Overexpression of Interleukin (IL)-7 Leads to IL-15–independent Generation of Memory Phenotype CD8+ T Cells (original) (raw)
Abstract
Transgenic (TG) mice expressing a high copy number of interleukin (IL)-7 cDNA under the control of the major histocomaptability complex (MHC) class II promoter display a 10–20-fold increase in total T cell numbers. Here, we show that the increase in T cell numbers in IL-7 TG mice is most apparent at the level of memory phenotype CD44hi CD122hi CD8+ cells. Based on studies with T cell receptor (TCR) TG mice crossed to IL-7 TG mice, increased levels of IL-7 may provide costimulation for TCR recognition of self-MHC ligands and thus cause naive CD8+ cells to proliferate and differentiate into memory phenotype cells. In addition, a marked increase in CD44hi CD122hi CD8+ cells was found in IL-7 TG IL-15− mice. Since these cell are rare in normal IL-15− mice, the dependency of memory phenotype CD8+ cells on IL-15 can be overcome by overexpression of IL-7.
Keywords: T lymphocytes, homeostasis, cytokines, mice, transgenic
Introduction
The overall size and composition of the mature T cell pool are closely regulated by homeostatic mechanisms (1). Recently, it has been shown that homeostasis of naive T cells is primarily controlled through TCR contact with self-MHC/peptide ligands and exposure to the cytokine IL-7 (2–5). Thus, in the absence of interaction with either of these ligands, naive T cells disappear and fail to undergo “homeostatic” proliferation in T cell–deficient conditions (2–5). Since the density of self-MHC/peptide ligands is constant, IL-7 may be the key factor in determining the overall size of the naive T cell pool. Accordingly, overproduction of IL-7 would presumably lead to a proportional enlargement of the naive T cell pool. In contrast to naive cells, homeostasis of memory T cells is known to be regulated independently of contact with MHC molecules (6, 7). In terms of cytokine requirements, none of the common γ chain (γc)* family of cytokines (IL-2, -4, -7, -9, -15) appears essential for memory CD4+ cells (8). By contrast, memory CD8+ cells are heavily dependent on IL-15 (9, 10). Thus, most memory CD8+ cells express elevated levels of the IL-15R CD122, undergo selective proliferation in response to IL-15, and are markedly depleted in IL-15– and IL-15Rα− mice (9–12).
IL-7 was initially described as a growth factor for B cell progenitors and was shown to be produced by bone marrow stromal cells (13). Subsequent studies showed that IL-7 is also synthesized by other tissues, including thymic and intestinal epithelial cells (14, 15). Furthermore, a generation of IL-7− and IL-7R− mice revealed that IL-7 has a nonredundant role in supporting early development of both B and T cells (16, 17). In addition to knockout mice, four independent lines of transgenic (TG) mice overexpressing IL-7 were generated in the early 1990s (18–21). Although most of the TG mice were found to possess increased numbers of B and T cells, a careful examination of the T cell pool was not performed.
To study the effect of IL-7 overproduction on T cell homeostasis, we analyzed a B6 TG line that expresses a high copy number of IL-7 cDNA under the control of the MHC class II promoter (21). Despite a marked (25–50-fold) elevation in IL-7 levels, these mice appear healthy and, unlike other lines (18, 20), remained free of dermatitis (21); tumor formation is low and restricted to B cells. Here, we show that the massive overproduction of T cells in IL-7 TG mice is largely skewed to memory-phenotype CD44hi CD122hi CD8+ cells. Based on the results of crossing IL-7 TG mice to TCR TG and IL-15− mice, IL-7 may play an important role in guiding both the generation and maintenance of memory CD8+ cells.
Materials and Methods
Mice.
IL-7 TG mice (21) were obtained from J. Andersson at the Basel Institute and maintained by breeding to C57BL/6 (B6) or B6.Ly 5.1+ mice. All mice used in the current study were hemizygous for the transgene. B6 and B6.PL congenic mice were purchased from The Scripps Research Institute (TSRI) breeding facility. Origins of OT-I, HY, IL-15−, and β2m−K−D− mice were described previously (3, 5).
FACS® Analysis.
Suspensions of thymus, LN, and spleen cells were double- and triple-stained as described previously (2) using various combinations of PE-anti-CD44, PE-anti-CD122, Cy5-anti-CD8, FITC-anti-CD8, Cy5-anti-CD4, Cy5-anti-Ly 5.1, and biotinylated T3.70 (all from eBioscience). Biotinylated anti–Thy-1.1, anti-γc and FITC-Vβ5, and PE-Vα2 antibodies were purchased from BD PharMingen. Biotinylated mAbs were detected with Cy5-conjugated streptavidin (Jackson ImmunoResearch Laboratories).
For intracellular cytokine staining, spleen cells (4 × 106 cells per milliliter) in complete medium containing 0.67 μls/ml Brefeldin A (GolgiStop; BD PharMingen) were incubated for 7 h in 24-well plates coated with anti-CD3 (2C11; eBioscience) mAb (0.5 μg/ml) and stained as described previously (22). Briefly, cells were first stained with Cy5-anti-CD8 and PE-anti-CD44, fixed with paraformaldehyde followed by permeabilization with saponin and stained with FITC-anti–IFN-γ mAb (eBioscience).
To assess homeostatic proliferation, small numbers (106 cells per mouse) of FACS®-sorted CD44hi CD8+ cells were labeled with CFSE (Molecular Probes) and injected into mice exposed to 600 cGy irradiation and analyzed 7 d later as described previously (2).
The rate of background T cell turnover in adult thymectomized mice was performed by adding BrdU (0.8 mg/ml) into the drinking water for 7 d and staining LN cells as described previously (23).
