Cdc42 and noncanonical Wnt signal transduction pathways cooperate to promote cell polarity (original) (raw)
Abstract
Scratch-induced disruption of cultured monolayers induces polarity in front row cells that can be visualized by spatially localized polymerization of actin at the front of the cell and reorientation of the centrosome/Golgi to face the leading edge. We previously reported that centrosomal reorientation and microtubule polarization depend on a Cdc42-regulated signal transduction pathway involving activation of the Par6/aPKC complex followed by inhibition of GSK-3β and accumulation of the adenomatous polyposis coli (APC) protein at the plus ends of leading-edge microtubules. Using monolayers of primary rodent embryo fibroblasts, we show here that dishevelled (Dvl) and axin, two major components of the Wnt signaling pathway are required for centrosome reorientation and that Wnt5a is required for activation of this pathway. We conclude that disruption of cell–cell contacts leads to the activation of a noncanonical Wnt/dishevelled signal transduction pathway that cooperates with Cdc42/Par6/aPKC to promote polarized reorganization of the microtubule cytoskeleton.
Introduction
Scratch-induced disruption of tissue culture monolayers has been used to study aspects of directed cell migration in a variety of cell types, including fibroblasts, astrocytes, endothelial cells, and epithelial cells. With most primary nontransformed cells, migration involves coordinated movement of the monolayer in a manner more similar to the morphogenetic movements seen during development, such as dorsal closure and convergent extension, than the movement of single cells such as in neutrophil chemotaxis. Disruption of the monolayer causes the loss of cell–cell contacts and a major consequence of this is to induce polarity in cells proximal to the scratch. One aspect of polarization is the formation of actin-rich protrusions, specifically at the front of the cell (Nobes and Hall, 1999). A second aspect of polarization involves the microtubule cytoskeleton and can be visualized as reorientation of the centrosome and Golgi to face the front of the cell. This involves the association of microtubule plus-end tips with plasma membrane complexes at the leading edge as well as movement of the nucleus to the back of the cell (Kupfer et al., 1982; Etienne-Manneville and Hall, 2001; Gomes et al., 2005). Numerous studies have now shown that the small GTPase Cdc42, or one of its close relatives, is required for polarization of the actin and microtubule cytoskeletons in astrocytes, primary fibroblasts, 3T3 fibroblasts, Vero epithelial cells, and endothelial cells (Nobes and Hall, 1999; Etienne-Manneville and Hall, 2001, 2003; Palazzo et al., 2001b; Tzima et al., 2003; Watanabe et al., 2004; Cau and Hall, 2005; Gomes et al., 2005).
Studies of the signaling pathways controlling microtubule polarization in different adherent cell types have identified a complex consisting of the scaffold protein Par6 and an atypical PKC (aPKC) downstream of Cdc42. Localized activation of Cdc42 leads to localized activation of the Par6/aPKC complex, and this has now been described in astrocytes (Etienne-Manneville and Hall, 2001, 2003), primary rat fibroblasts (Nobes and Hall, 1999; Cau and Hall, 2005), 3T3 fibroblasts (Gomes et al., 2005), and endothelial cells (Tzima et al., 2003). The Par6/aPKC complex has at least two crucial activities in this process. First, it is required for the accumulation of the tumor suppressor protein adenomatous polyposis coli (APC) at the plus-end tips of microtubules, specifically at the leading edge. In primary astrocytes, GSK-3β is phosphorylated at Ser9 by PKCζ (Etienne-Manneville and Hall, 2003), and this was assumed to be the likely mechanism for inhibition of kinase activity leading to APC accumulation. A second activity of the Par6/aPKC complex is to promote the accumulation of another tumor suppressor protein Dlg (Discs Large) in the plasma membrane at the leading edge. The subsequent association of microtubule-bound APC with membrane-bound Dlg is required for microtubule polarization and centrosome reorientation (Etienne-Manneville et al., 2005). It is likely that many other cellular activities are required for reorganization of the microtubule cytoskeleton; for example IQGAP, another Cdc42 effector, is required both for protrusion polarity as well APC and microtubule polarity (Watanabe et al., 2004) and the dynein/dynactin complex is required for centrosome reorientation and mDia and EB1, regulated by Rho, also contribute to APC localization and stabilization (Palazzo et al., 2001a; Wen et al., 2004).
In this report, we reexamined the significance of GSK-3 phosphorylation using fibroblasts derived from knock-in mice in which the phosphorylation sites of both GSK3α and β isoforms (Ser21 and Ser9, respectively) have been replaced with Ala (McManus et al., 2005). We find that GSK-3 phosphorylation is not required for Golgi/centrosome reorientation, but instead dishevelled (Dvl), axin, and Wnt ligands are required. It appears that a Cdc42/Par6/aPKC signaling pathway cooperates with a noncanonical Wnt signaling pathway to promote polarization of the microtubule cytoskeleton.
