïve T cells engaged an average density of approximately 60 molecules/µm2 when settled onto bilayers containing an initial agonist IEk density of 70 molecules/µm2. The lowest density of I-Ek in a cSMAC was 35 molecules/µm2 at an initial agonist density of 0·7 molecules/µm2. Recent findings suggest that some of the accumulated I-Ek was likely to be loaded with the K99A mutated MHCp.23 Naïve T cells failed to form an immunological synapse at an initial agonist MHCp density of 0·07 molecules/µm2 in the bilayer. Formation of immunological synapses by naïve T cells is therefore antigen dose-dependent, consistent with our previous results.27
Chemokines induce antigen-independent naïve T-cell adhesion
Surface-immobilized chemokines induce the firm adhesion of naïve T lymphocytes to integrin substrates both in static assays and under flow conditions.29,30 Chemokines can be immobilized to proteoglycans including purified heparin,28 as well as to heparan sulphate on the endothelial cell surface.31 In the light of these studies, we decided to address the effect of chemokines on naïve T-cell adhesion and immunological synapse formation by incubating the chemokine CXCL12 (SDF-1α), CCL21 (SLC, 6Ckine, TCA4) or CCL3 (MIP-1α) with planar phospholipid bilayers containing adsorbed heparin. Receptors for both CXCL1232 and CCL2133 are expressed on naïve T cells, while the receptors for CCL3 are not.34 Radioimmunoassays using 125I anti-CXCL12 monoclonal antibody for detection demonstrated that approximately 25 molecules/µm2 of the chemokine were adsorbed to the bilayers.
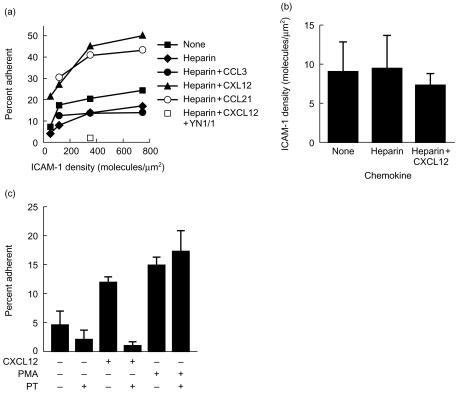
We tested the ability of CXCL12 and CCL21 to induce the adhesion of naïve T lymphocytes to ICAM-1-containing bilayers (Fig. 2). The percentage of naïve T cells adhering to ICAM-1-containing bilayers increased from 7% at 50 molecules/µm2 ICAM-1 to 23% at 760 molecules/µm2. Adsorption of heparin alone to the ICAM-1 bilayers slightly decreased adhesion, possibly by increasing the negative charge of the bilayer. However, CXCL12 or CCL21 triggered a 3–4-fold increase in adhesion over the range of ICAM-1 densities tested (Fig. 2a). This increased adhesion was mediated by the LFA-1–ICAM-1 interaction since pretreatment of the bilayers with the ICAM-1 blocking antibody YN1/1 eliminated all T-cell adhesion in the presence or absence of the chemokines. CCL3, whose receptors are not expressed on naïve T cells, did not increase naïve T-cell adhesion to ICAM-1 (Fig. 2a). The densities of engaged ICAM-1 receptors in the spontaneous and chemokine-stimulated contacts were similar (Fig. 2b). The chemokine-stimulated adhesion was dependent on G-protein signalling, since pretreatment of the T cells with pertussis toxin inhibited chemokine-induced, but not PMA-induced, T-cell adhesion (Fig. 2c). It is notable that most of the basal adhesion in the absence of an added chemokine was also pertussis toxin sensitive. This consistent trend suggests that the basal adhesion was in part attributable to signalling through G-protein coupled receptors. Therefore, chemokines adsorbed to the bilayers increased the proportion of cells that adhered, but did not increase the number of LFA-1–ICAM-1 interactions.
Figure 2.