Effect of cytokines in vitro was assessed by culturing CD4+ and CD8+ cells (2 × 106 cells per milliliter), purified as described previously (2), for 5 d in complete medium containing IL-7 and/or IL-15 at 20 ng/ml. Some cultures contained mAbs to IL-7R (A7R34) (24) and IL-2Rβ (TM-β1) (25) at 50 μg/ml. The in vivo effect of blocking IL-7R was measured by injecting mice intraperitoneally with either 200 μg of salt-precipitated A7R34 ascites or 200 μg of rat IgG every other day over a 7-d period. In vitro–cultured cells and LN and spleen cells from antibody-injected mice were analyzed by triple staining with FITC-anti-CD4, Cy5-anti-CD8 and PE-anti-CD44, or PE-anti-CD122 mAbs.
CDR3 Lengths Analysis of TCR Chains.
Spectratyping of TCR-Vα and -Vβ repertoire was performed on purified CD4+ and CD8+ cells as described previously (26). Briefly, after cDNA synthesis from total RNA, the RNA was denatured and reverse transcribed. Small aliquots of the cDNA were amplified by PCR using primers previously described to analyze the repertoire of Vα and Vβ chains (27). The PCR product was then subjected to run-off reaction with fluorescent-tagged primers and analyzed by capillary electrophoresis in an automated DNA sequencer (Applied Biosystems) and the distribution of CDR3 lengths determined.
Results and Discussion
Characterization of T Cells in IL-7 TG Mice.
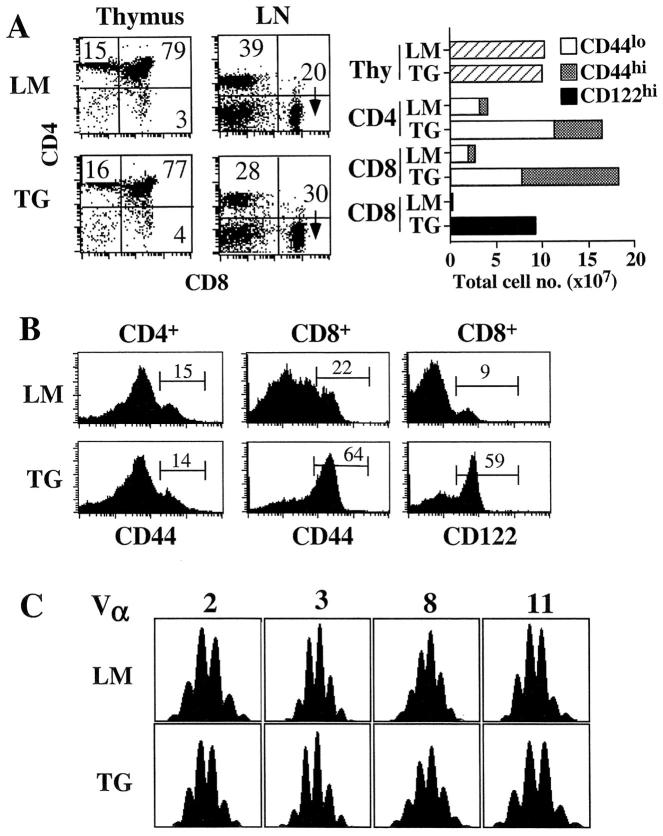
As reported previously (21), young adult IL-7 TG mice were found to contain massive numbers of T (and B) cells (10–20 times above normal) in the secondary lymphoid tissues, despite the presence of a relatively normal sized thymus (Fig. 1 A). The increase in peripheral T cell numbers was more pronounced for CD8+ cells than CD4+ cells and resulted in a reduction in the CD4/CD8 ratio. The increase in total T cell numbers and the preferential effect of IL-7 on CD8+ cells are likely to be a consequence of IL-7 acting directly on mature T cells as similar effects were observed in normal mice given repeated injection of recombinant IL-7 (28–30). Strikingly, the majority (55–70%) of CD8+ cells in IL-7 TG mice had a memory (CD44hi and CD122hi) phenotype, whereas most CD4+ cells had a naive (CD44lo) phenotype (Fig. 1 B). For CD8+ cells, the increase in total cell numbers was ∼50-fold for CD122hi cells and ∼25-fold for CD44hi cells but only ∼4-fold for naive CD44lo cells. For CD4+ cells, CD44hi cells were elevated by ∼5-fold and CD44lo cells by ∼4-fold (Fig. 1 A). Expression of other markers, such as CD25, CD62L, CD69, IL-7Rα, on TG T cells was comparable to wild-type T cells (data not shown).
Figure 1.
Characterization of T cells in young adult IL-7 TG mice. (A) T cell subset distribution and cell numbers in the thymus, LN, and spleen in IL-7 TG and LMs. Cells were triple-stained for CD4, CD8, CD44, or CD122 and analyzed by flow cytometry as described in Materials and Methods. Total numbers of thymocytes and indicated naive and memory phenotype CD4+ and CD8+ cells, combined from spleen and pooled LNs, IL-7 TG, and LMs are shown. Data are representative of >20 TG and LM mice. (B) Expression of CD44 and CD122 on gated LN CD4+ and CD8+ cells from IL-7 TG and LMs. (C) Randomly distributed CDR3 lengths of TCR-Vα chains expressed on CD8+ cells from IL-7 TG mice. CDR3 lengths on most Vα and all Vβ chains were determined on purified CD8+ cells from IL-7 TG and LM mice as described in Materials and Methods. Representative data for indicated Vα chains are shown; all analyzed chains were randomly distributed in both TG and LM cells.