Results and discussion
GSK-3 phosphorylation is not required for centrosome/Golgi reorientation
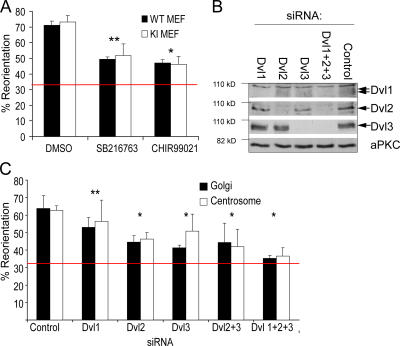
We previously reported that localized inhibition of GSK-3β is required for centrosome/Golgi reorientation and that GSK-3β is phosphorylated downstream of Cdc42/Par6/PKCζ in migrating astrocytes and fibroblasts (Etienne-Manneville and Hall, 2003; Cau and Hall, 2005). To investigate whether phosphorylation is the mechanism of GSK-3 inhibition, we analyzed primary embryonic fibroblasts derived from double knock-in mice in which Ser21 of GSK-3α and Ser9 of GSK-3β have been replaced with nonphosphorylatable Ala residues (to be referred to as GSK-3SA) (McManus et al., 2005). After scratching a monolayer, GSK-3SA cells showed no defect in reorientation of the centrosome or the Golgi compared with littermate wild-type fibroblasts (GSK-3WT), both exhibiting ∼70% reorientation as early as 2 h after wounding (Fig. 1 A). The experiment is scored such that 33% corresponds to random orientation. Furthermore, small molecule inhibitors of GSK-3 blocked centrosome and Golgi reorientation in GSK-3SA and GSK-3WT cells to the same extent (Fig. 1 A). We conclude that although phosphorylation of GSK-3 occurs as a consequence of inducing directed cell migration, the spatially localized inhibition of GSK-3 occurs through a mechanism that does not involve GSK-3 phosphorylation.
Figure 1.
GSK-3 inhibition is mediated by dishevelled and not by phosphorylation. (A) Golgi reorientation toward the scratch was quantified in GSK-3WT and GSK-3SA MEFs. Cells were pretreated for 1 h with indicated inhibitors, or 0.1% DMSO as control: 20 μM SB216763 or 5 μM CHIR99021 (both are GSK-3 inhibitors). *, P < 0.001; **, P < 0.01 in _t_ test when compared with DMSO control. (B) Dvl1, 2, and 3 are efficiently depleted using specific siRNAs, also in combination (depletion >95%), compared with nonspecific control siRNA oligo. aPKC serves as loading control. Arrows indicate position of Dvl protein. (C) Golgi and centrosome reorientation were measured in cells depleted of single Dvl isoforms or their combinations. Red bar marks basal level expected from random Golgi/centrosome reorientation (33%). *, P < 0.01; **, P > 0.05, in t test when compared with control.
Dishevelled and axin are required for centrosome/Golgi reorientation
An alternative mechanism of inhibiting GSK-3 activity is seen during Wnt signaling and involves protein–protein interactions mediated by dishevelled (Dvl). Wnt ligands induce the interaction of Dvl with the large scaffold protein axin, leading to dissociation of GSK-3 from a complex containing axin, APC, and β-catenin (Doble and Woodgett, 2003). To determine whether Dvl is required for centrosome reorientation, all three Dvl isoforms were depleted in rat embryo fibroblasts (REFs) using specific siRNA (Fig. 1 B). Depletion of single Dvl isoforms had varying partial effects, but depletion of all three Dvl isoforms completely inhibited centrosome and Golgi reorientation (Fig. 1 C; red line represents random orientation). This suggests that localized inhibition of GSK-3 is mediated by Dvl.
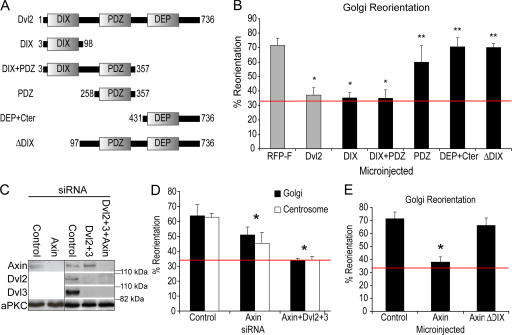
To analyze which domains of Dvl are required for reorientation, full-length Dvl2 or different Dvl domain constructs were microinjected into the nuclei of leading edge cells immediately after scratching a REF monolayer (Fig. 2, A and B). Cells expressing full-length Dvl2 exhibited complete loss of reorientation (Fig. 2 B). Overexpression of Dvl also blocked centrosome reorientation in GSK-3SA knock-in cells (unpublished data). This suggests that overexpression of Dvl interferes with the polarized inhibition of GSK-3, as has been observed in other situations (Itoh and Sokol, 1997; Boutros et al., 1998; Mlodzik, 2002). Constructs containing the DIX domain (DIX and DIX+PDZ) were also able to block reorientation, while the PDZ alone, DEP domain, or ΔDIX had no effect on reorientation (Fig. 2 B), suggesting that Dvl regulates reorientation through its DIX domain.
Figure 2.