Effect of chemokine on naïve T-cell interaction with ICAM-1. (a) Effect of chemokine on naïve T-cell adhesion to ICAM-1. Planar phospholipid bilayers containing the indicated densities of ICAM-1 were formed. The bilayers were then incubated with heparin at 2 mg/ml for 20 min, washed thoroughly, and then incubated for 10 min with the chemokine CCL3, CXCL12 or CCL21 at 1 µg/ml each. Unbound chemokine was removed by thorough washing. Alternatively, the bilayers were left untreated. Bilayers were pretreated with the ICAM-1 blocking antibody YN1/1 at 10 µg/ml to determine the requirement for the LFA-1–ICAM-1 interaction. Naïve T cells were then settled onto the bilayers. The T cells were allowed to interact with the bilayers for 20 min, after which the unbound cells were removed by gentle washing, and the percentage of input cells that remained bound was determined. (b) Pertussis toxin inhibits chemokine-induced adhesion. Naïve T cells were treated with 100 ng/ml pertussis toxin for 2 hr at 37°. These cells were then settled onto bilayers containing 50 molecules/µm2 ICAM-1 with or without adsorbed CXCL12 as in (a). Some cells were also pretreated with 50 ng/ml PMA at 37° for 10 min. The percentage of adherent cells was determined as in (a). (c) Effect of chemokines on naïve T-cell engagement of ICAM-1. Naïve T cells were incubated on bilayers containing 115 molecules/µm2 ICAM-1. CXCL12 was adsorbed to the bilayers as in (a). Images were captured after 20 min of T-cell interaction with the bilayers. Results shown are from single experiments that are representative of three experiments for each panel. The effect of pertussis toxin on basal adhesion was significant at _P_≤0·025 based on the Mann–Whitney test.
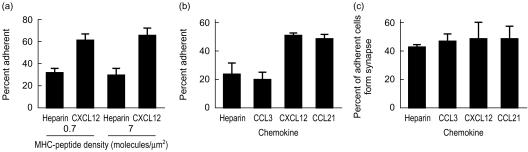
Like chemokines, inclusion of agonist MHCp molecules in the planar phospholipid bilayers increased the number of adherent naive T lymphocytes (Fig. 3). Inclusion of 0·7 molecules/µm2 agonist IEk in the bilayers resulted in a 3–4-fold increase in the number of adherent naïve T cells compared to adhesion to bilayers containing ICAM-1 alone (Fig. 3a). The effect of agonist MHCps and chemokines on the percentage of adhering cells was nearly additive (Fig. 3a). CXCL12 or CCL21 adsorbed to the bilayers increased the number of adherent naïve T cells (Fig. 3b). As expected, CCL3 had no effect on naïve T-cell adhesion (Fig. 3b). CXCL12 and CCL21 did not increase the proportion of adhering cells that formed immunological synapses on substrates with ICAM-1 and agonist MHCp (Fig. 3c). However, since more total cells adhered (Fig. 3b), these chemokines increased the total number of immunological synapses to 25%. This proportion of immunological synapse formation approaches the proportion of T-cell blasts that form immunological synapses in the absence of chemokine.35 Therefore, both antigen and chemokines increased the proportion of adherent T cells in an additive manner.
Figure 3.
Effect of chemokine on naïve T-cell interaction with antigen-containing bilayers. (a) Percentage naïve T cells adhering to bilayers. GPI-IEk was used at the indicated densities. (b) Percentage of naïve T cells adhering to bilayers. GPI-IEk was used at 0·7 molecules/µm2. Heparin and chemokines were adsorbed to the bilayers as in Fig. 2. (c) Percentage adherent cells that formed an immunological synapse. The results are representative of three experiments.