To determine whether the enlargement of the mature T cell pool is due to an oligoclonal expansion, the TCR repertoire of purified IL-7 TG CD4+ and CD8+ cells was examined. A previous study did not reveal any significant skewing of the TCR-Vβ repertoire (21), but it was still possible that an oligoclonal expansion could be revealed from analysis of the CDR3 lengths. Examination of all Vβ chains and the majority of Vα chains revealed that the CDR3 lengths were randomly distributed for all chains similar to T cells from littermates (LMs) (Fig. 1 C).
The experiments discussed below address the issue of why IL-7 TG mice show a preferential elevation of memory phenotype CD8+ cells. Why these mice also show a milder increase in naive phenotype cells, despite having a normal thymus, will be the subject of another paper.
Functional Capacity of IL-7 TG CD44hi CD8+ Cells.
By two different parameters, TG CD44hi CD8+ cells closely resembled CD44hi CD8+ cells from wild-type mice. First, as measured by BrdU incorporation in thymectomized mice, the turnover of TG CD44hi CD8+ (and CD44hi CD4+) cells was relatively slow, in fact slower than in LMs; this finding applied both in adult (8-wk) mice (Fig. 2 A) and also in neonatal (3-wk) mice (data not shown). Hence the increase in CD44hi CD8+ cells in IL-7 TG mice did not reflect an increase in their rate of division. However, total numbers of proliferating T cells in IL-7 TG mice were 7–10-fold higher than in wild-type mice. Second, as measured by division of CFSE-labeled cells, sorted TG CD44hi CD8+ cells transferred to irradiated B6 hosts behaved similarly to normal B6 CD44hi CD8+ cells in their rapid rate of homeostatic proliferation in these T cell–depleted hosts (Fig. 2 B). Moreover, like normal CD44hi CD8+ cells (Fig. 2 C), but unlike naive CD44lo CD8+ cells (6), IL-7 TG CD44hi CD8+ cells underwent efficient homeostatic proliferation in MHC class I− (β2m−D−K−) mice (Fig. 2 C).
Figure 2.
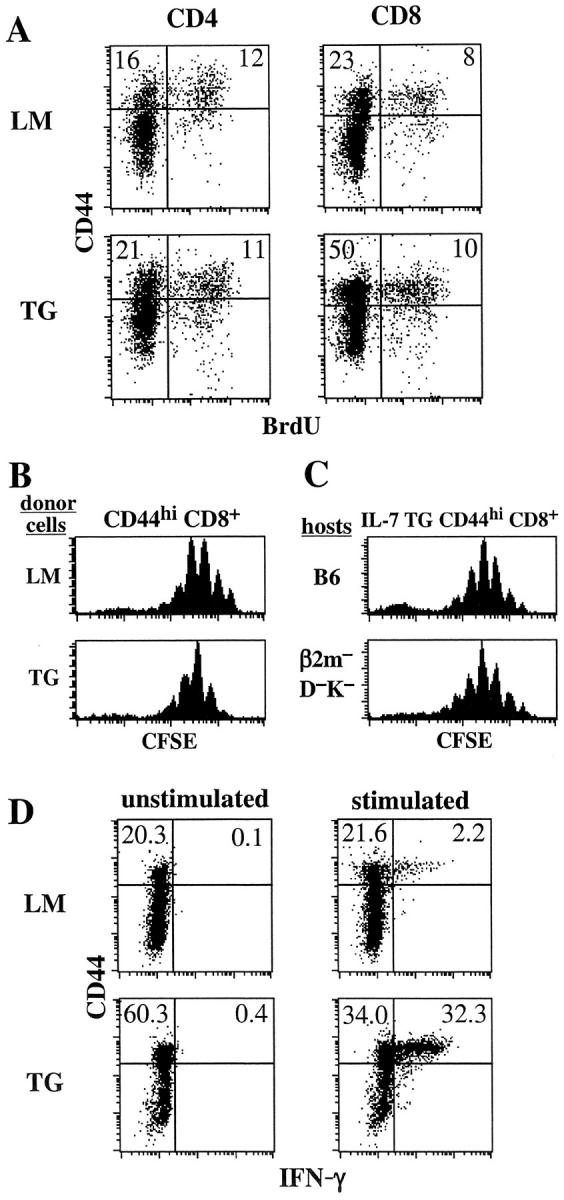
CD44hi CD8+ cells from IL-7 TG mice functionally resemble CD44hi CD8+ cells from wild-type mice. (A) Background turnover over of T cells IL-7 TG mice. Adult thymectomized IL-7 TG and LMs were given BrdU in the drinking water for 7 d and LN cells were triple stained for BrdU, CD44, and either CD4 or CD8 as described in Materials and Methods. (B) IL-7 TG CD44hi CD8+ cells undergo the same rate of homeostatic proliferation in T cell–depleted hosts as wild-type CD44hi CD8+ cells. CD44hi CD8+ cells from Ly 5.1+ IL-7 TG and LM mice purified by cell sorting were CFSE labeled and injected into irradiated (600 cGy) B6 mice. 7 d after transfer donor cells in host LN were analyzed for CFSE expression by staining for Ly5.1+ and CD8+. Shown are gated donor LMs and TG CD8+ cells. (C) Homeostatic proliferation of IL-7 TG CD44hi CD8+ cells is MHC independent. Sorted and CFSE-labeled CD44hi CD8+ cells from Ly 5.1+ IL-7 TG mice were injected into irradiated B6 and MHC class I− (β2m_–_D−K−) mice and analyzed 7 d later as in B. (D) IL-7 TG CD44hi CD8+ cells synthesize IFN-γ upon activation. Spleen cells from IL-7 TG and LMs were stimulated with anti-CD3 mAb in vitro for 7 h in the presence of Brefeldin A and stained for CD8, CD44, and intracellular IFN-γ as described in Materials and Methods. Shown are gated CD8+ cells. All data are representative of results from three experiments.