Axin and dishevelled are required for reorientation in a DIX-dependent mechanism. (A) The different Dvl2 constructs used in microinjection experiments. (B) Golgi and centrosome reorientation were measured in cells microinjected with RFP-F control, or various Dvl2 domain-constructs. *, P < 0.01; **, P > 0.05, in t test when compared with control. (C) Axin is efficiently depleted with siRNA, also when combined with Dvl2 and 3 (right panel). aPKC serves as loading control. (D) Golgi and centrosome reorientation were measured in axin-depleted cells and in cells depleted of axin together with Dvl2 and 3. (E) Golgi reorientation was measured in cells microinjected with full-length axin or axinΔDIX. Red bar marks basal level expected from random Golgi/centrosome reorientation (33%). *, P < 0.005 in t test when compared with control.
The DIX domain interacts with Dvl itself and with axin (Kishida et al., 1999). To examine whether axin is required for reorientation, siRNA was used (Fig. 2, C and D). Interestingly, axin depletion blocked reorientation to a similar degree as Dvl2 or Dvl3 depletion (Fig. 2 D). Moreover, when axin was depleted together with Dvl2 and Dvl3, reorientation was completely inhibited, suggesting that Dvl2 and Dvl3 cooperate with axin, likely through a direct interaction. Axin overexpression after microinjection of an expression construct also blocked reorientation, whereas expression of axin lacking the DIX domain did not (Fig. 2 E), emphasizing the important role for the DIX domain in this process.
Wnt ligands are required for reorientation upstream of dishevelled
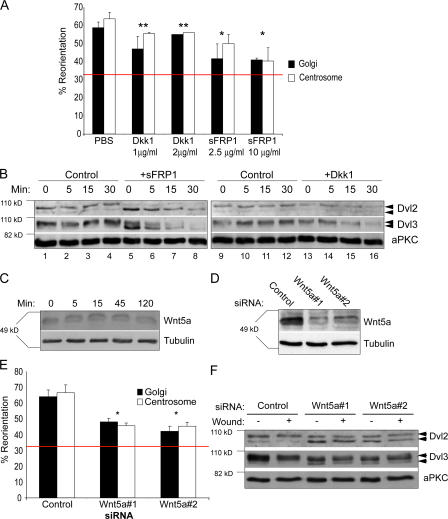
Because Dvl and axin are key components of Wnt signaling pathways and are also required for centrosome/Golgi reorientation, we tested whether Wnt ligands themselves might be involved. Wnt signaling was blocked using recombinant soluble Frizzled-related protein 1 (sFRP1), a naturally occurring antagonist of the Wnt pathway, which acts by binding to Wnt ligands and sequestering them away from frizzled receptors (Jones and Jomary, 2002). sFRP1 treatment led to substantial inhibition of centrosome/Golgi reorientation, suggesting that Wnts are involved in reorientation (Fig. 3 A). In contrast, recombinant Dickkopf1 (Dkk1) protein, another antagonist that specifically inhibits a subset of Wnt ligands that are required only for the canonical Wnt pathway, did not significantly block reorientation, implying that one or more Wnts belonging to the noncanonical family are responsible (Kawano and Kypta, 2003). Importantly, both antagonists blocked β-catenin stabilization in L-cells treated with Wnt3a, confirming their activity as negative regulators of Wnt signaling (unpublished data).
Figure 3.
Wnt5a is required for reorientation and Dvl phosphorylation. (A) Cells were pretreated for 1 h with the indicated concentrations of recombinant Dkk1 or sFRP1 proteins (these concentrations were based on previous reports [Endo et al., 2005] and were also determined by titration) or with PBS as control. Golgi and centrosome reorientation were measured 4.5 h after wounding. (B) Dvl2 and Dvl3 phosphorylation can be detected as a band-shift in Western blots preformed on 7.5% SDS/polyacrylamide gels, indicated by arrowheads. 5 μg/ml sFRP1 or 1 μg/ml Dkk1 was used. aPKC serves as loading control. (C) Wnt5a protein can be detected in fibroblast cell extracts by Western blot. Protein levels do not change over the time course of migration. Tubulin serves as loading control. (D) Wnt5a protein can be efficiently depleted using two different siRNA oligos. Tubulin serves as loading control. (E) Centrosome and Golgi reorientation were measured in Wnt5a-depleted cells. Red bar marks basal level expected from random Golgi/centrosome reorientation (33%). (F) Dvl2 and Dvl3 phosphorylation was visualized in cells treated with control or two different Wnt5a siRNA oligos. Arrows indicate differently phosphorylated forms of Dvl and aPKC serves as loading control.
Dvl activity has been reported to be controlled by multiple phosphorylation events (Willert et al., 1997; McKay et al., 2001; Sun et al., 2001), which can be observed as a series of band shifts on Western blots (Gonzalez-Sancho et al., 2004; Endo et al., 2005). To examine whether the block in centrosome reorientation by sFRP1 impacts Dvl phosphorylation, we examined Dvl2 and Dvl3 mobility on 7.5% polyacrylamide-SDS gels (Fig. 3 B). Dvl2 and Dvl3 appear as multiple bands even in unscratched, confluent REF monolayers, suggesting some constitutive phosphorylation (Fig. 3 B, top panel lanes 1 and 9). Addition of sFRP-1, but not Dkk-1 caused a significant increase in gel mobility (Fig. 3 B, lanes 5 and 13, respectively), suggesting that Dvl is phosphorylated in monolayers and that this is dependent on a constitutive, noncanonical-like Wnt activity. Scratching the monolayer resulted in no major changes in the mobility of Dvl.
