Thymic emigration revisited (original) (raw)
Abstract
Conventional αβ T cell precursors undergo positive selection in the thymic cortex. When this is successful, they migrate to the medulla and are exposed to tissue-specific antigens (TSA) for purposes of central tolerance, and they undergo maturation to become functionally responsive T cells. It is commonly understood that thymocytes spend up to 2 wk in the medulla undergoing these final maturation steps before emigrating to peripheral lymphoid tissues. In addition, emigration is thought to occur via a stochastic mechanism whereby some progenitors leave early and others leave late—a so-called “lucky dip” process. However, recent research has revealed that medullary thymocytes are a heterogeneous mix of naive αβ T cell precursors, memory T cells, natural killer T cells, and regulatory T cells. Given this, we revisited the question of how long it takes naive αβ T cell precursors to emigrate. We combined the following three approaches to study this question: BrdU labeling, intrathymic injection of a cellular tag, and RAG2p-GFP reporter mice. We established that, on average, naive αβ T cell precursors emigrate only 4–5 d after becoming single-positive (SP) thymocytes. Furthermore, emigration occurs via a strict “conveyor belt” mechanism, where the oldest thymocytes leave first.
Positive selection occurs in the thymic cortex. This signaling event causes up-regulation of CCR7 and migration of the progenitor to the thymic medulla (1). During medullary residency, thymocytes are exposed to tissue-specific antigens (TSAs) expressed by medullary thymic epithelial cells (mTECs). They also undergo receptor tuning and final differentiation to become a functional naive T cell. Ultimately, such cells emigrate from the thymus via a mechanism that requires KLF2-mediated up-regulation of the sphingosine-1 phosphate receptor (S1P1) (2, 3). This entire process is commonly believed to take ∼14 d (4).
The time course of medullary residency and emigration have been primarily examined using either single-dose administration of BrdU to track a cohort of cells, or continuous labeling using either BrdU or tritiated thymidine (5–8). Recent thymic emigrants (RTEs) were detected after only 2 d of continuous BrdU administration (5). However, continuous labeling studies showed that complete labeling of the mature single-positive (SP) thymocyte pool took up to 20 d (7). An asynchronous, or “lucky dip,” mechanism was proposed to reconcile these data, and because RTEs were felt to be of various ages based on their expression of heat-stable antigen (HSA) and Qa-2 (9). A lengthy medullary residency period has been speculated to be important in allowing thymic progenitors sufficient time to survey the TSAs displayed by mTECs, as individual antigens are often expressed only in a small fraction of total mTECs (10).
Since these studies were published, it has become apparent that the medulla is also important for the generation of various antigen-experienced populations: NKT cells, T reg cells, and CD8αα T cells (11). Although such populations may be selected in the cortex, their final maturation requires mTECs and is disrupted in mouse strains that lack a normal mTEC compartment (12). Furthermore, it is not clear if all NKT cells, T reg cells, and CD8αα T cells present in the thymus were newly developed because NK1.1pos NKT cells have been shown to be retained in the thymus long term (13), and T reg cells have been shown to be capable of reentering the thymus (14). Indeed, activated memory T cells have also been shown to be able to recirculate back to the thymus (15, 16), as can naive T cells during various periods of life (17, 18). Given this heterogeneity of SP thymocytes, we decided to revisit the question of how long it takes a naive αβ T cell progenitor to exit the thymus.
An important tool that was not previously available is the RAG2p-GFP (also called NG-BAC) mouse. This mouse carries a bacterial artificial chromosome transgene encoding a modified RAG1/2 locus, where GFP is inserted after the RAG2 promoter (19). The RAG genes are highly expressed in double-positive (DP) progenitors, but then are rapidly suppressed after selection. However, the half-life of GFP is such that it lingers long after RAG gene expression is terminated (20). Because of this, GFP expression in such mice has the potential to act as a “molecular timer” of thymocyte maturation. Using this tool and careful exclusion of nonconventional αβ T cells, as well as antigen-experienced T cells, by phenotypic gating, we were able to determine the kinetics of thymic emigration of naive αβ T cells. Surprisingly, we found that naive SP thymocytes emigrate only 4–5 d after entering the SP pool, via a highly ordered process where only the oldest and most mature cells exit the thymus.