By the above parameters, TG CD44hi CD8+ cells closely resembled normal B6 CD44hi CD8+ cells. However, different results were obtained for IFN-γ synthesis. Thus, after in vitro stimulation with anti-CD3 mAb, the proportion of IFN-γ synthesizing cells was much higher for TG CD44hi CD8+ cells (49%) than for normal B6 CD44hi CD8+ cells (9%) (Fig. 3 D). Since IL-7 has costimulatory activity for T cell stimulation and function (30, 31), this difference could be a reflection of prior exposure of the TG cells to high levels of IL-7 in vivo.
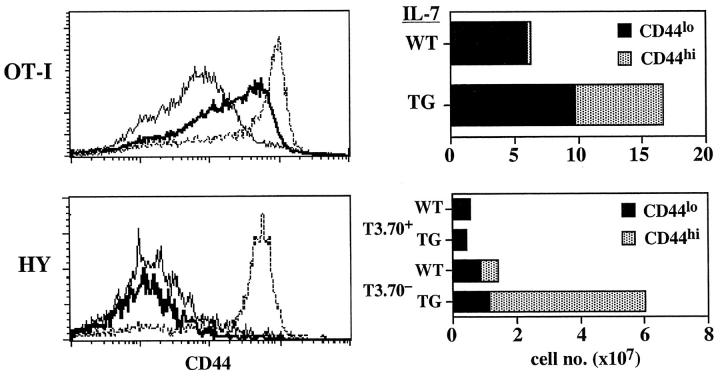
Figure 3.
Differential effect of IL-7 overproduction on OT-I and HY TCR TG CD8+ cells. OT-I and HY TCR TG mice were bred to express the IL-7 transgene; LN and spleen cells from 4-wk-old mice double TG mice were stained for CD8, Vα2, and CD44 for OT-I mice and CD8, T3.70, and CD44 for HY female mice. Shown on the left side for OT-I cells are the profiles of CD44 on gated CD8+ Vα2+ cells from normal OT-I TG mice (thin line) and IL-7 TG OT-I mice (thick line); CD44 levels on polyclonal CD8+ cells (broken line) from IL-7 TG mice are shown for comparison. For HY cells, shown are CD44 levels on gated T3.70+ cells from normal HY TG mice (thin line), IL-7 TG HY mice (thick line) and T3.70− cells from IL-7 TG HY mice (broken line). The total numbers (pooled from LN and spleen) of recovered CD44lo and CD44hi populations of CD8+ cells from the OT-I and HY mice are shown on the right side. Similar results were found when the cells were analyzed in terms of CD122 expression (not shown). Data are representative of results from four to six individuals for each type of mice.
Generation of TCR TG CD44hi CD8+ Cells in IL-7 TG Mice.
T cells with a memory phenotype usually originate from naive T cells responding to foreign antigen, but can also arise from naive T cells undergoing homeostatic proliferation to self-ligands (32). To examine which ligands, self or foreign, cause production of memory phenotype CD8+ cells in IL-7 TG mice, we crossed two TCR TG lines, OT-I and HY, to IL-7 TG mice.
For the OT-I line, nearly all (∼95%) of the CD8+ cells in this line are clonotype-positive (Vα2+ Vβ5+) cells and have a naive CD44lo phenotype (data not shown). These naive cells appear to have significant affinity for self-MHC/peptide ligands because exposure to these ligands after transfer to T cell–depleted hosts causes strong homeostatic proliferation of the cells and a switch to a memory phenotype (3). When OT-I mice were crossed to IL-7 TG mice, thus producing OT-I IL-7 TG mice, total numbers of CD8+ cells in these mice were increased about threefold relative to OT-I LMs that lacked the IL-7 transgene (Fig. 3). Significantly, numbers of CD44hi CD8+ cells were ∼20-fold higher in OT-I IL-7 TG mice than in normal OT-I LMs. In addition, in contrast to OT-I LMs, many of the “CD44lo” cells in OT-I IL-7 TG mice were actually CD44int rather than CD44lo (Fig. 3). This finding is of interest because OT-I cells show a similar CD44int/hi phenotype after homeostatic proliferation in T cell–depleted hosts (3). Since nearly all (∼95%) of the CD8+ cells in OT-I IL-7 TG mice were Vα2+ Vβ5+, therefore, it would seem highly likely that the stimulus for expansion of CD44int/hi cells in these mice was provided by self-MHC/peptide ligands rather than foreign antigens. Thus, the implication is that high levels of IL-7 in OT-I IL-7 TG mice act as a costimulator to drive naive OT-I cells to undergo proliferation and differentiation in response to self-MHC/peptide ligands. Despite the high penetrance of the OT-I transgene, however, one caveat is that the IL-7 TG OT-I mice were not in a background (recombination activating gene [RAG]− or TCR-α−) that precludes expression of endogenous TCR chains. Therefore, the contribution of foreign antigens in upregulation of CD44 cannot be totally excluded.
If self-MHC/peptide ligands are driving production of CD44hi CD8+ cells in IL-7 TG mice, the capacity of high levels of IL-7 to augment T cell reactivity to self-MHC/peptide ligands might not apply to T cells with “below average” affinity to self-ligands. To assess this possibility we examined the HY TCR TG line. As shown previously, the TCR clonotype-positive cells in this line (detected by T3.70 mAb) fail to undergo homeostatic proliferation in T cell–depleted hosts (2), presumably because the TCR affinity of T3.70+ cells for self-ligands is quite low. HY T cells do undergo homeostatic proliferation but this applies only to the T3.70− subset (2). As shown in Fig. 3, HY IL-7 TG mice showed a marked expansion of CD44hi CD8+ cells. Significantly, almost all of these cells were T3.70−; there was no expansion of T3.70+ cells and these cells retained a naive CD44lo phenotype. Thus, expansion of T3.70− but not T3.70+ cells in HY IL-7 TG mice correlated closely with the capacity of T3.70− but not T3.70+ cells to undergo homeostatic proliferation in T cell–depleted hosts.