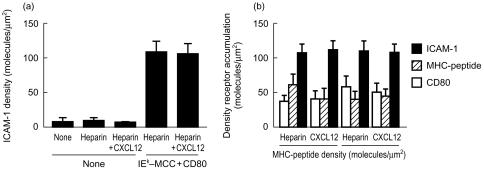
Chemokines cause the redistribution of surface receptors, including CD43 and ICAMs, on responding cells. We examined the effect of chemokines on the interactions of LFA-1, CD28 and the TCR in the immunological synapse. In the absence of antigen, LFA-1–ICAM-1 interactions were not increased by CXCL12 adsorption to the substrate (Fig. 4a), although the number of cells adhering was increased as noted above. In contrast, agonist MHCp stimulated an 8–10-fold increase in the density of LFA-1–ICAM-1 interactions (Fig. 4a). This increase in the density of engaged LFA-1 receptors occurred over a range of initial MHCp densities in the bilayer (Fig. 1b). The density of bound CD28–CD80 interactions was also increased by the presence of agonist MHCp as previously noted.27 These major changes in LFA-1 and CD28 interactions triggered by agonist MHCp were detected in the absence of chemokines. The presence of CXCL12 on the substrate did not have any effect on the densities of bound ICAM-1, CD80, and IEk MCC 91-103 per synapse (Fig. 4b). Overall, chemokines increased the proportion of T cells that form a stable interaction in the absence and presence of agonist MHCp in the bilayers containing ICAM-1 and CD80. Thus, chemokines presented in this manner recruited additional cells into adhesive interactions, but the molecular details of the adhesion and immunological synapse assembly were unchanged.
Figure 4.
Effect of chemokines on accumulation of receptors within the T-cell contact area. (a) T cells were allowed to interact with bilayers containing ICAM-1 with or without adsorbed CXCL12 and 0·7 molecules/µm2 GPI-IEk. The density of bound ICAM-1 in the contact areas was determined. (b) Density accumulated GPI-IEk (horizontal striped bars), GPI-CD80 (black bars) and ICAM-1 (diagonally striped bars). The results shown in each panel are representative of three experiments.
Effect of chemokine on antigen-independent and -dependent cell–cell conjugate formation
T cells cluster with antigen-presenting cells both in vitro and in vivo. Previous studies have indicated that resting T cells adhere to DCs by an antigen-independent mechanism.16,36,37 Maximal interaction is achieved at 20 min after the initial incubation of T cells with DCs. This transient antigen-independent adhesion could allow T cells to scan DCs for surface-presented agonist MHCp complexes.
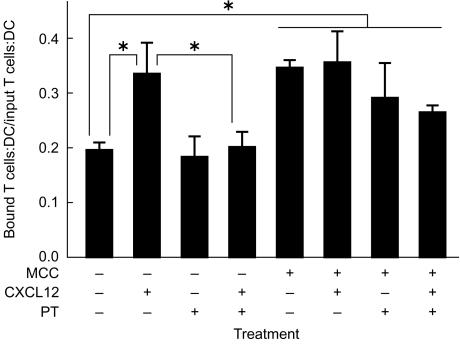
In order to determine whether chemokines also increase the interaction of naïve T cells with DCs, conjugate assays were performed. Cell tracker green-labelled naïve T cells were allowed to interact with unpulsed or antigen-pulsed bone marrow-derived DCs, and their interaction was monitored by mixed fluorescence and transmission microscopy. Comparison of the number of input DCs and T cells to the number of remaining DC–T cell conjugates revealed that chemokines increase the antigen-independent interaction of naïve T cells with DCs. While 20% of input naïve T cells bound to DCs in the absence of chemokines, the population of adherent naïve T cells was increased to 35% when the DCs were preincubated with chemokine (Fig. 5). This chemokine-induced adhesion was reversed by pretreatment of the T cells with pertussis toxin (Fig. 5), indicating that G-protein signalling mediates the chemokine-induced adhesion. However, unlike the situation for adhesion to ICAM-1 substrates, the basal adhesion was not reduced by pertussis toxin treatment of the naïve T cells.
Figure 5.