RESULTS AND DISCUSSION
SP thymocytes are a heterogeneous mixture of various cell types
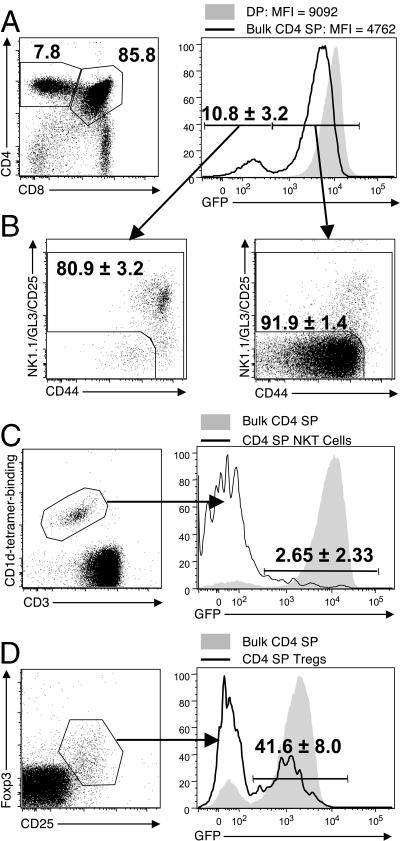
For the following studies, we used RAG2p-GFP mice where GFP expression is initiated coincidentally with RAG2. However, the GFP protein lingers after RAG expression is terminated during positive selection at the DP stage, creating a situation where the level of GFP can indicate how much time has passed since positive selection. Thus, DP thymocytes express a uniformly high level of GFP, whereas SP thymocytes show a reduced level (Fig. 1 A) (20). In fact, the level of GFP expressed on CD4 SP thymocytes is heterogeneous, with a prominent population of GFP-negative cells (10.8 ± 3.2%; Fig. 1 A). This is consistent with heterogeneity in the “age” of SP thymocytes relative to their differentiation from DP precursors, and would suggest that some SP thymocytes are retained long-term in the thymus. However, the CD4 SP thymocyte pool includes not only bona fide naive αβ thymocytes, but also NKT cells, T reg cells, γδ T cells, and recirculating memory cells. In total, these nonconventional thymocyte populations account for 13.5 ± 1.3% of the total CD4 SP pool (unpublished data), which is in agreement with published data (21, 22). We used a “dump” strategy (NK1.1, GL3, and CD25), along with CD44, to exclude NKT cells, γδ T cells, T reg cells, and memory T cells, respectively, and found that the majority (80.9 ± 3.2%) of the GFPneg cells were CD44hi and/or dumppos, whereas the GFPpos thymocytes were primarily (91.9 ± 1.4%) a CD44/dumpneg phenotype (henceforth called “naive”; Fig. 1 B). When NKT cells were specifically gated, >95% were GFPneg (Fig. 1 C), and T reg cells were ∼60% GFPneg (Fig. 1 D). This low GFP level indicates that the majority of NKT cells and T reg cells have either: (a) recirculated from the periphery, (b) divided extensively, or (c) been retained for long periods in the thymus. Thymic reentry has been reported for T reg cells (14), and long retention periods have been reported for NKT cells (13). However, it has also been shown that the NKT cells that emigrate from the thymus are NK1.1neg immature cells (23, 24). When we gated on NK1.1neg CD1d/αGalCer teteramerpos cells, we found that their GFP profile more closely resembled conventional naive αβ T cells (unpublished data). Although our approach does not distinguish between cell division, long thymic residency, and thymic reentry in these NKT cells and T reg cells, we emphasize that their presence in previous experiments would certainly have complicated interpretation of the results. For future flow cytometry experiments, we used a dump channel consisting of NK1.1, GL3, and CD25, along with CD44, to remove these cell populations from our analyses.
Figure 1.
CD4 SPs are a heterogeneous population. (A) GFP expression in DP and bulk CD4 SP thymocytes. (B) A dump channel consisting of NK1.1, GL3, and CD25 was used to identify nonconventional αβ thymocytes, and CD44 was used to identify memory T cells. (left and right) GFPneg and GFPpos gated CD4 SP thymocytes, respectively. (C) GFP expression in CD1d/αGalCer tetramerpos CD3pos CD4 SP NKT cells compared with total CD4 SPs in the thymus. (D) GFP expression in Foxp3pos CD25pos CD4 SP T reg cells compared with total CD4 SPs in the thymus. Numbers on plots indicate the percentage of the parent gate with the SD from multiple experiments, where indicated. The mice used were 6–11 wk old.
Using this dump strategy, we found that the majority of naive CD4 SPs (98.5%) were uniformly GFPpos. The very small GFPneg population (1.5 ± 0.4%) could theoretically represent thymocytes that were retained in the organ for a long time. Alternatively, they could represent NK1.1neg NKT cells or, more likely, naive peripheral T cells that recirculated back to the thymus from the periphery during the neonatal period, as naive T cells were shown to recirculate to the thymus in neonatal and old mice (17, 18).