Collectively, these data on OT-I and HY mice suggest that a significant proportion of the expanded CD44hi CD8+ cells in IL-7 TG mice are derived from precursor cells stimulated by self-MHC/peptide ligands. This notion is consistent with the finding that the TCR repertoire of TG CD8+ cells appears very diverse (Fig. 1 C). In addition, however, a proportion of the expanded CD44hi CD8+ cells may arise through the action of IL-7 on naive CD8+ cells responding to foreign antigens. In this respect, we have observed that IL-7 has marked costimulatory activity for T cells responding to foreign antigens in vivo. Thus, injection of male splenic APC into IL-7 TG HY mice led to much greater expansion of T3.70+ cells followed by establishment of a larger pool of T3.70+ memory CD44hi CD8+ cells than was observed in HY mice lacking IL-7 transgene (data not shown). Although the relative contribution made by foreign versus self-antigens in the production of CD44hi CD8+ cells in IL-7 TG mice is unknown it is notable that the marked increase in CD44hi CD8+ cell numbers in these mice is apparent even in neonatal (1.5 wk) mice (data not shown). With high IL-7 levels, CD44hi CD8+ cells arise very early in life and accumulate progressively.
IL-15–independent Generation of CD44hi CD8+ Cells in IL-7 TG Mice.
As mentioned earlier (see Introduction), survival of most CD44hi CD8+ cells in T cell–sufficient mice has recently been shown to be heavily dependent on IL-15 (9–11). IL-15 dependency is particularly pronounced for CD122hi CD44hi CD8+ cells, as these cells are conspicuously absent in IL-15− mice (unpublished data). To determine whether the generation and/or the maintenance of CD44hi CD8+ cells in IL-7 TG mice is IL-15 dependent, IL-7 TG mice were crossed with IL-15− mice. Surprisingly, comparison of 4-wk-old IL-15− IL-7 TG mice with age-matched control IL-15+ IL-7 TG LMs revealed that IL-15 is dispensable for generation of CD122hi CD44hi CD8+ cells in IL-7 TG mice. Thus, in terms of both total cellularity and phenotype, including both CD44 and CD122 expression, the peripheral CD8+ cell compartment was comparable in IL-15+ IL-7 TG versus IL-15− IL-7 TG mice (Fig. 4) . The proportions of CD122hi CD8+ and CD44hi CD81 cells in cell cycle, as determined by BrdU labeling, were also comparable between IL-15+ versus IL-15_−_ IL-7 TG mice (data not shown). It should be mentioned that IL-15 deficiency was accompanied by a slight reduction in the total number of CD8+ cells and a minor increase in the total number of CD4+ cells (Fig. 4). The point to emphasize, however, is that numbers of IL-15 responsive CD122hi CD8+ cells rose from nearly undetectable levels in IL-15− mice to very high levels in IL-7 TG IL-15− mice (Fig. 4).
Figure 4.
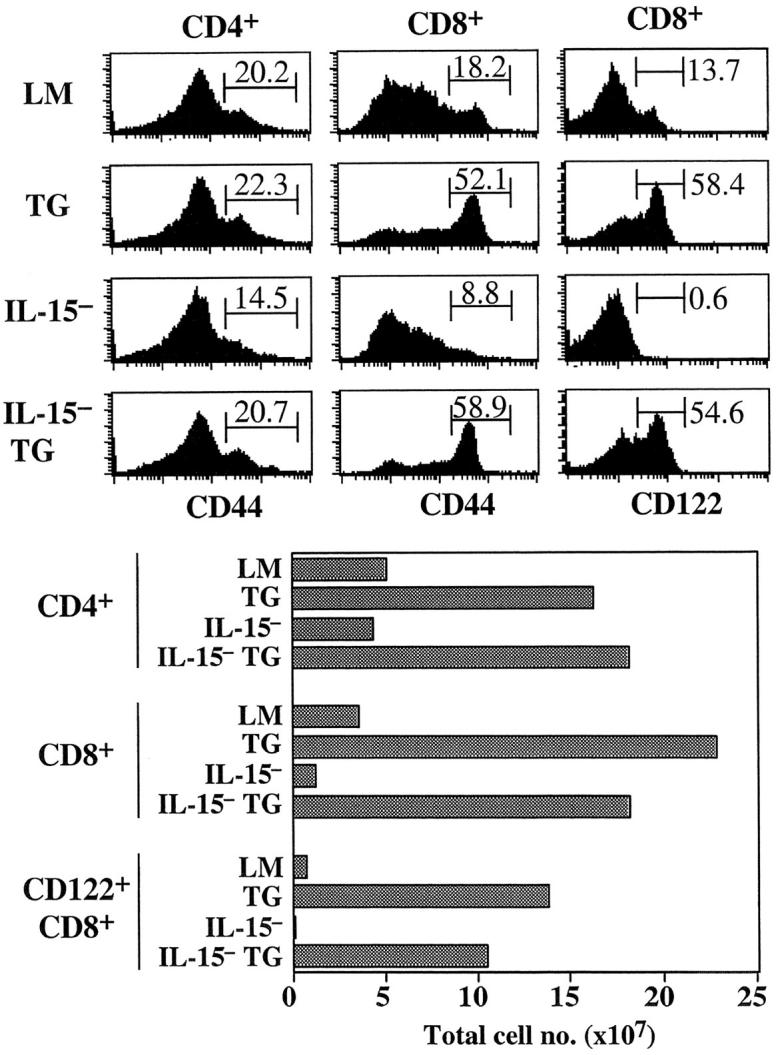
IL-15 is not required for generation of CD44hi CD8+ cells in IL-7 TG mice. LN cells from young adult IL-7 TG mice bred to an IL-15− background were stained for CD4, CD8, and CD44 or CD122 and analyzed. Shown are histograms of CD44 and CD122 on gated CD4+ and CD8+ cells as compared with the same cells from age-matched control LMs, IL-7 TG, and IL-15− mice. Total numbers of CD4+, CD8+, and CD122hi CD8+ cells from spleen and LNs from the mice are shown below. Data are representative of results from six to eight individuals for each type of mice.
