Constitutively active β-catenin confers multi-lineage differentiation potential on lymphoid and myeloid progenitors (original) (raw)
. Author manuscript; available in PMC: 2007 Apr 10.
Summary
β-catenin mediated Wnt signaling may contribute to the self-renewal of hematopoietic stem cells and proliferation in some malignancies. We now show that expression of constitutively active β-catenin in normal lymphoid or myeloid progenitors generated uncommitted cells with multi-lineage differentiation potential. Inappropriate gene expression occurred in cells destined to produce either cell type and caused corresponding changes in their characteristics. For example, forced activation of β-catenin quickly increased C/EBPα while reducing EBF and Pax-5 in lymphoid progenitors that then generated myeloid cells. Inversely, EBF dramatically increased in transduced myeloid progenitors and lymphocytes were produced. The results indicate that ectopic activation of β-catenin de-stabilizes lineage fate decisions and confers some, but not all stem cell properties on committed progenitors.
Introduction
Traditional models of blood cell formation depict the production of lineage committed progenitor cells from multipotential hematopoietic stem cells (HSC), with progressive loss of differentiation options and self-renewal activity (Orkin, 2000; Kincade et al., 2002). Hematopoietic cell lineages are specified in a gradual process involving intrinsic and extrinsic factors. Intermediate and committed progenitors are characterized by patterns of gene expression that are determined and maintained by a combination of transcription factors and chromatin remodeling events. Substantial progress has been made in learning how a small number of early-acting transcription factors initiate the formation of lineage committed progenitors (Warren and Rothenberg, 2003). Low level expression of several of these occurs during a “priming” phase (Miyamoto et al., 2002), and subsequent events depend on combinations and doses of transcription factors in individual cells. In addition, cross competition between transcription factors ensures that differentiating cells lack properties of more than one lineage.
Although progression in one of the major lineages is thought to be unidirectional, there is growing evidence for at least latent plasticity at early stages. For example, lymphoid progenitors can convert to myeloid lineage cells by ectopic activation of IL-2 or GM-CSF signaling pathways (Kondo et al., 2000; Iwasaki-Arai et al., 2003) or erythrocyte/megakaryocytes by enforced expression of GATA-1 (Iwasaki et al., 2003) Lymphoid lineage cell lines switch to macrophages under certain conditions (Boyd and Schrader, 1982; Klinken et al., 1988; Borzillo et al., 1990). Furthermore, normal and malignant CD5+ B lymphocytes can develop macrophage-like characteristics on fibroblasts (Borrello and Phipps, 1995). A recent study showed that mature B lymphocytes can be converted to macrophages under experimental circumstances (Xie et al., 2004). Inversely, there is one example where macrophage tumor cells re-acquired lymphoid characteristics by manipulation of E2A (Kee and Murre, 1998). Finally, primitive progenitors converted to definitive multipotent hematopoietic cells by ectopic expression of HoxB4 (Kyba et al., 2002) or Myb-Ets from the E26 leukemia virus (McNagny and Graf, 2003).
Mechanisms for the earliest steps in normal lineage commitment remain incompletely understood, but recent studies indicate that the Wnt family of ligands, receptors and signal transmitting molecules may be involved (Staal and Clevers, 2005). At least 19 Wnt proteins bind to one of 10 Frizzled receptors, leading to inactivation of the glycogen synthase kinase 3β (GSK3β). In the absence of this signaling, GSK3β normally phosphorylates β-catenin, targeting it for destruction. Unphosphorylated β-catenin translocates to the nucleus, where it modulates activity of the TCF/LEF family of transcription factors and may induce chromatin remodeling for target gene expression through association with Brg1, a component of SWI/SNF chromatin remodeling complex (Nusse, 2005). This canonical pathway for Wnt family signaling can be simulated by introduction of a non-degradable form of β-catenin. Enforced expression of such a stable β-catenin in hematopoietic stem cells of bcl-2 transgenic mice promoted stem cell self-renewal (Reya et al., 2003). Wnt signaling is normally reduced as stem cells convert to committed myeloid progenitor cells (Reya et al., 2003). Also, purified Wnt3a protein promotes HSC self-renewal in vitro (Willert et al., 2003). Conditional deletion of β-catenin from adult hematopoietic stem cells in vivo did not cause a self-renewal defect (Cobas et al., 2004). However, Plakoglobin (γ-catenin) has overlapping functions, and could potentially substitute for β-catenin in hematopoietic cells (Reya and Clevers, 2005). In addition to HSC, embryonic stem cells, epidermal stem cells, and epithelial stem cells seem to be responsive to, or dependent on, Wnt/β-catenin signaling to maintain them in an undifferentiated, proliferating state (Willert et al., 2003; Korinek et al., 1998; Sato et al., 2004; Gat et al., 1998; Zhu and Watt, 1999). Furthermore, activation of β-catenin in granulocyte-macrophage progenitors from the blast-crisis stage of chronic myelogenous leukemia (CML) appears to enhance their self-renewal and leukemic potential (Jamieson et al., 2004). Such observations also indicate that self-renewal programs may be re-activated in committed hematopoietic progenitors as a result of excess β-catenin stabilization.
Manipulation of self-renewal properties represents a major goal of stem cell studies, and it was important to learn the consequences of inappropriate stimulation of the Wnt/β-catenin pathway in normal hematopoietic cells. We now show that enforced expression of a constitutively active form of β-catenin confers multi-lineage differentiation potential on lymphoid and myeloid progenitors through inappropriate gene expression. Activation of β-catenin signaling in committed progenitors may re-activate part of the stem cell program, leading to lineage infidelity/instability.
Results
Expression of active β-catenin in lymphoid progenitors expands cells with multi-lineage differentiation potential
In a companion study, introduction of stable β-catenin allowed long-term growth of primitive hematopoietic cells without loss of differentiation potential (Baba et al. manuscript submitted). We have now investigated the influence of this treatment on highly enriched Lin−IL-7Rα+c-KitloSca-1lo lymphoid progenitors. Cells transduced with control vector or stable β-catenin were prepared and placed in OP9 stromal cell co-cultures along with SCF, FL and IL-7. Under these conditions, lymphoid progenitors receiving the control vector generated almost pure populations of CD19+ lymphocytes (Figure 1A), along with very small numbers of CD11b/Mac-1+ CD11c+ dendritic cells (data not shown). In contrast, introduction of stable β-catenin caused substantial increases in dendritic cells and myeloid cells bearing either Gr-1 or F4/80 (Figure 1A). Examination of cytocentrifuge slides revealed cells with dendritic and granulocyte/monocyte morphologies (Figure 1B). Absolute numbers of CD19+ lymphocytes were reduced by 15.6-fold at two weeks, and β-catenin stimulated expansion of total nucleated cells beyond this time (Figure 1C).
Figure 1. Expression of constitutive stable β-catenin in lymphoid progenitors confers myeloid lineage differentiation potential.