Chemokine enhances antigen-independent DC–T cell conjugates, but not antigen-dependent conjugates. DCs differentiated from the bone marrow of B10.Br mice were cultured in media alone or with 1 µm MCC 91-103 for 24 hr (MCC). These DCs were then attached to coverslips coated with poly l-lysine in a flow chamber and were then left untreated or incubated for 20 min with 1 µg/ml CXCL12 as indicated. T cells were purified from 2B4 mice and then left untreated or treated for 2 hr at 37° with 100 ng/ml pertussis toxin (PT). The T cells were then injected into the flow chamber and allowed to interact with the DCs for 20 min at 37°. Images were acquired. Then, unbound cells were washed from the flow chamber with a flow rate that gave a shear stress of ∼ 1 dyne/cm2 and postwash images were acquired. The ratio of bound T cells and DCs to input T cells and DCs was determined. The results shown are an average of results from three experiments. The *indicate significant differences at P<0·005 in the _t_-test and _P_≤0·025 in the Mann–Whitney test. Other differences were not statistically significant.
To assay the effect of chemokine on antigen-dependent adhesion of naïve T cells, bone marrow-derived DCs were pulsed at day 6 of the culture with 1 µm MCC 91-103 peptide. After a day to allow for effective loading of MHCp, these cells were attached to poly l-lysine coated coverslips and left untreated or incubated with CXCL12. Naïve IEk MCC 91-103-specific 2B4 T cells were allowed to interact with the antigen-pulsed DCs, and the percentage of adherent cells was calculated. Increased numbers of naïve antigen-specific T cells interacted with antigen-pulsed DCs compared to unpulsed DCs (Fig. 5). This adhesion was resistant to pretreatment of the T cells with pertussis toxin. Incubation of the antigen-pulsed DCs with CXCL12 had no effect on the number of adherent resting T cells. Again, this adhesion was resistant to pretreatment of the T cells with pertussis toxin. In contrast to antigen-presenting planar phospholipid bilayers, antigen-pulsed DCs engaged in similar levels of interaction with naïve T cells with equal efficiency in the presence or absence of chemokine (Fig. 5).
Discussion
We investigated the role of surface-adsorbed chemokines in naïve T-cell adhesion to supported planar bilayers and DCs. Our hypothesis was that chemokines initiate the antigen non-specific adhesion of naïve T cells to APC that is permissive for the interaction of TCR with MHCp. We found that CXCL12 and CCL21 significantly stimulated adhesion to ICAM-1-containing supported planar bilayers and increased the efficiency of mature immunological synapse formation on supported planar bilayers containing ICAM-1, CD80 and agonist MHCp. It was noted also that much of the basal adhesion was pertussis toxin sensitive. This suggested that chemokine activity encountered in vivo may have been responsible for some of the adhesive activity of the ex vivo naïve T cells. We also found that adsorption of CXCL12 to the surface of DCs increased the efficiency of naïve T cell adhesion. However, this effect was not significant in the presence of agonist MHCp. More importantly, the basal adhesion of naïve T cells to DCs was not pertussis toxin sensitive, in contrast to the basal adhesion of naïve T cells to ICAM-1 alone, which was repeatedly found to be pertussis toxin sensitive. This indicates there are two potential pathways for initiating adhesion between naïve T cells and DCs: one that is chemokine-dependent and one that is chemokine-independent.
Since naïve T cells do not efficiently adhere to bilayers containing the LFA-1 ligand ICAM-1 alone, the LFA-1–ICAM-1 interaction is not sufficient for the Gαi independent adhesion of T cells to DCs. Naïve T cells are relatively non-motile in vitro and their integrins are maintained in a low-activity state in the absence of stimulation. However, in order to respond to foreign antigen, T cells must interact with APCs. Adhesion of T cells to APCs is induced by TCR interaction with agonist MHCp through inside-out signalling.11,12 Both the TCR and the MHC are small integral membrane proteins that face several barriers to their interaction, including small size, low affinity and small numbers of agonist MHCps.38 Therefore, their interaction requires the formation of intimate contacts between T cells and APCs. Using a very simple system with only ICAM-1 as a ligand, we found that chemokines are sufficient to induce a significant increase in LFA-1–ICAM-1 mediated adhesion. Further, much of the basal adhesion was consistently found to be reduced by pertussis toxin, suggesting that a Gαi coupled signalling process maintains the basal adhesion. When the planar bilayers were replaced with more complex DCs, chemokines attached to the surface of DCs increased the basal adhesion of naïve T cells. However, the basal adhesion in this case was never reduced by pertussis toxin. Thus, DCs elaborate a chemokine- and Gαi-independent mechanism for initiating interactions.