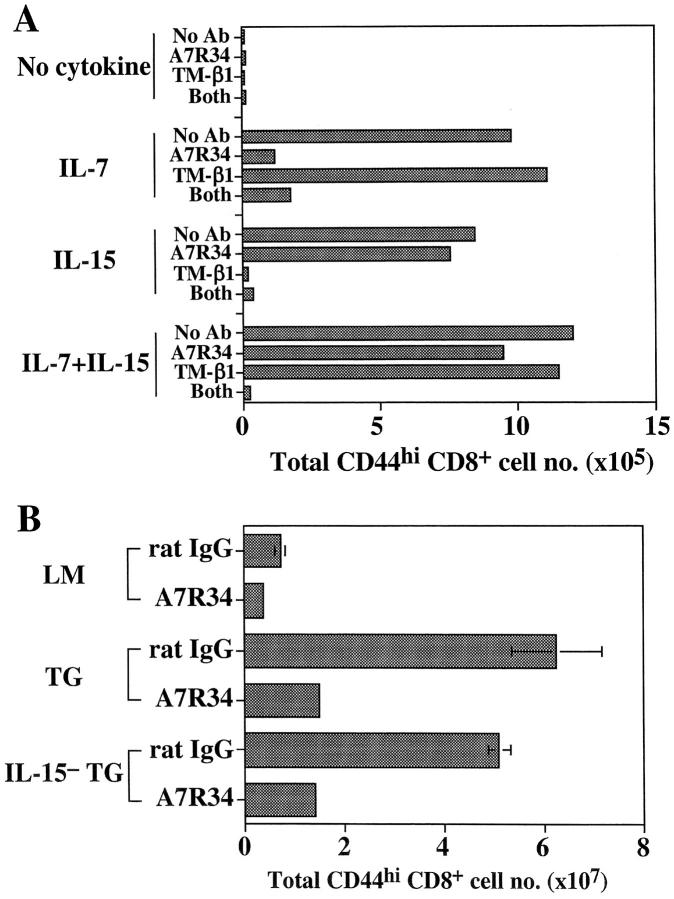
Since nonTG IL-15− mice are virtually devoid of CD122hi CD8+ cells (reference 11 and Fig. 4), the above finding with IL-7 TG IL-15− mice indicates that high concentrations of IL-7 can compensate for absence of IL-15 in promoting survival of CD44hi CD122hi CD8+ cells. In support of this idea, a submitogenic dose (20 ng/ml) of either IL-7 or IL-15 maintained viability of CD44hi CD8+ cells in vitro for 5 d, whereas these cells died in the absence of cytokines (Fig. 5 A). This finding applied equally to T cells purified from IL-7 TG or wild-type mice. Whereas IL-7 was also able to enhance survival of other T cell subsets, the antiapoptotic function of IL-15 was largely restricted to CD44hi CD8+ cells, presumably because the IL-15 receptor, CD122, is expressed at a much higher level on CD44hi CD8+ cells than on other T cell subsets (9).
Figure 5.
Role of IL-7 in supporting survival of CD44hi CD8+ cells in vitro and in vivo in IL-7 TG mice. (A) IL-7 can maintain survival of CD44hi CD8+ cells in vitro. Aliquots of purified IL-7 TG LN T cells were incubated with the indicated cytokines at (20 ng/ml) either alone or in the presence of anti–IL-7Rα mAb (A7R34), anti-CD122 mAb (TM-β1), or both mAbs at 50 μg/ml. The cells were harvested 5 d later and stained for CD4, CD8, and CD44, and the numbers of viable CD44hi CD8+ cells were calculated. (B) Blocking the IL-7R leads to depletion of CD44hi CD8+ cells in IL-7 TG mice. Groups of two to three LMs, IL-7 TG, and IL-15− IL-7 TG mice were injected three times with either 200 μg of anti–IL-7Rα mAb (A7R34) or rat IgG at 2-d intervals. 2 d after the last injection, LN and spleen cells were collected and stained for CD4, CD8, and CD44. The total numbers of CD44hi CD8+ cells recovered from spleens and pooled LN are shown. Another experiment showed similar results.
Although IL-7 is presumed to mediate its effect through the IL-7R, it is conceivable that the high levels of IL-7 available in IL-7 TG mice might act through weak cross-reactive binding to the IL-15R. This possibility seems unlikely for two reasons. First, the capacity of IL-7 to rescue CD44hi CD8+ cells from spontaneous death in vitro was strongly inhibited by anti–IL-7Rα mAb, and the presence of anti-CD122 mAb did not diminish the effect of IL-7 (Fig. 5 A). This finding also applied when the concentration of IL-7 was raised to a high level (data not shown). Second, injecting either IL-7 TG or IL-15− IL-7 TG mice for 1 wk with anti–IL-7Rα mAb (200 μg every other day) caused a marked reduction in total numbers of CD44hi CD8+ cells in the mice (Fig. 5 B). A partial reduction in CD44hi CD8+ cells numbers was also observed in control LM mice (Fig. 5 B). The reduction in cell numbers in these mice is unlikely to be due to antibody-mediated opsonization as depletion of other IL-7Rhi cells, e.g., CD44hi CD4+ cells and mature B cells, was minimal (data not shown).