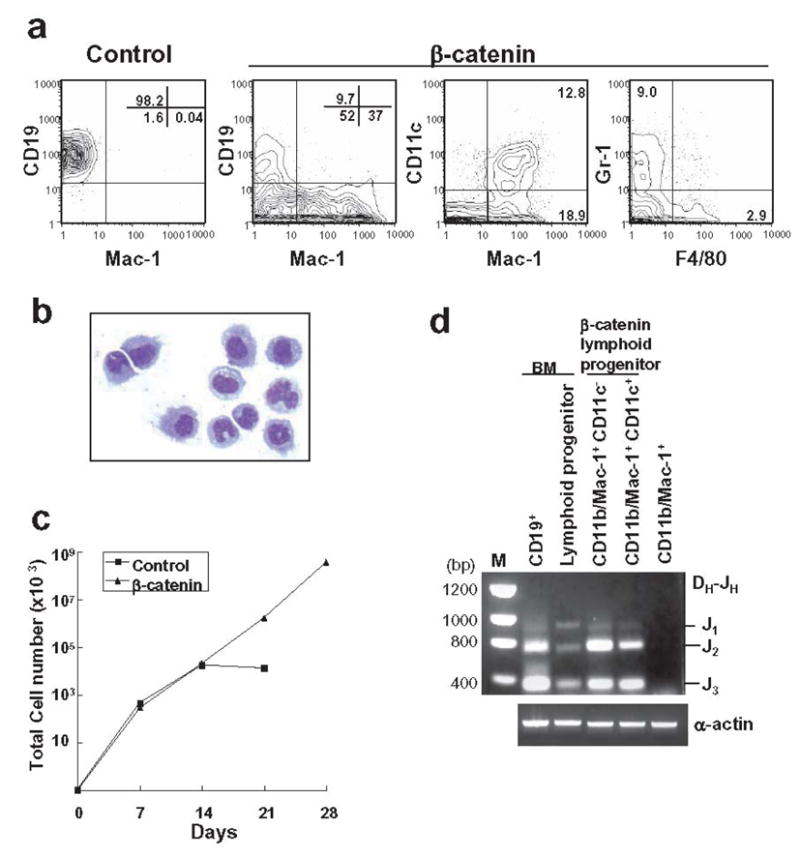
(A) Transduced GFP+ lymphoid progenitors with either control vector or stable β-catenin were sorted and co-cultured for 14 days on OP9 stromal cells with SCF, FL and IL-7. Flow cytometry was then used to detect various myeloid and B lineage lymphoid cells. (B) Mac-1+ cells were sorted after OP9 co-culture and a May-Grunwald-Geimsa stained cytospin preparation is shown. (C) After the first 7 days, and at 7 day intervals, cells were harvested and counted, and then 10,000 were re-plated on fresh OP9 stromal cells. (D) CD11b/Mac-1+ cells with or without the CD11c dendritic marker were recovered from 14 day cultures and purified genomic DNA was evaluated for IgH gene rearrangements. Freshly sorted CD19+ cells and Lin− c-KitLo Sca-1Lo IL-7Rα+ lymphoid progenitors from bone marrow were used as positive controls and compared to CD11b/Mac-1+ cells generated from HSC fraction in culture.
Cells with a lymphoid ancestry would be expected to have undergone immunoglobulin gene rearrangements and this point was investigated with sorted cells expressing myeloid (CD11b/Mac-1+ CD11c−) and dendritic (CD11b/Mac-1+ CD11c+) markers. Ig DH-JH rearrangement products were found in lymphoid progenitors that had converted to both of these cell types following transduction with β-catenin (Figure 1D). None were found when a non-transduced stem cell rich fraction was used to generate CD11b/Mac-1+ cells in culture (Figure 1D).
OP9 stromal cell co-cultures of β-catenin transduced lymphoid progenitors contained CD19− CD11b/Mac-1− cells with many primitive characteristics. That is, they were c-kit+Sca-1low/− CD43+ AA4.1+ Ter-119− CD3− DX-5− NK1.1− CD11c− Gr-1− and only expressed the CD45R/B220+marker that is frequently used to subdivide B lineage lymphocytes (data not shown). We sorted and sub-cultured these CD19− CD11b/Mac-1− cells on OP9 stromal cells with FL and IL-7 to assess their differentiation potential. SCF was omitted at this stage because preliminary experiments demonstrated that this cytokine promoted retention of primitive characteristics. After ten days, CD19+ and CD11b/Mac-1+ cells were again present (Figure 2A). Small numbers of CD3+ T lineage cells were generated when the cells were transferred to Delta-like 1 transduced OP9 (OP9-DL1) stromal cells (Schmitt and Zúñiga-Pflücker, 2002) and TCR γ/δ+ lymphocytes predominated over TCR α/β+ cells at three weeks of culture (data not shown).
Figure 2. Expression of constitutive stable β-catenin in lymphoid progenitors confers multi-lineage differentiation potential.

(A) CD19− Mac-1− cells in 14 day primary cultures initiated with stable β-catenin were sorted and tested for differentiation potential. Cells sub-cultured onto OP9 with FL and IL-7 generated both CD19+ B and CD11b/Mac-1+ lineage cells (left two panels). CD3ε T lineage cells emerged within 3 weeks onto OP9 stromal cells transduced with the Delta-like1 Notch ligand (OP9-DL1) (right two panels). (B) Lymphoid progenitors transduced with control vector or β-catenin were co-cultured on OP9 stromal cells in the presence of SCF, FL and IL-7. After 14 days, 1x106 cells were injected into sublethally irradiated NOD/SCID mice. After 5 weeks, cells were isolated from spleens before analysis by flow cytometry.
Bone marrow cells cultured for two weeks were transferred to sub-lethally irradiated NOD/SCID mice as an additional test of differentiation potential. No donor-type CD45.2 marked cells were found in recipients of cultures established from control vector transduced cells (Figure 2B). In contrast, CD19+ CD45R/B220+ lymphocytes were present in spleens of mice five weeks after receiving stable β-catenin transduced cells. Small numbers of CD11b/Mac-1+ myeloid cells were also present. Donor type T cells were not observed in the spleen or thymus (Figure 2B and data not shown), even though the five week interval is sufficient for T lymphopoiesis from stem cells (Spangrude and Scollay, 1990).
These results demonstrate that lymphoid committed progenitors can be expanded in culture for at least four weeks under the influence of ectopic β-catenin, but became less restricted with respect to fate. Primitive CD19− CD11b/Mac-1− cells recovered from these cultures had myeloid, as well as B and T lineage differentiation potential.
Ectopic activation of β-catenin in myeloid progenitors confers multi-lineage differentiation potential
A reporter system was previously used to determine that β-catenin activity is detectable in Lin− c-Kithi Sca-1+ HSC, but not in Lin− c-Kithi Sca-1− progenitors (Reya et al., 2003). Down-regulation of Wnt pathway signaling might accompany, or be a requirement for lineage progression. Therefore, we investigated whether ectopic β-catenin would influence self-renewal and lympho-/myeloid development in myeloid progenitors. Purified Lin−IL-7Rα −c-KithiSca1− myeloid progenitors were transduced with stable β-catenin or control vector before culturing on OP9 stromal cells with SCF, FL and IL-7. After 10 days, cells expressing control vector generated only CD11b/Mac-1+ myeloid lineage cells. Artificial expression of stable β-catenin allowed myeloid progenitors to generate not only myeloid cells but also CD19+ B lineage cells under these conditions (Figure 3A). In addition, the β-catenin transduced cells had a growth advantage over the control vector expressing cells (Figure 3B).
Figure 3. Ectopic activation of β-catenin in myeloid progenitors confers multi-lineage differentiation potential.