GFP intensity acts as a “molecular timer” to follow development
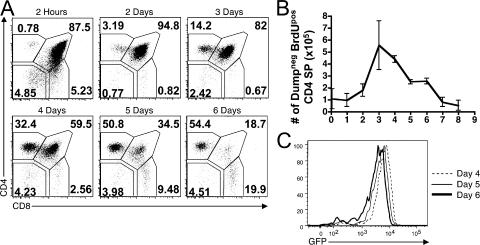
We first sought to confirm that declining GFP levels in SP thymocytes correlated with increasing age. To do this, we performed a BrdU pulse-chase time course. 2 mg BrdU was injected into RAG2p-GFP mice at the indicated times before harvest, after which, BrdUpos cells were analyzed by flow cytometry. Shortly after BrdU administration (2 h to 1 d), cells were still transitioning from the DN to DP pool and there were very few BrdUpos cells present in the SP compartments (Fig. 2 A). By day 3, a notable population of labeled CD4 SPs began to appear. The absolute number of BrdUpos CD4 SP thymocytes reached a maximum at 3–4 d after BrdU injection (Fig. 2 B). The number of BrdUpos CD4 SPs began to decrease after day 4, which was presumably caused by a combination of medullary negative selection and emigration to the periphery, and was essentially below the limit of detection by day 8 (Fig. 2 B). These kinetics are similar to those previously published (6). We also examined the mean fluorescent intensity (MFI) of GFP in BrdUpos CD4 SPs at various time points, and found that it declined progressively with time, demonstrating that GFP acts as a molecular timer for differentiation in these mice (Fig. 2 C). Furthermore, based on the decrease in GFP intensity over this time period, we estimated that the half-life of GFP is ∼56 h in vivo (unpublished data).
Figure 2.
GFP intensity decreases during thymocyte residency time. 2 mg of BrdU was injected at the indicated time point before harvest. (A) The expression of CD4 and CD8 on dumpneg BrdUpos thymocytes from the indicated time point. (B) The absolute number of dumpneg BrdUpos CD4 SP thymocytes was calculated for each time point. Data represent the mean ± the SD from two to three experiments. (C) The GFP MFI of dumpneg BrdUpos CD4 SPs from the indicated time points confirms that GFP intensity decreases with time.
Emigration occurs via a synchronous mechanism
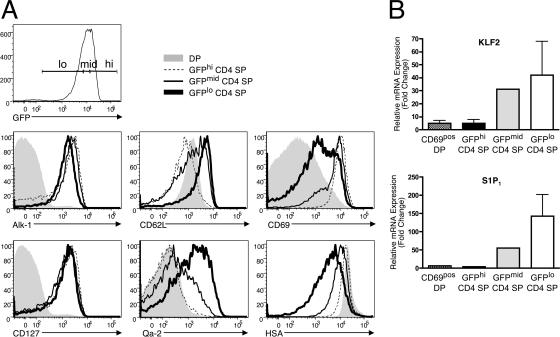
We next sought to determine which cells emigrate from the thymus, and if emigration occurs via a lucky dip mechanism or if it functions more similarly to a conveyor belt. To do this, we divided the naive GFPpos CD4 SP thymocytes into three equal bins, which we called GFPhi, GFPmid, and GFPlo (Fig. 3 A). We then examined the three GFP subsets for their expression of molecules known to be changed after positive selection. As expected, some molecules, such as Alk-1 and CD127 (IL-7Rα), were up-regulated after positive selection and maintained throughout the lifespan of CD4 SP thymocytes (Fig. 3 A). However, several molecules known to correlate with thymocyte maturity were changed most dramatically only in the oldest, GFPlo thymocytes. Specifically, HSA was down-regulated the most in GFPlo thymocytes, as was CD69. Likewise, CD62L and Qa-2 were expressed most highly in GFPlo thymocytes (Fig. 3 A). TCRβpos CD8 SPs were also examined, and similar results were obtained, with the exception that GFP, CD69, HSA, and Qa-2 expression indicated that the cells were older and more mature, confirming that CD8 SPs take longer to transition from the DP stage (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20070601/DC1). Using multicolor cell sorting and quantitative RT-PCR, we examined the mRNA expression of KLF2 and S1P1, which are two molecules that are required for emigration from the thymus (2, 3). We saw that these molecules were most highly up-regulated in GFPlo thymocytes (Fig. 3 B). Collectively, these data suggest that GFPlo thymocytes bear the most mature phenotype, and that these cells have an “exit” phenotype.
Figure 3.
GFPlo CD4 SPs are the most mature and have an “exit” phenotype. (A) Naive CD4 SP thymocytes were divided into three equal bins based on GFP intensity. The three bins, along with DP thymocytes, were then analyzed for their expression of the indicated surface molecule. Representative data from 4–5 experiments is shown. (B) Dumpneg cells from the indicated subset were FACS-sorted, RNA was isolated and used for cDNA synthesis, and quantitative RT-PCR was performed for KLF2 and S1P1. Data represent the mean fold change ± the SD from two independent sorts relative to CD69neg DPs and normalized using primers for β-catenin. GFPmid CD4 SPs were only sorted once.