We found that up to 23% of freshly isolated splenic T cells adhered to ICAM-1 presented in supported planar bilayers. Since the LFA-1–ICAM-1 interaction is tightly regulated, this raises a question as to what stimulates this adhesion. Unlike earlier studies with peripheral blood lymphocytes,11 the splenic T cells were taken from a tissue and some of these cells may have recently extravasated or may even have been actively migrating in the tissue at the time of isolation. The activation responsible for the extravasation and migration process may have a long enough half-life to be evident in the isolated cell. It is notable that the density of LFA-1–ICAM-1 interactions, which was not measured in prior studies, was very low with resting and solid phase chemokine-stimulated cells. Thus, chemokine-stimulated adhesion may be sufficient to provide contact for potential detection of agonist MHCp, but weak enough to allow slow T-cell migration through the lymph nodes at an average of ∼1 µm/min. This number is estimated based on the ability of T cells to traverse a distance of ∼0·5 mm through the T-cell zone once or twice daily.39
Immobilized chemokines induce the firm adhesion of naïve T lymphocytes in static assays as well as under flow conditions.29,30 Firm adhesion is induced by a chemokine-regulated increase in integrin avidity.13,30 Our results demonstrate that immobilized chemokines increase the number of naïve T cells that interact with an ICAM-1 substrate. This adhesion is mediated by the LFA-1–ICAM-1 interaction, since pretreatment of the bilayers with an ICAM-1 blocking antibody inhibits all adhesion. In addition, ICAM-1 is the only adhesion ligand in the planar bilayer system. This adhesion also depends on G-protein-mediated signalling, since pretreatment of the T cells with pertussis toxin eliminates chemokine-induced adhesion. However, the increase in adhesion is independent of any increase in the number of bound ICAM-1 receptors on a per cell basis. Similarly, chemokines increase the population of naïve T cells that interact with DCs in an antigen-independent fashion.
Increased numbers of naïve T cells also interact with antigen-presenting planar phospholipid bilayers containing immobilized chemokines. However, the proportion of adherent cells that form immunological synapses is unchanged by exposure to chemokine. Therefore, the total number of immunological synapses was increased by chemokine treatment. While chemokines induce the redistribution of membrane receptors on responding cells,40,41 the accumulation of bound ICAM-1, CD80 and IEk receptors within the immunological synapse was unchanged in the presence or absence of chemokines. This may be attributable to the very transient nature of the chemokine signal that is received in contact with the chemokine coated surface. This stimulation may be sufficient to trigger adhesion, but may not last long enough to increase accumulation of LFA-1–ICAM-1 interactions. Signalling through CXCR4 is turned off by TCR signalling and this may have a role in the failure of CXCL12 to regulate molecular redistribution in the synapse.42,43 In contrast, sustained signalling through the TCR in the immunological synapse maintains a high level of LFA-1–ICAM-1 accumulation in the pSMAC. This higher level of LFA-1–ICAM-1 interaction may also lead to greater signalling through LFA-1, which may contribute to full T-cell activation.
When DCs were prepared as in our experiments, we did not find evidence that endogenous chemokines were presented on the cell surface, at least as detected through pertussis toxin-sensitive adhesion. Cultured DCs prepared from bone marrow produce a number of chemokines.44 Paradoxically, these chemokines are more potent in attracting memory T cells as opposed to naïve T cells. DCs have been reported to produce CXCL12 in vivo, but it is not clear if this applies to cultured DCs.45 If the DCs are producing CXCL12 then it is not secreted at a high enough level to bind to the cell surface or interfere with the effect of the exogenous CXCL12 that is adsorbed to the cell surface. We still have much to learn about the organization of soluble and surface-adsorbed chemokines in lymph nodes. Such knowledge will be important to design additional experiments to test the importance of chemokines in initiating interactions of T cells and DCs. The ability of CXCL12 to increase T-cell adhesion to DCs probably results from the effect of the chemokine on T cells. However, it is also possible that CXCL12 binding to CXCR4 on the DCs may have some pertussis toxin-sensitive effects that lead to changes in the ability of DCs to capture T cells.