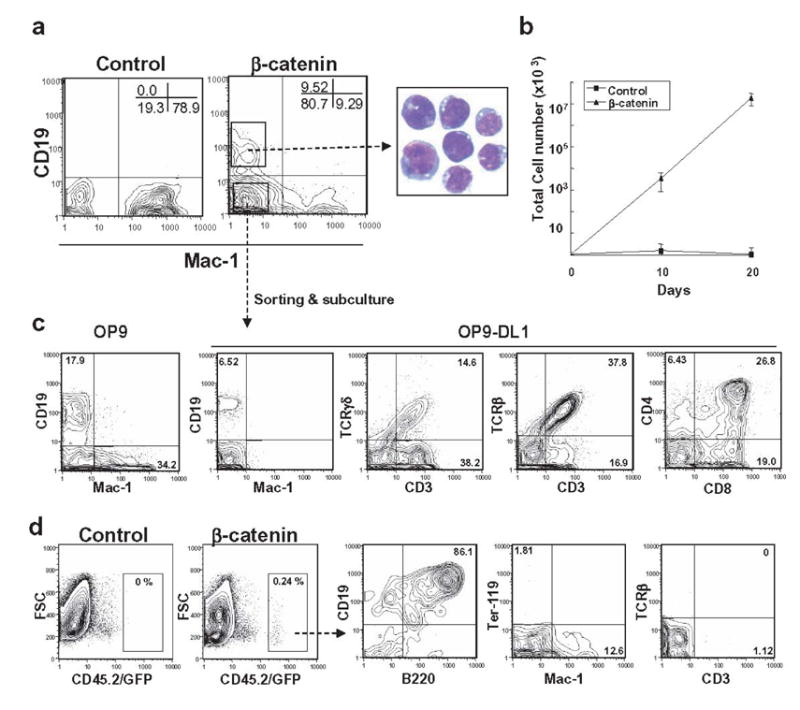
(A) GFP+ myeloid progenitors transduced with control vector or stable β-catenin were sorted and co-cultured on OP9 stromal cells in the presence of SCF, FL and IL-7. B (CD19) or myeloid (Mac-1) lineage differentiation was assessed by flow cytometry at 10 days. May-Grunwald-Geimsa staining of sorted CD19+ cells is shown. (B) After the first 10 days, and at 10 day intervals, cells were harvested and counted, and then 10,000 were re-plated on fresh OP9 stromal cells. (C) CD19− Mac-1−cells derived from stable β-catenin expressing myeloid progenitors were sorted and allowed to differentiate to CD19+ and Mac-1+ cells on OP9 stromal cells in the presence of FL and IL-7 during an additional 10 days (left panel). Sorted CD19− Mac-1−cells also gave rise to αβT and γδT cells when held for 3 weeks on OP9-DL1 stromal cells in the presence of FL and IL-7 (right three panels). (D) Myeloid progenitors transduced with control vector or β-catenin were co-cultured on OP9 stromal cells for 10 days in the presence of SCF, FL and IL-7 and 1 x106 cells were injected into sublethally irradiated NOD/SCID mice. After 5 weeks, cells were isolated from spleen and analyzed by flow cytometry.
To determine if differentiation was an ongoing process, we purified the predominant CD19− Mac-1− fraction from primary cultures of β-catenin transduced myeloid progenitors. Flow cytometry revealed that the sorted cells were Lin− c-kit+ Sca-1low/− CD45R/B220+ CD43+ AA4.1+ Ter-119− CD3− DX-5− NK1.1− CD11c− Gr-1− at that time (data not shown). Ten days after sub-culture on OP9 stromal cells with only FL and IL-7, B lineage lymphoid and myeloid cells were again made under these conditions while sub-culture on OP9-DL1 cells supported impressive generation of T lineage lymphocytes (Figure 3C). Freshly isolated myeloid progenitors did not generate T lineage cells on OP9-DL1 under these conditions (data not shown). Unlike the situation with transduced lymphoid progenitors, TCRα/β+ cells predominated over lymphocytes with γ/δ receptors. As recently found in another study with freshly harvested hematopoietic cells (Huang et al., 2005) Delta-like 1 Notch ligand suppressed myelopoiesis as well as B lymphopoiesis.
Myeloid progenitors were then transduced with constitutively active β-catenin and expanded on OP9 stromal cells with cytokines for 10 days before transfer to sub-lethally irradiated NOD/SCID mice. As was the case with transduced lymphoid progenitors, donor type B lineage lymphocytes and myeloid cells, but not T cells were recovered from recipient spleens (Figure 3D) or thymus (data not shown).
These observations suggested that committed myeloid progenitors might become lineage unstable as they are expanded under the influence of stable β-catenin. The point was further investigated with progenitors subdivided according to other parameters (Akashi et al., 2000). A Lin− c-KitHi Sca-1− FcγRLo CD34− subset previously designated megakaryocyte/erythrocyte progenitor (MEP) did not survive transduction with either the control or β-catenin containing vectors. However, Lin− c-KitHi Sca-1− FcγRLo CD34+ common myeloid progenitors (CMP) thrived after this treatment and generated both lymphoid and myeloid progeny in 10 day stromal cell co-cultures (Figure 4). In contrast, a very similar Lin− c-KitHi Sca-1− FcγRHi CD34+ granulocyte/macrophage progenitor (GMP) subset did not expand as well and appeared to be more myeloid lineage restricted. Both of these subsets produced many CD19− CD11b/Mac-1− cells in response to β-catenin, while few grew from cells given the control vector.
Figure 4. One subset of myeloid progenitors efficiently gives rise to B lineage cells following β-catenin activation.

Lin− c-KitHi Sca-1− CD34+ FcγRII/IIILo CMP and Lin− c-KitHi Sca-1− CD34+ FcγRII/IIIHi GMP were sorted and transduced with either control vector or stable β-catenin. GFP+ cells were then isolated and co-cultured on OP9 stromal cells in the presence of SCF, FL and IL-7 for 10 days. The bar graphs indicate yields, i.e., numbers of CD19+ B, CD11b/Mac-1+ myeloid lineage or CD19− CD11b/Mac-1− undifferentiated cells recovered per input cell.
We conclude that stable β-catenin permits substantial growth of at least two subsets of largely restricted myeloid progenitors and causes one of them to become lineage unstable.
Stable β-catenin disrupts lineage restriction in lymphoid and myeloid progenitors
Clonal studies were then conducted to learn if active β-catenin selects for rare contaminating cells or confers lineage instability to cells that should have restricted fates. We sorted single cells transduced with control or active β-catenin into 96-well plates containing OP9 stromal cells, SCF, FL and IL7. After 12 days, flow cytometry was used to discern four distinct phenotypes (Figure 5). Some cultures maintained primitive properties and expressed neither CD19 nor CD11b/Mac-1. Other cultures contained both B and myeloid lineage cells (B+M), while still others had restricted B or myeloid lineage cells (B or M). Although β-catenin expression did not substantially affect the plating efficiency of HSC enriched Lin− c-KitHi Sca-1+ IL-7Rα − marrow cells, numbers of clones with primitive cells were increased. Outgrowth of bipotent, B/myeloid cells was also increased, suggesting that β-catenin enhanced uncommitted cells rather than committed B or myeloid progenitors.
Figure 5. Altered lineage fates of lymphoid and myeloid progenitors resulting from activation of β-catenin.
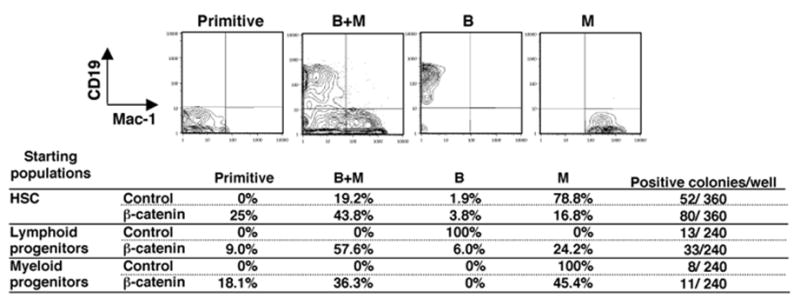
Single sorted GFP+ cells from a HSC enriched fraction, lymphoid progenitors or myeloid progenitors transduced with control vector or stable β-catenin were cultured on OP9 stromal cells in 96-well plates for 12 days in the presence of SCF, FL and IL-7. Positive readout wells (more than 40 cells) were determined by microscopic observation and phenotypes were determined by flow cytometry (representative examples shown on top row). The frequencies of wells with each of these differentiation patterns are shown along with total numbers of clones observed.