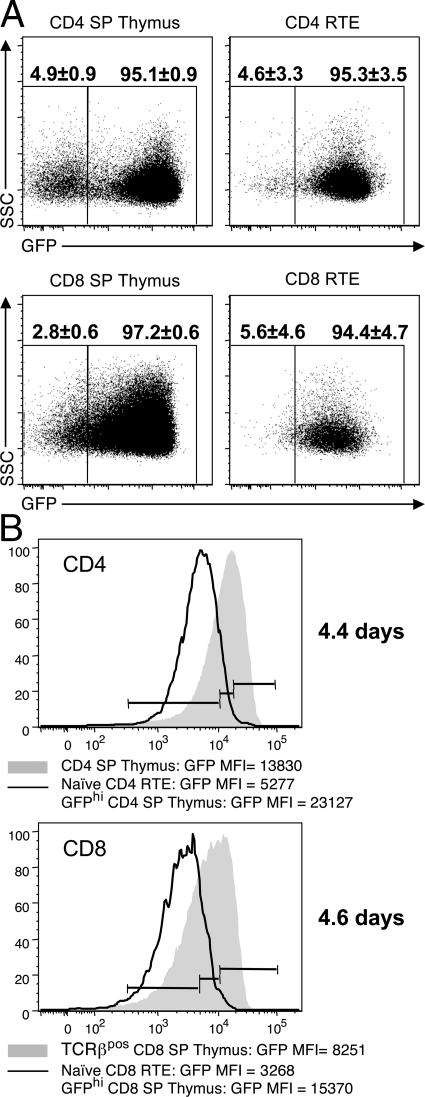
To study emigrants directly, we performed intrathymic injection of a biotinylating agent, which allowed us to track RTEs in the periphery (Fig. 4). Examination of the SApos CD4 and CD8 SPs showed that the majority of RTEs are uniformly GFPpos, but a considerable population of GFPneg cells could also be seen, especially in CD4 SPs (Fig. 4 A). However, when we examined these GFPneg cells further, they were found to be mostly CD44 and/or dumppos (not depicted), which was similar to the thymus (Fig. 1 B). This provides further evidence that the CD4 SP pool is heterogeneous and that not all T cells emigrating from the thymus are bona fide, naive αβ T cells. When we compared the GFP intensity of naive RTEs to that of the SP thymocytes, we saw a distinct shift (Fig. 4 B), and the majority of RTEs fell within the GFPlo bin. Furthermore, the expression of HSA and Qa-2 on naive phenotype RTEs was uniform and correlated with the GFPlo thymocytes (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20070601/DC1). These data confirm that emigration from the thymus occurs via a conveyor belt mechanism, where only the oldest thymocytes express the molecules necessary to leave the thymus and are represented among the recently emigrated cells.
Figure 4.
Thymic emigration occurs after only 4–5 d. 50 μg of biotin was injected intrathymically and lymph node RTEs were visualized 12, 24, 48, and 72 h later by staining with streptavidin-conjugated Pacific Orange. The 12-h time point is shown. (A) GFP expression in bulk SApos CD4 SP and CD8 SP thymocytes and bulk CD4 and CD8 RTEs. (B) The expression of GFP in naive CD4 and CD8 RTEs compared with naive CD4 and CD8 SP thymocytes, respectively. Gates were drawn on naive CD4 and CD8 SP thymocytes, as seen in Fig. 3. The MFI for the indicated subsets is shown below the histogram. The CV values were as follows: CD4 SP, 57.59; CD4 RTE, 59.29; CD8 SP, 73.44; and CD8 RTE, 74.68. For comparison, DP thymocytes were 34.52. The MFI values were used to estimate the average difference in age between the youngest (GFPhi) SP thymocyte and a RTE, as described in the Materials and methods (shown on right, in days).
Emigration of naive T cells occurs after only 4–5 d
To estimate how long it takes an SP thymocyte to emigrate, we sought to measure the decay of GFP between the GFPhi SPs present in the thymus, and RTE in the periphery. To translate this into time we determined the half-life of GFP in RAG2p-GFP SP thymocytes cultured in vitro, and found it to be 54 h (see Materials and methods and Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20070601/DC1). This is similar to the half-life we estimated in vivo (56 h). Thus, we compared the mean fluorescence of GFP in GFPhi SP thymocytes (those just entering the medullary SP thymocyte pool) to that seen in RTEs 0–12 h after emigration (Fig. 4 B). From this, we calculated that RTEs are only 4.4 (CD4) or 4.6 d (CD8) older than GFPhi thymocytes on average. Interestingly, CD8 SP thymocytes have a lower GFP intensity than CD4 SPs, again suggesting that they take longer to transition from the DP stage. However, the difference in GFP intensity was the same when comparing both GFPhi CD4 or CD8 SP thymocytes and their respective population of RTEs, indicating that medullary residency time is equal for CD4 and CD8 SPs. This timeframe for emigration is also in agreement with the BrdU pulse-chase data, where the majority (85%) of BrdUpos CD4 SPs were gone by 4 d after their peak (Fig. 2 B).