Although we found that chemokines increased the number of cells able to interact with antigen-presenting planar phospholipid bilayers, we found that naïve T cells were able to adhere to DC equally efficiently in the presence or absence of chemokine. Naïve T cells were able to initiate their interaction with DCs without any additional chemokine signal. Activated DC are thought to be the most effective APCs for naïve T cells. These cells express high levels of costimulatory molecules. Therefore, abundant cell surface molecules such as CD86, CD48 and DC-specific ICAM-3 grabbing non-integrin (SIGN) (which are not present in our planar bilayer system) may be sufficient to induce the initial antigen-independent adhesion of naïve T cells, which is then stabilized by engagement of antigen.6 Recent studies have also indicated that DCs have unique properties compared to other APCs. They actively participate through the rearrangement of their actin cytoskeleton in the stimulation of T cells,46 and they induce an antigen-independent Ca2+ signal as well as antigen-independent polarization of a number of signalling molecules.15 It is likely that the antigen-independent adhesion of T cell to DCs that we detected is similar or identical to the process described by Trautmann et al.15 Our experiments suggest that this interaction is not chemokine-dependent and can be augmented by either chemokine or antigen receptor signals. While it has been proposed that DC-Sign has a role in the initial antigen non-specific interaction of DCs and T cells, it is not known if this mechanism accounts for all of the antigen non-specific adhesion. It will be important to fully characterize the chemokine- and antigen-independent mechanisms that initiate interactions of naïve T cells and DCs.
Acknowledgments
We thank O. Kanagawa and E. Unanue for animals and reagents. We thank R. Houdei for technical assistance. We are grateful to A. Shaw for providing laboratory space for S.K.B. to complete these experiments. We also acknowledge valuable discussions with A. Shaw, P. Allen, T. Baranski, J. Green and A. Chan. We thank S. Y. Tseng and A. Tager for critical reading of the manuscript. This work was supported by grants from the NIH and Irene Diamond.
References
- 1.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241–51. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 2.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–6. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 3.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–7. doi: 10.1126/science.285.5425.221. 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Inaba K, Steinman RM. Accessory cell–T lymphocyte interactions. Antigen-dependent and -independent clustering. J Exp Med. 1986;163:247–61. doi: 10.1084/jem.163.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauss P, Selz F, Cavazzana-Calvo M, Fischer A. Characteristics of antigen-independent and antigen-dependent interaction of dendritic cells with CD4+ T cells. Eur J Immunol. 1995;25:2285–94. doi: 10.1002/eji.1830250826. [DOI] [PubMed] [Google Scholar]
- 7.Ingulli E, Mondino A, Khoruts A, Jenkins MK. In vivo detection of dendritic cell antigen presentation to CD4 (+) T cells. J Exp Med. 1997;185:2133–41. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanchez-Madrid F, del Pozo MA. Leukocyte polarization in cell migration and immune interactions. Embo J. 1999;18:501–11. doi: 10.1093/emboj/18.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dustin ML, Cooper JA. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nature Immunol. 2000;1:23–9. doi: 10.1038/76877. [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson PC. The locomotor capacity of human lymphocytes and its enhancement by cell growth. Immunology. 1986;57:281–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Dustin ML, Springer TA. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341:619–24. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- 12.van Kooyk Y, van de Wiel-van Kemenade P, Weder P, Kuijpers TW, Figdor CG. Enhancement of LFA-1-mediated cell adhesion by triggering through CD2 or CD3 on T lymphocytes. Nature. 1989;342:811–3. doi: 10.1038/342811a0. [DOI] [PubMed] [Google Scholar]