Lin− c-KitLo Sca-1Lo IL-7Rα+ lymphoid progenitors transduced with the control vector exclusively gave rise to CD19+ B cells. In striking contrast, lymphoid cells receiving active β-catenin generated all possible combinations of progeny. Similarly, cells that were largely restricted to a myeloid fate generated mixed populations of lymphoid and myeloid cells when transduced with stable β-catenin.
We conclude that restricted progenitors become unstable under the influence of constitutively active β-catenin, generating primitive cells as well as those corresponding to other lineages.
Active β-catenin changes gene expression patterns in committed progenitors
The above findings suggest that active β-catenin can destabilize fate determination in committed progenitor cells, and we used semi-quantitative RT-PCR to evaluate the expression of selected genes 48 hr after control vector or stable β-catenin transduction. Of particular interest were transcription factors known to be associated with lineage choice selection and self-renewal in primitive cells. Three substantial changes were found in lymphoid progenitors. EBF levels declined approximately 70%, while Pax-5 was extinguished and C/EBPα transcripts increased approximately 12 fold (Figure 6A). A dramatic induction in EBF was the only significant change found in myeloid progenitors transduced with β-catenin (Figure 6B). We were surprised not to find changes in HoxB4, Notch-1 or PU.1. A larger screen for gene expression was then made with the same samples (Supplementary Figure 1). Interpretation of some patterns is complicated by the fact that control transduced cells differed from freshly isolated progenitors. However, it is interesting that β-catenin may prolong expression of Gfi-1, Bmi-1 and Aiolos in cultured lymphoid cells, as well as Bmi-1 and the Id-3 transcriptional repressor in myeloid progenitors. We conclude that ectopic β-catenin results in inappropriate expression of several genes known to control differentiation patterns.
Figure 6. Stable β-catenin alters lineage-associated gene patterns.

(A, B) Progenitors of the indicated types were transduced with control vector or stable β-catenin for 48 hours before sorting for GFP+ cells. Freshly isolated lymphoid progenitors and myeloid progenitors were used as controls. Semi-quantitative RT-PCR was carried out to amplify transcripts for the indicated genes in each population. The bar graphs represent the results of [α 32P] dCTP incorporation on the linear parts of PCR amplification curves normalized relative to β-actin control values as detailed in Materials and Methods.
Discussion
Defining properties of committed progenitors include finite renewal capacity and some loss of differentiation options. The transition of self-renewing, pluripotent stem cells to myeloid progenitors is accompanied by down-regulation of Wnt signaling (Reya et al., 2003) and we have now explored the outcome of reactivated β-catenin in that cell type. In parallel, stable β-catenin was expressed in lymphoid progenitors to determine if this mediator of Wnt signaling influenced their properties. The treatment gave a growth advantage to, and revealed latent differentiation potential in both cell types. While β-catenin altered expression of some early acting transcription factors and restored multiple differentiation options, individual cells that resulted did not have markers associated with two lineages. These findings help to define the activities of one member of a complex signaling pathway and suggest how it might contribute to hematopoietic malignancies.
There have been many reports where lymphoid cells acquired myeloid characteristics under experimental circumstances (Boyd and Schrader, 1982; Klinken et al., 1988; Borzillo et al., 1990). In one recent example, sIgM+ B cells were converted to macrophages by strong over-expression of C/EBPα or C/EBPβ (Xie et al., 2004). That would appear to be a clear case of lineage conversion, since single cells with lymphoid and myeloid characteristics were found during the transition. The same was true with cloned tumor cell lines that lost differentiated properties and acquired many new ones (Graf, 2002). Other investigators have shown that progenitors largely committed to a lymphoid fate could be re-directed to become granulocytes and macrophages by artificial expression of receptors for growth and differentiation factors (Kondo et al., 2000; Iwasaki-Arai et al., 2003). In addition, lymphoid progenitors became megakaryocytes and erythroid cells as a result of GATA-1 over-expression (Iwasaki et al., 2003). These studies demonstrate that progenitors retain latent differentiation potential, and that characteristics of fully mature lymphocytes are maintained by a few critical transcription factors. Those regulators both maintain expression of lymphoid genes and repress those corresponding to alternate fates. The many instances of lymphoid to myeloid conversion could also be interpreted in terms of the latter representing a default state.
Given this extensive literature, it was not surprising that stable β-catenin conferred myeloid potential on lymphoid progenitors. While the transduction also gave cells a growth advantage, our clonal analyses argue against the possibility that rare multipotential cells contaminated the highly enriched suspensions and simply expanded with time. Furthemore, the presence of Ig DH-JH rearrangement products in dendritic and myeloid cells provides strong evidence that they arose from progenitors that were originally lymphoid committed. Flow cytometry revealed that the transduced cells were Lin− c-kit+Sca-1low/−CD45R/B220+CD43+AA4.1+ 10 days after transduction and culture initiation. CD45R/B220 is not a B lineage restricted marker, and individual cells with combined lymphoid and myeloid properties were not found in any of our experiments. This suggests that active β-catenin did not cause generalized gene expression, but rather permitted latent differentiation options to be exercised.
EBF is essential for initiating and maintaining B lymphopoiesis (Lin and Grosschedl, 1995; Maves and Schubiger, 2003; Singh et al., 2005) so rapid down-regulation of this transcription factor is consistent with at least some cells in the population becoming myeloid competent. More dramatic was the extinction of Pax-5, a factor required for intermediate and late stages of B lymphocyte formation (Nutt et al., 1999; Rolink et al., 1999). Note that the most primitive, early lymphoid progenitors within normal bone marrow are Pax-5− (Igarashi et al., 2002). C/EBPα levels increased under the influence of β-catenin and C/EBPα is reported to suppress Pax-5 activity (Xie et al., 2004).
In contrast to the lymphoid to myeloid conversion, there are few examples where non-lymphoid cells or committed progenitors were spontaneously or experimentally turned into lymphocytes. Kee and Murre manipulated a macrophage-like variant of the 70Z/3 pre-B lymphoma (Kee and Murre, 1998). They found that lymphoid characteristics were dramatically restored by expression of the E2A transcription factor. Rare, CD45R/B220− cells in bone marrow that express CD19 retain some potential to generate macrophages and B lineage lymphocytes (Montecino-Rodriguez et al., 2001). While this shows that display of CD19 is not necessarily a definitive lymphoid characteristic, the findings do not prove that committed lymphoid or myeloid progenitors spontaneously undergo lineage conversion. It is clear in our study that at least two major subsets of T cells (α/β and γ/δ TCR+ cells), as well as CD19+ B lineage lymphocytes were generated from myeloid progenitors in response to stable β-catenin. The Lin− c-KitHi Sca-1− FcγRLo CD34+ population referred to as common myeloid progenitors was particularly prone to generating lymphocytes under these conditions. This potential was present to a lower degree in a companion Lin− c-KitHi Sca-1− FcγRHi CD34+ granulocyte/macrophage progenitor subset.
It is to be expected that lymphoid related genes would be expressed in cultures that produce lymphocytes, but EBF was detectable in myeloid progenitors within 48 hrs of stable β-catenin introduction. This may represent a key event because Pax-5 transcripts were not found and myeloid permissive C/EBPα was not suppressed at that time. It is noteworthy that HOXB4 and Notch 1 levels were unchanged in either cell type, although this has been shown in another context to be a target of Wnt pathway signaling (Reya et al., 2003). Also, no significant changes were found in PU.1, although doses of this transcription factor are key in lymphoid versus myeloid differentiation decisions (DeKoter and Singh, 2000). A very recent study demonstrated that while critical amounts of PU.1 may be needed to produce lymphoid progenitors, it is not required to sustain that lineage (Iwasaki et al., 2005). Transcripts for GATA-3, Aiolos, Gfi-1, Bmi-1 and Id-3 were lower in cells that had been transduced with control vector than in the freshly isolated progenitors, but normal to high in β-catenin expressing progenitors. The Bmi-1 and Gfi-1 members of the polycomb group of genes are thought to have key roles retaining stem cell properties (Cellot and Sauvageau, 2005; Iwama et al., 2004) and it will be interesting to learn if they are targets of Wnt pathway signaling in other cell types.