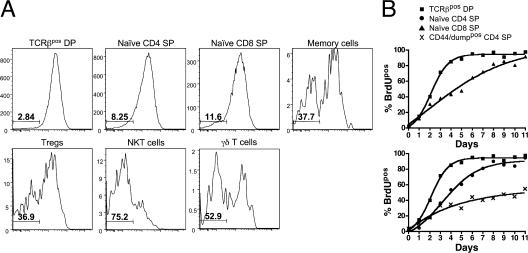
Our data are in agreement with Lucas et al., who showed similar kinetics for thymic development (6), and also Tough and Sprent, who showed a linear accumulation of labeled cells in the periphery after only a few days (5). In this study, we have extended those results by making use of RAG2p-GFP mice and 10-parameter flow cytometry to reexamine thymic emigration in more precise detail. Our data are in apparent opposition to results obtained by Egerton et al. who showed, with continuous 3H labeling, that complete labeling of the CD4 SP pool required 20 d (7), and that RTEs were of various stages of maturity (9). To reconcile these data, Scollay and Godfrey proposed a “lucky dip” model where the average residency time of SP thymocytes is ∼14 d (25), which has long remained dogma. However, at that time the degree of heterogeneity in the CD4 SP pool was not appreciated. Their analysis of CD4pos CD8neg CD3pos cells would have included T reg cells, NKT cells, γδ cells, and mature recirculating cells. In an attempt to reconcile these findings, we performed continuous BrdU labeling studies and found that naive CD4 and CD8 SPs approached complete labeling after only 8–9 d, which was just 4 d after TCRβpos DPs were completely labeled (Fig. 5). However, CD44/dumppos cells had only reached ∼50% BrdUpos in this timeframe, and when specifically gated, T reg cells, γδ T cells, NKT cells, and memory T cells all had a large fraction of BrdUneg cells, even at day 11.
Figure 5.
Continuous labeling shows rapid turnover of naive CD4 and CD8 SPs compared with CD44/dumppos CD4 SPs. 1 mg BrdU was injected intraperitoneally at the indicated time point before harvest, and mice were continuously given 0.8 mg/ml BrdU drinking water, which was changed daily until harvest. (A) The BrdU profile of the indicated subset of cells after 11 d of BrdU labeling. The percentage of BrdUneg cells is indicated. (B) The turnover kinetics of the indicated population of thymocytes over time.
It has also been observed by several groups that mature SPs can undergo a minimal amount of proliferation (26–29). Our analysis of the few cycling SPs defined at 1 h after BrdU injection confirmed that most of these are naive αβ thymocytes and that more proliferation is seen in CD8 SPs, compared with CD4 SPs (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20070601/DC1). This difference between CD4 and CD8 SPs likely accounts for the slightly different labeling kinetics observed in continuous labeling experiments (Fig. 5 B). Furthermore, we note that the BrdUpos SP thymocytes do not represent any specific maturation state based on GFP expression (Fig. S4, top) or any other maturation marker, e.g., Qa-2 or HSA (not depicted). Neither our estimation of medullary residency time based on the GFP intensity of RTEs compared with SP thymocytes nor previous estimates based on BrdU 3H incorporation take into account this putative division of SPs after positive selection. However, we note that because cell division would further dilute GFP, our data is, if anything, an overestimate of medullary residency time, and emigration may happen even more quickly. In summary, our data show that naive αβ T cells emigrate quickly and synchronously. They further suggest that the T cells that “emigrate” late are mostly a heterogeneous mix of NKT cells, γδ T cells, T reg cells, and memory T cells. In fact, it is probable that these cells, or a substantial proportion of them, are recirculating, as opposed to emigrating late.
Given our finding that medullary residency is much shorter than previously thought, a pertinent question is asked—is this a problem for central tolerance? Not only do SP thymocytes require interaction with the medulla to tune their TCR and lose responsiveness to self-ligands (30), but they also undergo negative selection to TSA in the medulla. Furthermore, SP thymocytes are only competent to undergo clonal deletion at the semimature stage (HSAhi and Qa-2neg), which is roughly the first two thirds of their residency time (unpublished data). Given that some TSAs are expressed only by rare mTECs (10), and that loss of tolerance to just one TSA is capable of causing autoimmunity (31), a short medullary residency time might be a point of weakness in the central tolerance arsenal. A comprehensive understanding of TCR tuning and medullar deletion (including AIRE-mediated), must take into account this kinetic aspect.