Our observation that the elevation of T cell numbers in IL-7 TG mice is skewed toward CD8+ cells is consistent with prior evidence on the effects of short-term injection of IL-7 into normal mice (28–30). Although these latter data indicated that IL-7 acted largely on peripheral T cells (rather than thymocytes), the issue of whether IL-7 stimulated naive T cells or memory T cells or both subsets was not resolved. The data in this paper suggest that, in IL-7 TG mice, elevated levels of IL-7 boost homeostasis of memory CD8+ cells through two different mechanisms.
First, the data on IL-7 TG TCR TG mice suggest that high levels of IL-7 amplify TCR recognition of self-MHC ligands by naive CD8+ cells. For these cells, TCR recognition of self-ligands in the presence of background levels of IL-7 normally induces a covert signal that is sufficient to keep naive CD8+ cells alive, but without inducing proliferation or a change in surface phenotype. With high levels of IL-7, however, TCR signaling is enhanced and contact with self-ligands causes naive CD8+ cells to proliferate and differentiate into memory phenotype cells.
Second, the high levels of IL-7 contribute to survival and turnover of established memory phenotype CD8+ cells. As discussed earlier, the background turnover and survival of CD44hi CD8+ cells is normally controlled by IL-15; IL-7 is not important, probably because levels of IL-7 in the peripheral lymphoid tissues of normal mice are too low to play a significant role in homeostasis. However, in IL-7 TG mice one can envisage that IL-7 and IL-15 both contribute to homeostasis. The presence of large numbers of CD44hi CD122hi CD8+ cells in IL-15_−_IL-7 TG mice but not normal IL-15− mice is then readily explained. Here, high levels of IL-7 compensate for the lack of IL-15 and homeostasis of CD44hi CD8+ cells remains normal. The finding that the total numbers of CD44hi CD8+ cells in IL-15− IL-7 TG mice dropped sharply after treatment with anti–IL-7R mAb is consistent with this idea. However, the finding that anti–IL-7R mAb treatment had a similar effect on IL-15+ IL-7 TG mice is surprising because here we expected the cells to be rescued by IL-15. It is conceivable, however, that the massive overproduction of CD122hi CD8+ cells in IL-7 TG mice depletes IL-15, thus causing the cells to be heavily dependent on IL-7.
In addition to memory phenotype CD8+ cells, IL-7 TG mice clearly contain increased numbers of naive T cells, including both CD4+ and CD8+ cells. Likewise, naive T cell increase in number after injection of IL-7 (28–30). How IL-7 leads to expansion of naive T cells is unknown, although increases in cell survival, proliferation, and release of new T cells from the thymus are all likely possibilities. Resolving this important issue will have to await further investigation.
Acknowledgments
We are grateful to Drs. P. Marrack and J. Andersson for sending A7R34 hybridomas and the IL-7 TG mice, respectively. We also thank J. Kuhns, M. Chan, and D. Kim for various supports. This is publication number 14506-IMM from TSRI.
This work was supported by U.S. Public Health Service grants AI21487, CA38355, and AI46710 (to J. Sprent) and AI41079, AI45809, and AG20186 (to C.D. Surh). J.T. Tan and W.C. Kieper are supported by U.S. Public Health Service Institute National Research Service Award HL07196 and AI07244, respectively. C.D. Surh is a Scholar of the Leukemia and Lymphoma Society.
Footnotes
*
Abbreviations used in this paper: γc, common γ chain; LM, littermate; TG, transgenic.
References
- 1.Goldrath, A.W., and M.J. Bevan. 1999. Selecting and maintaining a diverse T-cell repertoire. Nature. 402:255–262. [DOI] [PubMed] [Google Scholar]
- 2.Ernst, B., D.-S. Lee, J.M. Chang, J. Sprent, and C.D. Surh. 1999. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 11:173–181. [DOI] [PubMed] [Google Scholar]
- 3.Goldrath, A.W., and M.J. Bevan. 1999. Antagonist ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 11:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schluns, K.S., W.C. Kieper, S.C. Jameson, and L. Lefrancois. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1:426–432. [DOI] [PubMed] [Google Scholar]
- 5.Tan, J.T., E. Dudl, E. LeRoy, R. Murray, J. Sprent, K.I. Weinberg, and C.D. Surh. 2001. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc. Natl. Acad. Sci. USA. 98:8732–8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murali-Krishna, K., L.L. Lau, S. Sambhara, F. Lemonnier, J. Altman, and R. Ahmed. 1999. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 286:1377–1381. [DOI] [PubMed] [Google Scholar]
- 7.Swain, S.L., H. Hu, and G. Huston. 1999. Class II-independent generation of CD4 memory T cells from effectors. Science. 286:1381–1383. [DOI] [PubMed] [Google Scholar]
- 8.Lantz, O., I. Grandjean, P. Matzinger, and J.P. Di Santo. 2000. γ chain required for naive CD4+ T cell survival but not for antigen proliferation. Nat. Immunol. 1:54–58. [DOI] [PubMed] [Google Scholar]
- 9.Zhang, X., S. Sun, I. Hwang, D.F. Tough, and J. Sprent. 1998. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 8:591–599. [DOI] [PubMed] [Google Scholar]