These observations should be considered in context with a parallel study, where we introduced stable β-catenin to more primitive cells (Baba et al., manuscript submitted). In that case, long-term cultured, multipotential cells could be established and maintained in the presence of just two cytokines, SCF and IL-6. Of particular note, the cells were not obviously transformed after many passages and retained the ability to differentiate in response to normal environmental cues. While this might be interpreted in terms of retention of stem cell properties, the present findings suggest that it may also be possible to induce some stem cell characteristics in partially committed progenitors by ectopic expression of stable β-catenin.
In that sense, our results could represent an example of retrodifferentiation of hematopoietic cells (Graf, 2002). However, β-catenin transduced progenitors did not reconstitute T cells when transplanted to immunodeficient mice. This is despite the fact that the same manipulated progenitors generated T lineage cells in the OP9-DL1 co-culture system, and the β-catenin dependent TCF/LEF genes are important for T cell formation (Reya and Clevers, 2005; Verbeek et al., 1995). We had the same experience with β-catenin transduced HSC, and it is possible the cultured cells are unable to home to the recipient thymuses (Baba, manuscript submitted). The findings are compatible with another study, where thymuses with experimentally elevated β-catenin were only 10% normal size due to reduced proliferation and increased thymocyte death (Gounari et al., 2001). This situation does contrast with long-term cultures prepared from Pax-5 or E2A targeted cells (Nutt et al., 1999; Rolink et al., 1999; Ikawa et al., 2004). Thus, β-catenin is not sufficient for committed cells to re-aquire all stem cell properties.
Two other reports demonstrated the conversion of progenitors to multipotential cells. One showed that HoxB4 over-expression induced the expansion of definitive HSC and conferred long-term, multi-lineage potential to primitive hematopoietic cells derived from yolk sac (Kyba et al., 2002). The other study demonstrated that Myb-Ets oncoprotein of E26 leukemia virus converted primitive erythroid cells into proliferating definitive multi-potent cells (McNagny and Graf, 2003). Although our study did not detect any difference in HoxB4 expression levels, it will be interesting to determine whether stable β-catenin expression induces Myb-Ets target genes.
Recently, a somewhat different concept has emerged from fate switch studies in Drosophila. Imaginal disc cells are rigidly determined to form specific structures late in larval development. However, they can switch fates during regenerative cell divisions (Maves and Schubiger, 2003). In addition, ectopic expression of the Wingless (Wg) member of the Wnt family can induce trans-determination with unique cell cycle state and cell size, but not retro-differentiation (Sustar and Schubiger, 2005). This work, together with our findings suggests that Wnt/Wg signaling allows cells to acquire an apparently novel multi-potential state without conversion to true stem cells.
While these observations may not pertain to normal physiological conditions, they should be relevant to malignancy, inflammation or pathogenic infections (Reya and Clevers, 2005). Disregulation of the Wnt/β-catenin pathway may be particularly important in leukemia (Serinsoz et al., 2004; Chung et al., 2002; Jamieson et al., 2004; Lu et al., 2004; Shackelford et al., 2003). Although we saw no indication that stable β-catenin was sufficient for leukemic transformation of progenitor cells, it will be important to learn if the Wnt signaling pathway contributes to any of the malignant examples of lineage instability. For example, myeloid leukemia can undergo blast crisis with lymphoid characteristics including rearrangement of TCR or Ig genes (Cheng et al., 1986; Boggs, 1974; Stass et al., 1984) Conversely, switching of acute lymphoblastic leukemia cells to myeloid lineages has also been reported (Hershfield et al., 1984; Stass et al., 1984; Nosaka et al., 1988). This behavior has been referred to as “lineage infidelity” (Smith et al., 1983; Schmidt and Przybylski, 2001).
Several reports suggest that β-catenin may be involved in chromatin remodeling. For example, β-catenin physically interacts with CBP/p300 which is known to function as a transcriptional coactivator (Takemaru and Moon, 2000; Hecht et al., 2000). CBP/p300 has intrinsic histone acetyltransferase (HAT) activities that alter chromatin structure by covalent modification of nucleosomal histones, that in turn play a major role in regulation of gene expression (Ogryzko et al., 1996). Furthermore, Brg1 has been identified as a binding partner of β-catenin, a component of the SWI/SNF chromatin remodeling complex that can induce the expression of target genes by facilitating DNA accessibility (Barker et al., 2001). This complex has been shown to regulate T lymphocyte development in the thymus (Chi et al., 2002; Gebuhr et al., 2003; Chi et al., 2003). More recently, it has been shown that Pygopus associates with β-catenin through the Lgs/Bcl-9 adaptor protein (Kramps et al., 2002). The complex of Pygopus-Lgs/Bcl-9 allows β-catenin to localize to the nucleus, which may be required for maximum activation of β-catenin and LEF/TCF-dependent promoters/genes (Thompson et al., 2002; Parker et al., 2002; Belenkaya et al., 2002). Interestingly, the Pygopus protein contains a PHD domain, often found in chromatin remodeling factors that are thought to alter chromatin structure and thereby allow activation or repression of specific genes (Aasland et al., 1995). This body of work suggests mechanisms through which enforced β-catenin activation could disrupt chromatin status and cause instability of gene expression.
Our findings demonstrate that manipulation of β-catenin can directly influence the survival and differentiation of normal hematopoietic progenitors. This information is not necessarily relevant to physiologic processes, but could be important as attempts are made to exploit the Wnt signaling pathway for tissue regeneration (Moon et al., 2004).
Experimental Procedures
Mice and Cell lines
C57BL/6 (CD45.2 alloantigen positive) and NOD/SCID mice were purchased from the Jackson Labs (Bar Harbor) and maintained in our laboratory animal facility. The murine OP9 and OP9-DL1 stromal cell lines were obtained from Dr. Juan Carlos Zúñiga-Pflücker at the University of Toronto and maintained in α-MEM supplemented with 20% FCS.
Antibodies
Anti-CD45RA (14.8) mAb developed in our laboratory and the anti-CD11b/Mac-1 (M1/70) mAb were used as culture supernatants of the respective hybridomas. Purified anti-erythroid (Ter-119) and anti-Gr-1 (Ly-6G; RB6-8C5) antibodies, FITC-conjugated anti-Ter-119, anti-Gr-1, anti-CD11b/Mac-1, anti-CD45R/B220 (RA3/6B2), anti-CD19 (ID3), anti-CD2 (LFA-2), anti-CD3 (145-2C11), anti-CD34 (RAM34) and anti-CD8α (53−6.7) antibodies, PE-conjugated anti-IL-7Rα chain (SB/199), anti-Sca-1 (Ly6A/E; E13-161.7), anti-FcγRII/III (2.4G2), anti-CD19, anti-Gr-1, anti-CD11c (HL3), anti-CD45R/B220 (RA3/6B2), anti-pan NK cell (DX5/CD49), anti-TCRβ (H57-597), anti-TCRγδ (GL3), anti-CD43 (S7), anti-C1qRp (AA4.1), anti-CD135 (Flk2/Flt3, Ly-72), anti-CD4 (L3T4), biotin-conjugated anti-Sca-1, anti-VCAM-1 (429 MVCAM.A), APC-conjugated anti-c-Kit (2B8), anti-CD11b/Mac-1 (M1/70), anti-CD3 (145-2C11), anti-CD8α (53-6.7) mAb were all purchased from BD PharMingen. APC-conjugated anti-F4/80 was purchased from eBioscience. A phycoerythrin-Texas red tandem-conjugated streptavidin (PE-Texas Red-streptavidin) was purchased from Caltag.