Wnt5a is a potential ligand for controlling microtubule polarity
Recent reports describe Wnt5a as a regulator of noncanonical Wnt signaling, specifically during events requiring cell migration in vertebrates (Myers et al., 2002; De Calisto et al., 2005; Kim et al., 2005; Matsui et al., 2005; Okuse et al., 2005; Qiang et al., 2005; Nishita et al., 2006). This prompted us to examine a possible role for Wnt5a in polarized cell migration. Wnt5a protein could be detected by Western blot, though its levels remained constant throughout a 2-h time course after scratching, confirming that primary fibroblasts constitutively produce Wnt5a (Fig. 3 C). To address the role of Wnt5a in centrosome/Golgi reorientation, Wnt5a was depleted using two different specific siRNA oligonucleotides (Fig. 3 D). Both siRNA oligonucleotides led to substantial inhibition of reorientation, suggesting that Wnt5a is likely to be the major Wnt ligand regulating polarity in these cells (Fig. 3 E). siRNA-mediated depletion of two other Wnt ligands, Wnt1 and Wnt3a, did not block reorientation (unpublished data). Furthermore, Dvl mobility on gels was significantly increased by both Wnt5a siRNA oligos, suggesting that Dvl phosphorylation is dependent on Wnt5a (Fig. 3 F). Although we cannot exclude the possibility that other Wnts are expressed and involved, these data support an important role for Wnt5a in polarization of the centrosome/Golgi.
Wnt and dishevelled signaling are required for APC recruitment to microtubule plus tips
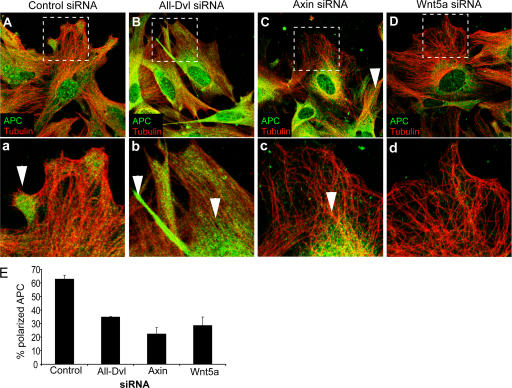
A key step in the pathway controlling centrosome/Golgi reorientation is APC recruitment to microtubule plus ends (Etienne-Manneville and Hall, 2003; Etienne-Manneville et al., 2005). In control scratched monolayers, APC is localized along microtubules but is enriched at the leading edge (Fig. 4 A). In Dvl-depleted cells, APC is mislocalized; it is found along all microtubules at the back as well as the front of the cell and is enriched in the perinuclear region (Fig. 4 B). APC localization was also greatly affected by axin or Wnt5a depletion (Fig. 4, C and D, respectively), showing loss of microtubule tip accumulation and concentration of APC in the perinuclear region. APC localization in cells treated with sFRP1 was similar to that of cells treated with Wnt5a siRNA (unpublished data). Quantifying the effects of siRNA on APC localization demonstrates that Dvl, axin, and Wnt5a are all required for proper APC polarization (Fig. 4 E).
Figure 4.
Wnt signaling is required for the accumulation of APC on microtubules at the leading edge. (A–D) Confocal images of cells treated with control siRNA (A); all three Dvl isoform siRNAs (B); Axin siRNA (C); Wnt5a siRNA (D) and stained for APC (green) and microtubules (red). Higher magnification of areas marked with dashed-line squares are shown in bottom panel (a–d). Arrowheads point to regions of APC accumulation, at the front of control-siRNA cells, at the back of All-Dvl-siRNA cells, or at the perinuclear region of Axin-siRNA or Wnt5a-siRNA cells. (E) APC polarization was quantified as described in Materials and methods.
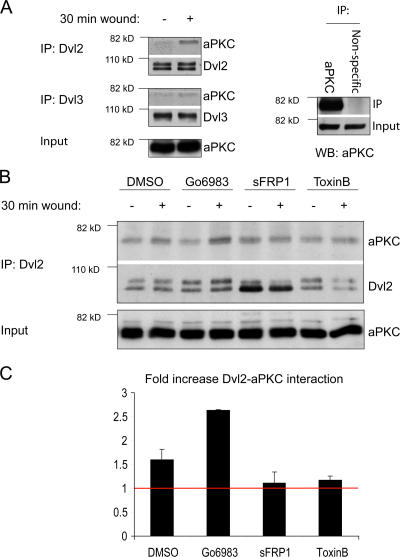
aPKC interacts with Dvl2 after scratching
Dvl can be regulated by protein–protein interactions, leading to changes in its subcellular localization and subsequent complex formation (Cliffe et al., 2003; Ciani et al., 2004; Dollar et al., 2005; Park et al., 2005). Because we have previously shown that the Par6/aPKC/Cdc42 complex is also required for polarized APC accumulation, we looked for a biochemical interaction between these two signaling pathways. Dvl2 was immunoprecipitated from cells pretreated with an aPKC inhibitor (Gö6983), a Wnt inhibitor (sFRP1), or a Cdc42 inhibitor (Toxin B10463). In untreated cells, aPKC could be found in a complex with Dvl2 and Dvl3, and this was increased upon scratching (Fig. 5 A). Although the increase is modest, scratching induces localized changes in signaling activities at the front of the cell and this is very different, for example, from adding a soluble agonist. When Wnt signaling or Cdc42 activity was blocked, the induced, but not the basal level of this interaction was lost, suggesting both Wnt and Cdc42 activities are required to promote a Dvl2/aPKC interaction after scratching (Fig. 5, B and C). In contrast, aPKC inhibitors did not block this interaction, suggesting aPKC activity was not required for Dvl2/aPKC complex formation.