- 13.Constantin G, Majeed M, Giagulli C, Piccio L, Kim JY, Butcher EC, Laudanna C. Chemokines trigger immediate beta2 integrin affinity and mobility changes. differential regulation and roles in lymphocyte arrest under flow. Immunity. 2000;13:759–69. doi: 10.1016/s1074-7613(00)00074-1. [DOI] [PubMed] [Google Scholar]
- 14.Tang HL, Cyster JG. Chemokine up-regulation and activated T cell attraction by maturing dendritic cells. Science. 1999;284:819–22. doi: 10.1126/science.284.5415.819. [DOI] [PubMed] [Google Scholar]
- 15.Delon J, Bercovici N, Raposo G, Liblau R, Trautmann A. Antigen-dependent and -independent Ca2+ responses triggered in T cells by dendritic cells compared with B cells. J Exp Med. 1998;188:1473–84. doi: 10.1084/jem.188.8.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Revy P, Sospedra M, Barbour B, Trautmann A. Functional antigen-independent synapses formed between T cells and dendritic cells. Nat Immunol. 2001;2:925–31. doi: 10.1038/ni713. [DOI] [PubMed] [Google Scholar]
- 17.Weiner OD, Servant G, Welch MD, Mitchison TJ, Sedat JW, Bourne HR. Spatial control of actin polymerization during neutrophil chemotaxis. Nat Cell Biol. 1999;1:75–81. doi: 10.1038/10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freiß A. Intergiditating reticulum cells in the popliteal lymph node. Cell Tissue Res. 1976;170:43–60. doi: 10.1007/BF00220109. [DOI] [PubMed] [Google Scholar]
- 19.Negulescu PA, Krasieva TB, Khan A, Kerschbaum HH, Cahalan MD. Polarity of T cell shape, motility, and sensitivity to antigen. Immunity. 1996;4:421–30. doi: 10.1016/s1074-7613(00)80409-4. [DOI] [PubMed] [Google Scholar]
- 20.Wei X, Tromberg BJ, Cahalan MD. Mapping the sensitivity of T cells with an optical trap: polarity and minimal number of receptors for Ca (2+) signaling. Proc Natl Acad Sci USA. 1999;96:8471–6. doi: 10.1073/pnas.96.15.8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dustin ML, Bromley SK, Kan Z, Peterson DA, Unanue ER. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc Natl Acad Sci USA. 1997;94:3909–13. doi: 10.1073/pnas.94.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wulfing C, Sumen C, Sjaastad MD, Wu LC, Dustin ML, Davis MM. Costimulation and endogenous MHC ligands contribute to T cell recognition. Nat Immunol. 2002;3:42–7. doi: 10.1038/ni741. 10.1038/ni741. [DOI] [PubMed] [Google Scholar]
- 24.Takei F. Inhibition of mixed lymphocyte response by a rat monoclonal antibody to a novel murine lymphocyte activation antigen (MALA-2) J Immunol. 1985;134:1403–7. [PubMed] [Google Scholar]
- 25.Razi-Wolf Z, Freeman GJ, Galvin F, Benacerraf B, Nadler L, Reiser H. Expression and function of the murine B7 antigen, the major costimulatory molecule expressed by pertoneal exudate cells. Proc Natl Acad Sci USA. 1995;89:4210–4. doi: 10.1073/pnas.89.9.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reay PA, Matsui K, Haase K, Wulfing C, Chien YH, Davis MM. Determination of the relationship between T cell responsiveness and the number of MHC-peptide complexes using specific monoclonal antibodies. J Immunol. 2000;164:5626–34. doi: 10.4049/jimmunol.164.11.5626. [DOI] [PubMed] [Google Scholar]
- 27.Bromley SK, Iaboni A, Davis SJ, Whitty A, Green JM, Shaw AS, Weiss A, Dustin ML. The immunological synapse and CD28–CD80 interactions. Nat Immunol. 2001;2:1159–66. doi: 10.1038/ni737. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka Y, Adams DH, Hubscher S, Hirano H, Siebenlist U, Shaw S. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1 beta. Nature. 1993;361:79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- 29.Campbell JJ, Hedrick J, Zlotnik A, Siani MA, Thompson DA, Butcher EC. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381–4. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- 30.Tangemann K, Gunn MD, Giblin P, Rosen SD. A high endothelial cell-derived chemokine induces rapid, efficient, and subset-selective arrest of rolling T lymphocytes on a reconstituted endothelial substrate. J Immunol. 1998;161:6330–7. [PubMed] [Google Scholar]