Lastly, the means by which thymocytes leave the organ is poorly understood. Selected thymocytes migrate to the medulla in a CCR7-dependent manner. Thus, it would stand to reason that emigration occurs via an exit route from the medulla. However, CCR7-deficient mice appear to have normal thymic emigration, even though they show poor accumulation of SP thymocytes in the medulla (1, 32). This suggests that migration to the medulla is not a prerequisite for emigration, and that exit routes from the thymus are present in both the cortex and the medulla. It is possible that emigration occurs either through the lymphatic system, or through blood vessels (33–35). Perivascular spaces were proposed as a site for emigration, but this has not been proven (35, 36). It is our hope that this study of the kinetics and phenotype of emigrating thymocytes will lead to greater understanding of this process in the future.
MATERIALS AND METHODS
Mice.
RAG2p-GFP mice have been previously described (19), and they were provided by P. Fink (University of Washington, Seattle, WA). All animals were maintained and treated in accordance with federal guidelines approved by the University of Minnesota Institutional Animal Care Committee.
Antibodies and flow cytometry.
All fluorochrome-conjugated and biotinylated antibodies were purchased from BD Biosciences, eBioscience, Invitrogen, or BioLegend, except for biotinylated mCD1d/αGalCer monomers, which were obtained from the National Institutes of Health Tetramer Facility (Atlanta, GA). Tetramers were formed by mixing monomers with streptavidin PE at a molar ratio of 1:6. Anti-BrdU (PRB-1) was purchased from Phoenix Flow Systems. Conventional surface staining was performed by staining cells with antibody for 30 min on ice in FACS buffer (PBS, 1% FCS, and 0.02% azide, pH 7.2) and washing two times in FACS buffer after each antibody incubation. Intracellular Foxp3 staining was performed using the Mouse Regulatory T Cell Staining kit (eBioscience). Cell events were collected using an LSR-II cytometer (BD Biosciences) and analyzed with FlowJo software (Tree Star, Inc.).
BrdU labeling.
BrdU pulse-chase time courses were performed as previously described (6). 100 μl of 10 mg/ml BrdU (Sigma-Aldrich; B5002) was injected intraperitoneally twice at 4-h intervals for a total of 2 mg/mouse at the indicated time point before harvest. Thymi were harvested and surface stained as described in the previous paragraph, followed by intracellular staining for BrdU using the APC BrdU flow kit (BD Biosciences), with the substitution of the anti-BrdU antibody mentioned in the previous paragraph. Continuous BrdU labeling was performed similarly with the following exceptions, as previously described (37). Groups of 3 mice were injected intraperitoneally with 1 mg BrdU at the indicated time point before harvest, and then continuously given 0.8 mg/ml BrdU in sterile drinking water that was changed daily until harvest.
Cell sorting and quantitative RT-PCR.
Thymocyte subsets were purified by FACS-sorting on a FACSVantage (Becton Dickinson). RNA was isolated from sorted populations by oligo(dT) and random hexamer priming using the RNeasy kit (Qiagen), and cDNA was produced using the SuperScriptIII Platinum Two-Step qRT-PCR kit (Invitrogen). qRT-PCR was performed with FastStart SYBR Green Master Mix kit (Roche) using the SmartCycler real-time PCR machine (Cepheid). Fold changes were calculated using the ΔΔCt method with CD69neg DP samples as the baseline sample and β-catenin as the reference gene.
Intrathymic biotin injection.
Animals were sedated and given an intrathymic injection of 10 μl of a 5 mg/ml solution of sulfo-NHS-LC biotin (Pierce Chemical Co.). At 12, 24, 48, and 72 h later, thymus, lymph nodes, and spleen were stained with streptavidin-conjugated Pacific Orange and other markers and analyzed by flow cytometry. Use of this in vivo biotinylation approach was based on the studies of Rybak et al. (38).
Determination of GFP half-life and medullary residency time.
Bulk thymocytes were harvested from RAG2p-GFP mice and cultured at a concentration of 106 cells/ml in RPMI-based media with 10 ng/ml human IL-7 (National Institutes of Health). The GFP intensity of Annexin V–negative CD4 SPs was assessed after 0, 9, 24, 33, 48, and 57 h of culture. The decay of GFP appeared to be linear, and a linear trend line was applied and used to calculate the time at which the GFP intensity had decreased by one half. To calculate medullary residency time, the GFP MFI fold change from naive GFPhi SP thymocytes compared with naive RTEs was determined and converted to log(2), to calculate the difference between these two populations in number of half-lives. This number was then multiplied by the half-life of GFP (54 h) and divided by 24 to convert to days.
Online supplemental material.
Fig. S1 shows that GFPlo CD8 SPs are the most mature and have an “exit” phenotype. Fig. S2 shows that RTEs have uniform expression of maturation markers. Fig. S3 shows the half-life of GFP. Fig. S4 shows that proliferation of naive SP thymocytes is increased in CD8s compared with CD4s. The online version of this article is available at http://www.jem.org/cgi/content/full/jem.20070601/DC1.