Figure 5.
Dvl2 and aPKC form a complex after scratching in a Wnt- and Cdc42-dependent manner. (A) Endogenous Dvl was immunoprecipitated using monoclonal antibodies against Dvl2 or Dvl3 from cell extracts 30 min after scratching. Top panel shows coimmunoprecipitated aPKC and immunoprecipitated Dvl2 (Western blotting was done using a rabbit antibody against Dvl2), and middle panel shows the coimmunoprecipitated aPKC and immunoprecipitated Dvl3. aPKC levels in 10% of total input extract were used as loading control (bottom panel). Control immunoprecipitation (right panel) using a nonspecific antibody (mouse anti-Myc) demonstrates the specificity of aPKC immunoprecipitations. (B) Endogenous Dvl2 was immunoprecipitated from extracts of cells that had been pretreated for 1 h with 8 μM Gö6983 (aPKC inhibitor), 5 μg/ml sFRP1 (Wnt inhibitor), or 5 ng/ml Toxin B10463 (Rho GTPase inhibitor) and then scratched and left for 30 min. Top two panels show coimmunoprecipitated aPKC and immunoprecipitated Dvl2. aPKC levels in 10% of total input extract were used as loading control. (C) Quantification of fold increase in Dvl2-aPKC interaction after scratching cell monolayer is shown. aPKC levels were normalized to Dvl2 levels (i.e., the sum of the two migrating species). Average of three independent experiments is shown. Red bar marks basal (un-induced) levels.
In conclusion, whereas Dvl, axin, APC, and GSK-3 participate in canonical Wnt signaling to promote a transcriptional response, the Wnt5a/dishevelled effects described here represent a noncanonical pathway that is independent of transcription and involves polarization of microtubules. Although Wnt5a is constitutively expressed in these cells, the scratch-induced generation of a new leading edge leads to spatially localized activation of the Cdc42/Par6/aPKC complex and these two pathways are both required to control polarity. The regulation of polarity by Wnt5a could therefore occur by (a) its polarized secretion, (b) polarized activation of its receptor, or (c) polarized activation of the downstream signal transduction pathway (Strutt, 2002, 2003; Wu et al., 2004). The biochemical link between Cdc42/Par6/aPKC and Wnt5a/Dvl is not clear, though we have identified an interaction between aPKC and Dvl2, which is increased soon after scratching. The results described here provide new insights into the role of noncanonical Wnt pathways in establishing microtubule polarity and identify a potential link between Cdc42 and Wnt signaling pathways, whereby polarization of Wnt signaling can occur in a Cdc42-dependent manner, in response to an external cue.
Materials and methods
Reagents and antibodies
The following antibodies were used: mouse anti-Dvl1, rabbit anti-Dvl2 (used for Western blot), mouse anti-Dvl2 (used for immunoprecipitation), mouse anti-Dvl3, rabbit anti-aPKC, (Santa Cruz Biotechnology, Inc.), rabbit anti-axin (Zymed Laboratories), goat anti-Wnt5a (R&D Systems), mouse anti-p115 (Golgi marker), mouse anti-tubulin (used for IF), mouse anti-myc, (Cancer Research UK, London), rabbit anti-pericentrin (Covance), rat anti–α-tubulin (used for Western blot) (Harlan), and rabbit anti-APC (a gift from Inke Nathke, University of Dundee, UK). Secondary antibodies coupled to HRP were from Jackson ImmunoResearch Laboratories. Secondary antibodies used for immunofluorescence and fluorescent phalloidin were from Molecular Probes. The following reagents were used: recombinant human sFRP-1 and recombinant human Dkk-1 (R&D Systems), Gö6983, Toxin B10463 (Calbiochem), SB216763 (Tocris), and CHIR99021 (a gift from Philip Cohen, University of Dundee, UK).
cDNA constructs and cloning procedures
Full-length cDNA of mouse Dvl2 was a gift from Trevor Dale (Institute of Cancer Research, UK). Deletion mutants of Dvl2 were made using PCR amplification of fragments of interest and subcloning into pRK5myc. All sequences were confirmed by sequencing (MWG Biotech). RFP-F, a farnesylated form of RFP that is targeted to the membrane, was described previously (Cau and Hall, 2005). Axin constructs were a gift from Robert Kypta (Hammersmith Hospital, UK).