- 31.Middleton J, Neil S, Wintle J, Clark-Lewis I, Moore H, Lam C, Auer M, Hub E, Rot A. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385–95. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 32.Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–30. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA 1997. 1998;95:258–63. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sallusto F, Kremmer E, Palermo B, et al. Switch in chemokine receptor expression upon TCR stimulation reveals novel homing potential for recently activated T cells. Eur J Immunol. 1999;29:2037–45. doi: 10.1002/(SICI)1521-4141(199906)29:06<2037::AID-IMMU2037>3.0.CO;2-V. 10.1002/(SICI)1521-4141(199906)29:06<2037::AID-IMMU20373.3.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 35.Johnson KG, Bromley SK, Dustin ML, Thomas ML. A supramolecular basis for CD45 tyrosine phosphatase regulation in sustained T cell activation. Proc Natl Acad Sci USA. 2000;97:10138–43. doi: 10.1073/pnas.97.18.10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inaba K, Steinman RM. Accessory cell–T lymphocyte interactions: Antigen-dependent and -independent clustering. J Exp Med. 1986;163:247–61. doi: 10.1084/jem.163.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inaba K, Romani N, Steinman RM. An antigen-independent contact mechanism as an early step in T cell-proliferative responses to dendritic cells. J Exp Med. 1989;170:527–42. doi: 10.1084/jem.170.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaw AS, Dustin ML. Making the T cell receptor go the distance. A topological view of T cell activation. Immunity. 1997;6:361–9. doi: 10.1016/s1074-7613(00)80279-4. [DOI] [PubMed] [Google Scholar]
- 39.Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, Itano A, Pape KA. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- 40.Serrador JM, Nieto M, Alonso-Lebrero JL, et al. CD43 interacts with moesin and ezrin and regulates its redistribution to the uropods of T lymphocytes at the cell-cell contacts. Blood. 1998;91:4632–44. [PubMed] [Google Scholar]
- 41.Serrador JM, Alonso-Lebrero JL, del Pozo MA, Furthmayr H, Schwartz-Albiez R, Calvo J, Lozano F, Sanchez-Madrid F. Moesin interacts with the cytoplasmic region of intercellular adhesion molecule-3 and is redistributed to the uropod of T lymphocytes during cell polarization. J Cell Biol. 1997;138:1409–23. doi: 10.1083/jcb.138.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peacock JW, Jirik FR. TCR activation inhibits chemotaxis toward stromal cell-derived factor-1. Evidence for reciprocal regulation between CXCR4 and the TCR. J Immunol. 1999;162:215–23. [PubMed] [Google Scholar]
- 43.Guinamard R, Signoret N, Masamichi I, Marsh M, Kurosaki T, Ravetch JV. B cell antigen receptor engagement inhibits stromal cell-derived factor (SDF)-1alpha chemotaxis and promotes protein kinase C (PKC)-induced internalization of CXCR4. J Exp Med. 1999;189:1461–6. doi: 10.1084/jem.189.9.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lieberam I, Forster I. The murine beta-chemokine TARC is expressed by subsets of dendritic cells and attracts primed CD4+ T cells. Eur J Immunol. 1999;29:2684–94. doi: 10.1002/(SICI)1521-4141(199909)29:09<2684::AID-IMMU2684>3.0.CO;2-Y. 10.1002/(SICI)1521-4141(199909)29:09<2684::AID-IMMU26843.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 45.Pablos JL, Amara A, Bouloc A, et al. Stromal-cell derived factor is expressed by dendritic cells and endothelium in human skin. Am J Pathol. 1999;155:1577–86. doi: 10.1016/S0002-9440(10)65474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al-Alwan MM, Rowden G, Lee TD, West KA. The dendritic cell cytoskeleton is critical for the formation of the immunological synapse. J Immunol. 2001;166:1452–6. doi: 10.4049/jimmunol.166.3.1452. [DOI] [PubMed] [Google Scholar]