Supplemental Material
[Supplemental Material Index]
Acknowledgments
We would like to thank X-.J. Ding, B. Goudy, and A. Gerjets for technical assistance, and S. Jameson, M. Sandau, L. Bursch, J. Bauer, and H. Cohen for critical review of this manuscript.
This work was supported by the National Institutes of Health (grants RO1AI39560 and RO1AI50105 to K.A. Hogquist and T32AI07313 to T.M. McCaughtry).
The authors have no conflicting financial interests.
References
- 1.Ueno, T., F. Saito, D.H. Gray, S. Kuse, K. Hieshima, H. Nakano, T. Kakiuchi, M. Lipp, R.L. Boyd, and Y. Takahama. 2004. CCR7 signals are essential for cortex–medulla migration of developing thymocytes. J. Exp. Med. 200:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlson, C.M., B.T. Endrizzi, J. Wu, X. Ding, M.A. Weinreich, E.R. Walsh, M.A. Wani, J.B. Lingrel, K.A. Hogquist, and S.C. Jameson. 2006. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 442:299–302. [DOI] [PubMed] [Google Scholar]
- 3.Matloubian, M., C.G. Lo, G. Cinamon, M.J. Lesneski, Y. Xu, V. Brinkmann, M.L. Allende, R.L. Proia, and J.G. Cyster. 2004. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 427:355–360. [DOI] [PubMed] [Google Scholar]
- 4.Petrie, H.T., and J.C. Zuniga-Pflucker. 2007. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu. Rev. Immunol. 25:649–679. [DOI] [PubMed] [Google Scholar]
- 5.Tough, D.F., and J. Sprent. 1994. Turnover of naive- and memory-phenotype T cells. J. Exp. Med. 179:1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucas, B., F. Vasseur, and C. Penit. 1993. Normal sequence of phenotypic transitions in one cohort of 5-bromo-2'-deoxyuridine-pulse-labeled thymocytes. Correlation with T cell receptor expression. J. Immunol. 151:4574–4582. [PubMed] [Google Scholar]
- 7.Egerton, M., R. Scollay, and K. Shortman. 1990. Kinetics of mature T-cell development in the thymus. Proc. Natl. Acad. Sci. USA. 87:2579–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huesmann, M., B. Scott, P. Kisielow, and H. von Boehmer. 1991. Kinetics and efficacy of positive selection in the thymus of normal and T cell receptor transgenic mice. Cell. 66:533–540. [DOI] [PubMed] [Google Scholar]
- 9.Kelly, K.A., and R. Scollay. 1990. Analysis of recent thymic emigrants with subset- and maturity-related markers. Int. Immunol. 2:419–425. [DOI] [PubMed] [Google Scholar]
- 10.Derbinski, J., A. Schulte, B. Kyewski, and L. Klein. 2001. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat. Immunol. 2:1032–1039. [DOI] [PubMed] [Google Scholar]
- 11.Baldwin, T.A., K.A. Hogquist, and S.C. Jameson. 2004. The fourth way? Harnessing aggressive tendencies in the thymus. J. Immunol. 173:6515–6520. [DOI] [PubMed] [Google Scholar]
- 12.Hogquist, K.A., T.A. Baldwin, and S.C. Jameson. 2005. Central tolerance: learning self-control in the thymus. Nat. Rev. Immunol. (Paris). 5:772–782. [DOI] [PubMed] [Google Scholar]
- 13.Berzins, S.P., F.W. McNab, C.M. Jones, M.J. Smyth, and D.I. Godfrey. 2006. Long-term retention of mature NK1.1+ NKT cells in the thymus. J. Immunol. 176:4059–4065. [DOI] [PubMed] [Google Scholar]
- 14.Bosco, N., F. Agenes, A.G. Rolink, and R. Ceredig. 2006. Peripheral T cell lymphopenia and concomitant enrichment in naturally arising regulatory T cells: the case of the pre-Talpha gene-deleted mouse. J. Immunol. 177:5014–5023. [DOI] [PubMed] [Google Scholar]
- 15.Reinhardt, R.L., A. Khoruts, R. Merica, T. Zell, and M.K. Jenkins. 2001. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 410:101–105. [DOI] [PubMed] [Google Scholar]
- 16.Agus, D.B., C.D. Surh, and J. Sprent. 1991. Reentry of T cells to the adult thymus is restricted to activated T cells. J. Exp. Med. 173:1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hale, J.S., T.E. Boursalian, G.L. Turk, and P.J. Fink. 2006. Thymic output in aged mice. Proc. Natl. Acad. Sci. USA. 103:8447–8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Surh, C.D., J. Sprent, and S.R. Webb. 1993. Exclusion of circulating T cells from the thymus does not apply in the neonatal period. J. Exp. Med. 177:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu, W., H. Nagaoka, M. Jankovic, Z. Misulovin, H. Suh, A. Rolink, F. Melchers, E. Meffre, and M.C. Nussenzweig. 1999. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature. 400:682–687. [DOI] [PubMed] [Google Scholar]