Cell culture and microinjection
Primary rat embryonic fibroblasts (REF) were prepared as described previously (Cau and Hall, 2005). Primary mouse embryonic fibroblasts (MEF) were provided by McManus et al.(2005). Only cells from passage 2–3 were used in all experiments. Both cell types were maintained in DME supplemented with 10% fetal calf serum, streptomycin, and penicillin at 37°C. REFs were maintained in 10% CO2 and MEF in 5% CO2. Microinjections were performed as previously described (Cau and Hall, 2005). In brief, confluent cell monolayers grown on glass coverslips were scratched with a P2 tip. 30 min later, cells along the wound edge were microinjected with 0.4 μg/μl cDNA of interest plus 0.15 μg/μl pRFP-F, which served as an injection tracer and membrane marker. Cells were fixed 4.5 h later.
Quantification of Golgi, centrosome, and APC polarization
Polarization was determined 4.5 h or 2 h after scratching of REF or MEF monolayers, respectively. Golgi was detected using antibodies against p115 and centrosome was detected using antibodies against pericentrin. First row cells showing the Golgi/centrosome located in front of the nucleus and in the 120° sector facing the wound, were counted as oriented (Etienne-Manneville and Hall, 2001; Cau and Hall, 2005). For APC polarization, cells exhibiting front accumulation of APC were counted as polarized. Cells exhibiting uniform distribution or perinuclear staining were counted as nonpolarized. For drug-treated or siRNA-treated cells at least 100 cells were counted per coverslip. For microinjected coverslips at least 50 cells were counted per coverslip. Each data point represents at least three independent experiments. Error bars represent SD and statistical tests were performed using a two-tailed t test with equal variances.
RNAi experiments and nucleofections
Unless otherwise specified, all double-stranded predesigned HPLC-grade siRNA oligos were obtained from MWG Biotech. siRNA treatment was performed as previously described (Etienne-Manneville and Hall, 2001; Cau and Hall, 2005). In brief, a total of 600 pmol of siRNA oligos were introduced into 1.5 × 106 cells by nucleofection (Amaxa) using solution V, program G-09. Cells were replated at a density of 5 × 104/cm2 and assayed 72 h later. Efficient depletion was observed 2–3 d after nucleofection, and was quantified by Western blot. The following siRNA oligos were used: rat Dvl1 (Ambion) GGAGGAGAUCUUCGACGACtt, rat Dvl2 GUACCAGCUUAGGAGAUUCtt, rat Dvl3 GGAAGAGAUCUCGGAUGACtt, rat axin GUAUCGUUGUGGCCUACUAtt, rat Wnt5a (#1) UAACACGUAUUCGAACUUAtt, rat Wnt5a (#2) GCACAGUGGACAACACUUCtt, nonspecific control AGGUAGUGUAAUCGCCUUGtt.
Coimmunoprecipitation experiments and Western blots
Confluent monolayers grown on 60-mm dishes and scratched 24 times using a multi-channel pipette fitted with four P2 tips. When drug treatments were performed, cells were preincubated with drugs in complete media for 1 h (or 3 h for Toxin B10463). Cells were washed with ice-cold PBS containing 1 mM sodium orthovanadate and lysed in IP lysis buffer (10 mM Tris, pH 7.4, 140 mM NaCl, 5 mM EDTA, 25 mM NaF, 10 mM sodium pyrophosphate, 1% NP-40, supplemented freshly with 1 mM sodium orthovanadate, 5 mM sodium glycerophosphate, 2 mM PMSF, and one Roche Complete tablet/50 ml). Extracts were cleared by centrifugation at 14,000 rpm for 15 min at 4°C. Protein concentration was measured using Bradford assay (Bio-Rad Laboratories). For coimmunoprecipitation, 200-μg protein extracts were precleared for 1 h with protein G-Sepharose (Sigma-Aldrich). Extracts were then incubated for 2 h with 1 μg of antibody, and then with protein G-Sepharose for an additional hour. Beads were washed four times with IP lysis buffer before loading. For straight Western blot, equal protein amounts ranging between 10–20 μg were typically loaded. Standard SDS-PAGE and Western blot techniques were used.
Immunoprecipitation of Dvl2 was performed using a monoclonal antibody, which preferentially recognizes the nonphosphorylated form of Dvl2 by Western blot (Gonzalez-Sancho et al., 2004), although in our hands it can immunoprecipitate both phosphorylated and nonphosphorylated forms. Western blot for Dvl2 was performed using a rabbit antibody, which recognizes both forms.
Immunofluorescence and confocal microscopy
Cells growing on glass coverslips were washed once in PBS and fixed with 4% paraformaldehyde in PBS (pH 7.4) for 15 min at room temperature, or in methanol, prechilled to −20°C, 5 min at −20°C. Cells were blocked and permeabilized in 0.2% saponin/2%BSA/PBS for 30 min at room temperature or overnight at 4°C. Primary antibodies were incubated overnight in PBS/2%BSA/0.2% saponin. Coverslips were washed in PBS and incubated with secondary antibodies for 1 h at room temperature. Hoechst 33342 (Molecular Probes) was used at 1 μg/ml for nuclear staining.