Cell sorting
Bone marrow cells were harvested and enriched for lineage negative cells by incubation with antibodies to Gr-1 and CD11b/Mac-1 for myeloid cells, CD45R/B220 for B lineage cells and Ter-119 for erythroid cells, followed by negative selection using the MACS cell separation system (Miltenyi Biotec). These partially lineage depleted cells were further stained with FITC-labeled lineage markers Gr-1, CD11b/Mac-1, CD2, CD19, CD3, CD8α, Ter-119, CD45R/B220 and PE-anti-IL-7Rα chain, APC-anti-c-kit (2B8) and biotin-anti-Sca-1 antibodies followed by streptavidin PE-Texas Red. Lymphoid progenitors and myeloid progenitors were sorted as Lin−IL-7Rα+c-KitlowSca1low and Lin−IL-7Rα −c-KitHiSca1−, respectively. To further subdivide myeloid progenitors, the sorted Lin−IL-7Rα −Sca1−c-KitHi population was re-stained with FITC-anti-CD34 and PE-anti-FcγRII/III antibodies, and sorted as Lin−IL-7Rα −Sca1−c-KitHiCD34+FcγRII/IIIlo (CMP), Lin−IL-7Rα −Sca1−c-KitHiCD34+FcγRII/IIIHi (GMP), and Lin−IL-7Rα −Sca1−c-KitHiCD34−FcγRII/IIIlo (MEP) (Akashi et al., 2000). All sorts were performed either with a MoFlo (Cytomation) or FacsAria (Becton Dickinson).
Retrovirus production and infection
A retroviral vector containing a cDNA encoding HA-tagged stable β-catenin has been described in detail elsewhere (Baba et al., manuscript submitted). A cDNA encoding HA-tagged stable β-catenin with substitute mutations of S33A, S37A, T41A, S45A (a gift from Dr. Shinichi Hayashi) was cloned into the LZRS-IRES-GFP retroviral vector (a gift from Dr. Hergen Spits). The resulting vector and a control lacking β-catenin were transfected into EcoPack2 (BD Biosciences) by FuGENE6 (Roche) and transfected cells were selected with 2 μg/ml puromycin (Sigma). Supernatants were harvested 24 hr after changing media and immediately used for infections.
For infection of progenitors, sorted cells were deposited into single wells of 24-well plates at 1x104 to 5x105 cells per well in X-VIVO15 medium (Bio Whittaker) containing 1 % detoxified bovine serum albumin (Stem Cell Technologies), 2 mM L-glutamine, 5 x 10−5 M 2-mercaptoethanol, 100 U/ml penicillin and 100 μg/ml streptomycin plus 20 ng/ml recombinant mouse stem cell factor (SCF), 100 ng/ml Flk2/Flt3 ligand (FL), and 1ng/ml IL-7 for lymphoid progenitors or 20 ng/ml SCF for myeloid progenitors. Equal volumes of virus supernatant containing growth factor and 8 μg/ml polybrene (Sigma) were added. Spin infections were then conducted for 2 hr at 2000 rpm in a centrifuge at 32° C and the plates were incubated at 37° C for 5hr. Culture media was replaced with fresh media containing fresh virus supernatant, growth factor and polybrene, and then spin infection was repeated as before. After infection, cells were incubated at 37° C overnight. Culture media was replaced the following day with fresh media containing growth factors and incubated for an additional 24 hr. For infection of the HSC enriched fraction, sorted cells (Lin− c-kithi Sca-1+ IL-7Rα −) were pre-stimulated in X-VIVO15 medium containing 1% detoxified bovine serum, 2 mM L-glutamine, 5 x 10−5 M 2-mercaptoethanol, 100 U/ml penicillin and 100 μg/ml streptomycin in the presence of 20 ng/ml SCF, 100 ng/ml FL, and 20 ng/ml thrombopoietin (TPO) for 16 hr to 18 hr. Spin infection was then conducted with the same cytokines. After infection, GFP positive cells were purified by MoFlo. All cytokines were purchased from R&D Systems.
Culture assays and Flow Cytometry
To evaluate B and myeloid lineage cells differentiation, single cells or 1000 cells of each sorted fraction were co-cultured with OP9 cells in single wells of 96-well or 24-well plates in the presence of SCF (20 ng/ml), FL (100 ng/ml) and IL-7 (1 ng/ml) for the indicated periods. To evaluate T lineage cell differentiation potential, 10,000 CD19− Mac-1− cells were recovered and sorted from primary OP9 cultures and then sub-cultured with OP9-DL1 stromal cells in the presence of FL (100 ng/ml) and IL-7 (1 ng/ml) for 3 weeks. At the end of culture, cells were counted excluding stromal cells and then subjected to flow cytometry. A biotinylated anti-VCAM-1 mAb was used to exclude potential contamination of VCAM-1+ stromal cells in the analyzed populations and 7AAD was used to exclude dead cells. Flow cytometry was performed on a FACSCalibur, and the data were analyzed with Flowjo software (Treestar).
Transplantation of cultured hematopoietic cells
The sorted progenitors were transduced with control vector or stable β-catenin before being cultured on OP9 stromal cells in the presence of SCF, FL and IL-7. Then, 1 x 106 cultured cells were transferred intravenously into sublethally irradiated (200 rad) NOD/SCID mice. Spleens were harvested from these mice and analyzed by flow cytometry five weeks post-injection. CD45.2 and GFP were used to distinguish host cells from donor cells.
Ig gene rearrangement assay
Genomic DNA was isolated from sorted cells using a DNeasy Tissue Kit (QIAGEN). PCR reactions were conducted as described elsewhere (Igarashi et al., 2002). The primers used were DHL(5’) and J3(3’) to detect DH-JH rearrangement and α-actin as described (Igarashi et al., 2002). PCR products were electrophoresed through 1.4% agarose gels in TAE buffer and products were visualized by ethidium bromide staining.
Semi-quantitative RT-PCR Analysis of Gene Expression
The mRNAs were isolated from sorted cells using MicroPoly(A) Pure (Ambion). cDNA was prepared from DNase I-treated mRNA using oligo-dT and Moloney murine leukemia virus reverse transcriptase (Invitrogen). PCR reactions were conducted in buffer containing 200 μM dATP, dGTP, dTTP, 100 μM dCTP and 0.5 μCi [α 32P] dCTP. Aliquots were removed at cycle 25, 28 and 31 for β-actin and cycles 32, 35 and 38 for all others to insure that PCR remained within the exponential range of amplifications. Five micro liter aliquots were denatured in a formamide-loading buffer and applied to a 6 % polyacrylamide gel containing 7 M urea. Incorporation of [α 32P] dCTP into PCR product bands was quantified by PhosphoImager (Molecular Dynamics). Primer sequences and amplification conditions are available from the authors on request.
Acknowledgments
We thank Viji Dandapani, Jacob Bass and Diana Hamilton for expert technical assistance. In addition, we appreciate the secretarial help provided by Shelli Wasson. This work was supported by grants AI20069, AI58162 from the National Institutes of Health and P20-RR15577 from the COBRE Program of the National Center for Research Resources. P.W.K. holds the William H. and Rita Bell Endowed Chair in Biomedical Research.