- 20.Boursalian, T.E., J. Golob, D.M. Soper, C.J. Cooper, and P.J. Fink. 2004. Continued maturation of thymic emigrants in the periphery. Nat. Immunol. 5:418–425. [DOI] [PubMed] [Google Scholar]
- 21.Fontenot, J.D., J.L. Dooley, A.G. Farr, and A.Y. Rudensky. 2005. Developmental regulation of Foxp3 expression during ontogeny. J. Exp. Med. 202:901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gapin, L., J.L. Matsuda, C.D. Surh, and M. Kronenberg. 2001. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat. Immunol. 2:971–978. [DOI] [PubMed] [Google Scholar]
- 23.Benlagha, K., T. Kyin, A. Beavis, L. Teyton, and A. Bendelac. 2002. A thymic precursor to the NK T cell lineage. Science. 296:553–555. [DOI] [PubMed] [Google Scholar]
- 24.Pellicci, D.G., K.J. Hammond, A.P. Uldrich, A.G. Baxter, M.J. Smyth, and D.I. Godfrey. 2002. A natural killer T (NKT) cell developmental pathway involving a thymus-dependent NK1.1−CD4+ CD1d-dependent precursor stage. J. Exp. Med. 195:835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scollay, R., and D.I. Godfrey. 1995. Thymic emigration: conveyor belts or lucky dips? Immunol. Today. 16:268–273. [DOI] [PubMed] [Google Scholar]
- 26.Le Campion, A., B. Lucas, N. Dautigny, S. Leaument, F. Vasseur, and C. Penit. 2002. Quantitative and qualitative adjustment of thymic T cell production by clonal expansion of premigrant thymocytes. J. Immunol. 168:1664–1671. [DOI] [PubMed] [Google Scholar]
- 27.Ernst, B., C.D. Surh, and J. Sprent. 1995. Thymic selection and cell division. J. Exp. Med. 182:961–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penit, C., and F. Vasseur. 1997. Expansion of mature thymocyte subsets before emigration to the periphery. J. Immunol. 159:4848–4856. [PubMed] [Google Scholar]
- 29.Le Campion, A., F. Vasseur, and C. Penit. 2000. Regulation and kinetics of premigrant thymocyte expansion. Eur. J. Immunol. 30:738–746. [DOI] [PubMed] [Google Scholar]
- 30.Eck, S.C., P. Zhu, M. Pepper, S.J. Bensinger, B.D. Freedman, and T.M. Laufer. 2006. Developmental alterations in thymocyte sensitivity are actively regulated by MHC class II expression in the thymic medulla. J. Immunol. 176:2229–2237. [DOI] [PubMed] [Google Scholar]
- 31.DeVoss, J., Y. Hou, K. Johannes, W. Lu, G.I. Liou, J. Rinn, H. Chang, R. Caspi, L. Fong, and M.S. Anderson. 2006. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J. Exp. Med. 203:2727–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurobe, H., C. Liu, T. Ueno, F. Saito, I. Ohigashi, N. Seach, R. Arakaki, Y. Hayashi, T. Kitagawa, M. Lipp, et al. 2006. CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity. 24:165–177. [DOI] [PubMed] [Google Scholar]
- 33.Kotani, M., K. Seiki, A. Yamashita, and I. Horii. 1966. Lymphatic drainage of thymocytes to the circulation in the guinea pig. Blood. 27:511–520. [PubMed] [Google Scholar]
- 34.Ernstrom, U., L. Glyllensten, and B. Larsson. 1965. Venous output of lymphocytes from the thymus. Nature. 207:540–541. [DOI] [PubMed] [Google Scholar]
- 35.Kato, S. 1997. Thymic microvascular system. Microsc. Res. Tech. 38:287–299. [DOI] [PubMed] [Google Scholar]
- 36.Ushiki, T. 1986. A scanning electron-microscopic study of the rat thymus with special reference to cell types and migration of lymphocytes into the general circulation. Cell Tissue Res. 244:285–298. [DOI] [PubMed] [Google Scholar]
- 37.Kamath, A.T., S. Henri, F. Battye, D.F. Tough, and K. Shortman. 2002. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 100:1734–1741. [PubMed] [Google Scholar]
- 38.Rybak, J.N., A. Ettorre, B. Kaissling, R. Giavazzi, D. Neri, and G. Elia. 2005. In vivo protein biotinylation for identification of organ-specific antigens accessible from the vasculature. Nat. Methods. 2:291–298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Material Index]