Confocal images were taken with a MRC1024 (Bio-Rad Laboratories) confocal OptiphotII (Nikon) microscope using a 60× objective (NA 1.4) and a Kr/Ar laser. Immunofluorescent images were taken with Axioplan microscope using a 63× oil immersion objective (NA 1.4). Images were acquired with an ORCA-ER (Hamamatsu) camera using Openlab software (Improvision). Images were analyzed and processed for presentation using brightness and contrast adjustments (same settings for all images) with ImageJ and Adobe Photoshop CS.
Acknowledgments
We thank D. Alessi (University of Dundee, UK) for GSK-3 knockin cells and I. Nathke (U. Dundee, UK), T. Dale (Institute of Cancer Research, UK), Philip Cohen (U. Dundee, UK), and Robert Kypta (Hammersmith Hospital, UK) for reagents. We also thank Julien Cau (Centre National de la Recherche Scientifique, Montpellier, France) for advice and discussion and Lisa Clark for cloning of Dvl2 constructs.
This work was supported by a Cancer Research UK Program Grant (A. Hall), a Susan G. Komen Breast Cancer Foundation Fellowship Award (A. Hall and K. Schlessinger), and a Louis-Jeantet Foundation Award (A. Hall).
E.J. McManus' present address is Division of Molecular Medicine, Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia.
A. Hall's present address is Memorial Sloan-Kettering Cancer Center, New York, NY 10021.
Abbreviations used in this paper: APC, adenomatous polyposis coli; aPKC, atypical protein kinase C; Dkk1, Dickkopf 1; Dvl, Dishevelled; MEF, mouse embryonic fibroblasts; REF, rat embryonic fibroblasts; sFRP1, soluble frizzled related protein 1.
References
- Boutros, M., N. Paricio, D.I. Strutt, and M. Mlodzik. 1998. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 94:109–118. [DOI] [PubMed] [Google Scholar]
- Cau, J., and A. Hall. 2005. Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J. Cell Sci. 118:2579–2587. [DOI] [PubMed] [Google Scholar]
- Ciani, L., O. Krylova, M.J. Smalley, T.C. Dale, and P.C. Salinas. 2004. A divergent canonical WNT-signaling pathway regulates microtubule dynamics: dishevelled signals locally to stabilize microtubules. J. Cell Biol. 164:243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliffe, A., F. Hamada, and M. Bienz. 2003. A role of Dishevelled in relocating Axin to the plasma membrane during wingless signaling. Curr. Biol. 13:960–966. [DOI] [PubMed] [Google Scholar]
- De Calisto, J., C. Araya, L. Marchant, C.F. Riaz, and R. Mayor. 2005. Essential role of non-canonical Wnt signalling in neural crest migration. Development. 132:2587–2597. [DOI] [PubMed] [Google Scholar]
- Doble, B.W., and J.R. Woodgett. 2003. GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116:1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollar, G.L., U. Weber, M. Mlodzik, and S.Y. Sokol. 2005. Regulation of Lethal giant larvae by Dishevelled. Nature. 437:1376–1380. [DOI] [PubMed] [Google Scholar]
- Endo, Y., V. Wolf, K. Muraiso, K. Kamijo, L. Soon, A. Uren, M. Barshishat-Kupper, and J.S. Rubin. 2005. Wnt-3a-dependent cell motility involves RhoA activation and is specifically regulated by dishevelled-2. J. Biol. Chem. 280:777–786. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville, S., and A. Hall. 2001. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 106:489–498. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville, S., and A. Hall. 2003. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 421:753–756. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville, S., J.B. Manneville, S. Nicholls, M.A. Ferenczi, and A. Hall. 2005. Cdc42 and Par6-PKC{zeta} regulate the spatially localized association of Dlg1 and APC to control cell polarization. J. Cell Biol. 170:895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes, E.R., S. Jani, and G.G. Gundersen. 2005. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell. 121:451–463. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Sancho, J.M., K.R. Brennan, L.A. Castelo-Soccio, and A.M. Brown. 2004. Wnt proteins induce dishevelled phosphorylation via an LRP5/6-independent mechanism, irrespective of their ability to stabilize beta-catenin. Mol. Cell. Biol. 24:4757–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, K., and S.Y. Sokol. 1997. Graded amounts of Xenopus dishevelled specify discrete anteroposterior cell fates in prospective ectoderm. Mech. Dev. 61:113–125. [DOI] [PubMed] [Google Scholar]
- Jones, S.E., and C. Jomary. 2002. Secreted Frizzled-related proteins: searching for relationships and patterns. Bioessays. 24:811–820. [DOI] [PubMed] [Google Scholar]
- Kawano, Y., and R. Kypta. 2003. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 116:2627–2634. [DOI] [PubMed] [Google Scholar]