- 10.Ku, C.C., M. Murakami, A. Sakamoto, J. Kappler, and P. Marrack. 2000. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 288:675–678. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy, M.K., M. Glaccum, S.N. Brown, E.A. Butz, J.L. Viney, M. Embers, N. Matsuki, K. Charrier, L. Sedger, C.R. Willis, et al. 2000. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191:771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lodolce, J.P., D.L. Boone, S. Chai, R.E. Swain, T. Dassopoulos, S. Trettin, and A. Ma. 1998. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 9:669–676. [DOI] [PubMed] [Google Scholar]
- 13.Namen, A.E., S. Lupton, K. Hjerrild, J. Wignall, D.Y. Mochizuki, A. Schmierer, B. Mosley, C.J. March, D. Urdal, and S. Gillis. 1988. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 333:571–573. [DOI] [PubMed] [Google Scholar]
- 14.Gutierrez, J.C., and R. Palacios. 1991. Heterogeneity of thymic epithelial cells in promoting T-lymphocyte differentiation in vivo. Proc. Natl. Acad. Sci. USA. 88:642–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe, M., Y. Ueno, T. Yajima, Y. Iwao, M. Tsuchiya, H. Ishikawa, S. Aiso, T. Hibi, and H. Ishii. 1995. Interleukin 7 is produced by human intestinal epithelial cells and regulates the proliferation of intestinal mucosal lymphocytes. J. Clin. Invest. 95:2945–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Freeden-Jeffry, U., P. Vieira, L.A. Lucian, T. McNeil, S.E.G. Burdach, and R. Murray. 1995. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 181:1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peschon, J.J., P.J. Morrissey, K.H. Grabstein, F.J. Ramsdell, E. Maraskovsky, B.C. Gliniak, L.S. Park, S.F. Ziegler, D.E. Williams, C.B. Ware, et al. 1994. Early lymphocyte expansion is severly impaired in interleukin 7 receptor-deficient mice. J. Exp. Med. 180:1955–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uehira, M., H. Matsuda, I. Hikita, T. Sakata, H. Fujiwara, and H. Nishimoto. 1993. The development of dermatitis infiltrated by γδ T cells in IL-7 transgenic mice. Int. Immunol. 5:1619–1627. [DOI] [PubMed] [Google Scholar]
- 19.Samaridis, J., G. Casorati, A. Traunecker, A. Iglesias, J.C. Gutierrez, U. Muller, and R. Palacios. 1991. Development of lymphocytes in interleukin 7-transgenic mice. Eur. J. Immunol. 21:453–460. [DOI] [PubMed] [Google Scholar]
- 20.Rich, B.E., J. Campos-Torres, R.I. Tepper, R.W. Moreadith, and P. Leder. 1993. Cutaneous lymphoproliferation and lymphomas in interleukin 7 transgenic mice. J. Exp. Med. 177:305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mertsching, E., C. Burdet, and R. Ceredig. 1995. IL-7 transgenic mice: analysis of the role of IL-7 in the differentiation of thymocytes in vivo and in vitro. Int. Immunol. 7:401–414. [DOI] [PubMed] [Google Scholar]
- 22.Prussin, C. 1997. Cytokine flow cytometry: understanding cytokine biology at the single-cell level. J. Clin. Immunol. 17:195–204. [DOI] [PubMed] [Google Scholar]
- 23.Tough, D.F., and J. Sprent. 1994. Turnover of naive- and memory-phenotype T cells. J. Exp. Med. 179:1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sudo, T., S. Nishikawa, N. Ohno, N. Akiyama, M. Tamakoshi, and H. Yoshida. 1993. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc. Natl. Acad. Sci. USA. 90:9125–9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka, T., M. Tsudo, H. Karasuyama, F. Kitamura, T. Kono, M. Hatakeyama, T. Taniguchi, and M. Miyasaka. 1991. A novel monoclonal antibody against murine IL-2 receptor β-chain. Characterization of receptor expression in normal lymphoid cells and EL-4 cells. J. Immunol. 147:2222–2228. [PubMed] [Google Scholar]
- 26.Gapin, L., J.L. Matsuda, C.D. Surh, and M. Kronenberg. 2001. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat. Immunol. 2:971–978. [DOI] [PubMed] [Google Scholar]
- 27.Casanova, J.L., P. Romero, C. Widmann, P. Kourilsky, and J.L. Maryanski. 1991. T cell receptor genes in a series of class I major histocompatibility complex-restricted cytotoxic T lymphocyte clones specific for a Plasmodium berghei nonapeptide: implications for T cell allelic exclusion and antigen-specific repertoire. J. Exp. Med. 174:1371–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrissey, P.J., P. Conlon, K. Charrier, S. Braddy, A. Alpert, D. Williams, A.E. Namen, and D. Mochizuki. 1991. Administration of IL-7 to normal mice stimulates B-lymphopoiesis and peripheral lymphadenopathy. J. Immunol. 147:561–568. [PubMed] [Google Scholar]
- 29.Komschlies, K.L., T.A. Gregorio, M.E. Gruys, T.C. Back, C.R. Faltynek, and R.H. Wiltrout. 1994. Administration of recombinant human IL-7 to mice alters the composition of B-lineage cells and T cell subsets, enhances T cell function, and induces regression of established metastases. J. Immunol. 152:5776–5784. [PubMed] [Google Scholar]
- 30.Geiselhart, L.A., C.A. Humphries, T.A. Gregorio, S. Mou, J. Subleski, and K.L. Komschlies. 2001. IL-7 administration alters the CD4:CD8 ratio, increases T cell numbers, and increases T cell function in the absence of activation. J. Immunol. 166:3019–3027. [DOI] [PubMed] [Google Scholar]
- 31.Morrissey, P.J., R.G. Goodwin, R.P. Nordan, D. Anderson, K.H. Grabstein, D. Cosman, J. Sims, S. Lupton, B. Acres, and S.G. Reed. 1989. Recombinant interleukin 7, pre-B cell growth factor, has costimulatory activity on purified mature T cells. J. Exp. Med. 169:707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Surh, C.D., and J. Sprent. 2000. Homeostatic T cell proliferation. How far can T cells be activated to self-ligands? J. Exp. Med. 192:F9–F14. [DOI] [PMC free article] [PubMed] [Google Scholar]