References
- Aasland R, Gibson TJ, Stewart AF. The PHD finger: implications for chromatin-mediated transcriptional regulation. Trends Biochem Sci. 1995;20:56–59. doi: 10.1016/s0968-0004(00)88957-4. [DOI] [PubMed] [Google Scholar]
- Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- Barker N, Hurlstone A, Musisi H, Miles A, Bienz M, Clevers H. The chromatin remodelling factor Brg-1 interacts with β-catenin to promote target gene activation. EMBO J. 2001;20:4935–4943. doi: 10.1093/emboj/20.17.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenkaya TY, Han C, Standley HJ, Lin X, Houston DW, Heasman J, Lin X. pygopus Encodes a nuclear protein essential for wingless/Wnt signaling. Development. 2002;129:4089–4101. doi: 10.1242/dev.129.17.4089. [DOI] [PubMed] [Google Scholar]
- Boggs DR. Editorial: Hematopoietic stem cell theory in relation to possible lymphoblastic conversion of chronic myeloid leukemia. Blood. 1974;44:449–453. [PubMed] [Google Scholar]
- Borrello MA, Phipps RP. Fibroblasts support outgrowth of splenocytes simultaneously expressing B lymphocyte and macrophage characteristics. J Immunol. 1995;155:4155–4161. [PubMed] [Google Scholar]
- Borzillo GV, Ashmun RA, Sherr CJ. Macrophage lineage switching of murine early pre-B lymphoid cells expressing transduced fms genes. Mol Cell Biol. 1990;10:2703–2714. doi: 10.1128/mcb.10.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd AW, Schrader JW. Derivation of macrophage-like lines from the pre-B lymphoma ABLS 8.1 using 5-azacytidine. Nature. 1982;297:691–693. doi: 10.1038/297691a0. [DOI] [PubMed] [Google Scholar]
- Cellot S, Sauvageau G. Gfi-1: another piece in the HSC puzzle. Trends Immunol. 2005;26:68–71. doi: 10.1016/j.it.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Cheng GY, Minden MD, Toyonaga B, Mak TW, McCulloch EA. T cell receptor and immunoglobulin gene rearrangements in acute myeloblastic leukemia. J Exp Med. 1986;163:414–424. doi: 10.1084/jem.163.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi TH, Wan M, Lee PP, Akashi K, Metzger D, Chambon P, Wilson CB, Crabtree GR. Sequential roles of Brg, the ATPase subunit of BAF chromatin remodeling complexes, in thymocyte development. Immunity. 2003;19:169–182. doi: 10.1016/s1074-7613(03)00199-7. [DOI] [PubMed] [Google Scholar]
- Chi TH, Wan M, Zhao K, Taniuchi I, Chen L, Littman DR, Crabtree GR. Reciprocal regulation of CD4/CD8 expression by SWI/SNF-like BAF complexes. Nature. 2002;418:195–199. doi: 10.1038/nature00876. [DOI] [PubMed] [Google Scholar]
- Chung EJ, Hwang SG, Nguyen P, Lee S, Kim JS, Kim JW, Henkart PA, Bottaro DP, Soon L, Bonvini P, Lee SJ, Karp JE, Oh HJ, Rubin JS, Trepel JB. Regulation of leukemic cell adhesion, proliferation, and survival by β-catenin. Blood. 2002;100:982–990. doi: 10.1182/blood.v100.3.982. [DOI] [PubMed] [Google Scholar]
- Cobas M, Wilson A, Ernst B, Mancini SJ, MacDonald HR, Kemler R, Radtke F. β-catenin is dispensable for hematopoiesis and lymphopoiesis. J Exp Med. 2004;199:221–229. doi: 10.1084/jem.20031615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKoter RP, Singh H. Regulation of B lymphocyte and macrophage development by graded expression of PU.1. Science. 2000;288:1439–1441. doi: 10.1126/science.288.5470.1439. [DOI] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Gebuhr TC, Kovalev GI, Bultman S, Godfrey V, Su L, Magnuson T. The role of Brg1, a catalytic subunit of mammalian chromatin-remodeling complexes, in T cell development. J Exp Med. 2003;198:1937–1949. doi: 10.1084/jem.20030714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gounari F, Aifantis I, Khazaie K, Hoeflinger S, Harada N, Taketo MM, von BH. Somatic activation of β-catenin bypasses pre-TCR signaling and TCR selection in thymocyte development. Nat Immunol. 2001;2:863–869. doi: 10.1038/ni0901-863. [DOI] [PubMed] [Google Scholar]
- Graf T. Differentiation plasticity of hematopoietic cells. Blood. 2002;99:3089–3101. doi: 10.1182/blood.v99.9.3089. [DOI] [PubMed] [Google Scholar]
- Hecht A, Vleminckx K, Stemmler MP, van RF, Kemler R. The p300/CBP acetyltransferases function as transcriptional coactivators of β-catenin in vertebrates. EMBO J. 2000;19:1839–1850. doi: 10.1093/emboj/19.8.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield MS, Kurtzberg J, Harden E, Moore JO, Whang-Peng J, Haynes BF. Conversion of a stem cell leukemia from a T-lymphoid to a myeloid phenotype induced by the adenosine deaminase inhibitor 2′-deoxycoformycin. Proc Natl Acad Sci USA. 1984;81:253–257. doi: 10.1073/pnas.81.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Garrett KP, Pelayo R, Zúñiga-Pflücker JC, Petrie HT, Kincade PW. Propensity of adult lymphoid progenitors to progress to DN2/3 stage thymocytes with Notch receptor ligation. J Immunol. 2005 doi: 10.4049/jimmunol.175.8.4858. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi H, Gregory SC, Yokota T, Sakaguchi N, Kincade PW. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity. 2002;17:117–130. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- Ikawa T, Kawamoto H, Wright LY, Murre C. Long-term cultured E2A-deficient hematopoietic progenitor cells are pluripotent. Immunity. 2004;20:349–360. doi: 10.1016/s1074-7613(04)00049-4. [DOI] [PubMed] [Google Scholar]
- Iwama A, Oguro H, Negishi M, Kato Y, Morita Y, Tsukui H, Ema H, Kamijo T, Katoh-Fukui Y, Koseki H, van LM, Nakauchi H. Enhanced self-renewal of hematopoietic stem cells mediated by the polycomb gene product Bmi-1. Immunity. 2004;21:843–851. doi: 10.1016/j.immuni.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Mizuno S, Wells RA, Cantor AB, Watanabe S, Akashi K. GATA-1 converts lymphoid and myelomonocytic progenitors into the megakaryocyte/erythrocyte lineages. Immunity. 2003;19:451–462. doi: 10.1016/s1074-7613(03)00242-5. [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Somoza C, Shigematsu H, Duprez EA, Iwasaki-Arai J, Mizuno S, Arinobu Y, Geary K, Zhang P, Dayaram T, Fenyus ML, Elf S, Chan S, Kastner P, Huettner CS, Murray R, Tenen DG, Akashi K. Distinctive and indispensable roles of PU.1 in maintenance of hematopoietic stem cells and their differentiation. Blood. 2005;106:1590–1600. doi: 10.1182/blood-2005-03-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki-Arai J, Iwasaki H, Miyamoto T, Watanabe S, Akashi K. Enforced granulocyte/macrophage colony-stimulating factor signals do not support lymphopoiesis, but instruct lymphoid to myelomonocytic lineage conversion. J Exp Med. 2003;197:1311–1322. doi: 10.1084/jem.20021843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating A, Sawyers CL, Weissman IL. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- Kee BL, Murre C. Induction of early B cell factor (EBF) and multiple B lineage genes by the basic helix-loop-helix transcription factor E12. J Exp Med. 1998;188:699–713. doi: 10.1084/jem.188.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincade PW, Owen JJT, Igarashi H, Kouro T, Yokota T, Rossi MID. Nature or Nurture? Steady state lymphocyte formation in adults does not recapitulate ontogeny. Immunol Rev. 2002;187:116–125. doi: 10.1034/j.1600-065x.2002.18710.x. [DOI] [PubMed] [Google Scholar]