- Kim, H.J., J.R. Schleiffarth, J. Jessurun, S. Sumanas, A. Petryk, S. Lin, and S.C. Ekker. 2005. Wnt5 signaling in vertebrate pancreas development. BMC Biol. 3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida, S., H. Yamamoto, S. Hino, S. Ikeda, M. Kishida, and A. Kikuchi. 1999. DIX domains of Dvl and axin are necessary for protein interactions and their ability to regulate beta-catenin stability. Mol. Cell. Biol. 19:4414–4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer, A., D. Louvard, and S.J. Singer. 1982. Polarization of the Golgi apparatus and the microtubule-organizing center in cultured fibroblasts at the edge of an experimental wound. Proc. Natl. Acad. Sci. USA... 79:2603–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui, T., A. Raya, Y. Kawakami, C. Callol-Massot, J. Capdevila, C. Rodriguez-Esteban, and J.C. Izpisua Belmonte. 2005. Noncanonical Wnt signaling regulates midline convergence of organ primordia during zebrafish development. Genes Dev. 19:164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay, R.M., J.M. Peters, and J.M. Graff. 2001. The casein kinase I family in Wnt signaling. Dev. Biol. 235:388–396. [DOI] [PubMed] [Google Scholar]
- McManus, E.J., K. Sakamoto, L.J. Armit, L. Ronaldson, N. Shpiro, R. Marquez, and D.R. Alessi. 2005. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 24:1571–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlodzik, M. 2002. Planar cell polarization: do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet. 18:564–571. [DOI] [PubMed] [Google Scholar]
- Myers, D.C., D.S. Sepich, and L. Solnica-Krezel. 2002. Bmp activity gradient regulates convergent extension during zebrafish gastrulation. Dev. Biol. 243:81–98. [DOI] [PubMed] [Google Scholar]
- Nishita, M., S.K. Yoo, A. Nomachi, S. Kani, N. Sougawa, Y. Ohta, S. Takada, A. Kikuchi, and Y. Minami. 2006. Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J. Cell Biol. 175:555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes, C.D., and A. Hall. 1999. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol. 144:1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuse, T., T. Chiba, I. Katsuumi, and K. Imai. 2005. Differential expression and localization of WNTs in an animal model of skin wound healing. Wound Repair Regen. 13:491–497. [DOI] [PubMed] [Google Scholar]
- Palazzo, A.F., T.A. Cook, A.S. Alberts, and G.G. Gundersen. 2001. a. mDia mediates Rho-regulated formation and orientation of stable microtubules. Nat. Cell Biol. 3:723–729. [DOI] [PubMed] [Google Scholar]
- Palazzo, A.F., H.L. Joseph, Y.J. Chen, D.L. Dujardin, A.S. Alberts, K.K. Pfister, R.B. Vallee, and G.G. Gundersen. 2001. b. Cdc42, dynein, and dynactin regulate MTOC reorientation independent of Rho-regulated microtubule stabilization. Curr. Biol. 11:1536–1541. [DOI] [PubMed] [Google Scholar]
- Park, T.J., R.S. Gray, A. Sato, R. Habas, and J.B. Wallingford. 2005. Subcellular localization and signaling properties of dishevelled in developing vertebrate embryos. Curr. Biol. 15:1039–1044. [DOI] [PubMed] [Google Scholar]
- Qiang, Y.W., K. Walsh, L. Yao, N. Kedei, P.M. Blumberg, J.S. Rubin, J. Shaughnessy Jr., and S. Rudikoff. 2005. Wnts induce migration and invasion of myeloma plasma cells. Blood. 106:1786–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt, D.I. 2002. The asymmetric subcellular localisation of components of the planar polarity pathway. Semin. Cell Dev. Biol. 13:225–231. [DOI] [PubMed] [Google Scholar]
- Strutt, D. 2003. Frizzled signalling and cell polarisation in Drosophila and vertebrates. Development. 130:4501–4513. [DOI] [PubMed] [Google Scholar]
- Sun, T.Q., B. Lu, J.J. Feng, C. Reinhard, Y.N. Jan, W.J. Fantl, and L.T. Williams. 2001. PAR-1 is a Dishevelled-associated kinase and a positive regulator of Wnt signalling. Nat. Cell Biol. 3:628–636. [DOI] [PubMed] [Google Scholar]
- Tzima, E., W.B. Kiosses, M.A. del Pozo, and M.A. Schwartz. 2003. Localized cdc42 activation, detected using a novel assay, mediates microtubule organizing center positioning in endothelial cells in response to fluid shear stress. J. Biol. Chem. 278:31020–31023. [DOI] [PubMed] [Google Scholar]
- Watanabe, T., S. Wang, J. Noritake, K. Sato, M. Fukata, M. Takefuji, M. Nakagawa, N. Izumi, T. Akiyama, and K. Kaibuchi. 2004. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev. Cell. 7:871–883. [DOI] [PubMed] [Google Scholar]
- Wen, Y., C.H. Eng, J. Schmoranzer, N. Cabrera-Poch, E.J. Morris, M. Chen, B.J. Wallar, A.S. Alberts, and G.G. Gundersen. 2004. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat. Cell Biol. 6:820–830. [DOI] [PubMed] [Google Scholar]
- Willert, K., M. Brink, A. Wodarz, H. Varmus, and R. Nusse. 1997. Casein kinase 2 associates with and phosphorylates dishevelled. EMBO J. 16:3089–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J., T.J. Klein, and M. Mlodzik. 2004. Subcellular localization of frizzled receptors, mediated by their cytoplasmic tails, regulates signaling pathway specificity. PLoS Biol. 2:E158. [DOI] [PMC free article] [PubMed] [Google Scholar]