- Klinken SP, Alexander WS, Adams JM. Hemopoietic lineage switch: v-raf oncogene converts Eμmyc transgenic B cells into macrophages. Cell. 1988;53:857–867. doi: 10.1016/s0092-8674(88)90309-1. [DOI] [PubMed] [Google Scholar]
- Kondo M, Scherer DC, Miyamoto T, King AG, Akashi K, Sugamura K, Weissman IL. Cell-fate conversion of lymphoid-committed progenitors by instructive actions of cytokines. Nature. 2000;407:383–386. doi: 10.1038/35030112. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, Murone M, Zullig S, Basler K. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear β-catenin-TCF complex. Cell. 2002;109:47–60. doi: 10.1016/s0092-8674(02)00679-7. [DOI] [PubMed] [Google Scholar]
- Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- Lin H, Grosschedl R. Failure of B-cell differentiation in mice lacking the transcription factor EBF. Nature. 1995;376:263–267. doi: 10.1038/376263a0. [DOI] [PubMed] [Google Scholar]
- Lu D, Zhao Y, Tawatao R, Cottam HB, Sen M, Leoni LM, Kipps TJ, Corr M, Carson DA. Activation of the Wnt signaling pathway in chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 2004;101:3118–3123. doi: 10.1073/pnas.0308648100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maves L, Schubiger G. Transdetermination in Drosophila imaginal discs: a model for understanding pluripotency and selector gene maintenance. Curr Opin Genet Dev. 2003;13:472–479. doi: 10.1016/j.gde.2003.08.006. [DOI] [PubMed] [Google Scholar]
- McNagny KM, Graf T. E26 leukemia virus converts primitive erythroid cells into cycling multilineage progenitors. Blood. 2003;101:1103–1110. doi: 10.1182/blood-2002-04-1050. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Iwasaki H, Reizis B, Ye M, Graf T, Weissman IL, Akashi K. Myeloid or lymphoid promiscuity as a critical step in hematopoietic lineage commitment. Dev Cell. 2002;3:137–147. doi: 10.1016/s1534-5807(02)00201-0. [DOI] [PubMed] [Google Scholar]
- Montecino-Rodriguez E, Leathers H, Dorshkind K. Bipotential B-macrophage progenitors are present in adult bone marrow. Nat Immunol. 2001;2:83–88. doi: 10.1038/83210. [DOI] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and β-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- Nosaka T, Ohno H, Doi S, Fukuhara S, Miwa H, Kita K, Shirakawa S, Honjo T, Hatanaka M. Phenotypic conversion of T lymphoblastic lymphoma to acute biphenotypic leukemia composed of lymphoblasts and myeloblasts. Molecular genetic evidence of the same clonal origin. J Clin Invest. 1988;81:1824–1828. doi: 10.1172/JCI113526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- Nutt SL, Heavey B, Rolink AG, Busslinger M. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature. 1999;401:556–562. doi: 10.1038/44076. [DOI] [PubMed] [Google Scholar]
- Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- Orkin SH. Diversification of haematopoietic stem cells to specific lineages. Nat Rev Genet. 2000;1:57–64. doi: 10.1038/35049577. [DOI] [PubMed] [Google Scholar]
- Parker DS, Jemison J, Cadigan KM. Pygopus, a nuclear PHD-finger protein required for Wingless signaling in Drosophila. Development. 2002;129:2565–2576. doi: 10.1242/dev.129.11.2565. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- Rolink AG, Nutt SL, Melchers F, Busslinger M. Long-term in vivo reconstitution of T-cell development by Pax5-deficient B-cell progenitors. Nature. 1999;401:603–606. doi: 10.1038/44164. [DOI] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Schmidt CA, Przybylski GK. What can we learn from leukemia as for the process of lineage commitment in hematopoiesis? Int Rev Immunol. 2001;20:107–115. doi: 10.3109/08830180109056725. [DOI] [PubMed] [Google Scholar]
- Schmitt TM, Zúñiga-Pflücker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- Serinsoz E, Neusch M, Busche G, Wasielewski R, Kreipe H, Bock O. Aberrant expression of β-catenin discriminates acute myeloid leukaemia from acute lymphoblastic leukaemia. Br J Haematol. 2004;126:313–319. doi: 10.1111/j.1365-2141.2004.05049.x. [DOI] [PubMed] [Google Scholar]
- Shackelford J, Maier C, Pagano JS. Epstein-Barr virus activates β-catenin in type III latently infected B lymphocyte lines: association with deubiquitinating enzymes. Proc Natl Acad Sci USA. 2003;100:15572–15576. doi: 10.1073/pnas.2636947100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Medina KL, Pongubala JM. Contingent gene regulatory networks and B cell fate specification. Proc Natl Acad Sci U S A. 2005;102:4949–4953. doi: 10.1073/pnas.0500480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LJ, Curtis JE, Messner HA, Senn JS, Furthmayr H, McCulloch EA. Lineage infidelity in acute leukemia. Blood. 1983;61:1138–1145. [PubMed] [Google Scholar]
- Spangrude GJ, Scollay R. Differentiation of hematopoietic stem cells in irradiated mouse thymic lobes: Kinetics and phenotype of progeny. J Immunol. 1990;145:3661–3668. [PubMed] [Google Scholar]
- Staal FJ, Clevers HC. WNT signalling and haematopoiesis: a WNT-WNT situation. Nat Rev Immunol. 2005;5:21–30. doi: 10.1038/nri1529. [DOI] [PubMed] [Google Scholar]
- Stass S, Mirro J, Melvin S, Pui CH, Murphy SB, Williams D. Lineage switch in acute leukemia. Blood. 1984;64:701–706. [PubMed] [Google Scholar]
- Sustar A, Schubiger G. A transient cell cycle shift in Drosophila imaginal disc cells precedes multipotency. Cell. 2005;120:383–393. doi: 10.1016/j.cell.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Takemaru KI, Moon RT. The transcriptional coactivator CBP interacts with β-catenin to activate gene expression. J Cell Biol. 2000;149:249–254. doi: 10.1083/jcb.149.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B, Townsley F, Rosin-Arbesfeld R, Musisi H, Bienz M. A new nuclear component of the Wnt signalling pathway. Nat Cell Biol. 2002;4:367–373. doi: 10.1038/ncb786. [DOI] [PubMed] [Google Scholar]
- Verbeek S, Izon D, Hofhuis F, Robanus-Maandag E, te RH, van de WM, Oosterwegel M, Wilson A, MacDonald HR, Clevers H. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature. 1995;374:70–74. doi: 10.1038/374070a0. [DOI] [PubMed] [Google Scholar]
- Warren LA, Rothenberg EV. Regulatory coding of lymphoid lineage choice by hematopoietic transcription factors. Curr Opin Immunol. 2003;15:166–175. doi: 10.1016/s0952-7915(03)00011-6. [DOI] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, III, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Xie H, Ye M, Feng R, Graf T. Stepwise reprogramming of B cells into macrophages. Cell. 2004;117:663–676. doi: 10.1016/s0092-8674(04)00419-2. [DOI] [PubMed] [Google Scholar]
- Zhu AJ, Watt FM. β-catenin signalling modulates proliferative potential of human epidermal keratinocytes independently of intercellular adhesion. Development. 1999;126:2285–2298. doi: 10.1242/dev.126.10.2285. [DOI] [PubMed] [Google Scholar]